Simple Summary
Several literature reviews of arboviruses in Nigeria have demonstrated a high prevalence of chikungunya and dengue antibodies in individuals with either febrile or non-febrile illness. The current study reveals a substantial burden of undetected arboviral cocirculating antibodies of these mosquito-borne diseases in the country. This underscores the importance of incorporating multiplex diagnostic testing and surveillance efforts to accurately determine the full extent of the arbovirus disease burden in Nigeria and Sub-Saharan Africa.
Abstract
Chikungunya and dengue are arboviral diseases transmitted by mosquitoes that have been increasingly recognized as public health concerns in Sub-Saharan Africa. Several studies conducted in Nigeria and other West African countries have revealed the seroprevalence burden and cocirculation of antibodies against mosquito-borne infections, thereby revealing a significant burden and clinical outcome complexities that have largely gone undetected. The current research study has important implications for disease surveillance, prevention strategies, and healthcare planning in Nigeria and other Sub-Saharan countries. A cross-sectional study was conducted on 871 outpatients and pregnant women from three regions of Nigeria. CHIKV and DENV immunoblot molecular diagnostic assays were used to analyze the serum samples for the presence of arboviral antibody serological markers IgG (Mikrogen Diagnostik, Germany) with DENV nonstructural protein 1 and DENV Equad and CHIKV virus-like particles (VLPs), according to the manufacturer’s instructions. A total of 871 participants were recruited from three geographical regions in Nigeria. Among them, 17.5% (152/871) were from Abia (southern Nigeria), 34.4% (300/871) were from Kaduna (northern Nigeria), and 48.1% (419/871) were from Nasarawa (central Nigeria). The ages of the participants ranged from 0 months to 80 years, with a mean age of 36.6 years. Of the 871 subjects, 71.0% (619/871) were female, and 29.0% (252/871) were male. The overall cohort detectable antibody seropositivity against CHIKV was 64.9% (565/871), 95% CI (61.74–68.06); DENV, 44.7% (389/871), 95% CI (41.41–47.99); and CHIKV-DENV cocirculation antibodies, 31.6% (95% CI 29–35). This study highlighted the unpredictably high seroprevalence, expansion, magnitude, and undetected burden of chikungunya and dengue in Nigeria.
1. Background
Dengue and chikungunya fever are arboviral infections that are endemic in the tropical and subtropical regions of Nigeria and other Sub-Saharan states [1,2,3,4,5,6,7,8]. Global and local outbreaks of these infections are driven by multiple factors, such as urbanization, increased travel and commerce, climate change, and economic development.
These infections are transmitted by a common mosquito vector and cocirculate in many geographical regions of Nigeria [5]. Dengue virus belongs to the family Flaviviridae and the genus Flavivirus. It is a small, enveloped RNA virus with a single-stranded positive-sense RNA genome of 10.6 kb. There are four strains of dengue virus (DENV 1–4) that offer only transient protection to each other [6,7,8,9,10]. There are four to five genotypes within each serotype of the dengue virus. Chikungunya virus (CHIKV) is a member of the Togaviridae family and belongs to the genus Alphavirus. It has a linear positive-sense RNA genome of 1.8 Kb length [9,11,12,13,14,15]. There are three genotypes of CHIKV: Eastern, Central South African [ECSA], West African, and Asian [6,10]. These two viral infections are diagnosed by virus isolation, genome detection (RT–PCR), and antibody detection (IgM or IgG ELISA) [6,16,17,18]. DENV viral infection can also be detected by antigen detection nonstructural protein (NS1) ELISA. Cross-reactivity and prolonged detection of virus-specific antibodies complicate the interpretation of serological test results, and their utility depends on the patient’s current and previous flavivirus exposure. Therefore, it is important to consider the epidemiological and clinical context when testing for antibodies against CHIKV, ZIKV, and DENV [WHO]. There are now two licensed vaccines for dengue but none for chikungunya arboviral diseases [19,20,21,22]. No antiviral drugs are available for these infections. CHIKV fever is sometimes misdiagnosed as a dengue infection because of its many common clinical presentations [1,2,3,4,23]. DENV fever has a high incidence rate in Nigeria; therefore, symptomatic patients are frequently tested for dengue and rarely for CHIKV [1,2,3,4]. In endemic areas, such as Nigeria, patients suspected of having DENV and/or CHIKV infections should be tested for both viruses. Studies involving larger patient groups are needed to determine the full pathogenicity and severity of dual arboviral infections [4,18,24]. This is crucial for the timely and accurate diagnosis of viral infections and the correlation of disease severity with mono- or dual infections, which will help guide patient care and management. Studies on arboviral coinfections in endemic regions of Nigeria are needed to determine the epidemiological and evolutionary characteristics of these emerging viruses. These data will also be useful for designing and implementing effective control measures. A few studies from African countries have shown the existence of co-infections with these viruses [24,25,26]. In Nigeria, the true disease seroprevalence, hidden endemicity, and burden of dengue and chikungunya viral infections remain unknown because most of these studies involved a relatively small number of patients. Therefore, the present study assessed the seropositivity, hidden endemicity, and burden of chikungunya, dengue, and chikungunya-dengue cocirculation in three Nigerian regions.
2. Methods
2.1. Study Site and Design
We conducted a cross-sectional study in three university teaching hospitals located in three geographical regions of Nigeria: the Federal Medical Centre, Keffi, located in Nasarawa State, central Nigeria; Abia State University Teaching Hospital, Aba, located in southern Nigeria; and Baru-Diko Teaching Hospital, Kaduna, located in northern Nigeria (Figure 1) [1]. The three study regions have a combined population of over 30 million people; 45% of the population lives in urban areas, 40% in rural areas, and 15% in slums or informal settlements [1,3]. The average annual temperatures in these areas range from 21 °C to 27 °C, whereas in the interior lowlands, temperatures are generally above 27 °C. The mean annual precipitation is 1165.0 mm [1,3,6,7,8]. It rains throughout the year in most parts of southern and central Nigeria, with most rainfall occurring between April and October and minimal rainfall occurring between November and March in the north. The main occupation of people in these regions is farming [1].
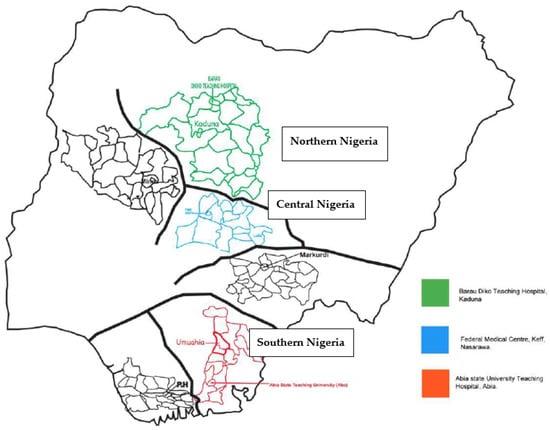
Figure 1.
Arbovirus study sites in Nigeria.
2.2. Study Population
The study population comprised outpatients, pregnant women who were enrolled for antenatal care, and patients presenting with illness at the rapid-access healthcare and antiretroviral (people living with AIDS) units of hospitals between December 2020 and November 2021. These hospitals were purposefully selected to reflect diversity in terms of different cultures, religions, ethnicities, ecology, topographical and vegetation features, and human activities. The inclusion criteria were all outpatients within an age range of 0 months to 80 years who agreed to participate in the study and signed the consent form, including children whose parents or guardians gave consent, while the exclusion criteria were participants who were already undergoing treatment for malaria, those who refused to sign the consent form, and seriously ill patients who were hospitalized [1].
A structured clinical research questionnaire was used to obtain information, including questions on demographics, medical history, vital signs and symptoms, clinical evaluation, data on hospitalization, and a summary form [1]. All study participants were screened for malaria- and chikungunya-dengue-related symptoms (fever, headaches, rashes, joint pain, conjunctivitis, and muscular pain). Detailed protocol information was made available and fully explained to the participants in English and their respective local languages before enrolment. The study participants signed an informed consent form after enrolment [1]. Participants who could not read and write were asked to provide verbal consent and then to thumbprint, indicating that they were willing to participate in the study.
2.3. Total Number of Samples Collected
The sample size calculation (based on a 40% expected proportion of CHIKV and DENV infections in a total population of five hundred thousand patients with a confidence interval of 95% and a p-value of 0.05) showed a minimum sample size of 384 serum samples, which we increased to 871 samples to be able to analyze subgroups according to regions [1].
Venous blood samples (5 mL) were collected from each participant. Additionally, a local clinical diagnostic laboratory technician (located in the hospital who collected patient blood samples daily) collected 110 blood samples, along with the clinical history, from the blood banks of the three hospitals. We tested all serum samples at the study site for malaria parasites using a rapid antigen test kit (SD BIOLINE Malaria Differential P.f/Pan Ag RDT (HRP II+ pLDH, Abbott, Libertyville, IL, USA), according to the manufacturer’s instructions [1]. In summary, 5 µL of blood sample was transferred into the sample well using the appropriate device included in the kit, and five drops of lysis buffer were added to the buffer well. The results were read visually after 15–20 min. Samples were shipped on dry ice to the Institute of Virology in Freiburg, Germany. The samples were stored at −20 °C in preparation for testing for chikungunya and Zika antibodies [1].
2.4. Laboratory Procedure
For CHIKV and DENV, analyses were performed using the immunoblot assay recomLine Tropical Fever for the presence of arboviral antibody serological marker IgG immunoblot (Mikrogen Diagnostik, Neuried, Germany) with DENV Nonstructural protein 1 (NS 1), DENV Equad (variant of the envelope protein with designated mutations to increase specificity), and CHIKV virus-like particle (VLP), according to the manufacturer’s instructions [1,27]. This test is highly specific because of targeted mutations. In summary, test strips were loaded with DENV and CHIKV antigens and incubated with diluted serum in a dish for 1 h. The strips were then washed three times. Peroxidase-conjugated anti-human antibodies (IgG-specific) were added, incubated for 45 min, and washed three times. Insoluble bands developed at the sites on the test strips occupied by antibodies 8 min after the addition of the coloring solution.
2.5. Statistical Tests
Statistical analyses were performed using SPSS version 28. Descriptive statistics were employed for the analysis of results, and we tested for associations between demographics and CHIKV and DENV seropositivity using correlation with the results deemed statistically significant at a p-value ≤ 0.05 and odds ratios (ORs) at a confidence interval (CI) of 95%.
2.6. Ethics Statement
The study protocol was reviewed and approved by the local ethics committee on human research at the Universitatsklinikum, Freiburg [No. 140/19], and the local ethics committee on human research at the Tertiary Hospitals and National Ethics Committee on Human Research of Nigeria [No KF/REC/02/21]. All experiments were performed in accordance with relevant guidelines and regulations.
3. Results
3.1. Arbovirus Serology
In epidemiological studies, serological tests are essential because of the mild and nonspecific symptoms of arboviral disease. However, these serological test interpretations may be complicated by the notorious cross-reactive antibodies between flaviviruses and alphaviruses. Therefore, the test interpretation in the current study may be presented as flavivirus seropositive for DENV and alphavirus seropositive for CHIKV infection.
3.2. Seropositivity of Chikungunya (Alphaviruses) and Dengue (Flaviviruses) in the Study Regions
A total of 871 participants were recruited from the three geographical regions. Among these, 17.5% (152/871) were from Abia (southern Nigeria), 34.4% (300/871) were from Kaduna (northern Nigeria), and 48.1% (419/871) were from Nasarawa (central Nigeria). The ages of the participants ranged from 0 months to 80 years, with a mean age of 36.6 years. Of the 871 subjects, 71.0% (619/871) were females, and 29.0% (252/871) were males. The overall cohort detectable antibody seropositivity against CHIKV was 64.9% (565/871), 95% CI (0.61–0.68), and that against DENV was 44.7% (389/871), 95% CI (0.44–0.46). It was observed that 31.6% (95% CI (0.31–0.32) of the population was seropositive for CHIKV-DENV cocirculating antibodies (Table 1).

Table 1.
Sociodemographic characteristics and antibody seropositivity of chikungunya, dengue, and chikungunya–dengue cocirculation.
3.3. Sociodemographic Characteristics and Seropositivity of CHIKV, DENV, and CHIKV-DENV in the Study Population Regions
The subgroup analysis revealed considerably higher antibody seropositivity against CHIKV in the central [69.5% (291/419); 95% CI (0.95–1.58) OR = 1.2; p = 0.00)] and northern regions than in the southern region. Antibody seropositivity rates against DENV and CHIKV-DENV were higher in the central and northern regions. The predicted odds of CHIKV infection in the central region were 1.2 times the odds of other regions (p = 0.00) (Table 1).
3.4. Sex Seropositivity of Chikungunya and Dengue Arboviral Infection
A significant level of detectable antibodies against CHIKV [65.1% (403/619); 95% CI (0.81–1.25); OR = 1.0], DENV [46.4% (287/619); 95% CI (0.87–1.31); OR = 1.1], and CHIKV-DENV [34.7% (215/619); 95% CI (1.19–2.70); OR = 1.2, p = 0.00] was observed in females compared with males (Table 1).
3.5. Place-Specific Seropositivity of Chikungunya and Dengue
In the current study, the highest levels of detectable antibodies against CHIKV [70.8% (75/106); 95% CI (0.84–2.04); OR = 1.3, p = 0.03)], DENV [55.7% (59/106); 95% CI (1.04–2.33); OR = 1.6, p = 0.02], and CHIKV-DENV-seropositive participants [45.3% (48/106); 95% CI (1.19–2.70); OR = 1.7, p = 0.00] were observed in slums and urban populations. The odds of CHIKV, DENV, and CHIKV-DENV infections were higher in the slums than in other groups (Table 1).
Seropositivity of Chikungunya and Dengue in Pregnant and Nonpregnant Participants
CHIKV antibody seropositivity was slightly higher among nonpregnant participants [65.8% (420/638); 95% CI (0.87–1.29)], whereas CHIKV-DENV detectable antibodies were evidently more prevalent among pregnant women [36.1% (84/233); 95% CI (0.90–1.65)] (Table 1).
3.6. HIV Status-Specific Seropositivity of Chikungunya and Dengue in the Population
Seropositive antibodies against CHIKV [74.4% (209/281); 95% CI (1.16–2.13); OR = 1.6, p = 0.00] and CHIKV-DENV [53.0% (149/281); 95% CI (1.85–3.21); OR = 2.5, p = 0.00] were notably higher among HIV-negative participants, whereas DENV-seropositive antibodies were remarkably higher among HIV-positive individuals [64.9% (230/590); 95% CI (0.64–0.97); OR = 0.8, p = 0.00]. There was a statistically significant difference between HIV status and CHIKV and DENV seropositivities (Table 1).
3.7. Marital Status-Related Seroprevalence of Chikungunya and Dengue
There was a marginally higher level of detectable antibodies against alphaviruses (CHIKV) [64.9% (95% CI (0.81–1.24)], flaviviruses (DENV) [46.0% (95% CI (42–49)], and alpha-flaviviruses (CHIKV-DENV) [35.0% (95% CI (29–41)] among married participants than among single individuals (Table 1).
3.8. Malaria Status-Specific Seroprevalence of Chikungunya and Dengue Infection
Malaria-positive participants showed marginal antibody seropositivity against alphaviruses (CHIKV) [65.0% (410/631); 95% CI (0.81–1.24)], whereas alpha- and flavivirus (CHIKV-DENV) detectable antibodies were highest among HIV-negative participants [35.0% (84/240); 95% CI (0.86–1.57)] (Table 1).
3.9. Outpatients and Blood Bank-Specific Seropositive Antibodies against Chikungunya and Dengue
Alphavirus-seropositive antibodies against CHIKV [77.3% (85/110); 95% CI (2.64–4.71); OR = 4.2, p = 0.00] and flavivirus-seropositive antibodies against DENV [62.7% (69/110); 95% CI (1.38–3.13); OR = 2.1, p = 0.00] were significantly higher in serum samples from the blood bank than in those from outpatients. There was a statistically significant difference between blood source and alphavirus/flavivirus antibody seropositivity in the study population (Table 1).
3.10. Clinical Signs and Symptoms Presented by Chikungunya and Dengue Mono-Infected Patients
The participants in this study who were seropositive for alphaviruses and flaviviruses (CHIKV and DENV) presented with undifferentiated clinical signs and symptoms associated with acute febrile illness (AFI) during the study. Table 2 shows that the only difference between alphavirus (CHIKV)- and flavivirus (DENV)-seropositive participants was the extent of clinical manifestation, which was more severe in alphavirus (CHIKV)-seropositive participants than in flavivirus (DENV)-seropositive participants and vice versa (Table 2).

Table 2.
Clinical signs and symptoms presented by CHIKV- and DENV-mono-infected patients.
3.11. Age-Specific Seroprevalence of Chikungunya, Dengue, and Chikungunya–Dengue Antibodies
The highest seropositivity for alphaviruses (CHIKV) was observed in the 50- to 59-year-old age group [75.6% (62/82); 95% CI (66–85)], and the lowest detectable antibody seropositivity was observed in the 70- to 79-year-old age group [25.0% (2/8); 95% CI (2–68)]. Significant antibody seropositivity against flaviviruses (DENV) [61.2% (120/196); 95% CI (54–68)] was observed in the 20–29-year-old age group, and the lowest detectable antibodies were recorded in the 80-year-old age group [16.7% (1/6)]. Alpha- and flavivirus (CHIKV-ZIKV)-seropositive [8.5% (7/82); 95% CI (2–15)] antibodies were minimally higher in the 50–59 years age group compared to all the other age groups (Figure 2, Table 3).
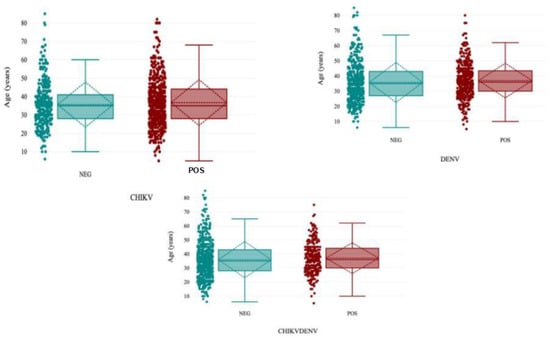
Figure 2.
Distribution of CHIKV, DENV, and CHIKV-DENV cocirculating antibody seropositivity in the population.

Table 3.
Age-specific seropositivity of chikungunya, dengue, and chikungunya–dengue antibodies.
3.12. Monthly Distribution of CHIKV and DENV Antibody Seropositivity during Sampling Period
The distribution of CHIKV and DENV antibody seropositivity during the sampling period was higher in the southern regions than in other regions (97.6%). The seropositivity was particularly high in October (97.6%) and September (91.80%) compared to all other months of the year (Figure 3).
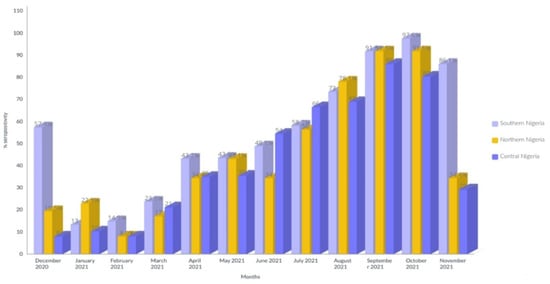
Figure 3.
Distribution of CHIKV and DENV antibody seropositivity during sampling period.
4. Discussion
The findings of our study are significant given that the transmission and geographic spread of DENV (flaviviruses) and CHIKV (alphaviruses) are not well documented in Nigeria. This study has significant limitations. The cross-reactivity of IgG antibodies between flaviviruses and alphaviruses is well established and a confounding factor in serological studies investigating the seropositivity of arboviruses. All samples that tested positive for both DENV and CHIKV were classified as positive for flavivirus or alphavirus, respectively. Due to the large sample size, it was impractical to conduct additional testing using techniques such as the plaque reduction neutralization test (PRNT) or other seroneutralization tests. Seropositivity for the two arboviruses was detected in the absence of antibodies against other flaviviruses or alphaviruses. Consequently, the circulation of each of the two targeted arboviruses was confirmed. The presence of IgG antibodies indicated that the participants were previously infected with CHIKV and DENV, as determined by immunoblot serology. IgG antibodies can be detected for years or even over a lifetime [9]; therefore, in the current study, we could not determine when individuals were infected with the two arboviruses.
We assessed the seropositivity, hidden burden (as they usually remain undetected by health services), and endemicity of CHIKV (alphavirus), DENV (flavivirus), and CHIKV-DENV cocirculating antibodies in three regions of Nigeria. There was a significant level of detectable antibodies against CHIKV (64.9%) and DENV (44.7%) and moderate or low seropositivity against CHIKV-DENV (31.6%). In addition, 65.1%, 45.5%, and 30.3% of CHIKV-seropositive, DENV-seropositive, and CHIKV-DENV-seropositive patients, respectively, were also positive for the malaria antigen. The varied seropositivity results recorded in the three regions could be attributed to the common vector of transmission (Aedes spp.) [1,2,3,4] occurring in the three geographic locations simultaneously, differences in climate (increasing reproductive activities and shortening the extrinsic cycle of CHIKV and DENV in the vector), vegetation index, meteorological factors, vector reproductive indices, attack rates, the sampled population, molecular diagnostic tools or assays employed [1,2,3,4], and the sampling period of our study. The multifariousness of detectable antibodies in the participants across the various geographical regions may also be explained by antibody cross-reactivity, cocirculation of CHIKV-DENV, past exposure, differences in human population, various human activities that may result in flooding (which favors the emergence and survival of CHIKV and DENV), changes in microhabitats such as refuse disposal or dumpsites, unplanned urbanization, and poor housing infrastructure in the three regions may also favor transmission dynamics of the mosquito-borne vectors of CHIKV and DENV [12,13]. There was a lack of or limited molecular laboratory diagnostic facilities or testing tools and manpower during patient assessment in various hospitals located in the three regions. The absence of epidemiological serosurveillance facilities and current health systems may also be responsible for the hidden seropositivity and endemicity. The seropositivity rates recorded in the present study were remarkably extensive compared to those reported in other seropositive studies conducted in other parts of Nigeria and the rest of the world [1,2,3,4,5,6,7,8,9,10,13,22].
CHIKV- and DENV-seropositive antibodies were more prevalent among the older age groups in various regions. This could be explained by past CHIKV and DENV exposure and immunosenescence in older age. Furthermore, older people maintain sedentary lifestyles because they sit for long periods in unscreened places, thus causing exposure to Aedes mosquito bites (the day-feeding activity of Aedes aegypti). This may also be attributed to the sociocultural habits of the study participants [19].
The high seropositivity of CHIKV and DENV among female participants could be ascribed to the higher number of females recruited in this study than males. Additionally, females in the three regions engage in outdoor activities, such as farming, trading, fetching or storing water in open domestic storage tanks, ponds, and streams, and gathering wood for fuel, thereby exposing them to the day-feeding activity of Aedes spp., which prefer human hosts in the presence of other alternative hosts [9,10].
In the present study, the levels of detectable antibodies against CHIKV and DENV were high among slums and urban dwellers. This could be due to several factors, such as increased vector exposure in relation to socioeconomic activities close to mosquito breeding habitats, uncontrolled urbanization favoring mosquito breeding habitats, and cultural and behavioral practices. This could also be explained by the poor sewage and drainage infrastructure, poor housing, and unhygienic conditions of the slum environment, compounded by unwholesome waste disposal practices in various slums and urban areas in the three regions. Rural–urban migration, expansion of agricultural activities to sylvatic areas in various community settings, and political fatigue may also be drivers of the high seropositivity rates among participants in these three geographical areas [1,2,8,10,26,28,29].
CHIKV and DENV antibodies were more prevalent in nonpregnant women. These differences could be explained by the good health-seeking behaviors of pregnant women during pregnancy compared with the poor health behaviors of nonpregnant women, resulting in weak or reduced immune responses to mosquito-borne infections [1,12,21].
There was ubiquitous and widespread seropositivity for CHIKV, DENV, and CHIKV-DENV antibodies among HIV-positive women, but the reasons for this are unclear. Studies have shown that HIV-positive or HIV-negative infections do not appear to have a greater risk of infection or complications from arboviral infections [18]. The low antibody seropositive rates of CHIKV and DENV among HIV-positive pregnant women could also be associated with strict adherence to antiretroviral therapy (ART) to suppress viral replication during antenatal care (most of the female study participants were recruited from antenatal care units of the university hospitals as a result, and they were undergoing antiviral treatment).
CHIKV, DENV, and CHIKV-DENV seropositivity rates were slightly higher among married women than among single women. This can be ascribed to family responsibilities involving outdoor and socioeconomic activities. This could also be attributed to poor socioeconomic status, such as poor housing, poor domestic water storage facilities, and petty trading, thereby exposing them to Aedes bites. However, single women’s activities are more related to the indoors [1,2,3,4,5,6,7,8].
There were marked antibodies against CHIKV, DENV, and CHIKV-DENV among the malaria-positive participants, but the exact reasons for this are unclear. Whether the presence of the malaria parasite reactivates or increases seropositivity or sensitivity to CHIKV, DENV, CHIKV, and DENV and increases malaria seropositivity or reactivation remains unclear [6,16,17,18]. The signs and symptoms observed in CHIKV-seropositive and DENV-seropositive patients were also observed in malaria-infected patients, suggesting that the majority of subjects in the present study had malaria.
5. Limitations of the Study
The study population was hospital-based; therefore, seroprevalence could not be a true reflection of what is obtainable in the general Nigerian population, and it could be underestimated. The non-use of seroneutralization tests or PCR and the deployment of a highly specific immunoblot molecular diagnostic test remain challenges in diagnosing alphaviruses and flaviviruses due to antibody cross-reactivity, which may complicate the interpretation of the present results. The interpretation of IgG antibodies may also be complicated (false positives or negatives) because of previous exposure to mosquito-borne arbovirus vaccination.
6. Conclusions
This study highlighted the unexpectedly high seroprevalence and hidden endemicity of CHIKV and DENV arboviral seropositivity in three regions of Nigeria. Several intrinsic and extrinsic factors are responsible for the high seroprevalence. It was also evident in the present study that these arboviral infections could go unnoticed, especially when causing fever, and will end up being treated as other common infections because of their clinical presentation. Therefore, regular epidemiological serosurveillance should be conducted in the major urban areas and slum cities in Nigeria. This will assist in predicting and controlling outbreaks of arboviruses. Molecular characterization and description of cocirculating strains are essential for studying the epidemiology of these rapidly evolving arboviruses, and this information is essential for designing strategies for outbreak control and other interventions.
Author Contributions
P.A.M.: Conceptualization, designed the project, collected the data, performed the statistical analysis, and contributed to writing the manuscript; funding acquisition. M.T.: Original draft of the manuscript and data analysis. T.N.: Contributed to writing the manuscript and statistical data analysis. P.E.A.: Contributed to manuscript writing and statistical analyses. C.M.B.: Statistical analysis and manuscript writing and editing. C.A.: Supervised the entire sample collection and contributed to the original draft of the manuscript and data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
WHO/NTD: Grant Number: 1013487-0.
Institutional Review Board Statement
Ethical approval was obtained from the Institutional Review Board (IRB) of the Federal Medical Centre, Keffi [No KF/REC/02/21], and Uniklinikum ethical committee, University of Freiburg [No. 140/19].
Informed Consent Statement
The study participants signed an informed consent form after enrollment. Pregnant subjects who could not read and write were asked to verbally consent and then to thumbprint, indicating that they were willing to participate in the study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank my supervisor, Marcus Panning, and the staff of Universitatsklinikum Freiburg, Institute of Human Virology, for their technical guidance on the success of this project.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AFLs | acute febrile illnesses |
| CHIKV | chikungunya virus |
| CI | confidence interval |
| DENV | dengue virus |
| pLDH | parasite lactate dehydrogenase |
| HRP | histidine-rich protein 2 |
| IgG | immunoglobulin G |
| RDT | rapid diagnostic test |
| RT–PCR | reverse transcription polymerase chain reaction |
| VLP | viral live particle |
References
- Asaga Mac, P.; Airiohuodion, P.E.; Yako, A.B.; Makpo, J.K.; Kroeger, A. The Seroprevalence and Hidden Burden of Chikungunya Endemicity and Malaria Mono- and Coinfection in Nigeria. Int. J. Environ. Res. Public Health 2022, 19, 8896. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, I.N.; Akande, A.O.; Muhammed, Y.; Rogo, L.D.; Oderinde, B.S. Prevalence Pattern of Chikungunya Virus Infection in Nigeria: A Four Decade Systematic Review and Meta-analysis. Pathog. Glob. Health 2020, 114, 111–116. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Dengue and Severe Dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 26 August 2020).
- Deeba, F.; Afreen, N.; Islam, A.; Naqvi, I.H.; Broor, S.; Ahmed, A.; Parveen, S. Coinfection with Dengue and Chikungunya Viruses. In Current Topics in Chikungunya; Rodriguez-Morales, A.J., Ed.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Pesko, K.; Westbrook, C.J.; Mores, C.N.; Lounibos, L.P.; Reiskind, M.H. Effects of infectious virus dose and blood meal delivery method on susceptibility of Aedes aegypti and Aedes albopictus to chikungunya virus. J. Entomol. 2019, 46, 395–399. [Google Scholar]
- Baba, M.; Logue, C.H.; Oderinde, B.; Abdulmaleek, H.; Williams, J.; Lewis, J.; Laws, T.R.; Hewson, R.; Marcello, A.; Agaro, P.D. Evidence of arbovirus coinfection in suspected febrile malaria and typhoid patients in Nigeria. J. Infect. Dev. Ctries. 2013, 7, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Ayorinde, A.F.; Oyeyiga, A.M.; Nosegbe, N.O.; Folarin, O.A. Asurvey of malaria and some arboviral infections among sus-pected febrile patients visiting a health centre in Simawa, Ogun State, Nigeria. J. Infect. Public Health 2016, 9, 52–59. [Google Scholar] [CrossRef][Green Version]
- Mala, W.; Wilairatana, P.; Kotepui, K.U.; Kotepui, M. Prevalence of Malaria and Chikungunya Co-Infection in Febrile Patients: A Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2021, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Adusei, J.A.; Narkwa, P.W.; Owusu, M.; Domfeh, S.A.; Alhassan, M.; Appau, E.; Salam, A.; Mutocheluh, M. Evidence of chikungunya virus infections among febrile patients at three secondary health facilities in the Ashanti and the Bono Regions of Ghana. PLoS Negl. Trop. Dis. 2021, 15, e0009735. [Google Scholar] [CrossRef]
- Olajiga, O.M.; Adesoye, O.E.; Emilolorun, A.P.; Adeyemi, A.J.; Adeyefa, E.O.; Aderibigbe, I.A.; Adejumo, S.A.; Adebimpe, W.O.; Opaleye, O.O.; Sule, W.F.; et al. Chikungunya Virus Seroprevalence and Associated Factors among Hospital Attendees in Two States of Southwest Nigeria: A Preliminary Assessment. Immunol. Investig. 2017, 46, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Gaviria, R.R.; Santhekadur, P. A case of coinfection with malaria and chikungunya in a returning traveler from Nigeria. J. Vector Borne Dis. 2021, 58, 178–180. [Google Scholar] [CrossRef]
- Doucoure, S.; Thiaw, O.; Wotodjo, A.N.; Bouganali, C.; Diagne, N.; Parola, P.; Sokhna, C. Anopheles arabiensis and Anopheles funestus biting patterns in Dielmo, an area of low-level exposure to malaria vectors. Malar. J. 2020, 19, 230. [Google Scholar] [CrossRef]
- Fagbami, A.H.; Onoja, A.B. Dengue haemorrhagic fever: An emerging disease in Nigeria, West Africa. J. Infect. Public Health 2018, 11, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Metz, S.W.; Gardner, J.; Geertsema, C.; Le, T.T.; Goh, L.; Vlak, J.M.; Suhrbier, A.; Pijlman, G.P. Effective Chikungunya Virus-like Particle Vaccine Produced in Insect Cells. PLoS Negl. Trop. Dis. 2013, 7, e2124. [Google Scholar] [CrossRef]
- Ingoba, L.; Vairo, A.A.S.M.P.F.; Haider, N.; Kock, R.; Ippolito, G.; Zumla, A.; Nguimbi, E.; Pallerla, S.R.; Ntoumi, T.P.V.F. Diagnosis of Chikungunya Virus in Febrile Patients from a Malaria Holoendemic Area. Int. J. Infect. Dis. 2021, 109, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Charrel, R.N.; De Lamballerie, X.; Raoult, D. Chikungunya outbreaks—The globalization of vector-borne diseases. N. Eng. J. Med. 2007, 356, 769–771. [Google Scholar] [CrossRef]
- Fritel, X.; Rollot, O.; Gérardin, P.; Gaüzère, B.-A.; Bideault, J.; Lagarde, L.; Dhuime, B.; Orvain, E.; Cuillier, F.; Ramful, D.; et al. Chikungunya virus infection during pregnancy, Réunion, France, 2006. Emerg. Infect. Dis. 2010, 16, 418–425. [Google Scholar] [CrossRef]
- Omatola, C.A.; Onoja, B.A.; Fassan, P.K.; Osaruyi, S.A.; Iyeh, M.; Samuel, M.A.; Haruna, P.U. Seroprevalence of chikungunya virus infection in five hospitals within Anyigba, Kogi State of Nigeria. Braz. J. Infect. Dis. 2020, 24, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rothan, H.A.; MBidokhti, R.M.; Byrareddy, S.N. Current concerns and perspectives on Zika virus coinfection with arboviruses and HIV. J. Autoimmun. 2018, 89, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ochieng, C.; Ahenda, P.; Vittor, A.Y.; Nyoka, R.; Gikunju, S.; Wachira, C.; Waiboci, L.; Umuro, M.; Kim, A.A.; Nderitu, L.; et al. Seroprevalence of Infections with Dengue, Rift Valley Fever and Chikungunya Viruses in Kenya, 2007. PLoS ONE 2015, 10, e0132645. [Google Scholar] [CrossRef]
- Anejo-Okopi, J.; Gotom, D.Y.; Chiehiura, N.A.; Okojokwu, J.O.; Amanyi, D.O.; Egbere, J.O.; Adetunji, J.; Ujah, O.I.; Audu, O. The seroprevalence of zika virus infection among HIV positive and HIV negative pregnant women in Jos, Nigeria. Hosts Viruses 2020, 7, 129–136. [Google Scholar] [CrossRef]
- Santhosh, S.R.; Dash, P.K.; Parida, M.M.; Khan, M.; Tiwari, M.; Rao, P.V.L. Comparative full genome analysis revealed E1: A226 V shift in 2007 Indian Chikungunya virus isolates. Virus Res. 2008, 135, 36–41. [Google Scholar] [CrossRef]
- Deeba, F.; Islam, A.; Kazim, S.N.; Naqvi, I.H.; Broor, S.; Ahmed, A.; Parveen, S. Chikungunya virus: Recent advances in epidemiology, host pathogen interaction and vaccine strategies. Pathog. Dis. 2016, 74, ftv119. [Google Scholar] [CrossRef] [PubMed]
- Afreen, N.; Deeba, F.; Naqvi, I.H.; Shareef, M.; Ahmed, A.; Broor, S.; Parveen, S. Molecular Investigation of 2013 Dengue Fever Outbreak from Delhi, India. PLoS Curr. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.mikrogen.de/english/products/product-overview/testsystem/tropical-fever-igg.html (accessed on 17 June 2024).
- Marinho, R.D.S.S.; Duro, R.L.S.; Santos, G.L.; Hunter, J.; da Aparecida Rodrigues Teles, M.; Brustulin, R.; de Padua Milagres, F.A.; Sabino, E.C.; Diaz, R.S.; Komninakis, S.V. Detection of coinfection with Chikungunya virus and Dengue virus serotype 2 in serum samples of patients in State of Tocantins, Brazil. J. Infect. Public Health 2020, 13, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Afreen, N.; Naqvi, I.H.; Broor, S.; Ahmed, A.; Parveen, S. Phylogenetic and Molecular Clock Analysis of Dengue Serotype 1 and 3 from New Delhi, India. PLoS ONE 2015, 10, e0141628. [Google Scholar] [CrossRef]
- Abdullahi, I.N.; Isong, I.K.; Emeribe, A.O.; Nwofe, J.O.; Shuaib, B.I.; Gwarzo, A.M.; Usman, Y.; Sadi, M.; Umeozuru, C.M.; Dangana, A.; et al. Dengue Virus is Hyperendemic in Nigeria from 2009 to 2020: A Contemporary Systematic Review. Infect. Chemother. 2021, 53, 284–299. [Google Scholar] [CrossRef]
- Nasir, I.A.; Agbede, O.O.; Dangana, A.; Baba, M.; Haruna, A.S. Dengue virus nonstructural Protein-1 expression and associated risk factors among febrile Patients attending University of Abuja Teaching Hospital, Nigeria. Virus Res. 2017, 230, 7–12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).