Abstract
Intestinal organoids are useful for the in vitro investigation of the properties of intestinal epithelial cells and their interaction with the gut microbiome. In this study, we cultured cecal and colonic organoids from common marmosets, which are highlighted as model nonhuman primates but are susceptible to gastrointestinal diseases. The organoids established were capable of passaging and long-term culture. The results of quantitative reverse transcription PCR and immunostaining showed that the organoids differentiated into major cell types (colonocytes, goblet cells, and enteroendocrine cells) in the intestinal epithelium, enabling the in vitro analysis of these cells in marmosets. The organoids could therefore represent a useful model for the investigation of gut physiology in relation to gastrointestinal diseases and host-microbiome interactions, further expanding medical, biological, and veterinary research in the future.
1. Introduction
The intestinal epithelium is a single layer of cells that covers the surface of the intestine. The intestinal lumen contains various substances, including nutrients, gut microbiota, and their metabolites. The intestinal epithelium interacts with these substances through intestinal epithelial cells (IECs), which consist of specialized differentiated cells such as absorptive cells (colonocytes in the large intestine, which are responsible for digesting and absorbing food and drugs), goblet cells (which secrete mucus), and enteroendocrine cells (which detect luminal chemicals and secrete gut hormones). Studying the functions and characteristics of IECs is crucial for understanding the physiology of the gut, including processes such as food digestion, nutrient sensing, drug metabolism, and host–microbiome interactions [1].
All cell types in the intestinal epithelium are derived from intestinal stem cells (ISCs), which are located at the base of the intestinal crypts. Findings related to ISC niche factors and the use of a laminin-rich extracellular matrix have enabled the in vitro maintenance of IECs in the form of intestinal organoids. These organoids are self-organized, three-dimensional (3D) structures consisting of a single layer of IECs [2,3]. Intestinal organoids are now widely used as in vitro models to study the characteristics and functions of IECs due to their ability to faithfully replicate IEC functions in vitro [4,5]. Additionally, advances in techniques such as genome editing [6] and coculture with microbes [7] have further enhanced the utility of intestinal organoids as powerful tools for studying the physiology of the gut.
This culture system has been successfully applied not only to humans and mice but also to various models and livestock animals such as macaques, canines, felines, bats, bovines, porcine animals, rabbits, horses, sheep, and chickens [8,9,10,11,12,13]. However, intestinal organoids from common marmosets (Callithrix jacchus) have not been reported thus far. The common marmoset is a small primate native to South America. In recent years, marmosets have been studied as small-bodied and highly reproductive non-human primate models for biomedical studies [14,15,16,17]. They are also utilized to study the physiology of the gut, particularly aspects related to the gut microbiome. For example, changes in the gut microbiome in relation to age [16] and diet [18] have been investigated in marmosets. Host–microbiome interactions in this species have also been studied using transcriptomic analyses [17]. Furthermore, the high susceptibility of common marmosets to gastrointestinal diseases is a problem when keeping them in captivity, prompting several studies that aimed to identify the causes of this susceptibility and develop treatments for these diseases [19,20,21]. Despite these advances, to the best of our knowledge, culture systems that are capable of providing in vitro models for studying the gut of the common marmoset have not yet been established. Therefore, establishing intestinal organoids from common marmosets would be valuable for experimentally verifying and advancing these studies.
In the present study, we established intestinal organoids from the cecum and colon of common marmosets. These intestinal segments were selected because marmosets are known to be hindgut fermenters with evolutionarily enlarged ceca [22]. The results of the RT-qPCR and immunostaining analyses of the established cecal and colonic organoids demonstrated that the organoids differentiated into major IEC subtypes. Intestinal organoids derived from common marmosets will serve as an excellent tool for studying the gut physiology of this species in the future.
2. Materials and Methods
2.1. Animals
Six common marmosets (0–3 years old) were used in this study. Detailed information about the individuals (individual number, age, sex, and methods of sampling) and the cell lines are listed in Table S1. The study was approved by the Animal Welfare and Animal Care Committee of the Center for the Evolutionary Origins of Human Behavior, Kyoto University (permission numbers 2023-134, approved 1 April 2023, and 2024-023, approved 1 April 2024).
2.2. Organoid Culture Media
Organoid culture media were prepared according to the method proposed by Fujii et al., 2018 [3]. The basal medium was prepared by supplementing Advanced Dulbecco’s Modified Eagle Medium/Ham’s F12 with 10 mM HEPES buffer, 2 mM L-Alanyl-l-Glutamine, 1 × B27 supplement, 1 × N2 supplement (all from Gibco, Waltham, MA, USA), 10 μg/mL Gentamycin, and 250 ng/mL Amphotericin B (both from Sigma-Aldrich, St. Louis, MO, USA). The growth medium was prepared by supplementing basal medium with 50% human Wnt3a conditioned medium (lab-made [23]), 10% human Rspondin-2 conditioned medium (lab-made [24]), 100 ng/mL recombinant human Noggin (Proteintech, Rosemont, IL, USA), 100 ng/mL recombinant human IGF-1 (FUJIFILM WAKO Pure Chemical Corporation, Osaka, Japan), 50 ng/mL recombinant human FGF-basic (FGF-2) (FUJIFILM WAKO Pure Chemical Corporation), 500 nM A83-01 (FUJIFILM WAKO Pure Chemical Corporation), 1 mM N-acetylcysteine (Sigma-Aldrich), 10 nM human [Leu15]-gastrin 1 (Sigma-Aldrich), 10 mM nicotinamide (FUJIFILM WAKO Pure Chemical Corporation), and 50 ng/mL recombinant human EGF (FUJIFILM WAKO Pure Chemical Corporation).
2.3. Crypt Isolation and Organoid Culture
Crypt isolation and organoid culture were performed as previously described [8,25], with some modifications. Briefly, the intestinal tissues were dissected, and the mucosal surfaces were washed with ice-cooled DPBS, supplemented with penicillin–streptomycin. The tissues were incubated with chelation buffer (DPBS with 2% D-glucitol, 1% sucrose, 1% BSA, penicillin-streptomycin) containing 2 mM of EDTA for 30–60 min. After rinsing with cold chelation buffer, the mucosal surfaces were scraped with sterilized forceps to isolate the intestinal crypts.
The obtained crypts were enriched via centrifugation (400× g, 5 min), embedded in Matrigel (Corning, NY, USA), and covered with growth medium. The medium was refreshed every 2 days. The organoids were passaged once at 7–9 days via physical dissociation using P10 pipette tips attached to P200 pipette tips, with a split ratio of 1:3–1:5 (mechanical passage), or via chemical dissociation using TrypLE Express (Gibco, Waltham, MA, USA) (chemical passage), with a seeding density of 1~2 × 104 cells per drop (25 μL). ROCK inhibitor Y-27632 (FUJIFILM WAKO Pure Chemical Corporation) was added for 2 days after passaging.
2.4. Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
The tissues were sampled and soaked in RNAlater (lab-made) for one night at 4 °C. The organoids were homogenized in buffer RLT in an RNeasy Plus Mini Kit (Qiagen GmbH, Hilden, Germany). After that, total RNA was isolated from the organoids and tissues using the RNeasy Plus Mini Kit, according to the manufacturer’s instructions. cDNA was synthesized using the PrimeScript RT reagent kit with gDNA Eraser (Takara, Shiga, Japan). Quantitative RT-PCR was performed with THUNDERBIRD NEXT SYBR qPCR Mix (Toyobo, Osaka, Japan) and the StepOne Plus System (Applied Biosystems, Foster City, CA, USA). Marker genes were selected according to the methods of previous studies [9,13]. The sequences of the primers used in this study are listed in Table S2. Statistical analysis was performed using ΔCt values [26].
2.5. Immunostaining of Organoids and Tissues
The tissues were fixed in a 4% paraformaldehyde 0.1 M phosphate buffer solution (4% PFA, Muto Pure Chemicals, Tokyo, Japan) overnight at 4 °C and embedded in paraffin. The organoids were gently washed in DPBS and fixed in 4% PFA for 20 min at room temperature. The fixed organoids were rinsed three times in DPBS, embedded in 3% (w/v) agarose, and then embedded in paraffin. The samples were sectioned at 5 μm, deparaffinized, and rehydrated in a graded alcohol series. Antigens were retrieved via tris-EDTA buffer (lab-made). Blocking was performed using 10% normal horse serum (S-2000; Vector Laboratories, Burlingame, CA, USA) diluted in PBS-T at room temperature for 1 h, and the sections were incubated overnight at 4 °C with primary antibodies (Table S3) diluted in blocking solution. The primary antibodies were detected using 488 or 555 Alexa secondary antibodies (1:1000) (Thermo Fisher Scientific) for 1 h at room temperature. The nuclei were stained with DAPI (Dojindo Molecular Technologies, Tokyo, Japan). The stained sections were mounted using Mountant Permafluor (TA-030-FM; Thermo Fisher Scientific, Waltham, MA, USA) and observed with a fluorescence microscope (LSM-510, Axioplan 2; Carl Zeiss Microscopy GmbH, Oberkochen, Germany) mounted on a cooled charge-coupled device camera system (Cool SNAP HQ; Photometrics, Tucson, AZ, USA).
3. Results
3.1. Common Marmoset Cecal and Colonic Organoids Were Successfully Cultured Using a Budding Structure
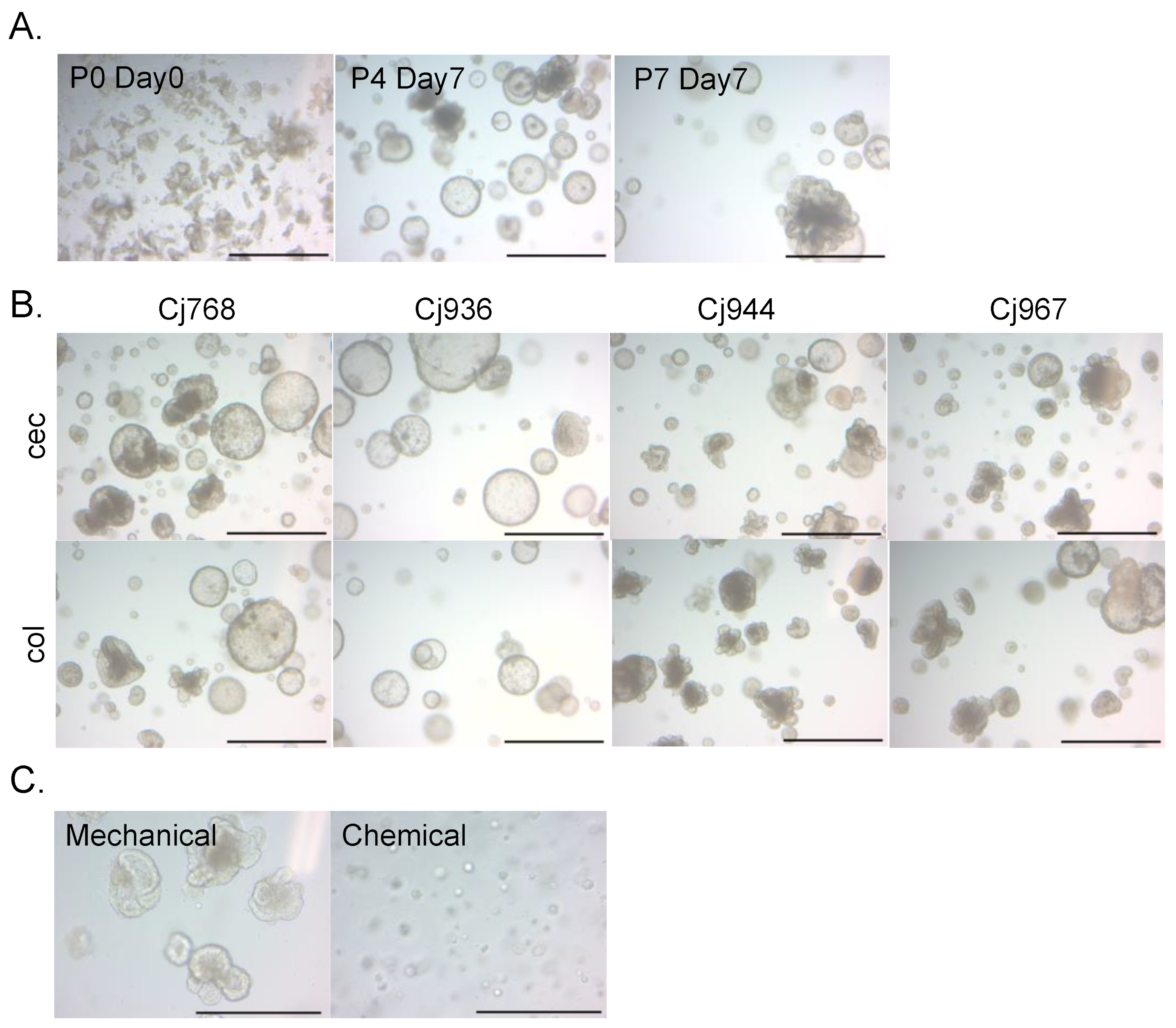
The cecal and colonic crypts of the common marmoset were embedded in Matrigel and cultured in medium containing ISC niche factors. After one week of culture, we observed the growth of organoid-like structures from the cecal and colonic crypts. The obtained organoids were capable of passaging and long-term expansion (Figure 1A). Cecal and colonic organoids were successfully established from one adult (Cj768) and three infant individuals (Cj936, 944, 967) (Figure 1B). The organoids could be both mechanically (physical dissociation via pipetting) and chemically (dissociation via digestive enzyme TrypLE Express) passaged. Since mechanically passaged organoids showed better growth (Figure 1C), we cultured organoids using the mechanical passaging method in the following experiments.
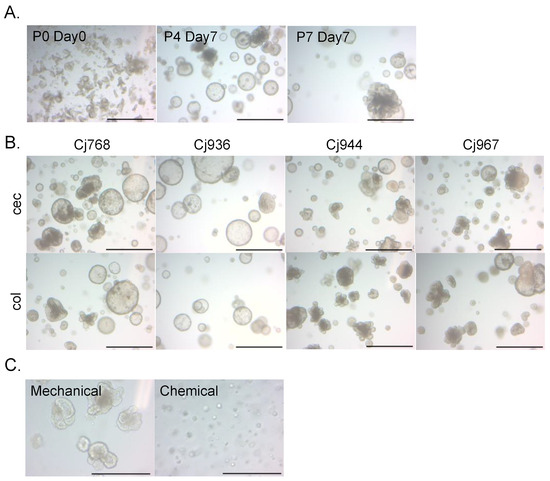
Figure 1.
Common marmoset intestinal organoids. (A) Representative images of cecal organoid growth (Cj768). The numbers after “P” indicate passage numbers (P0 = crypt culture). The numbers after “Day” indicate days passed from the latest passaging (Day0 = the day cells were passaged). (B) Representative images of organoids established in this study. The “cec” boxes show images of cecal organoids. The “col” boxes show images of colonic organoids. Scale bars in (A,B) = 1 mm. (C) Representative images of cecal organoids passaged using two different methods (Cj768). Images were captured 4 days after passaging. Scale bars in (C) = 500 μm.
3.2. Basic Characterization of Cecal Organoids by RT-qPCR
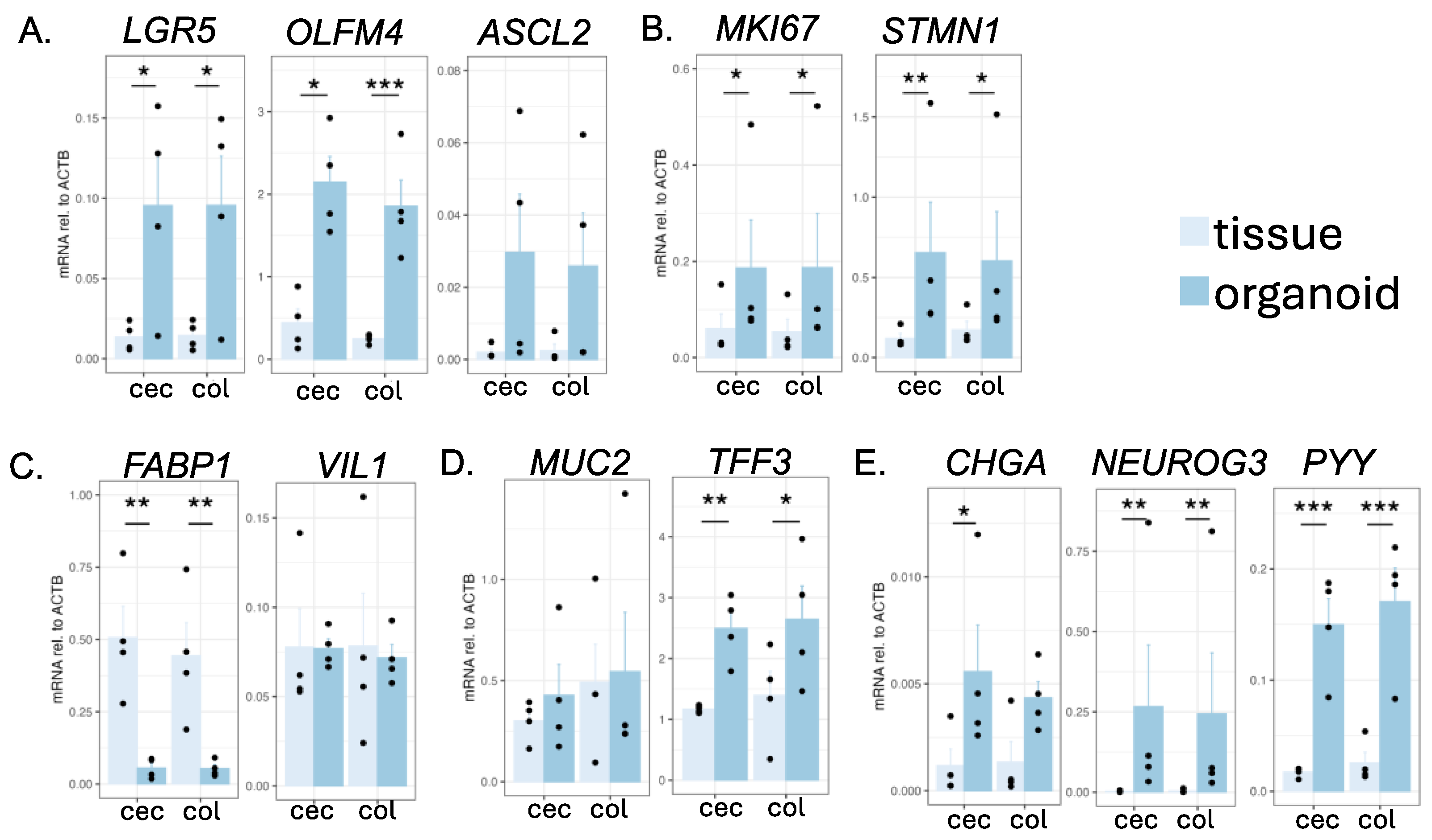
To identify which type of cells were contained in the cecal and colonic organoids, we performed quantitative reverse transcription PCR. We detected the expression of all the genes examined, suggesting that all major IEC subtypes were present in the cultured organoids (Figure 2). Almost all markers of ISC (Figure 2A) and transit-amplifying (TA) cells (Figure 2B) showed higher expression compared to that of the tissues. The colonocyte marker FABP1 showed significantly lower expression in the cells than in the tissues (Figure 2C), which is consistent with a previous study that showed the immaturity of enterocytes in this culture condition [3]. Notably, cecal and colonic organoids tended to show a higher expression of secretory cell markers (TFF3, NEUROG3, PYY) compared to that of the tissues.
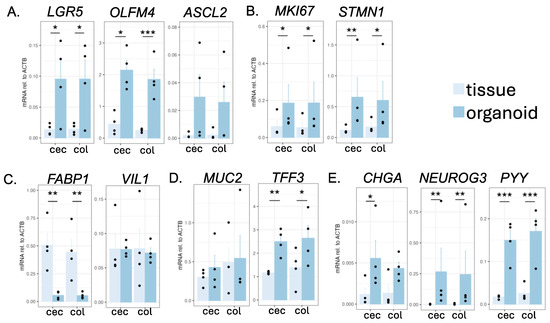
Figure 2.
Gene expression analysis of intestinal markers in the intestinal tissues and organoids of the common marmoset, as determined via RT-qPCR. The expression levels of marker genes of ISCs (A), TA cells (B), colonocytes (C), goblet cells (D), and enteroendocrine cells (E) were quantified. The expression levels were calculated via the ΔCt method, using ACTB as a housekeeping gene. Data are displayed as mean ± SE (N = 4 individuals). Welch’s t-test was performed (* p < 0.05, ** p < 0.01, *** p < 0.001). All samples listed in Supplementary Table S1 were used in this experiment.
3.3. Immunofluorescence of Cecal Organoids
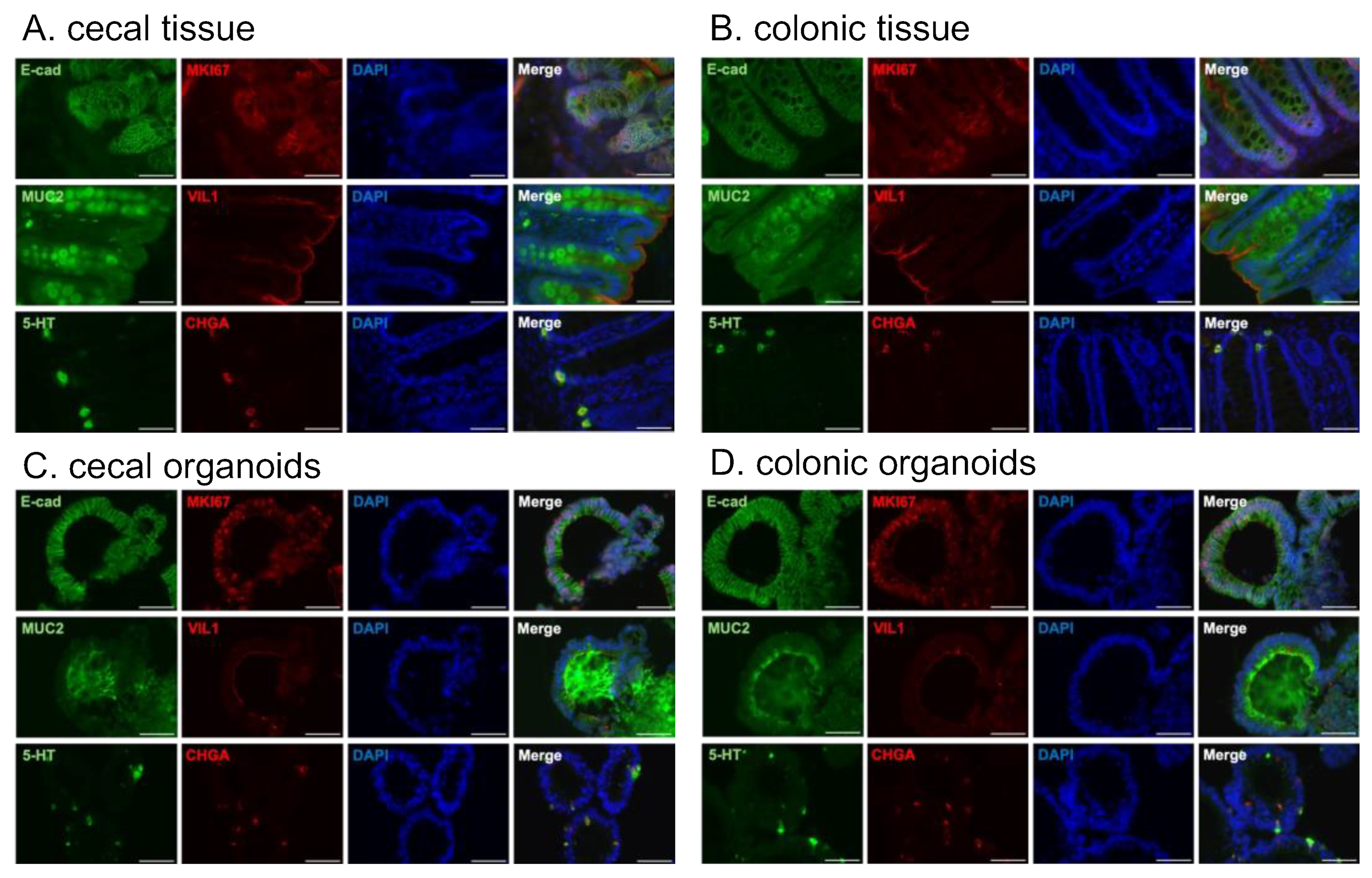
Finally, the structure and cellular distribution of the cecal and colonic organoids were confirmed via immunostaining. The specificity of the antibodies was validated using tissues (Figure 3A,B). Staining for the epithelial cell marker E-cadherin visualized the budding morphology of the established organoids (cecal: Figure 3C, colonic: Figure 3D, “E-cad”, Figure S1). Cells within the organoids oriented their VIL1-positive apical sides toward the inner lumen of the organoids (Figure 3C,D, “VIL1”).
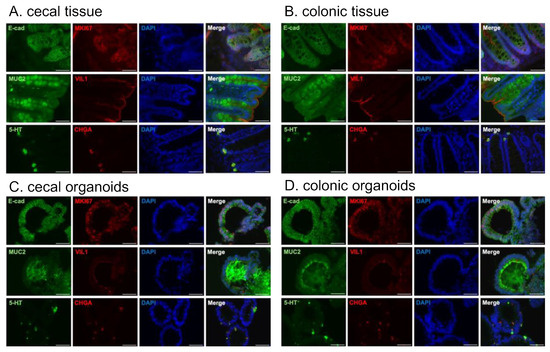
Figure 3.
Immunostaining of common marmoset (Cj768) cecal and colonic tissues and organoids. MKI67: proliferation marker, E-cadherin: epithelial cell marker, VIL1: colonocyte marker, MUC2: goblet cell marker, CHGA: enteroendocrine cell marker, and 5-HT: a type of gut hormone. (A,B) The specificity of the primary antibodies was confirmed using cecal (A) and colonic (B) tissue. (C) Representative images of stained cecal organoids. (D) Representative images of stained colonic organoids. Nuclei were stained with DAPI (blue). Scale bars = 50 μm.
The organoids abundantly contained MKI67-positive proliferating cells (Figure 3C,D, “MKI67”), indicating that the organoids were actively proliferating. MUC2-positive goblet cells and CHGA-positive enteroendocrine cells were also observed (Figure 3C,D). Most CHGA-positive cells were co-stained with 5-HT (serotonin), a type of gut hormone, consistent with the results observed in the tissue (Figure 3A–D, “5-HT”, “CHGA”, “Merge”).
4. Discussion
In this study, we succeeded in culturing intestinal organoids from common marmosets. Using the established organoids, we performed the basic characterization of cecal and colonic organoids. The cecal and colonic organoids exhibited a budding morphology, unlike the organoids obtained from the large intestines of macaques [8,9], which displayed a spheric morphology and contained few differentiated cells in the proliferation state.
The RT-qPCR results revealed that many of the marker genes for ISCs, TA cells, goblet cells, and enteroendocrine cells exhibited higher expression levels in the organoids than in the tissues. Immunostaining further confirmed that organoids include cells that can produce secretory substances such as MUC2 and 5-HT. These findings indicate that the organoids we established possess the ability to differentiate into secretory cells, while retaining proliferative capacity.
In the macaque colonic organoids established by Li et al. [9], the marker genes of the secretory cells (goblet and enteroendocrine cells) were expressed at lower levels than those in colonic tissue. The differences in the RT-qPCR results between Li et al.’s study and ours may due to either interspecies variations or differences in the culture conditions. The culture medium used by Li et al., which supplements media with the p38 inhibitor SB202190, is known to enhance organoid proliferation. In contrast, the medium we employed substitutes SB202190 with IGF-1 and FGF-2, which have been reported to promote the differentiation of ISCs into secretory cells, while maintaining the ability to self-renew in human [3] and canine [27] intestinal organoids. These differences in culture conditions are consistent with the discrepancies observed between our RT-qPCR data and those of Li et al. Although we are currently unable to disentangle the effects of interspecies differences from those of the culture conditions, we propose that the culture medium developed by Fujii et al. [3] is particularly suitable for establishing intestinal organoids that are rich in secretory cells from nonhuman primates.
The established cecal and colonic organoids, as well as the methods used to create them, can be used to study the gut physiology of marmosets. In recent years, many methods and technologies were developed to utilize intestinal organoids to study the interactions between gut and microbiome [27]. Understanding the host–microbiome interactions in the gut of marmosets is useful not only for biomedical studies, but also for understanding their feeding habits in the wild, exudativory [28,29]. Furthermore, it is known that intestinal diseases, such as marmoset wasting syndrome (WMS) [19] and marmoset duodenal dilation syndrome [20], are common in marmosets kept in captivity. As for WMS, previous studies have revealed that changes in the functions of IECs are observed in marmosets with this disease [19]. By culturing intestinal organoids from healthy and diseased marmosets and comparing their features, it may be possible to understand the molecular basis of these diseases.
5. Conclusions
We successfully established intestinal organoids from the common marmoset. The intestinal organoids established from the common marmoset could contribute to medical, biological, and veterinary research in the near future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/organoids4010003/s1. Table S1: Information about individuals and cell lines used in this study; Table S2: Primers for RT-qPCR; Table S3: Antibody list. Figure S1 shows the zoomed out view of the immunostaining results.
Author Contributions
Conceptualization, A.I. and H.I.; methodology, K.I.; data collection (organoid culture, qPCR, and immunostaining) and writing—original draft preparation, A.I.; writing—review and editing, H.I. and K.I.; funding acquisition, H.I. and K.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS Kakenhi, grant numbers 23K23937, 23K23557, and 24K21275, the Society for Research on Umami Taste and by Research Units for Exploring Future Horizons and Future Development Research Funding Program, Kyoto University Research Coordination Alliance.
Institutional Review Board Statement
The study was approved by the Animal Welfare and Animal Care Committee of the Center for the Evolutionary Origins of Human Behavior, Kyoto University (permit numbers 2023-134 and 2024-023), based on the Guidelines for Care and Use of Nonhuman Primates of the Primates Institute, Kyoto University (version 3; 9 June 2010).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank all those at Center for the Evolutionary Origins of Human Behavior, Kyoto University, who participated in the care and dissection of the animals used in this study. We thank H. Clevers for the Wnt3a-producing cell line and J. Whitsett for the R-spondin2-producing cell line.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Flier, L.G.v.d.; Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; Es, J.H.V.; Brink, S.V.d.; Houdt, W.J.V.; Pronk, A.; Gorp, J.V.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Matano, M.; Toshimitsu, K.; Takano, A.; Mikami, Y.; Nishikori, S.; Sugimoto, S.; Sato, T. Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition. Cell Stem Cell 2018, 23, 787–793.e6. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Noguchi, M.; Inoue, Y.; Sato, S.; Shimizu, M.; Kojima, H.; Okabe, T.; Kiyono, H.; Yamauchi, Y.; Sato, R. Organoid-derived intestinal epithelial cells are a suitable model for preclinical toxicology and pharmacokinetic studies. iScience 2022, 25, 104542. [Google Scholar] [CrossRef]
- Yamashita, T.; Inui, T.; Yokota, J.; Kawakami, K.; Morinaga, G.; Takatani, M.; Hirayama, D.; Nomoto, R.; Ito, K.; Cui, Y.; et al. Monolayer platform using human biopsy-derived duodenal organoids for pharmaceutical research. Mol. Therapy. Methods Clin. Dev. 2021, 22, 263–278. [Google Scholar] [CrossRef]
- Fujii, M.; Matano, M.; Nanki, K.; Sato, T. Efficient genetic engineering of human intestinal organoids using electroporation. Nat. Protoc. 2015, 10, 1474–1485. [Google Scholar] [CrossRef]
- Puschhof, J.; Pleguezuelos-Manzano, C.; Martinez-Silgado, A.; Akkerman, N.; Saftien, A.; Boot, C.; Waal, A.d.; Beumer, J.; Dutta, D.; Heo, I.; et al. Intestinal organoid cocultures with microbes. Nat. Protoc. 2021, 16, 4633–4649. [Google Scholar] [CrossRef]
- Inaba, A.; Kumaki, S.; Arinaga, A.; Tanaka, K.; Aihara, E.; Yamane, T.; Oishi, Y.; Imai, H.; Iwatsuki, K. Generation of intestinal chemosensory cells from nonhuman primate organoids. Biochem. Biophys. Res. Commun. 2021, 536, 20–25. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Zhang, M.; Wang, H.; Cui, A.; Zhao, J.; Ji, W.; Chen, Y.-G. Establishment of porcine and monkey colonic organoids for drug toxicity study. Cell Regen. 2021, 10, 32. [Google Scholar] [CrossRef]
- Chandra, L.; Borcherding, D.C.; Kingsbury, D.; Atherly, T.; Ambrosini, Y.M.; Bourgois-Mochel, A.; Yuan, W.; Kimber, M.; Qi, Y.; Wang, Q.; et al. Derivation of adult canine intestinal organoids for translational research in gastroenterology. BMC Biol. 2019, 17, 33. [Google Scholar] [CrossRef]
- Powell, R.H.; Behnke, M.S. WRN conditioned media is sufficient for in vitro propagation of intestinal organoids from large farm and small companion animals. Biol. Open 2017, 6, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, C.; Liu, X.; Chiu, M.C.; Zhao, X.; Wang, D.; Wei, Y.; Lee, A.; Zhang, A.J.; Chu, H.; et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat. Med. 2020, 26, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Mussard, E.; Pouzet, C.; Helies, V.; Pascal, G.; Fourre, S.; Cherbuy, C.; Rubio, A.; Vergnolle, N.; Combes, S.; Beaumont, M. Culture of rabbit caecum organoids by reconstituting the intestinal stem cell niche in vitro with pharmacological inhibitors or L-WRN conditioned medium. Stem Cell Res. 2020, 48, 101980. [Google Scholar] [CrossRef] [PubMed]
- Zühlke, U.; Weinbauer, G. The common marmoset (Callithrix jacchus) as a model in toxicology. Toxicol. Pathol. 2003, 31 (Suppl. S1), 123–127. [Google Scholar] [CrossRef]
- Inoue, T.; Yurimoto, T.; Seki, F.; Sato, K.; Sasaki, E. The common marmoset in biomedical research: Experimental disease models and veterinary management. Exp. Anim. 2023, 72, 140–150. [Google Scholar] [CrossRef]
- Reveles, K.; Patel, S.; Forney, L.; Ross, C. Age-related changes in the marmoset gut microbiome. Am. J. Primatol. 2019, 81, e22960. [Google Scholar] [CrossRef]
- Uehara, M.; Inoue, T.; Hase, S.; Sasaki, E.; Toyoda, A.; Sakakibara, Y. Decoding host-microbiome interactions through co-expression network analysis within the non-human primate intestine. mSystems 2024, 9, e01405–e01423. [Google Scholar] [CrossRef]
- Tang-Wing, C.; Mohanty, I.; Bryant, M.; Makowski, K.; Melendez, D.; Dorrestein, P.C.; Knight, R.; Caraballo-Rodríguez, A.M.; Jenné, C.A. Impact of diet change on the gut microbiome of common marmosets (Callithrix jacchus). mSystems 2024, 9, e00108-24. [Google Scholar] [CrossRef]
- Niimi, K.; Takahashi, E. Reduced differentiation of intestinal epithelial cells in wasting marmoset syndrome. J. Vet. Med. Sci. 2021, 83, 784–792. [Google Scholar] [CrossRef]
- Mineshige, T.; Inoue, T.; Yasuda, M.; Yurimoto, T.; Kawai, K.; Sasaki, E. Novel gastrointestinal disease in common marmosets characterised by duodenal dilation: A clinical and pathological study. Sci. Rep. 2020, 10, 3793. [Google Scholar] [CrossRef]
- Sheh, A.; Artim, S.C.; Burns, M.A.; Molina-Mora, J.A.; Lee, M.A.; Dzink-Fox, J.; Muthupalani, S.; Fox, J.G. Alterations in common marmoset gut microbiome associated with duodenal strictures. Sci. Rep. 2022, 12, 5277. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.F.; Martins, E.S. Gummivory and gut morphology in two sympatric callitrichids (Callithrix emiliae and Saguinus fuscicollis weddelli) from western Brazilian Amazonia. Am. J. Phys. Anthropol. 1992, 88, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Broutier, L.; Andersson-Rolf, A.; Hindley, C.J.; Boj, S.F.; Clevers, H.; Koo, B.-K.; Huch, M. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 2016, 11, 1724–1743. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.M.; Schreiner, C.M.; Wert, S.E.; Mucenski, M.L.; Scott, W.J.; Whitsett, J.A. R-spondin 2 is required for normal laryngeal-tracheal, lung and limb morphogenesis. Development 2008, 135, 1049–1058. [Google Scholar] [CrossRef]
- Inaba, A.; Arinaga, A.; Tanaka, K.; Endo, T.; Hayatsu, N.; Okazaki, Y.; Yamane, T.; Oishi, Y.; Imai, H.; Iwatsuki, K. Interleukin-4 Promotes Tuft Cell Differentiation and Acetylcholine Production in Intestinal Organoids of Non-Human Primate. Int. J. Mol. Sci. 2021, 22, 7921. [Google Scholar] [CrossRef]
- Rieu, I.; Powers, S.J. Real-Time Quantitative RT-PCR: Design, Calculations, and Statistics. Plant Cell 2025, 21, 1031. [Google Scholar] [CrossRef]
- Rubert, J.; Schweiger, P.; Mattivi, F.; Tuohy, K.; Jensen, K.; Lunardi, A. Intestinal Organoids: A Tool for Modelling Diet-Microbiome-Host Interactions. Trends Endocrinol. Metab. TEM 2020, 31, 848–858. [Google Scholar] [CrossRef]
- Guimarães-Lopes, V.d.P.; Gomes, M.R.V.S.; Kagueyama, M.; Faria, R.d.C.V.; Ribeiro Filho, O.P.; Melo, F.R.d.; Sartori, S.S.R. Histometric parameters of the large intestine of hybrid marmosets Callithrix sp. under the influence of seasonality. Anat. Histol. Embryol. 2021, 50, 888–896. [Google Scholar] [CrossRef]
- Power, M.L. Nutritional and Digestive Challenges to Being a Gum-Feeding Primate. In The Evolution of Exudativory in Primates; Burrows, A.M., Nash, L.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).