Abstract
This paper describes a study that for the first time addresses the physiological effects of an 8- week mechanically aided facial exercise program, using the Facial-Flex device (Facial Concepts, Inc., Blue Bell, PA) with four healthy individuals with no motor, speech, language, or hearing problems. For a variety of non-speech and speech tasks, upper and lower lip muscle activity (EMG) and upper and lower lip movements were recorded at two baseline sessions (separated by 1 week) and immediately after an 8-week training period. The results indicate that after the training period, all four subjects showed an increase in the number of task repetitions and the duration of isometric contraction using the Facial-Flex device with a fixed resistance (Linebaugh tests). However, with resped to physiological changes as related to the exercise program, the results were mixed. Only one subjed showed the expected significant increase in normalized EMG activity. This response was mirrored in a significant overall increase in movement range and peak velocity after the 8-week training period. Regarding the other three subjects, one subject showed no systematic training effect at all, whereas the remaining two subjects showed a significant increase in movement duration. Non-speech and speech tasks were found to be clearly different in their overall physiological characteristics; speech related movements were found to be more clearly defined in terms of larger amplitudes, shorter durations, higher peak velocities, and less variable movement cycles. The apparent discrepancy between the results of the Linebaugh tests and the physiological measures on specific oro-motor tasks warrants some caution in drawing conclusions on changes in the oro-motor system based on general performance measures. Further studies with well-defined clinical populations are needed to assess the usefulness of this device as an aid in the treatment of speech disorders based on motor system impairments.
INTRODUCTION
In many speech and swallowing disorders orofacial muscle strength and mobility is compromised. Current practice in speech therapy often involves different kinds of oro-motor exercises using a variety of devices (e.g., tongue depressors, straws) and techniques. Systematic research into the efficacy of such exercises for oro-motor rehabilitation is lacking, but a recent sbJdy by Robertson (2001), suggested that the combination of a standardized clinic-based program and home exercises was beneficial to a group of patients suffering from dysarthria following stroke.
The use of facial exercises to improve muscle strength and other oro-motor functions is a common approach in Orofacial Myofunctional therapy or OMT (Cooper, 1973; Deschenes, 1983; Garliner, 1975; Gaucher, 19TT; Hahn, 1991; Hanson, 1978; Hanson, 1988; Hockel, 1984; Padovan, 1995; Palacioz & Shannon, 1986; Paul-Brown & Clausen, 1999; Snow, 1983). In the past decades, OMT has been applied in a variety of settings, including orthodontics and dentistry (Daglio, Schwitzer, & Wuthrich, 1993; Gugino & Dus, 1998; Kondo & Aoba, 2000; Page, 1999; Ruf & Pancherz, 1999; Sasaki & Shibasaki, 1994; Uner, Darendeliler, & Bilir, 1999; Yamaguchi & Sebata, 1995); swallowing disorders (Gommerman & Hodge, 1995; Reinicke, Obijou, & Trankmann, 1998); and articulation problems (Bigenzahn, Fischman, & Mayrhofer-Krammel, 1992; Christensen & Hanson, 1981; Gommerman & Hodge, 1995; Landis, 1994). In many of these studies, OMT was found to have a positive impact, especially for non-speech oro-motor functions. For speech tasks, positive effects have been found when the training involved articulators with specific postural or myofunctional problems (Bigenzahn et al., 1992; Christensen et al., 1981; Umberger & Johnston, 1997).
Although the studies mentioned above provide some positive evidence for the use of oro-motor exercises with speech problems, the factors that influence their effectiveness and the potential underlying physiological mechanisms are not well documented (cf. Robertson, 2001). Some of the potential problems relate to the motivation of patients to actually do the exercises at home (Robertson, 2001; Zimmerman, 1988), and their interpretation of the instructions on how to perform them. With respect to the latter issue, mechanical devices are often used to restrict the degrees of freedom with respect to task execution (e.g., see Ruscello, 1995, for a discussion on the use of speech appliances). In a similar vein, the Facial Flex device (Facial Concepts Inc., Blue Bell, Pa; Figure 1) was introduced as having the potential to offer a more standardized way of exercising weak facial muscles as compared to the usual array of strengthening exercises used in OMT and similar approaches (Creed & Spiegel, n.d.).
The Facial-Flex is a lightweight mouthpiece that provides external resistance to oral movements; it was originally designed as a prosthetic training aid for patients with scarring and lip contracture due to orofacial bums. The amount of resistance can be changed using dental elastics of different strength with typical values of 4, 6, 8, and 14 ounces (oz.). The device is placed horizontally between the upper lip and lower lip, seated at the corners of the mouth. In this position, the elastic is minimally stretched (Figure 1, Open Mouth position). Subsequently, the subject has to bring the comers of the mouth together to form a rounded shape with the lips, similar to an /u/ sound configuration (Figure 1, Closed Mouth position). This position, at which the elastic is maximally stretched, is held for a short time (1-2 seconds) and gently released.
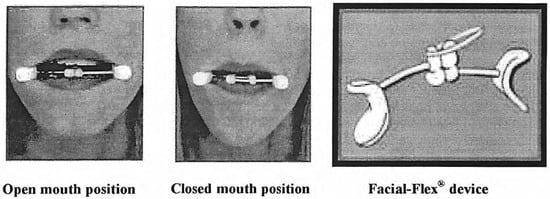
Figure 1.
Picture of Facial-Flex device and the open and closed mouth position while in use (printed with permission of Facial Concept, Inc.).
Figure 2 shows the force output (in Newton) required for a given amount of deflection in centimeters (which equals the amount of stretching) at a particular resistance, as determined by the manufacturer of the dental elastics (The data for the 16 ounce resistance were not provided by the manufacturer, but are based on a linear extrapolation of the data on the other resistances.) (Ultra Mold Corp., Yardley, PA). At maximum deflection, there is a distance of 5.5 cm between the comers of the mouth; fully relaxed the distance is 7.7 cm.
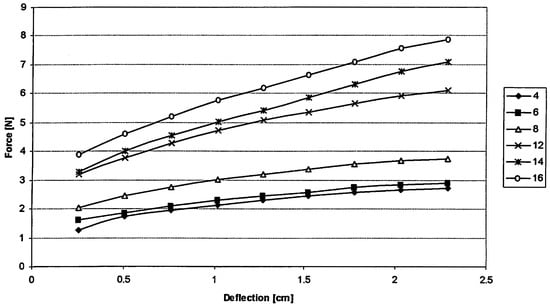
Figure 2.
Amount of deflection (in cm) in dental elastic versus force output (in Newton) at various elastic resistance values (4 - 16 oz.). (See text for more details).
To date, published data on the use of the Facial Flex show improved facial skin firmness and elasticity which, together with improvements on performance tests using the device (see next paragraph), are taken as evidence for increased facial muscle strength (Grove, Rimdzius, & Zerweck, 1994). The data from another study (Zide, Bradley, & Longaker, 2000) suggest that the use of the Facial Flex device could diminish upper lip tightness that occurs after lip augmentation procedures.
Typically, information on changes in the oro-motor system during and after the exercise program is assessed indirectly through the Linebaugh tests (Grove et al., 1994). The first of these tests requires subjects to perform a maximal number of repetitions (in two minutes). In the second test, they have to maintain a position in which the dental elastic is maximally stretched for as long as possible (isometric contraction). The assumption is that in doing better on both tests, the subject demonstrates an increase in muscle force output and resistance to fatigue (Grove et al., 1994). However, to our knowledge, the actual changes in muscle activity that may underlie the performance on the Linebaugh tests have never been documented. This is one aspect of what the current study was set out to do.
To this end, we used a similar approach as described in Kuehn, Moon and Folkins (1993) who looked at changes of levator muscle electromyography (EMG) activity as a function of intranasal air pressure. The pressure was used as a continuous resistance against velopharyngeal closure to strengthen the muscles involved in this activity. The results showed for speakers with and without a cleft palate that levator muscle activity increased with heightened intranasal air pressure (Kuehn et al., 1993). In a subsequent study (Kuehn & Moon, 1994), the authors demonstrated that changes in EMG were specific to velopharyngeal closure effort, and that speech tasks require only a small amount of force compared to a non-speech task as blowing on a straw. Specific changes in levator muscle activity as a function of vowel loudness, which was used to induce a generalized physical effort, were not found. Although lip muscles are different from muscles involved in velopharyngeal closing (Barlow, 1999), we would argue that we can apply the same principles in measuring task specific changes in muscle activity as a function of a strengthening exercise, in this case using the Facial-Flex device. More in general, the use of EMG seems appropriate in the context of the present study for several reasons.
First, surface EMG offers "easy access to the physiological process that causes the muscle to generate force, produce movement and accomplish the essential functions of everyday life" (p. 725, Kollmitzer, Ebenbichler, & Kopf, 1999). Second, surface EMG is commonly used in evaluations of muscle function in both normal and disordered speech (e.g., Forrest, Adams, McNeil, & Southwood, 1991; Kuehn et al., 1993; Van Lieshout, Peters, Starkweather, & Hulstijn, 1993; see also Luschei & Finnegan, 1997 for a review). Third, it proved to be a useful technique to evaluate the effects of myofunctional therapy on lip function in a non-speech task context (Schievano, Rontani, & Berzin, 1999). Without making further claims on the interpretation of surface EMG in terms of actual force produced, we adhere to the statement that in general "the size of the EMG signal bears a monotonic relationship to the degree by which the muscle has been activated" (p. 152, Luschei & Finnegan, 1997). Obviously, in some cases, in particular for facial muscles, the surface EMG electrodes might pick up activity from more than a single muscle (Blair & Smith, 1986). However, this is not a major issue, since our focus is not on a specific muscle, but rather on the combined muscle activity as related to a specific task execution. Fatigue could be an issue in the way it effects surface EMG signals, but for the kind of tasks used in this experiment, and in particular for speech tasks, this is not considered to be an immediate problem (Kuehn, & Moon, 1994; McHenry, Minton, Wilson, & Post, 1994).
Surface EMG can provide useful information about the amount (and nature) of muscle involvement, but it does not translate directly in an adequate description of the actual behavior of articulators. Therefore, we also recorded the movements of upper lip and lower lip before and after the training period. The mechanical aided resistance training requires subjects to execute lip movements in a strictly symmetrical fashion for 8 weeks, twice a day for about 10 minutes each. How this influences the nature of individual movements (duration, amplitude range, peak velocities, variability) and their coordination when embedded in meaningful oro-motor tasks is unknown. For example, Zanone and Kelso (1992) have shown that with respect to bimanual coordination, inherent differences between established movement patterns and the new patterns that have to be learned could induce qualitative changes in the a-priori existing coordinative patterns after training. In oro-motor research, a useful distinction that can be made with respect to potential differences in lip movement patterns is between speech and nonspeech tasks. This distinction is based on earlier findings that speech and nonspeech tasks put different demands on the oro-motor system (e.g., Kuehn, & Moon, 1994). If the inherent movement characteristics of the Facial-Flex exercise are incompatible with the existing patterns found in either task category, this should have an influence on the nature (and stability) of lip coordination. Movement characteristics are measured in terms of movement amplitude, duration, peak velocity, cyclic stability, and interlip coordination. Obviously, we do not exped our subjects to become unintelligible as a result of training, but if systematic changes do occur, they could provide important information with respect to their relevance in terms of treatment perspectives for clinical populations with oro-motor limitations (cf. Solomon, 2000).
To summarize, the main purpose.of this study is to document physiological changes for a variety of speech and non-speech tasks as they occur in surface EMG lip activity and articulatory movements after an intensive 8-week training period using the Facial-Flex. A second purpose is to compare these findings to the more general performance measures that are typically used to measure the outcomes of this exercise program. Such a comparison is deemed relevant to assess whether changes in general performance tasks using the device can be generalized to physiological changes in the articulators when used in an unrelated context. To establish preliminary reference data on these training effects, we performed this study using healthy young subjects. Intelligibility scores for speech tasks are not used, since none of the speakers had speech problems, and perceptual measures would not pick up any subtle changes in motor execution that might occur as a result of this training program. In general, a regular exercise regime for a prolonged period should have a measurable effed on lip muscles and their function, similar to the effects of muscle strengthening exercises that have been reported for velar (Kuehn, Moon, & Folkins, 1993) and limb muscles (Hartigan et al., 1989). It is expected that the data from the present study can provide preliminary information to clinicians and users about the possibilities and limitations of this exercise program in the rehabilitation of muscle related oro-motor problems.
METHODS
Subjects
Four subjects, two males (M1: 39 years; M2: 29 years) and two females (F1: 37 years; F2: 24 years), participated in this study. The number is small but not atypical for this type of study, mainly because participation required a strong commitment for a prolonged period of time and because of the amount of physiological data that needs to be processed and analyzed. None of the subjects reported any history of communication or swallowing problems and all had university level education. Subjects used Canadian English as their first language and were paid $125 CAD upon completion of the projed.
Tasks & Procedures
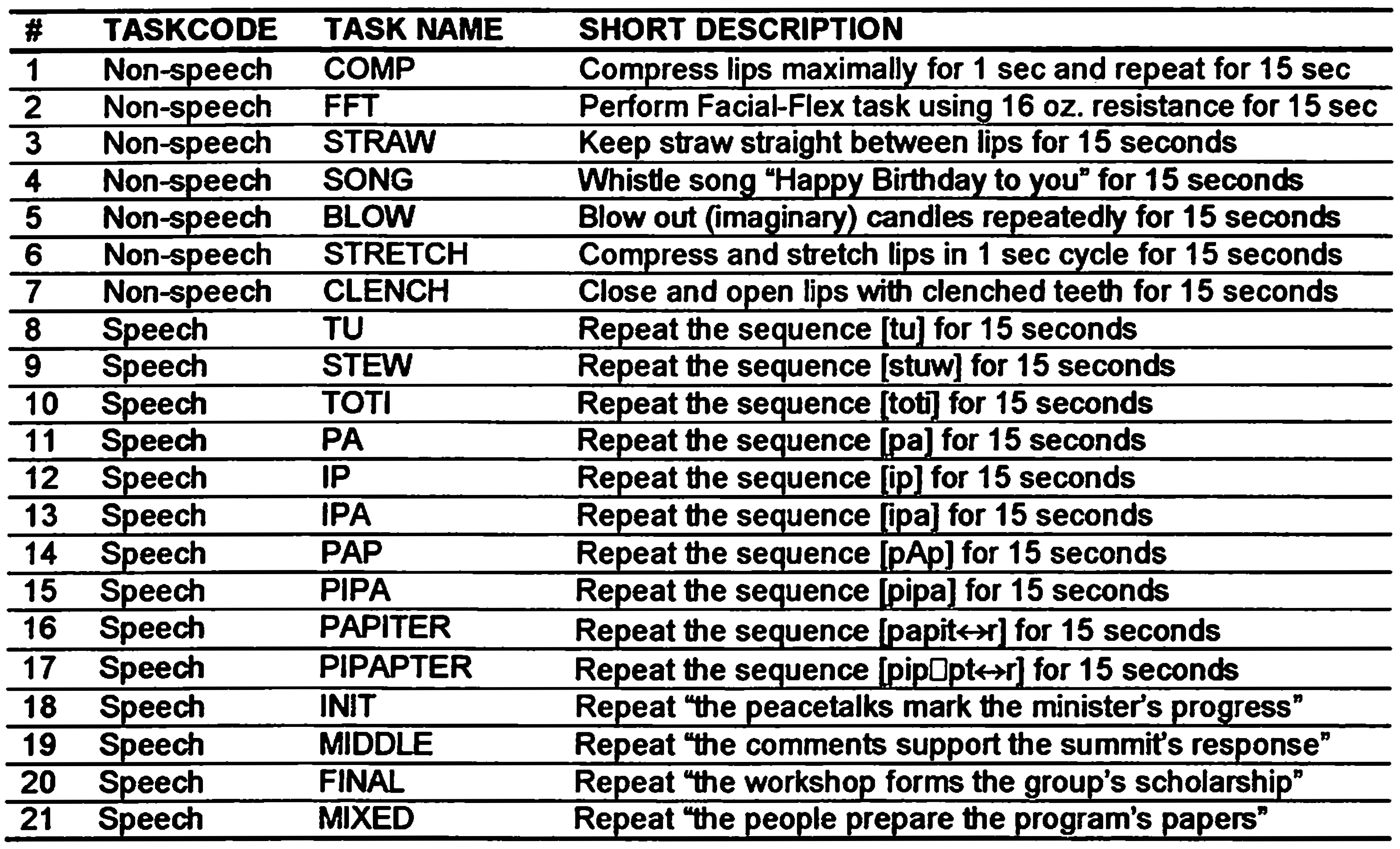
In the two sessions preceding the Facial-Flex exercise program (Baseline 1 and Baseline 2), as well as following the completion of the program (Post), all subjects performed a variety of speech and non-speech oro-motor tasks as listed in Table 1. These tasks are part of the EMMA Speech Motor Assessment (ESMA) protocol, commonly used in our lab to measure speech motor function (see van Lieshout & Moussa, 2000 for more details on the protocol).

Table 1.
Overview of non-speech and speech tasks used in this study, based on the EMMA Speech Motor Assessment (ESMA) protocol (van Ueshout and Moussa, 2000).
Table 1.
Overview of non-speech and speech tasks used in this study, based on the EMMA Speech Motor Assessment (ESMA) protocol (van Ueshout and Moussa, 2000).
Each task was repeated for 15 seconds in a single trial, the start and end of which were signaled to the subjects by simultaneously presented visual and auditory cues. The duration of 15 seconds was chosen to allow enough time to collect 5 repetitions for the sentence tasks (see Table 1) and at least 10 repetitions for the single word tasks, but not be too long for a subject to repeat the tasks on a single breath. Four seconds before the start of a trial, the task description (for non-speech tasks) or letter string (for speech tasks) was presented on a computer screen. In addition, 2 seconds into this preparation interval, a message appeared on the screen reminding the subject to take a deep breath before the onset of the task.
Surface electromyographic (EMG) and electromagnetic midsagittal articulography (EMMA) measurements were made at Baseline 1 (1 week before the start of the training period) and Baseline 2 (one day before the start of the training period). We also took baseline measurements for the Linebaugh tests at these sessions. Within two days following the completion of the program we measured EMG + EMMA data for the Post session. We took two baseline measures, since we know from recent work that when measured at different points in time, subjects may show variations in individual kinematic measures (Alfonso & van Lieshout, 1997). The two baseline sessions would allow us to monitor these changes and compare them to changes that occur after completion of the training program. During the 8-week training period, subjects did not see or practice any of the tasks used during the EMG + EMMA sessions, with exception of the Facial-Flex task itself. At the beginning of the first baseline session, subjects were familiarized with the device; to this end, they were shown an instructional video provided by the Facial Concepts Company, and given verbal instructions on the use of the device. However, they did not receive the device until the end of the second baseline session, to prevent them from exercising before the start of the training period.
Training-period The training period lasted for 8 weeks. During this time, the subjects used the Facial-Flex device with a constant resistance of 6 oz. according to recommendations in the literature (Grove et al., 1994). Subjects were asked to maintain a steady pace of one exercise cycle (closing + opening movement) per second during training. Furthermore, they were required to do 50 repetitions twice a day (morning and evening), every day. Both the resistance and the number of exercises were kept constant to ensure that all subjects were exposed to the same amount of training (cf. Robertson, 2001). They were also asked to keep a detailed log of their exercises and experiences. At the end of each week during this 8-week period, subjects came to the lab to provide feedback on their progress and to perform the Linebaugh tests. The information specified in the logs, as well as the weekly personal accounts confirmed that all subjects performed the exercises in agreement with the requirements as stated above. The Linebaugh tests were carried out using a 16 oz. resistance (2 dental elastics of 8 oz. combined). This amount was chosen because pilot testing showed that our subjects would easily max out on lower resistance values (the standard for these tests is 12 oz., cf. Grove et al., 1994). Subjects continued until they felt tired or discomfort, whichever came first. Normally, the tests are performed within a standard time frame of 2 minutes, but such a time restriction was too easy for our subjects. At each weekly session, the dental elastics of their device were replaced to prevent material wear out effects on the resistance offered to lip movements.
Instrumentation
EMG recordings were made with 10 mm (contact area 2 mm) Ag/AgCI surface electrodes (Gerionics), connected to low noise medical isolation amplifiers (lntronics IA296) and analogue filters (low pass at 1000 Hz, high pass at 10 Hz, and a notch filter at 60 Hz). The electrodes were positioned 1.5 cm to the left and right of the midsagittal line of the upper lip (UL) and lower lip (LL) at the vermilion border (cf. van Lieshout, Starkweather, Hulstijn, & Peters, 1995). EMG signals were digitized at 2000 Hz.
Upper lip (UL), lower lip (LL), tongue, and jaw positions were collected using the Electromagnetic Midsagittal Articulograph or EMMA system (AG100, Carstens Medizinelektronik GmbH, Germany). Sensor positions were sampled at a rate of 400 Hz. The UL and LL receivers were placed in the midsagittal line at the vermilion border. The tongue receivers were placed 1 cm behind the tongue tip (blade) and 3 cm behind that position (mid-tongue), and a third coil was placed as far back as possible on the surface of the tongue (tongue-back). The jaw receiver was placed on a custom-made thermo-plastic mould that was attached to the lower incisors in the midsagittal line. Head position reference coils were placed at the gums of the upper incisors, and the bridge of the nose. Before the start of an experimental session we collected data on the occlusal plane for each subject, using a custom made device with two receivers spaced 3 cm apart. These reference positions were used to rotate all data with respect to a standard X-axis as defined by the subject's occlusal plane (Westbury, 1994). Lower lip signals were corrected for jaw movements using an estimate of jaw rotation based on the principal component of the mandible sensor coil trajectory for each trial (Westbury, McClean, & Lindstrom, 2000). In this paper, we will focus on lower lip movements corrected for jaw influences using the method described above. Further details can be found elsewhere (van Lieshout and Moussa, 2000; van Lieshout, Rutjens, & Spauwen, 2002). Acoustic data were collected simultaneously with EMMA data using a sampling rate of 16,000 Hz. All further data processing was done in Matlab, version 5.3 (The MathWorks, Inc.).
Dependent variables
EMG The raw EMG signals were rectified, down sampled to 100 Hz (after appropriate filtering), and processed using a moving average window of 40 ms. From this signal, a 300 ms segment was selected from either the initial or final part of the trial, depending on which part showed EMG baseline activity (Baseline activity is defined as minimal EMG activity corresponding to a resting position of the lips (cf. Schievano et al, 1999)). For this interval, an average EMG level and standard deviation (SDn) was calculated; these values were used to define a threshold (= average baseline level + 1 SDn). Finally, a peak-picking algorithm was used to find peak amplitudes in the IEMG as related to the individual task performances within a trial. In addition, average IEMG amplitude was calculated as defined by the average signal amplitude above the calculated threshold for a given trial. Figure 3 shows an example of both raw EMG and IEMG signals. In the latter, the markers indicate peak values for UL and LL. In this report, the focus will be on the peak IEMG amplitudes, but the average IEMG amplitudes showed a similar pattern. IEMG peak values were normalized with respect to the average peak values found for the Facial-Flex task using a 16 oz. resistance (Task 2 in Table 1). This is in line with current standards in EMG research where "the EMG should be normalized [...], i.e., expressed in relation to a reference value obtained during standardized and reproducible conditions" (Burden & Bartlett, 1999, p. 247). It is also the same method used by Kuehn and Moon (1994) to normalize their raw EMG values. For this amount of resistance, subjects in this study theoretically had to produce a force of almost 8 N at maximum deflection (see Figure 2). Findings on inter-angle force (i.e., resultant force sampled between the comers of the mouth perpendicular to the midsagittal plane) indicate that such force levels are below the absolute maximum levels people potentially can generate at these interangle distances, but still much higher than typically found for speech and non-speech tasks (Barlow & Muller, 1991). Correspondingly, for each subject none of the EMG values during any other task exceeded the values found for the Facial-Flex task at the 16 oz. resistance.
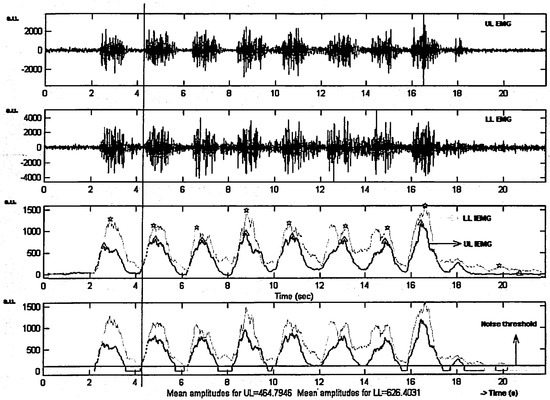
Figure 3.
Raw EMG Upper Lip (upper panel) and Lower Lip (second panel) and the integral of the rectified EMG over a time inteNal of 400 msec (IEMG) for both lips (third and fourth panel) for subject M2 while performing the FFT task. Markers in the third panel indicate peak IEMG values for UL (triangle) and LL (star). The horizontal line in the fourth panel indicates the baseline activity threshold used to calculate average IEMG across the whole trial. The vertical line indicates onset IEMG for UL and LL for the second repetition of the task.
EMMA – Individual movements Movement data were smoothed with an 11- point triangular filter (effective low pass frequency 27.5 Hz) before data processing. All movement signals were band-pass filtered between 0.1 Hz (removing slow varying drifts) and 6 Hz (all relevant frequencies were found below this cut-off point) using a 7th-order Hamming window Butterworth filter. Using upper and lower lip position and velocity signals, we calculated the following kinematic measures, separate for upper lip and lower lip:
- Movement amplitude (in mm): the range of movement between onset and offset of lip closing and opening [AMP]
- Peak velocity (in mmls): the maximum velocity achieved during lip closing and opening [PVEL]
- Movement duration (in msec): the duration of lip closing and opening [DUR]
- Normalized Time to peak velocity or Velocity Profile Symmetry index (in %): the interval between movement onset and the temporal location of the peak in the velocity profile normalized for movement duration [VPS]
These measures are routinely used in speech motor research (e.g., see Munhall, Ostry, & Parush, 1985; Van Lieshout, & Moussa, 2000) and provide a basic description of movement characteristics. In this study, we only focused on lip closing movements for two reasons. First, in reiterative tasks the differences between opening and closing movement are small (van Lieshout & Moussa, 2000). Second, closing movements are more sensitive to the effects of this type of muscle training, in the way they require Orbicularis Oris muscle activation (McClean & Clay, 1995).
The onset and offset of closing and opening movements are derived from automated peak-picking algorithms that detect peaks and valleys in the position and velocity signals using relative amplitude (proportion of maximum amplitude) and time (minimum interval between successive events) criteria. The parameters for the criteria were determined by calculating a cyclic spatio-temporal index or STI (cf. Smith, Goffman, Zelanznik, Ying & McGillen, 1995) with different parameter combinations. For each parameter combination, individual cycles (as defined by the peaks and valleys in the signal) were amplitude and time normalized and aligned with each other. Separate standard deviations for the overlapping segments are then computed at 2% intervals in relative time, the sum of which defines cSTI. This index provides a measure for the uniformity of a cyclic movement pattern. The computer selected the amplitude and time parameter combination that generated the lowest cyclic STI values. These values were used as a separate variable on movement stability (see van Lieshout et al., 2002, for more details).
EMMA – Inter-lip coordination patterns In order to quantify the nature and stability of inter-lip coordination before and after the training period we calculated continuous estimates of relative phase [PHI] and within-trial standard deviations of relative phase [SD PHI]. Relative phase reflects underlying (relatively) stable states in coordination as well as qualitative changes in these states without being influenced by ongoing transient changes in movement durations (Saltzman, Lofqvist, Kay, Kinsella-Shaw, & Rubin, 1998; van Lieshout et al., 2002). For the relative phase analysis, positive position and velocity signals of the upper lip and lower lip were amplitude normalized between O and 1; negative values were normalized between O and-1. From the normalized position and velocity functions, we calculated phase-time functions. Finally, relative phase values were derived by subtracting individual phase-time functions of upper and lower lip (Kelso et al., 1986; van Lieshout et al., 2002).
Data Analysis
IEMG and EMMA data were used to evaluate the effects of the 8-week training period using the Facial-Flex device. Given the small number of subjects, we performed separate analyses of variance for each subject, testing the effects of SESSION (Baseline 1, Baseline 2, & Post), TASK (non-speech & speech), and LIP (upper lip, lower lip) on the dependent variables as described above. When a significant main effect was found for SESSION, we used a Tukey-Kramer Multiple Comparison Test to test for differences between individual sessions. With a Bonferroni correction based on 8 individual tests per subject, the conservative level of significance is p < 0.006. However, to allow the reader a more tolerant inspection of potential relevant differences at or below the non-corrected level of significance (p < 0.05), F-values will be marked with non-corrected indices in the appropriate table, showing corrected significance values in bold.
Originally, we wanted to include all tasks for the TASK factor, but some tasks showed very little IEMG activity, so we had to select certain nonspeech and speech tasks for the IEMG comparisons. Specifically, we selected for the non-speech TASK category (normalized) peak IEMG values from tasks 1, 5, 6, and 7 as listed in Table 1. For the speech TASK category, we averaged across the same number of tasks—specifically, tasks 8, 10, 11, and 12 (Table 1). For subject M1 no upper lip EMG data was obtained, since his moustache interfered with the attachment of surface EMG electrodes.
For the kinematic and relative phase data, we selected tasks based on signal quality for upper and lower lip movements. For some of the nonspeech tasks, signals were extremely small and noisy, which forced us to exclude certain tasks used for statistical analysis. Specifically, we selected tasks 2, 5, and 7 for the nonspeech TASK category, whereas we were able to use a larger number of tasks for the speech TASK category (11, 12, 13, 15, 16, & 17). All analyses of variance were performed with the statistical software package NCSS (Hintze, 1998).
RESULTS
Linebaugh testing
Figure 4 shows the results (both individual and group mean data) for the four subjects on the Linebaugh tests. The inset shows the percentage change between the average baseline values and the values after 8 weeks of training.
A number of observations can be made. First, the two separate baseline measures indicate a stable baseline across time (one week apart) for both tests. Once the training started, subjects showed on average a stronger relative improvement (from average baseline to week 8) in the repetition task (Mean = 731.5 %) as compared to the compression task (Mean = 377.8 %). Individual differences are evident. For both tests, M1 showed the strongest relative performance increase, followed by F1, F2, and finally, M2. The latter subject only showed a relatively small improvement on both tests. Obviously, requiring subjects to perform these tests until they feel tired might result in different individual subjective perceptions of ''tiredness". This could clearly have influenced their performance on these tests. Still, in general all subjects showed improvement compared to their baseline performance, underscoring the statements from the subjects' logs and personal descriptions that they were committed to the prescribed exercise regime.
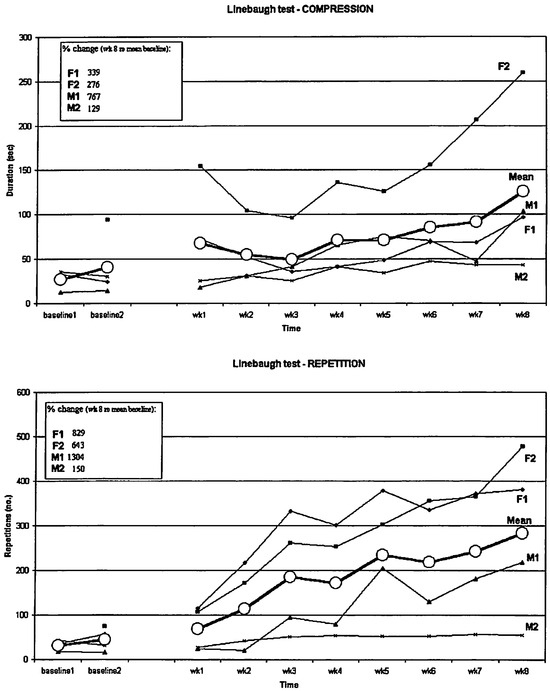
Figure 4.
Unebaugh tests results (repetition + compression). For subject F2 there were no Baseline 1 data on both tests. Inset shows percentage difference between the average value for the baseline sessions and the last session at week 8. (See text for more details).
IEMG-normalized peak values
Table 2 shows the means and standard deviations for the normalized peak IEMG values. Table 3 shows a summary of the ANOVA results for all dependent variables, with non-corrected significant p-values indicated by symbols (* p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001), and Bonferroni corrected significant p-values (p ≤ 0.006) indicated in bold.

Table 2.
SESSION (Baseline 1 = BL 1; Baseline 2 = BL2; & POST) by TASK (Non-speech = NS; Speech = SP) by LIP (Upper Lip = UL; Lower Lip = LL) means and standard deviations (SD) for normalized peak IEMG values (% of FFT @ 16 oz.). See text for details.
Table 2.
SESSION (Baseline 1 = BL 1; Baseline 2 = BL2; & POST) by TASK (Non-speech = NS; Speech = SP) by LIP (Upper Lip = UL; Lower Lip = LL) means and standard deviations (SD) for normalized peak IEMG values (% of FFT @ 16 oz.). See text for details.
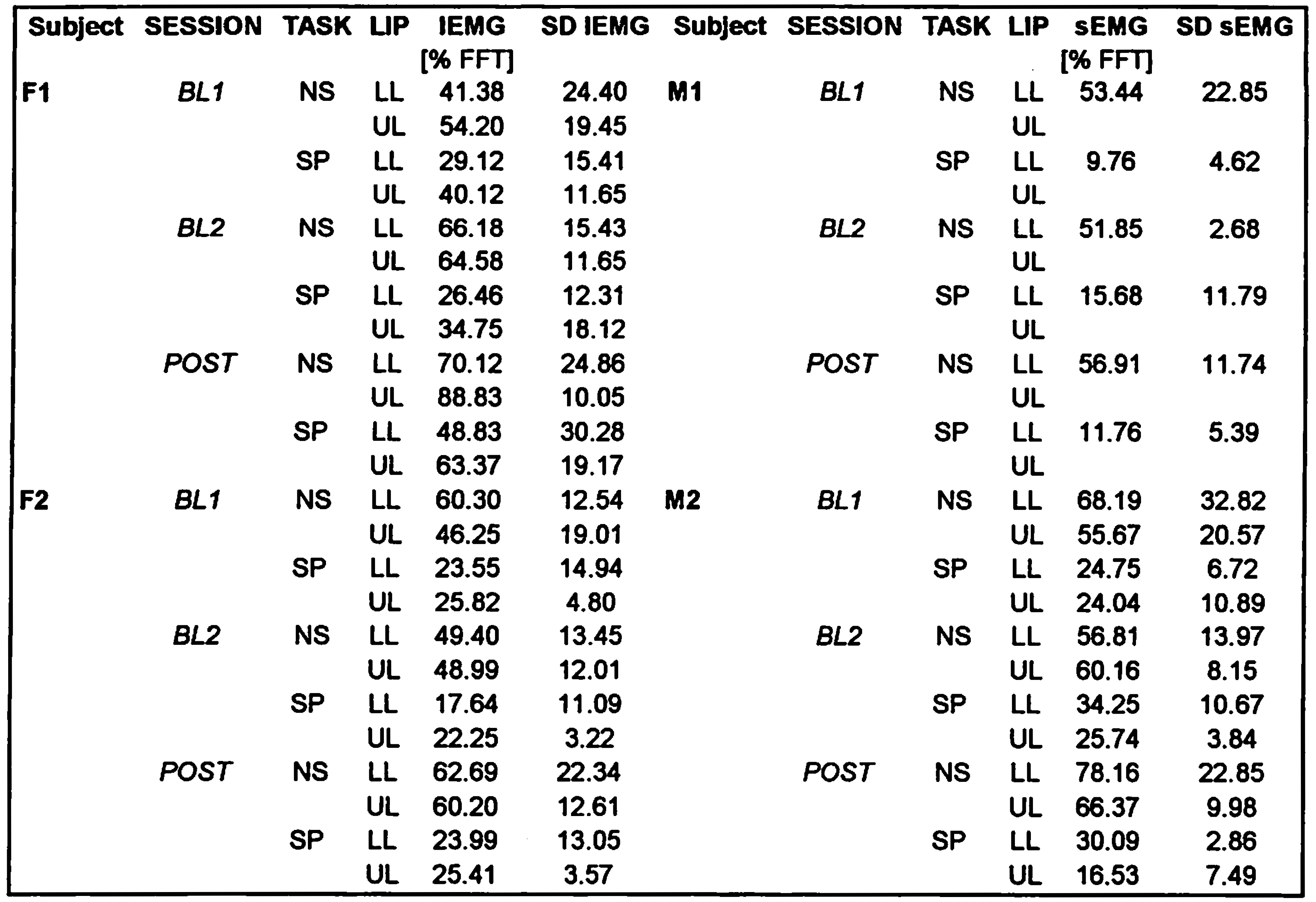
Subject F1 showed a significant main effect for session, which was based on a significant higher IEMG peak value at the POST session, compared to each of the two baseline sessions (see Figure 5). She also showed a main effect for TASK, indicating significantly lower IEMG values overall for the speech tasks compared to the nonspeech tasks. No other comparisons were found to be significant for this subject.
The situation was quite different for the other three subjects, who did not show a significant SESSION effect. This means that despite an 8-week training period with the Facial-Flex device, their normalized EMG activity did not show any systematic change towards higher values. All three subjects did show a significant main effect for TASK, which similar to subject F1, indicated on average lower peak IEMG values for the speech tasks, compared to the nonspeech tasks. It is also interesting to note that none of the subjects showed a significant effect for LIP, meaning that for normalized peak IEMG values, both lips show on average comparable values. Had we compared raw EMG values, lower lip IEMG would most likely have shown more activity than the upper lip (cf. Van Lieshout et al., 1993), but once normalized for their activity at a maximum voluntary contraction task (in this case the FFT task at a 16 oz. resistance), these differences disappear. Each lip seems to operate at a comparable range of its own potential muscle output, depending on the type of task. Across the four subjects, the upper lip shows an average normalized peak IEMG activity of around 61% on nonspeech tasks and 31% for speech tasks, whereas the lower lip shows 60% for the nonspeech tasks and around 25% for the speech tasks. These TASK differences for lip IEMG activity are very similar to the findings reported on speech versus nonspeech task differences in levator muscle activity (Kuehn, & Moon, 1994).
Kinematic data – individual movements Table 4 shows the means and standard deviations for the kinematic data. The results of the ANOVA comparisons are listed in Table 3.
Subject F1 showed a significant amplitude and peak velocity SESSION effect, both of which indicated that after 8 weeks of training with the Facial-Flex device, her lip movements were larger and with higher peak velocities compared to each of the two baseline sessions (see Figure 6). She also showed significant TASK effects for peak velocity, cSTI and VPS, with trends (p < .05) for movement duration and amplitude. In general, lip movements for speech tasks proved to be larger, faster, less variable, and more asymmetric compared to the nonspeech tasks (see Table 4). There were also main effects for LIP showing significantly larger and faster movements for the lower lip compared to the upper lip (see Table 4). For amplitude (F(1,42) = 10.56, p < 0.003) and peak velocity (F(1,42) = 13.12, p < 0.001), there was a significant interaction between the TASK and LIP factors, which indicated that the larger amplitude and higher peak velocities found for speech tasks were only seen for the lower lip data. Other test results did not reach the corrected p-value for this subject.

Table 3.
Summary of ANOVA results for all dependent variables, with tests for main effects of SESSION (Baseline 1 = BL1; Baseline 2 = BL2; & POST), TASK (Non-speech = NS; Speech = SP) and LIP (Upper Lip = UL; Lower Lip = LL). Non-corrected significant p-values are indicated by symbols (* p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001), and Bonferroni corrected significant p-values (p ≤ 0.006) are indicated in bold. Interaction effects are discussed in text.
Table 3.
Summary of ANOVA results for all dependent variables, with tests for main effects of SESSION (Baseline 1 = BL1; Baseline 2 = BL2; & POST), TASK (Non-speech = NS; Speech = SP) and LIP (Upper Lip = UL; Lower Lip = LL). Non-corrected significant p-values are indicated by symbols (* p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001), and Bonferroni corrected significant p-values (p ≤ 0.006) are indicated in bold. Interaction effects are discussed in text.
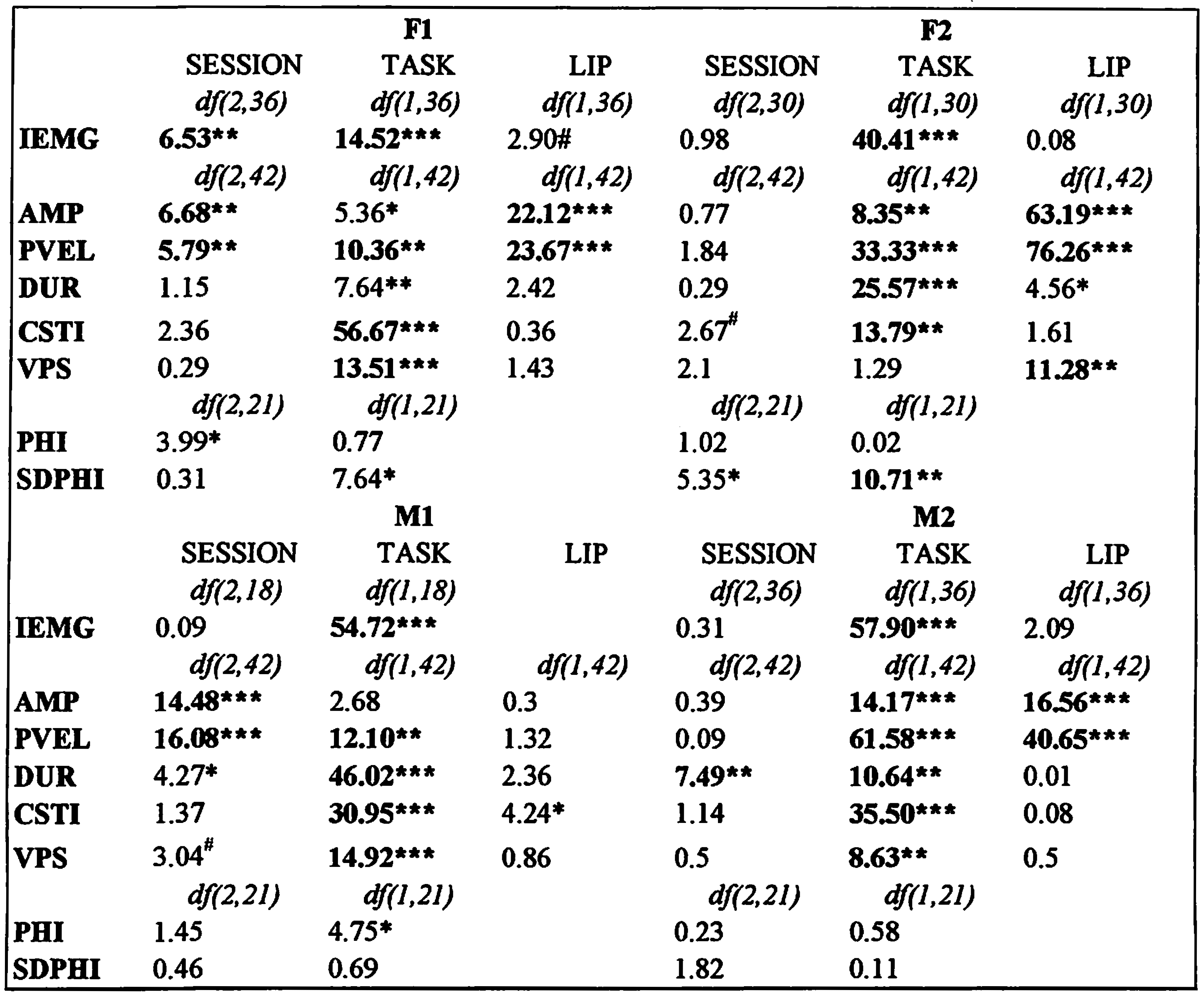
The SESSION effects for the other subjects were mixed. Subject F2 showed no significant SESSION effect, but subject M2 showed a significant increase in movement duration after 8 weeks of training. Post-hoc tests revealed that only the difference between the first baseline and the POST session was significant, since a slight increase in movement duration was already present in the second baseline session (see Table 4). Subject M1 showed significant SESSION main effects for amplitude and peak velocity. For both variables, the value at the POST session was smaller compared to the baseline values, however, only for peak velocity the POST value was actually significantly smaller compared to both. For amplitude, it was the value at baseline 2 that proved to be significantly higher compared to the other two sessions, whereas the baseline 1 and POST session values did not differ. In any case, this effect was clearly opposite to the SESSION effects found for subject F1. Subject M1 also showed an increase in movement duration for the POST session, similar to subject M2, but the effect did not reach the corrected p-value (Table 3).
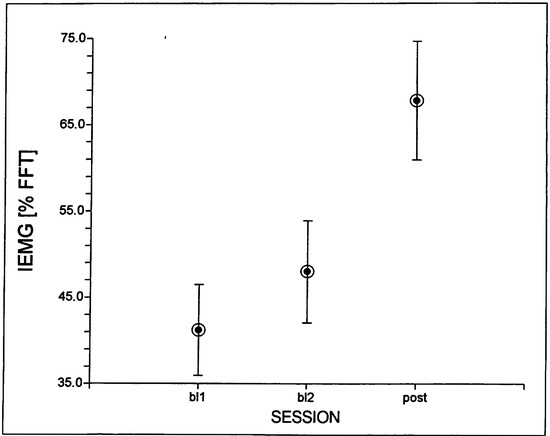
Figure 5.
Mean (and standard errors) SESSION values for normalized peak IEMG across both lips for subject F1.
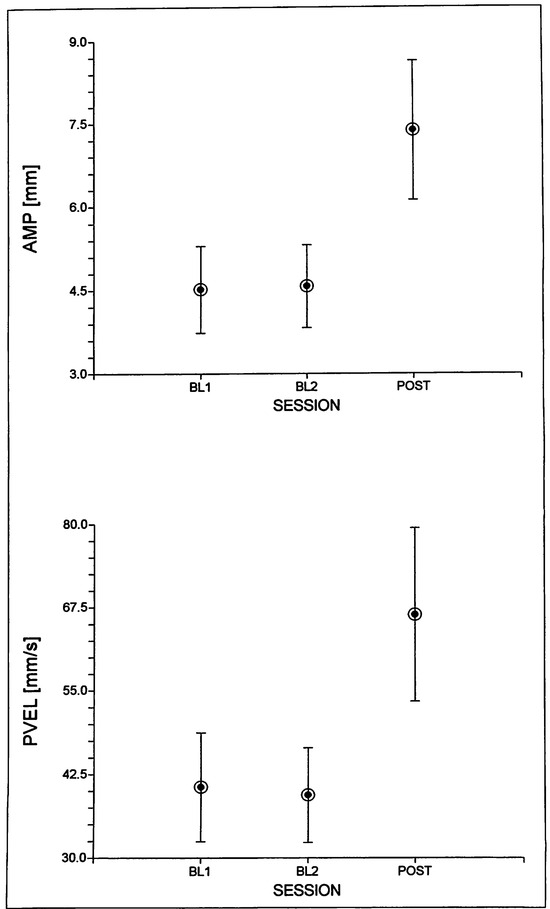
Figure 6.
Mean (and standard effors) SESSION values for movement amplitude [AMP] and peak velocity {PVELJ across both lips for subject F1.

Table 4.
SESSION (Base/me 1 = BL1; Base/me 2 = BL2; & POST) by TASK (Non-speech = NS; Speech = SP) by LIP (Upper Lip = UL; Lower Lip = LL) means and standard deviations (SD) for kinematic variables (see text for details).
Table 4.
SESSION (Base/me 1 = BL1; Base/me 2 = BL2; & POST) by TASK (Non-speech = NS; Speech = SP) by LIP (Upper Lip = UL; Lower Lip = LL) means and standard deviations (SD) for kinematic variables (see text for details).
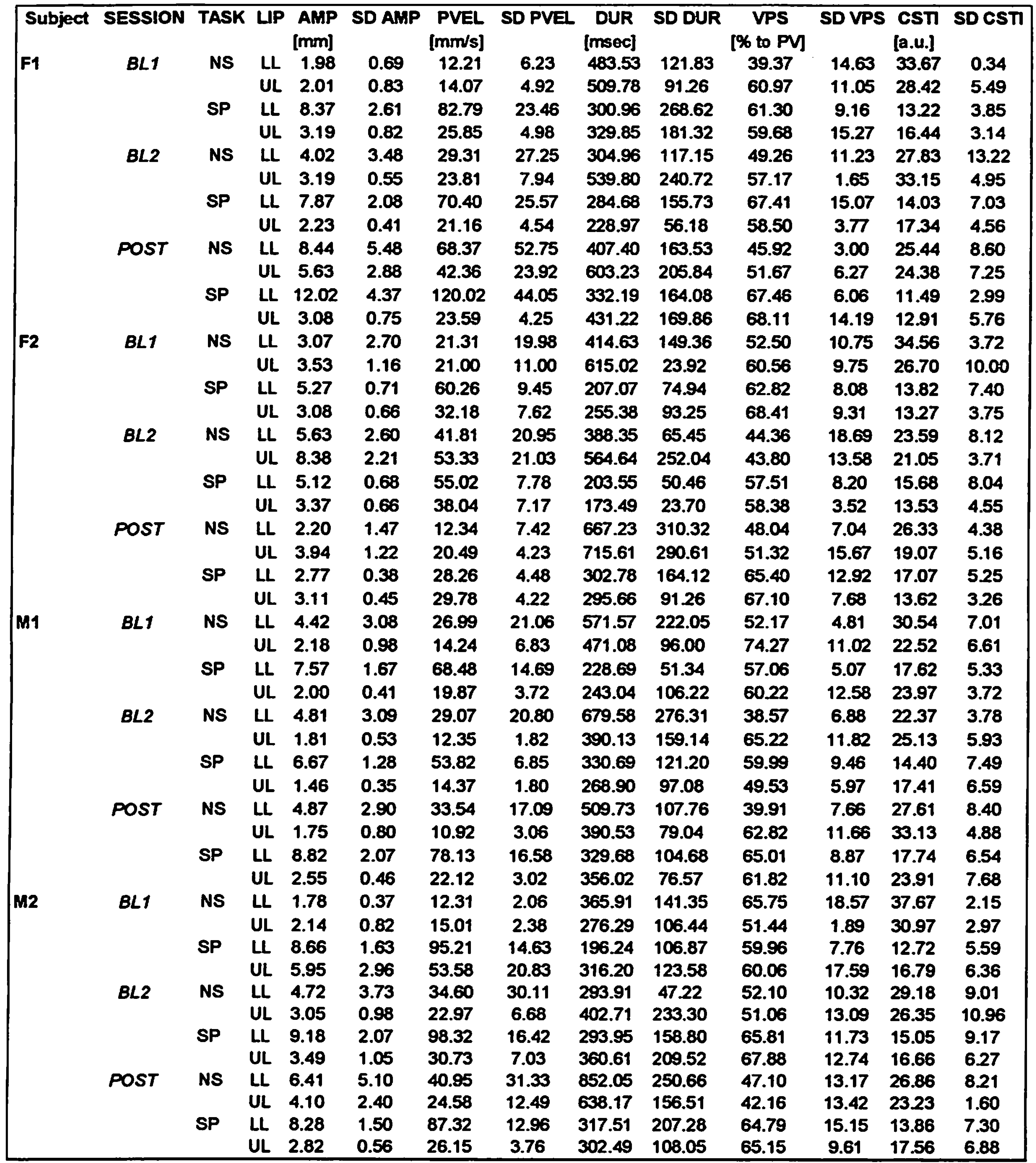
As for subject F1, TASK main effects were significant for the other three subjects in various kinematic variables. Speech movements in general were larger (except for M1), faster, shorter in duration, less variable, and more asymmetric (except for F2). In short, these TASK effects seem to replicate the TASK effects for F1 and convey a robust finding about kinematic differences between nonspeech and speech tasks (cf. Kuehn, & Moon, 1994).
Differences between upper lip and lower lip were evident for subjects F2 and M2, both showing larger and faster lower lip movements, similar to F1. For subject F2, the lower lip also showed a more symmetrical velocity profile. Subject M1 showed no significant LIP effects, only a trend for slightly more variable lower lip movements (Table 3). Subject M2 showed an interaction between TASK and LIP with a trend for amplitude (F(1,42) = 5.71, p < 0.05) and a significant effect for peak velocity (F(1,42) = 22.34, p < 0.001). This was similar to the findings for subject F1, with most of the increase in both variables for speech tasks seen in the lower lip.
Subject M1 also showed an interaction between both factors for peak velocity (F(1,42) = 8.92, p < 0.005), but not for amplitude. Subject F2 did not show a main effect for VPS in comparing nonspeech with speech tasks, but she did show an interaction between TASK and LIP (F(1,42) = 20.34, p < 0.001). For speech tasks, the velocity profiles of both lips became more symmetrical, compared to the asymmetrical profiles for both lips in the nonspeech tasks. Other effects were not found to be significant.

Table 5.
SESSION (Base/me 1 = BL1; Base/me 2 = BL2; & POST) by TASK (Non-speech= NS; Speech= SP) by LIP {Upper Lip = UL; Lower Lip = LL) means and standard deviations (SD) for relative phase variables (see text for details).
Table 5.
SESSION (Base/me 1 = BL1; Base/me 2 = BL2; & POST) by TASK (Non-speech= NS; Speech= SP) by LIP {Upper Lip = UL; Lower Lip = LL) means and standard deviations (SD) for relative phase variables (see text for details).
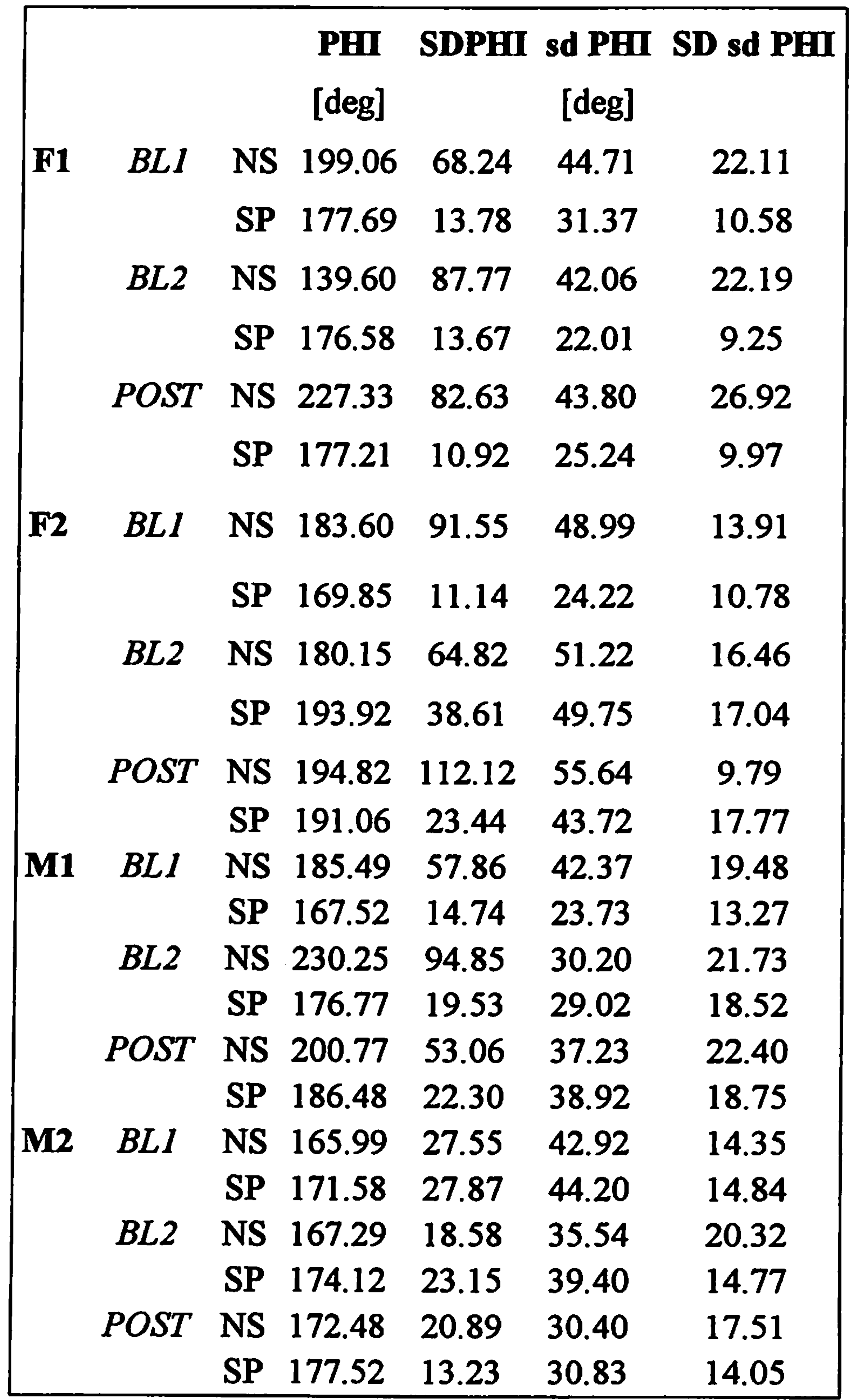
Kinematic data - lnterlip coupling Table 5 shows the means and standard deviations for the relative phase data. The results of the ANOVA comparisons are listed in Table 3.
None of the 4 subjects showed a significant SESSION effect for either relative phase or its within-trial variability. Subject F1 showed a trend for higher relative phase values after 8 weeks of training, but post-hoc tests revealed no significant differences between any of the sessions. Subject F2 showed a SESSION trend for the within-trial standard deviations in relative phase, but post-hoc tests revealed that this was based on lower SD values of relative phase at baseline 1.
TASK effects were not found for relative phase, except for a trend in subject M1 to show lower relative phase values in speech tasks. For the within-trial SD of relative phase, we did find a significant main effect for subject F2, showing less variable coordination patterns in the speech tasks. This was paralleled in a trend found for subject F1 for the same variable. No other effects were found to be significant
DISCUSSION
To summarize the main findings, the data showed that after an intensive 8-week training period using the Facial-Flex device, all subjects showed to some degree an improvement on the Linebaugh performance tests. This is normally taken as an indication for increase in strength and resistance to fatigue (cf. Grove et al., 1994). However, in terms of physiological effects, only one female subject (F1) showed an expected increase in normalized muscle· activity, which was paralleled in significant increases in lip movement amplitudes and peak velocities. The two male subjects showed no changes in normalized lip EMG activity, but they did show an overall increase in their lip movement durations after the 8-week period. The remaining female subject showed no training effect at all. We also found clear evidence for task related differences in oro-motor physiology, as in general speech tasks were executed with less muscle activity, greater amplitudes, faster speeds (and shorter durations), and less variable movement cycles compared to the nonspeech tasks. For two subjects (F1 and M2) these task effects were most prominent for the lower lip. Training effects were not evident for either the nature or variability of the coordination patterns between both lips. The two female subjects showed some evidence for less variable coordination patterns in the speech tasks compared to the nonspeech tasks.
With respect to subject F1, the findings from the general performance tests and from the physiological variables are in clear agreement. The Linebaugh test results indicate a steady improvement in both the number of repetitions and the maximum duration of holding the Facial Flex device in a position at which the dental elastic is fully stretched(= maximum resistance), although the latter effect was not as clear as the former. For this subject, the daily exercises for an 8-week period resulted in higher normalized EMG activity levels and movement amplitudes and peak velocities. Higher peak velocities are often associated with higher EMG levels and taken as an indication of physiological effort (McClean, & Runyan, 2000). So, in this sense we would argue that for subject F1 the exercise program gave rise to a stronger physiological effort in executing lip movements for both speech and nonspeech tasks. This is comparable to the findings described by Kuehn and colleagues (Kuehn, Moon, & Folkins, 1993; Kuehn, & Moon, 1994) who reported increased normalized EMG levels for velar movements against higher levels of resistance, which in their case involved an increase in air pressure.
For the other three subjects, the results were very mixed and none of them showed the same pattern of outcome as reported for subject F1. The two male subjects varied considerably in their results on the Linebaugh tests, with M1 showing the largest improvement and M2 showing the least. However, in terms of their physiological response to the exercise program, both showed a very similar effect in that their lip movements took longer to complete, regardless of type of task. This is a rather unexpected result, when compared to findings reported in the literature on the effects of strengthening exercises with respect to movement speed and/or duration. For example, a recent study (Kauranen, Siira, & Vanharanta, 1998) with healthy adult woman between 25 and 45 years involved a 10-week muscle strength-training program of the upper extremities. The results showed an increase in muscle activation, similar to the findings for lip muscle activity for subject F1 in the present study. However, Kauranen et al. also found an increase in the speed of (relative simple) motor functions like tapping. Likewise, data from a (elbow extensor) muscle-strengthening program also showed an increase of muscle strength and an increase in movement speed (Jaric, Ropret, Kukolj, & Ilic, 1995). Finally, a study on the effects of dynamic exercises against a constant load of 30-40% of maximal muscle strength reported an increase in muscle output and (ballistic) movement speed (Van Cutsem, Duchateau, & Hainaut, 1998). These authors related the increase in muscle contraction speed to changes in motor unit activation timing, the occurrence of extra motor interspike intervals (doublets) and an enhanced maximal firing rate.
The present study replicates these findings regarding increased muscle output for strengthening exercises for one subject (F1), but none of the subjects showed faster lip movements after training; on the contrary, movement duration increased in subjects M1 and M2. One possible reason for this unusual result may relate to the speed of movements at which the Facial-Flex exercise was performed. Subjects practiced at a relatively slow rate (about 1 Hz per exercise cycle of stretching and releasing the dental elastic) for 8 weeks in a row, twice a day. It is possible that there was some transfer of speed requirements from the FFT task to other tasks using the same muscles, even though the tasks used in the EMG + EMMA sessions were not practiced during the 8- week training period. It is unclear whether the fact that this effect only happened in the male subjects has any significance. With only 2 subjects in each group, we will not speculate on possible gender differences in this respect. Further studies with larger groups will have to address this issue in more detail.
Regarding interlip coordination, it is interesting to notice that despite the fact that the subjects had practiced between-lip movements in a particular and consistent manner for 8 weeks, this had no significant effect on either the nature or stability of lip coupling in other tasks. It is known from the literature that after people have learned a new type of coordination, this can influence their pre-existing coordination patterns as well (cf., Zanone & Kelso, 1992). If the same principle can be applied to speech motor control, our non-significant findings could indicate that the way the lips are moved during the exercise program is not sufficiently different from normal lip coordination as seen in our selection of nonspeech and speech oro-motor tasks. Thus, the dynamics of the system did not require a change in the nature or stability of the lip coupling for these latter tasks. Some support for this claim comes from the relative phase data for lip coordination during the execution of the Facial-Flex task with the 16 oz resistance. The average (across subjects) relative phase value for this task was 199.88 deg (SD = 84.43), which falls within the ranges of values found for the other nonspeech tasks (mean = 180.91 deg; SD = 64.56), and the speech tasks (mean = 178.38; SD = 20.54). Of course, this FFT task differs from the FFT task performed at home with only 6 oz of resistance, but in terms of lip coordination they are likely to be very similar if not identical given the constraints implemented by the layout of the device. Potentially, this finding could mean that if patients with speech disorders show aberrant lip interactions during speech production, the use of the Facial-Flex device might be helpful in the way it could enforce a pattern of coordination that falls within normal ranges. Obviously, the potential benefit of the Facial-Flex program to facilitate or change movement coordination requires further study in patient populations where lip coordination is indeed effected.
- Caveats with respect to training outcomes
The results of this study on the outcomes of an 8-week training period using the Facial-Flex exercise have to be taken with some caution. A number of issues need to be addressed before any firm conclusions can be drawn regarding the effectiveness of this device.
First, the data are based on a small group of normal speaking subjects and further testing with larger groups of people with and without speech disorders will be necessary to replicate the present positive and negative findings. However, it is clear from the present findings that individual differences will be prominent and have to be taken into account, regardless whether larger group comparisons would yield stronger effects.
Second, as mentioned in the method section, the device works with a fixed distance between fully stretched and fully relaxed dental elastic positions. However, individuals differ in the anatomical layout of their lip muscles, which in tum has an effect on the muscle length - force output relationship as defined by interangle span (Barlow & Muller, 1991). This means that given individual differences in actual muscle length at a fixed stretched position of the Facial-Flex device, the effective force generating capabilities for each subject at the training resistance of 6 oz. were potentially different For the three subjects in our study where training effects were minimal if not absent, the chosen resistance of 6 oz. may not have provided a sufficient "workout'. One way to control for individual differences in muscle force generating capabilities is to actually measure force output from the lips (cf. Barlow, 1998) and use that information to determine the optimal resistance for a given subject. That is, if the objective is to increase muscle strength proper (see caveat #4). The Linebaugh tests do not seem to provide a good substitute for this kind of information. For example, at the baseline sessions all our subjects showed a very similar performance (see Figure 4). In general, the performance on endurance tasks as used in the Linebaugh tests is not only determined by physicaVphysiological factors, but also, and perhaps even more, by psychological factors. For example, as already mentioned, people might differ widely in their willingness to continue with the test at certain levels of discomfort or fatigue. We think that an individualized approach using more objective assessments of force output might be a better way to optimize the effects of training, and we are currently exploring this aspect in a follow-up study with patients with facial muscle weakness.
Third, there is no simple reliable way to monitor the actual performance during the home exercises. Even though we are convinced based on the individual reports and logs of our subjects that they performed their exercises on a daily basis, we cannot be sure they performed them in the way that would guarantee the best outcome. This of course is a problem for all home-based exercise programs. In our view, the Facial-Flex program requires regular monitoring by clinicians to remind and help the clients in using the device in an appropriate manner and to keep them motivated. The weekly visits to the lab by our subjects certainly addressed both issues, and the logs as recommended by the Facial-Flex company play a critical role in this respect.
Fourth, an increase in relative muscle output may not always be the desired target for orofacial therapy. For example, a recent study describes how children with a habitual open-mouth posture were treated with a variety of orofacial exercises to induce less EMG activity during a closed lip position in order to promote nasal breathing (Schievano, Rontani, & Berzin, 1999). Another study used orofacial therapy to improve facial mobility, not muscle strength (Katsikitis & Pilowsky, 1995). Clearly, depending on the nature of the disorder (muscle weakness, rigidity, hypertension) different treatment approaches are needed, and this would include the potential use of the Facial-Flex device. For example, a recent paper suggests that the Facial-Flex exercise may counteract the negative effects of scarring tissue after lip surgery by enhancing lip mobility (Zide et al., 2000). In the latter case, the best approach is most likely to keep the resistance low and to put more emphasis on the rate and extension of the lip movements. It is also important to be aware of potential negative effects of prolonged fatiguing exercises on articulator control (e.g., Solomon, 2000) and avoid such situations, in particular with patients with neuromotor speech disorders.
Fifth, our study did not address the maintenance of exercise effects. Most likely, however, in order for these effects to endure, training needs to be continued and monitored on a regular basis (cf., Gommerman et al., 1995). What the most effective exercise regime will be to sustain training effects on the middle and long term is unclear at the moment, but obviously, this will depend on a number of factors, most of which are related to individual circumstances (role of spouses and other family members) and health care regulations to support follow-up visits (see also Robertson, 2001, on some of these issues).
Finally, our study only looked at lip data. The effects of the Facial-Flex program on other articulators have not yet been studied explicitly. The study by Grove et al. (1994) indicates global effects on facial musculature, but this was related to facial appearance (skin) and not to the actual functioning of the muscles.
Effects of tasks
Although not a primary focus for this study, a few words on TASK effects are in order. Foremost, it is obvious that non-speech tasks and speech tasks are very different in terms of muscle activation and kinematic behaviors. Non-speech tasks were found to show relatively higher muscle outputs, to be executed at a slower rate with smaller movement amplitudes, and to have more variable movement cycles compared to speech tasks. Such qualitative differences between both categories of tasks are also noted in the literature (e.g., Kuehn & Moon, 1994). As shown in the movement duration data for two subjects in this study, there is a potential for transfer of some of the Facial-Flex task characteristics to other tasks. The success of transfer of certain motor characteristics to a particular task as a whole depends on a number of factors (e.g., Schmidt, 1988), and these need to be critically assessed and addressed in light of the targets for a particular intervention (cf. Ruscello, 1995 for a similar discussion on the role of appliances in the treatment of phonological disorders). Otherwise, results may be less than satisfactory to both client and therapist. In general, the use of nonspeech tasks to improve speech function needs to be carefully evaluated (cf., Duffy, 1995), but not discarded a-priori without a better understanding of the control mechanisms underlying both.
CONCLUSIONS
Based on the IEMG and kinematic data from four subjects with no history of muscle weakness who used the Facial-Flex device with a fixed resistance (6 oz.) twice a day for 8 weeks, it was found that on average, their performance improved on the Linebaugh tests. This confirms earlier reports on the effects of using this device. The purpose of this study was to find a physiological underpinning for the effects of this exercise program and to compare these results to the general performance data from the Linebaugh tests. In general, one subject showed a clear match to her Linebaugh test results in making more effortful lip movements after training as indicated in increased normalized muscle activity, movement amplitude and peak velocity. The other subjects did not show such an effect, but two of them did show an increase in lip movement duration. In general, for these subjects the correspondence between Linebaugh test results and physiological changes was unclear. Speech and nonspeech task differences were significant and in line with earlier findings. In the discussion of our data, we highlight certain caveats as related to this study and emphasize the need for further studies with larger populations, in particular regarding speech disorders. However, given that, to our knowledge, no published information on the physiological effects of (mechanical) resistance exercises with facial muscles exists, these data may provide some useful information to consumers and dinicians regarding potential changes in muscle and kinematic function for oral tasks. It is our opinion that the device can have a place within the scope of a broader treatment approach. However, in light of our current observations, we do not recommend its use as a stand-alone training tool for the remediation of complex oro-motor functions in speech production.
Acknowledgments
This work was supported by an operating grant from Facial Concepts, Inc. (Blue Bell, PA, USA). The authors wish to thank Dr. Carol Leonard for her helpful comments and suggestions on an earlier draft of this paper.
References
- Alfonso, P. J., and P. van Lieshout. 1997. Spatial and temporal variability in obstruent gestural specification by stutterers and controls: Comparisons across sessions. In Speech production: Motor control, brain research and fluency disorders. Edited by W. Hulstijn, H. F. Peters and P. H. H. M. van Lieshout. Amsterdam: Elsevier Publishers, pp. 151–160. [Google Scholar]
- Barlow, S. M. 1999. Handbook of clinical speech physiology. San Diego, CA: Singular Publishing Group, Inc. [Google Scholar]
- Barlow, S. M., and E. M. Muller. 1991. The relation between interangle span and in vivo resultant force in the perioral musculature. Journal of Speech and Hearing Research 34: 252–259. [Google Scholar] [PubMed]
- Bigenzahn, W., L. Fischman, and U. Mayrhofer-Krammel. 1992. Myofunctional therapy in patients with orofacial dysfunctions affecting speech. Folia Phoniatrica 44: 238–244. [Google Scholar]
- Blair, C., and A. Smith. 1986. EMG recording in human lip muscles: Can single muscles be isolated? Journal of Speech and Hearing Research 29: 256–266. [Google Scholar] [PubMed]
- Burden, A., and R. Bartlett. 1999. Normalisation of EMG amplitude: an evaluation and comparison of old and new methods. Medical Engineering & Physics 21: 247–257. [Google Scholar]
- Christensen, M., and M. Hanson. 1981. An investigation of the efficacy of oral myofunctional therapy as a precursor to articulation therapy for pre-first grade children. Journal of Speech and Hearing Disorders 46: 160–165. [Google Scholar]
- Cooper, N. S. 1973. Myofunctional therapy. International Journal of Orthodontics 11: 81–87. [Google Scholar]
- Creed, J., and J. R. Spiegel. n.d.Using Facial Flex to assist treatment of articulation disorders. Available online: http://www.facialconcepts.com/corastudy.htm (accessed on 27 September 2001).
- Daglio, S., R. Schwitzer, and J. Wuthrich. 1993. Orthodontic changes in oral dyskinesia and malocclusion under the influence of myofunctional therapy. The International Journal of Orofacial Myology 19: 15–24. [Google Scholar]
- Deschenes, M. E. 1983. The pros and cons of myofunctional therapy. Of dubious value. The International Journal of Orofacial Myology 9: 21–23. [Google Scholar] [PubMed]
- Duffy, J. R. 1995. Motor speech disorders: Substrates, differential diagnosis. and management. St. Louis, MO: Mosby. [Google Scholar]
- Forrest, K., S. Adams, M.R. McNell, and H. Southwood. 1991. Kinematic, electromyographic, and perceptual evaluation of speech apraxia, conduction aphasia, ataxic dysarthria and normal speech production. In Dysarthria and apraxia of speech: Perspectives on management. Edited by C. A. Moore, K. M. Yorkston and D. R. Beukelman. Baltimore: Paul H. Brookes, pp. 147–171. [Google Scholar]
- Garliner, D. 1975. Myofunctional therapy. California Dental Association Journal 3: 34–41. [Google Scholar]
- Gaucher, H. 1977. Myofunctional therapy. Dental Journal 43: 568–572. [Google Scholar]
- Gommerman, S. L., and M. M. Hodge. 1995. Effects of oral myofunctional therapy on swallowing and sibilant production. The International Journal of Orofacial Myology 21: 9–22. [Google Scholar] [PubMed]
- Grove, G. L., B. S. Rimdzlus, and C. R. Zerweck. 1994. A mechanically aided resistance exercise program for sagging facial muscles. The Journal of Geriatric Dermatology 2: 152–158. [Google Scholar]
- Gugino, C. F., and I. Dus. 1998. Unlocking orthodontic malocclusions: An interplay between form and function. Seminars in Orthodontics 4: 246–255. [Google Scholar] [PubMed]
- Hahn, V. 1991. Myofunctional therapy-SO years old already and still misunderstood. Informationen Aus Orthodontie Und Kieferorthopadie 23: 517–524. [Google Scholar] [PubMed]
- Hanson, M. L. 1978. Oral myofunctional therapy. American Journal of Orthodontics 73: 59–67. [Google Scholar]
- Hanson, M. L. 1988. Orofacial myofunctional therapy: historical and philosophical considerations. The International Journal of Orofacial Myology 14: 3–10. [Google Scholar]
- Hartigan, C., J. A. Persing, S. C. Williamson, R. F. Morgan, A. Muir, and R. F. Edlich. 1989. An overview of muscle strengthening. The Journal of Burn Care & Rehabilitation 10: 251–257. [Google Scholar]
- Hintze, J. L. 1998. NCSS 2000 [Computer software]. Kaysville, Utah: NCSS. [Google Scholar]
- Hockel, J. L. 1984. Myofunctional therapy and the practice of gnathologic orthopedics. The International Journal of Orofacial Myology 10: 8–11. [Google Scholar]
- Jaric, S., R. Ropret, M. Kukolj, and D. B. llic. 1995. Role of agonist and antagonist muscle strength in perfonnance of rapid movements. European Journal of Applied Physiology and Occupational Physiology 71: 464–468. [Google Scholar]
- Katsikitis, M., and I. Pllowsky. 1995. A controlled study of facial mobility treatment in Parkinson's disease. Journal of Psychosomatic Research 40: 387–396. [Google Scholar]
- Kauranen, K. J., P. T. Siira, and H. V. Vanharanta. 1998. A 10-week strength training program: effect on the motor performance of an unimpaired upper extremity. Archives of Physical Medicine and Rehabilitation 79: 925–930. [Google Scholar] [CrossRef] [PubMed]
- Kollmitzer, J., G.R. Ebenbichler, and A. Kopf. 1999. Reliability of surface electromyographic measurements. Clinical Neurophysiology 110: 725–734. [Google Scholar] [CrossRef] [PubMed]
- Kondo, E., and T. J. Aoba. 2000. Nonsurgical and nonextraction treatment of skeletal Class Ill open bite: its long-term stability. American Journal of Orthodontics and Dentofacial Orthopedics 117: 267–287. [Google Scholar] [CrossRef]
- Kuehn, D. P., J. B. Moon, and J. W. Folkins. 1993. Levator veli palatini muscle activity in relation to intranasal air pressure variation. Cleft Palate-Craniofacial Journal 30: 1. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, D. P., and J. B. Moon. 1994. Levatorveli palatini muscle activity in relation to intraoral air pressure variation. Journal of Speech and Hearing Research 37: 1260–1270. [Google Scholar]
- Landis, C. F. 1994. Applications of orofacial myofunctional techniques to speech therapy. The International Journal of Orofacial Myology 20: 40–51. [Google Scholar]
- Luschei, E. S., and E. M. Finnegan. 1997. Electromyographic techniques for the assessment of motor speech disorders. In Clinical management of sensorimotor speech disorders. Edited by M.R. McNeil. New York: Thieme, pp. 149–176. [Google Scholar]
- McClean, M. D., and J. L. Clay. 1995. Activation of lip motor units with variations in speech rate and phonetic structure. Journal of Speech and Hearing Research 38: 772–782. [Google Scholar] [CrossRef]
- McClean, M. D., and C. M. Runyan. 2000. Variations in relative speeds of orofacial structures with stuttering severity. Journal of Speech, Language, and Hearing Research 43: 1524–1531. [Google Scholar] [CrossRef]
- McHenry, M. A., J. T. Minton, R. L. Wilson, and Y. V. Post. 1994. Intelligibility and nonspeech orofacial strength and force control following traumatic brain injury. Journal of Speech and Hearing Research 37: 1271–1283. [Google Scholar] [CrossRef]
- Munhall, K. G., D. J. Ostry, and A. Parush. 1985. Characteristics of velocity profiles of speech movements. Journal of Experimental Psychology: Human Perception and Performance 11: 457–474. [Google Scholar] [CrossRef]
- Padovan, B. A. 1995. Neurofunctional reorganization in myo-osteo-dentofacial disorders: complementary roles of orthodontics, speech and myofunctional therapy. The International Journal of Orofacial Myology 21: 33–40. [Google Scholar]
- Page, D. C. 1999. The new dental-medical renaissance. Medically efficacious functional jaw orthopedics. Functional Orthodontics 16: 16–5. [Google Scholar]
- Palacioz, D., and S. Shannon. 1986. Myofunctional therapy: recognition and treatment techniques. Dental Assistant 55: 14–16. [Google Scholar] [PubMed]
- Paul-Brown, D., and R. Clausen. 1999. Collaborative approach for identifying and treating speech, language, and orofacial myofunctional disorders. Alpha Omega Fraternity 92: 39–44. [Google Scholar]
- Reinicke, C., N. Obijou, and J. Trankmann. 1998. The palatal shape of upper removable appliances. Influence on the tongue position in swallowing. Journal of Orofacial Orthopedics 59: 202–207. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S. 2001. The efficacy of orofacial and articulation exercises in dysarthria following stroke. International Journal of Language and Communication Disorders 36: 292–297. [Google Scholar]
- Ruf, S., and H. Pancherz. 1999. Temporomandibular joint remodeling in adolescents and young adults during Herbst treatment: A prospective longitudinal magnetic resonance imaging and cephalometric radiographic investigation. American Journal of Orthodontics and Dentofacial Orthopedics 115: 607–618. [Google Scholar]
- Ruscello, D. M. 1995. Speech appliances in the treatment of phonological disorders. Journal of Communication Disorders 28: 331–353. [Google Scholar]
- Saltzman, E., A. Ltsfqvist, B. Kay, J. Kinsella-Shaw, and P. Rubin. 1998. Dynamics of intergestural timing: a perturbation study of lip-larynx coordination. Experimental Brain Research 123: 412–424. [Google Scholar]
- Sasaki, H., and Y. Shlbasaki. 1994. Application of myofunctional therapy in cases with craniomandibular disorders. The International Journal of Orofacial Myology 20: 27–31. [Google Scholar]
- Schievano, D., M. P. Rontani, and F. Berzin. 1999. Influence of myofunctional therapy on the perioral muscles. Clinical and electromyographic evaluations. Journal of Oral Rehabilitation 26: 564–569. [Google Scholar]
- Schmidt, R. A. 1988. Motor control and learning: A behavioral emphasis. Champaign, IL: Human Kinetics Publishers, Inc. [Google Scholar]
- Smith, A., L. Goffman, H. N. Zelaznik, G. S. Ying, and C. Mcglllem. 1995. Spatiotemporal stability and patterning of speech movement sequences. Experimental Brain Research 104: 493–501. [Google Scholar]
- Snow, M. L. 1983. The pros and cons of myofunctional therapy. Emerging specialty. The International Journal of Orofacial Myology 9: 17–20. [Google Scholar]
- Solomon, N. P. 2000. Changes in normal speech after fatiguing the tongue. Journal of Speech, Language, and Hearing Research 46: 1416–1428. [Google Scholar]
- Umberger, F., and R. G. Johnston. 1997. The efficiency of Oral Myofunctional and Coarticulation Therapy. The International Journal of Orofacial Myology 23: 3–9. [Google Scholar] [PubMed]
- Uner, O., N. Darendeliler, and E. Bilir. 1999. Effects of an activator on the masseter and anterior temporal muscle activities in Class II malocclusions. The Journal of Clinical Pediatric Dentistry 23: 327–332. [Google Scholar]
- Van Cutsem, M., J. Duchateau, and K. Hainaut. 1998. Changes in single motor unit behaviour contribute to the increase in contraction speed after dynamic training in humans. Journal of Physiology 513: 295–305. [Google Scholar] [PubMed]
- Van Lieshout, P. H. H. M., and W. Moussa. 2000. The assessment of speech motor behaviors using electromagnetic articulography. The Phonetician 81: 9–22. [Google Scholar]
- Van Lieshout, P. H. H. M., H. F. M. Peters, C. W. Starkweather, and W. Hulstijn. 1993. Physiological differences between stutterers and nonstutterers in perceptually fluent speech: EMG amplitude and duration. Journal of Speech and Hearing Research 36: 55–63. [Google Scholar]
- Van Lieshout, P. H. H. M., C. W. Starkweather, W. Hulstijn, and H. F. Peters. 1995. Effects of linguistic correlates of stuttering on EMG activity in nonstuttering speakers. Journal of Speech and Hearing Research 38: 360–372. [Google Scholar]
- Van Lieshout, P. H. H. M., C. Rutjens, and P. H. M. Spauwen. 2002. The dynamics of interlip coupling in speakers with a repaired unilateral cleft-lip history. Journal of Speech, Language, and Hearing Research 45: 5–19. [Google Scholar] [PubMed]
- Westbury, J. R. 1994. On coordinate systems and the representation of articulatory movements [letter]. Journal of the Acoustical Society of America 95: 2271–2273. [Google Scholar] [PubMed]
- Westbury, J. R., M. D. McClean, and M. J. Lindstrom. 2000. Tongues without jaws: Some consequences of ignoring jaw rotation. Conference on Motor Speech, San Antonio, February 2-5. [Google Scholar]
- Yamaguchi, H., and M. Sebata. 1995. Changes in oral functions and posture at rest following surgical orthodontic treatment and myofunctional therapy. Evaluation by means of video recording. The International Journal of Orofacial Myology 21: 29–32. [Google Scholar]
- Zanone, P. G., and J. A. S. Kelso. 1992. Evolution of behavioral attractors with learning: nonequilibrium phase transitions. Journal of Experimental Psychology: Human Perception and Performance 18, 2: 403–421. [Google Scholar] [PubMed]
- Zide, B. M., J. P. Bradley, and M. T. Longaker. 2000. Lip service for the stiff upper lip. Plastic and Reconstructive Surgery 105: 1154–1158. [Google Scholar]
- Zimmerman, J. B. 1988. Motivational considerations in orofacial myofunctional therapy. The International Journal of Orofacial Myology 14: 40–48. [Google Scholar]
© 2002 by the author. 2002 van Lieshout, P.H.H.M., Bose, A., Namasivayam, A.K.