Abstract
This report investigates the interaction between the metabolites of the highly virulent bacteria Salmonella enterica and RTGill-W1 cells, a cell line derived from rainbow trout gills. As a facultative intracellular pathogen, Salmonella enterica infects both animals and humans through many routes. Upon entering an organism it can cause severe infection and pathology, which is also influenced by the bacterial metabolites. Although no intracellular presence of the pathogen in the exposed cell line could be detected, a dose-dependent effect of the metabolites on the cell line was observed, as exposure to 5%, 10%, and 20% concentrations led to enhanced metabolic activity and increased cytoplasmic neutral lipid droplets accumulation, whereas the lower dosage of 2.5% induced a lower metabolic rate compared to control and no significant intracellular lipid accumulation. The combination of all of the metabolites might be speculated to have increased the metabolic rate and lipid droplet production at higher concentrations due to possessing a growth factor or an endocrine effect, or as a response to a toxin. This paper may be the first report investigating the effect of a complete bacterial metabolite mixture in cultured cells.
1. Introduction
Salmonella is a genus of the family Enterobacteriaceae, represented by rod-shaped Gram-negative bacteria [1]. As a common cause of foodborne infections, Salmonella can induce a variety of illnesses, from self-limiting gastroenteritis to the potentially fatal systemic illness known as typhoid fever [2]. The concerns regarding this bacterium are also heightened by the fact that it is responsible for over 20 million infections annually [3].
The pathogenicity of Salmonella is defined by its characteristic infection routes, with it being both an intracellular pathogen and an extracellular one [4]. In a pioneering study of the nontyphoidal serovar Typhimurium that took place in 1967, Takeuchi [5] published the first images of intracellular Salmonella enclosed by membrane-bound vacuoles in the initial stages of bacterial intestinal epithelium penetration. These Salmonella-containing vacuoles were considered to have differing biogenesis depending on the host cell type.
Although the mechanisms of intracellular invasion are still not yet fully understood, intracellular invasiveness of Salmonella occurs through the “trigger” and the “zipper” mechanisms, with Salmonella being the first bacteria shown to be able to induce both mechanisms to invade host cells [6]. This study found that other probable mechanisms were at work in invasion, and these might possibly involve the activity of metabolites. The trigger mechanism involves injecting bacterial effector proteins into the host cell using a type III secretion system, which causes cytoskeletal re-arrangements that lead to bacterial engulfment, and the zipper mechanism relies on the bacterial surface’s “resistance to complete killing” proteins (Rck) and phosphoglucomutase domain proteins (PagN) that interact with host cell receptors, triggering actin polymerization and membrane ruffling to engulf the bacteria [7].
Metabolites may play an unrecognized role, especially regarding host cell membranes before the attachment or engulfment of the bacteria, but this is unexplored [7,8]. In addition, bacterial metabolites may have some involvement in the biogenesis of Salmonella-containing Vacuoles before, during, and/or after their development. Epithelial cells have an endogenous protective barrier comprised of both cell and resident bacterial metabolites, but this would be reduced in cell cultures, which creates a need to understand the effect of the invasive bacterial metabolites.
Beyond its invasive capabilities, Salmonella produces a range of metabolites that play key roles in virulence, survival, and host manipulation independent of invasion [8]. These metabolites are short-chain fatty acids (e.g., acetate, butyrate), siderophores (e.g., enterobactin), and lipopolysaccharides. They can modulate host immune responses and thus enhance bacterial colonization [9]. Such bacterial metabolites play a significant role in the development and progression of septicemia, influencing the host’s immune response and causing organ dysfunction as well as critical imbalances in host endocrine and metabolic pathways [10]. The ability of these soluble factors to directly, or indirectly, alter host cell physiology is a critical aspect of pathogenesis. Metabolites need to be investigated as they may operate at the gill cells (or others) to change conditions for invasion independently of currently known infection means.
In this preliminary work, we focused on evaluating the effects of bacterial metabolites on cells by using a fish gill cell line, a model that has been unexplored in research on this topic until now to the best of our knowledge and according to a PubMed database search [11]. Gills are a vital detoxification organ of fish and, comparatively, the gill epithelium is the site of many processes that are mediated by renal and respiratory epithelia in terrestrial vertebrates [12]. Another highly important aspect of choosing this model is that gills, unlike other organs, may present enhanced bacterial resistance [13,14], as they are constantly exposed to the environment and can form symbiotic relationships with nitrogen cycle bacteria [15,16]. Given the presence of a mixture of bacterial metabolites, and the lack of any previous research, a test of cellular invasion by the bacteria was conducted to provide initial results. Specifically, this study aimed to determine the effects of the complete S. enterica metabolite mixture on RTGill-W1 cellular metabolic activity, viability, and lipid accumulation.
2. Materials and Methods
2.1. RTGill-W1 Culturing Conditions
RTGill-W1 (CRL 2523, ATCC), a cell line exhibiting epithelial-like morphology that was isolated from the gill of a normal rainbow trout [17], was cultured at 20 °C in Leibovitz media (L-15, L1518, Sigma, Bucharest, Romania) supplemented with 2% Ultroser G (15950-017, Sartorius, Göttingen, Germany) and 1% penicillin-streptomycin (P4333, Sigma) in 25 cm2 cell treated flasks (Cellbind, Corning, New York, NY, USA).
After reaching ≈70% confluency, the cells were detached from the flask surface with 1X trypsin and 1 × 104 cells/mL were used for the metabolic as well as for the live/dead assay. All cells used were incubated for 24 h in the above-stated conditions prior to exposure to the bacterial metabolites. All control cells were grown only in the fresh media.
2.2. Salmonella Enterica Culturing Conditions
Salmonella enterica subsp. enterica serovar Typhimurium (strain CDC 6516-60, 1408, ATCC) was grown in L-15 media at 20 °C for 48 h until O.D.600 of 0.218 was obtained. The same culture media as that used for the cell line was chosen for culturing S. enterica in order to avoid any possible interference caused by different media ingredients or metabolites of that media.
2.3. Cell Exposure to Bacteria or Metabolites
Supernatant of a log-phase S. enterica culture which grew to an optical density measured in absorbance at λmax = 600 nm (O.D.600) of 0.218 (1.09 × 108 cells) was centrifuged at 4000 rpm for 10 min and the supernatant was filtered through a 0.2 µm cellulose acetate filter (Isolab, Schweitenkirchen, Germany). The filtered supernatant was then diluted with fresh L-15 media to final concentrations of 2.5%, 5%, 10%, and 20% (v/v), and the gill cells were incubated in this media for 24 h at n = 3 biologic (independent) replicates. In addition to the O.D., we also found the culture to be 1.88 × 106 CFU/mL, which we determined through using the spread plate method [18] for 48 h at 20 °C.
To assess direct bacterial exposure, antibiotic-free RTGill-W1 cultures were aliquoted in a 96-well plate, inoculated with 10 µL of 10−2 log phase S. enterica culture, and incubated for 2 h at 20 °C. The cultures were then rinsed 5 times with PBS and staining was performed.
2.4. Microscopic Observation
The formation of cytoplasmic lipid droplets (usually neutral lipids such as triacylglycerols or cholesteryl esters) is a normal cellular process, as they serve as fatty acid energy reserves. However, abnormal accumulation of the cytoplasmic lipid droplets occurs in a variety of pathological conditions [19,20,21,22]. Nile red (N1142, Invitrogen, Waltham, MA, USA), solubilized in PBS, was used at 100 ng/mL as a fluorescent lysochrome for coloring both neutral and polar cytoplasmic lipid droplets, as described by Greenspan et al. [20]. The cells exposed to the various concentrations of metabolites were rinsed once with PBS and the fluorochrome was added, which was followed by a 10 min incubation in the dark. Microscopic observations were performed for neutral lipids (yellow) and for phospholipids (polar lipids, red).
The inoculated cultures were stained with 0.01% acridine orange (A.O., C.I. 46005, Carl Roth, Karlsruhe, Germany) and then counter-stained with 0.05% crystal violet (C.V.) to quench extracellular A.O.-stained bacteria. A.O./C.V. staining has been successfully used previously to evaluate the invasive abilities of Salmonella enteritidis on HeLa cells [23]. Microscopic observations were performed using an inverted Olympus IX71 microscope equipped with a Dapi/FITC fluorescence cube (U-N61002D, Olympus, Tokyo, Japan). The stained cultures were observed under 200× magnification for fluorescence, using a FITC/Texas Red filter (U-N61002, Olympus). Intracellular bacteria usually stain red while extracellular bacteria have the A.O. staining quenched by C.V. [23,24]. The A.O. staining is attributed to the increase in the amount of A.O. intercalating with the phosphate-sugar backbone of DNA [25].
2.5. Measurement of Cellular Metabolic Activity and Size
RTGill-W1 cells were aliquoted into cell-treated black fluorescence plates with a flat clear bottom (732-3731, VWR, Lutterworth, UK) and the metabolic rate following 24 h exposure was evaluated via the resazurin conversion to resorufin (Tox 8, Sigma) after 4 h of incubation at 20 °C (n = 3), under fluorescence readings set at λex = 550/λem = 590 [26], by using a plate reader (FlexStation 3, Molecular devices, San Jose, CA, USA).
Resazurin assay (also known as Alamar blue) [27] is a fluorometric metabolic indicator for detecting mitochondrial activity, which also implies the viability of the target cells. It acts as a non-toxic intermediate electron acceptor in the electron transport system between the final reduction of O2 and that of cytochrome oxidase [26]. The dye is a redox indicator, reduced by mitochondrial enzymes (NADH or NADPH dehydrogenase) to resorufin, which has fluorescence properties at λex = 550/λem = 590 and absorbance at λmax = 570 nm [28].
As both resazurin and resorufin are non-toxic, following the assay, the cultures were rinsed once with sterile phosphate buffer saline (PBS), trypsinized, and then evaluated with an automatic cell counter (Scepter 2.0, Merck, Darmstadt, Germany) equipped with 60 µm sensors and gated at 10.45–26.10 µm. Data analysis was performed with Scepter Pro 2.1.
2.6. Cellular Viability
Cells that were exposed to bacterial metabolites in identical conditions to those used in the metabolic assay were evaluated for viability (n = 3) using a live/dead cell viability assay (R37609, Thermofisher, Waltham, MA, USA). The spectrophotometric fluorescence readings were performed under dual wavelengths represented by λex = 360 nm/λem = 460 nm for live cells and λex = 504 nm/λem = 523 nm for all cell determinations.
2.7. Statistical Analysis
All statistical analyses were conducted using GraphPad Prism version 9.0 (GraphPad Software, San Diego, CA, USA). The selection of statistical models was based on the experimental design of each dataset. Cellular viability and morphological analyses (Figure 1) were assessed using a one-way ANOVA followed by Dunnett’s multiple comparison test to compare each treatment group with the corresponding control. The live/dead assay (Figure 2) was evaluated using a two-way analysis of variance (ANOVA) followed by Sidak’s multiple comparison test. Because the repeated-measures factor included only two levels, the assumption of sphericity was inherently met (ε = 1.0) and, therefore, the results were not affected by corrections for sphericity. A 95% confidence interval was applied to all comparisons. Statistical significance was established at p < 0.05 for all tests.
3. Results and Discussion
3.1. Cellular Metabolism
The resazurin results of cultures treated with bacterial metabolites showed a lower metabolic rate of the 2.5% group compared to the control, yet a dose-dependent higher rate at 10% and 20% (Figure 1). The results indicate a higher metabolic activity in the 10% and a statistically significant increase in the 20% groups. The unexpected reduction at the lowest metabolite dose (2.5%) may result from a mild toxic stress response in the cells that dampened their metabolic activity. The increased metabolic rate at higher concentrations may be due to the cellular defense mechanisms being activated. This increased metabolic activity is often a response to cellular stress, as cells attempt to detoxify or repair damage caused by the toxicants. For instance, some pollutants may generate intracellular ROS and trigger a metabolic shift towards lipogenesis in astrocytes and hepatocytes [29]. Another possible cause could be an increased NADH/NAD+ ratio, which occurs in hypoxia or electron transport chain dysfunction [30].
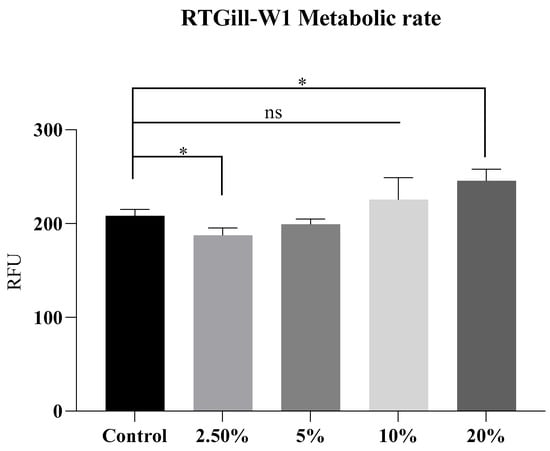
Figure 1.
Metabolic rate of RTGill-W1 cells exposed to S. enterica metabolites. * = p < 0.05; ns = non-significant, resazurin test assay. RFU = relative fluorescence units.
3.2. Cellular Viability and Morphology
The percentage of live cells differed insignificantly between groups (Figure 2); however, the ratio between live and all cells was high, with the control having the smallest difference between them. A high cell proliferation was observed in all other groups, although some mortality was observed. It is noteworthy that the viability remained high in all metabolite-treated groups, which contrasts with some studies that used purified bacterial metabolites, such as short-chain fatty acids, which often report reduced viability in mammalian cell cultures [31,32], highlighting a potential cell-type specific response.
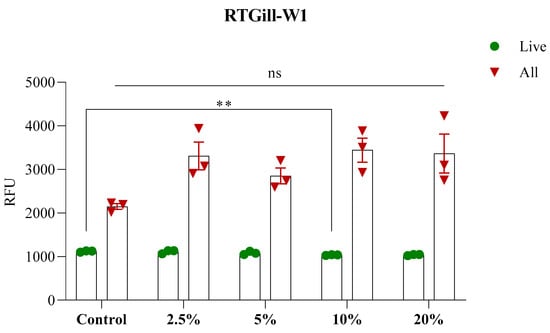
Figure 2.
Live/dead assay of cells exposed to S. enterica metabolites. The ratio between live (green circles) and all cells (red triangles). ** = p < 0.05; ns = non-significant.
Based on measurements performed in triplicate (Supplementary Data S1), the average diameter of control cells was 18.52 µm. The 2.5% group had an average of 17.80 µm, the 5% group had an average of 18.0 µm, the 10% group had an average of 18.51 µm, and the 20% group had an average of 18.95 µm (Figure 3). These data cannot be regarded as an indicator of cell health due to the small differences, and it is therefore inconclusive whether they can serve as a reference of RTGill-W1 size range. However, the fact that the size showed a correlation with the metabolic rate is suggestive of a biologic effect. The assay is useful for studying metabolic function as well as cell health [27,28].
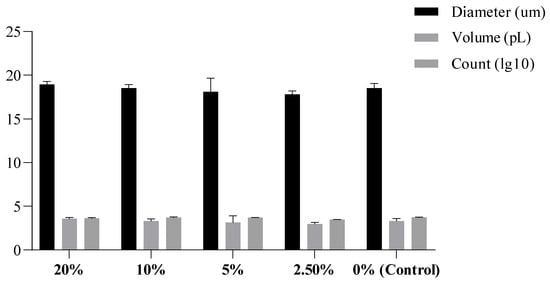
Figure 3.
Average of cell size and volume (n = 3 replicate treatments).
3.3. Microscopic Observations
RTGill-W1 cells inoculated with S. enterica, stained with A.O., and counter-stained with C.V. revealed no intracellular presence of S. enterica (Figure 4A,B). The 2 h incubation time appeared to be sufficient for some of the bacteria to attach to the substrate and remain after the PBS culture rinse. These results confirm that the observed cellular responses in metabolite-exposed cultures were due to extracellular bacterial factors (metabolites) rather than internalized bacteria.
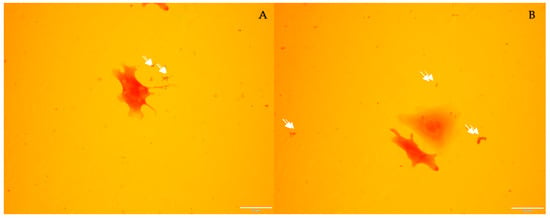
Figure 4.
Two photos (A,B) of S. enterica-inoculated RTGill-W1 culture rinsed with PBS after 2 h of incubation. Bacteria are indicated by white arrows. Images captured at 200× magnification.
For the intra-cellular lipid droplets (LDs) assay, the control group (Figure S1) showed a modest number and the lowest-intensity color of the LDs, followed by the 2.5% group (Figure S2). The 5% group started to reveal a mid-intensity color of the LDs and a higher number (Figure S3), while the 10% group peaked in color intensity and numbers (Figure S4). The 20% group, although it presented with only a mid-intensity, had a large number of LDs present. These data can be correlated with metabolic activity to some degree; however, further investigation is required to determine causation. In fact, Salmonella infection has been shown to induce host cell lipid droplet formation to benefit bacterial intracellular survival [8].
LDs occur normally in cells [33], as they serve as energy reserves for cell membranes and metabolism. LDs are enveloped by a single phospholipid membrane with neutral lipids inside, including glycerides, cholesterol, and sterols [34]. Ubiquitous in cells from archaea to eukaryotes, they are considered the earliest membranous organelles to evolve [34], as they emerged to allow cells to fix energy in the form of fats when the energy supply is rich and to break down these stored fats to sustain cellular life activities when the energy supply is low [35,36]. Excess accumulation of these droplets, however, might indicate metabolic disorders which can be followed by cell death [37]. In general, high accumulation is indicative of a variety of pathological states [20]. Under Nile red staining, a higher fluorescence intensity indicates a greater concentration of neutral lipids in the LD.
4. Conclusions
The preliminary results shown in this work demonstrate the particularly interesting behavior of RTGill-W1 in the presence of S. enterica metabolites. The lack of intracellular invasiveness of the bacterium suggests a possible potential natural resistance of the gill cell line (at least up to 2 h) to S. enterica intracellular invasiveness. As gills are known to normally host a diverse community of bacteria on their surface, this study further indicates the ability of gill cells to resist infection, which is significant as the gills are highly exposed to the environment. Still, such a claim should be vigorously investigated, as this is the first report of the RTGill-W1–Salmonella enterica interaction to the best of our knowledge.
The effects of S. enterica metabolites are, however, strongly indicative of a physiological cellular response, which was reflected by the generation of a disturbed and increased metabolic rate and the accumulation of neutral lipids. This is of particular interest for future cell-specific, physiological and immunological studies regarding S. enterica colonization and infection. Of particular note, the lower metabolic rate of the cells at 2.5% indicates a potential low-dose inhibitory effect of the metabolite mixture. The increased metabolism at higher metabolite concentrations may indicate an effect of one or more of the metabolites. Potential explanations could be that a metabolite is used as a growth supplement, that one or more metabolites has an endocrine-disrupting effect on the cells, or that the cells are mounting a compensatory response to a toxin. Similar action has been noted in gut microbiome studies and has been associated with endocrine tumorigenesis [38]. However, these interpretations are speculative and require further investigation.
This preliminary study has several limitations that should be acknowledged. The exact composition of the metabolite mixture was not characterized, and the observed effects could be due to a combination of specific (or several) bacterial metabolites, changes in media pH or osmolarity, nutrient depletion, or waste products. Furthermore, the absence of a conditioned media control (media incubated without bacteria) means we cannot fully isolate the effects of bacterial metabolites from changes inherent to the spent media itself.
Future work should focus on characterizing the supernatant using techniques like mass spectrometry to identify the active compound(s), implementing a conditioned media control, and investigating the specific signaling pathways involved in the observed lipid accumulation and metabolic shifts. Despite these limitations, our preliminary findings suggest that using the full spectrum of bacterial metabolites may be critical to understanding the complex effects of bacteria on host cells.
A brief mini review was completed using PubMed (PubMed.ncbi.nlm.nih.gov), searching for (“cell culture”) AND (“bacterial metabolites”), which returned only 13 references. Of these, only two reported bacterial metabolites as an additive to cell culture lines. One used only limited metabolites, short chain fatty acids (SCFAs), which resulted in a decrease in proliferation in intestinal cell enteroids (among some other effects) [31]. The other study was a review of studies (including gray literature) relating to using SCFAs in studying human oral epithelial cells, of which they found 10 articles with some relationship, and all found that SCFAs negatively affected cell viability [32]. A citation check did not return other papers, but it should be noted that several studies did co-culture bacteria with cells. However, these were excluded, as the direct impact of the bacteria results in other complications. These limited results indicate that this may be the first study using the complete bacterial metabolite spectrum to assess the effect on a cell line. Using the full range of bacterial metabolites may be critical to understanding the full effect of bacteria on cells, as the results of this preliminary study suggest.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/bacteria4040058/s1, Supplementary Data S1: cell counts; Supplementary Data S2: Figure S1: Control RTGill-W1 in S. enterica metabolite free media. Low intensity neutral lipid droplets in low number (arrows); Figure S2: RTGill-W1 exposed to 2.5% S. enterica metabolite. Medium intensity neutral lipid droplets in only one cell (arrows) and low intensity droplets in other cells; Figure S3: RTGill-W1 exposed to 5% S. enterica metabolite. Various medium intensity neutral lipid droplets in cells (white arrows); Figure S4: RTGill-W1 exposed to 10% S. enterica metabolite. Various high intensity neutral lipid droplets in cells (white arrows); Figure S5: RTGill-W1 exposed to 20% S. enterica metabolite. Various low and medium intensity neutral lipid droplets in cells (white arrows).
Author Contributions
Conceptualization, R.W. and A.J.; methodology, R.W. and A.J.; statistical analysis, B.-M.T.; data curation, A.J. and B.-M.T.; writing—original draft preparation, A.J., I.-T.G. and N.C.; writing—review and editing, R.W.; visualization, B.-M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable. The studies did not involve humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available from the corresponding author upon reasonable request.
Acknowledgments
We extend great appreciation to Christian Emilian Pop for his help in the design, execution, materials preparation and writing of this research paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- O’Beirne, D.; Francis, G.A. 12—Reducing pathogen risks in MAP-prepared produce. In Novel Food Packaging Techniques; Ahvenainen, R., Ed.; Woodhead Publishing: Cambridge, UK, 2003; pp. 231–275. [Google Scholar]
- Dougan, G.; Baker, S. Salmonella enterica Serovar Typhi and the Pathogenesis of Typhoid Fever. Annu. Rev. Microbiol. 2014, 68, 317–336. [Google Scholar] [CrossRef]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Finlay, B.B.; Falkow, S. Salmonella as an intracellular parasite. Mol. Microbiol. 1989, 3, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am. J. Pathol. 1967, 50, 109–136. [Google Scholar]
- Velge, P.; Wiedemann, A.; Rosselin, M.; Abed, N.; Boumart, Z.; Chaussé, A.M.; Grépinet, O.; Namdari, F.; Roche, S.M.; Rossignol, A.; et al. Multiplicity of Salmonella entry mechanisms, a new paradigm for Salmonella pathogenesis. Microbiol. Open 2012, 1, 243–258. [Google Scholar] [CrossRef]
- Mambu, J.; Virlogeux-Payant, I.; Holbert, S.; Grépinet, O.; Velge, P.; Wiedemann, A. An Updated View on the Rck Invasin of Salmonella: Still Much to Discover. Front. Cell Infect. Microbiol. 2017, 7, 500. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, X.; Zhang, N.; Zhu, L.; Lian, H. The interplay between Salmonella and host: Mechanisms and strategies for bacterial survival. Cell Insight 2025, 4, 100237. [Google Scholar] [CrossRef]
- LaRock, D.L.; Chaudhary, A.; Miller, S.I. Salmonellae interactions with host processes. Nat. Rev. Microbiol. 2015, 13, 191–205. [Google Scholar] [CrossRef]
- Antunes, L.C.M.; Arena, E.T.; Menendez, A.; Han, J.; Ferreira, R.B.R.; Buckner, M.M.C.; Lolić, P.; Madilao, L.L.; Bohlmann, J.; Borchers, C.H.; et al. Impact of Salmonella Infection on Host Hormone Metabolism Revealed by Metabolomics. Infect. Immun. 2011, 79, 1759–1769. [Google Scholar] [CrossRef]
- National Library of Medicine, National Institutes of Health. Available online: https://pubmed.ncbi.nlm.nih.gov/?term=RTGill-W1%3B+Salmonella&schema=all (accessed on 13 June 2025).
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef]
- Meng, S.; Xu, H.; Qin, L.; Chen, X.; Qiu, L.; Li, D.; Song, C.; Fan, L.; Hu, G.; Xu, P. The Gill-Associated Bacterial Community Is More Affected by Exogenous Chlorella pyrenoidosa Addition than the Bacterial Communities of Water and Fish Gut in GIFT Tilapia (Oreochromis niloticus) Aquaculture System. Biology 2023, 12, 1209. [Google Scholar] [CrossRef]
- O’Halloran, E.; Mooney, R.; Rodgers, K.; Henriquez, F.L. Microbial Interactions That Contribute to Gill Disease in Aquaculture. Parasitologia 2022, 2, 266–291. [Google Scholar] [CrossRef]
- van Kessel, M.A.H.J.; Mesman, R.J.; Arshad, A.; Metz, J.R.; Spanings, F.A.T.; van Dalen, S.C.M.; van Niftrik, L.; Flik, G.; Wendelaar Bonga, S.E.; Jetten, M.S.M.; et al. Branchial nitrogen cycle symbionts can remove ammonia in fish gills. Environ. Microbiol. Rep. 2016, 8, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Mes, W.; Lücker, S.; Jetten, M.S.M.; Siepel, H.; Gorissen, M.; Kessel, M.A.H.J.v. Gill-associated ammonia oxidizers are widespread in teleost fish. Microbiol. Spectr. 2024, 12, e00295-24. [Google Scholar] [CrossRef] [PubMed]
- Bols, N.C.; Barlian, A.; Chirino-Trejo, M.; Caldwell, S.J.; Goegan, P.; Lee, L.E.J. Development of a cell line from primary cultures of rainbow trout, Oncorhynchus mykiss (Walbaum), gills. J. Fish Dis. 1994, 17, 601–611. [Google Scholar] [CrossRef]
- Sanders, E.R. Aseptic laboratory techniques: Plating methods. J. Vis. Exp. 2012, 11, e3064. [Google Scholar] [CrossRef]
- Gluchowski, N.L.; Becuwe, M.; Walther, T.C.; Farese, R.V., Jr. Lipid droplets and liver disease: From basic biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 343–355. [Google Scholar] [CrossRef]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red: A selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef]
- Tian, J.; Du, Y.; Wang, B.; Ji, M.; Li, H.; Xia, Y.; Zhang, K.; Li, Z.; Xie, W.; Gong, W.; et al. Hif1α/Dhrs3a Pathway Participates in Lipid Droplet Accumulation via Retinol and Ppar-γ in Fish Hepatocytes. Int. J. Mol. Sci. 2023, 24, 10236. [Google Scholar] [CrossRef]
- Liu, X.H.; Pang, X.; Jin, L.; Pu, D.Y.; Wang, Z.J.; Zhang, Y.G. Exposure to acute waterborne cadmium caused severe damage on lipid metabolism of freshwater fish, revealed by nuclear lipid droplet deposition in hepatocytes of rare minnow. Aquat. Toxicol. 2023, 257, 106433. [Google Scholar] [CrossRef]
- Miliotis, M.D. Acridine orange stain for determining intracellular enteropathogens in HeLa cells. J. Clin. Microbiol. 1991, 29, 830–831. [Google Scholar] [CrossRef] [PubMed]
- Goldner, M.; Farkas-Himsley, H.; Kormendy, A.; Skinner, M. Bacterial Phagocytosis Monitored by Fluorescence and Extracellular Quenching: Ingestion and Intracellular Killing. Lab. Med. 1983, 14, 291–294. [Google Scholar] [CrossRef][Green Version]
- West, S.S. Quantitative microscopy in bacteriology. Ann. N. Y. Acad. Sci. 1969, 157, 111–122. [Google Scholar] [CrossRef]
- Braut-Boucher, F.; Aubery, M. Fluorescent Molecular Probes. In Encyclopedia of Spectroscopy and Spectrometry; Lindon, J.C., Ed.; Elsevier: Oxford, UK, 1999; p. 579. [Google Scholar]
- Rampersad, S.N. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef]
- Borra, R.C.; Lotufo, M.A.; Gagioti, S.M.; Barros Fde, M.; Andrade, P.M. A simple method to measure cell viability in proliferation and cytotoxicity assays. Braz. Oral Res. 2009, 23, 255–262. [Google Scholar] [CrossRef]
- Zhang, Y. Cell toxicity mechanism and biomarker. Clin. Transl. Med. 2018, 7, 34. [Google Scholar] [CrossRef]
- Lee, N.; Spears, M.E.; Carlisle, A.E.; Kim, D. Endogenous toxic metabolites and implications in cancer therapy. Oncogene 2020, 39, 5709–5720. [Google Scholar] [CrossRef]
- Pearce, S.C.; Weber, G.J.; van Sambeek, D.M.; Soares, J.W.; Racicot, K.; Breault, D.T. Intestinal enteroids recapitulate the effects of short-chain fatty acids on the intestinal epithelium. PLoS ONE 2020, 15, e0230231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Magrin, G.L.; Strauss, F.J.; Benfatti, C.A.M.; Maia, L.C.; Gruber, R. Effects of Short-Chain Fatty Acids on Human Oral Epithelial Cells and the Potential Impact on Periodontal Disease: A Systematic Review of In Vitro Studies. Int. J. Mol. Sci. 2020, 21, 4895. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thiam, A.R.; Farese, R.V., Jr.; Walther, T.C. The biophysics and cell biology of lipid droplets. Nat. Rev. Mol. Cell Biol. 2013, 14, 775–786. [Google Scholar] [CrossRef]
- Jin, Y.; Tan, Y.; Wu, J.; Ren, Z. Lipid droplets: A cellular organelle vital in cancer cells. Cell Death Discov. 2023, 9, 254. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Mizunoe, Y.; Kobayashi, M.; Hoshino, S.; Tagawa, R.; Itagawa, R.; Hoshino, A.; Okita, N.; Sudo, Y.; Nakagawa, Y.; Shimano, H.; et al. Cathepsin B overexpression induces degradation of perilipin 1 to cause lipid metabolism dysfunction in adipocytes. Sci. Rep. 2020, 10, 634. [Google Scholar] [CrossRef]
- Alza, N.P.; Conde, M.A.; Scodelaro-Bilbao, P.G.; Salvador, G.A. Neutral lipids as early biomarkers of cellular fate: The case of α-synuclein overexpression. Cell Death Dis. 2021, 12, 52. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, N.; Zhou, H.; Zhu, Y. The role of microbial metabolites in endocrine tumorigenesis: From the mechanistic insights to potential therapeutic biomarkers. Biomed. Pharmacother. 2024, 172, 116218. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).