Paneth Cells Are a Constitutive Source of IL-10 in Mouse Small Intestinal Organoids
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Crypt Isolation and Organoid Cultures
2.3. Cytokine Treatments of Enteroids
2.4. RNA Isolation and RT-qPCR
2.5. Immunofluorescent Staining
2.6. Adapting Enteroids to Monolayer Cultures
2.7. Protein Extraction
2.8. Western Blotting
2.9. Statistical Analyses
3. Results
3.1. Enteroid Culture Conditions and Intrinsic Expression of IL-10 and IL-10 Receptor mRNA
3.2. Paneth Cells Produce IL-10 Apically for Autocrine Activity
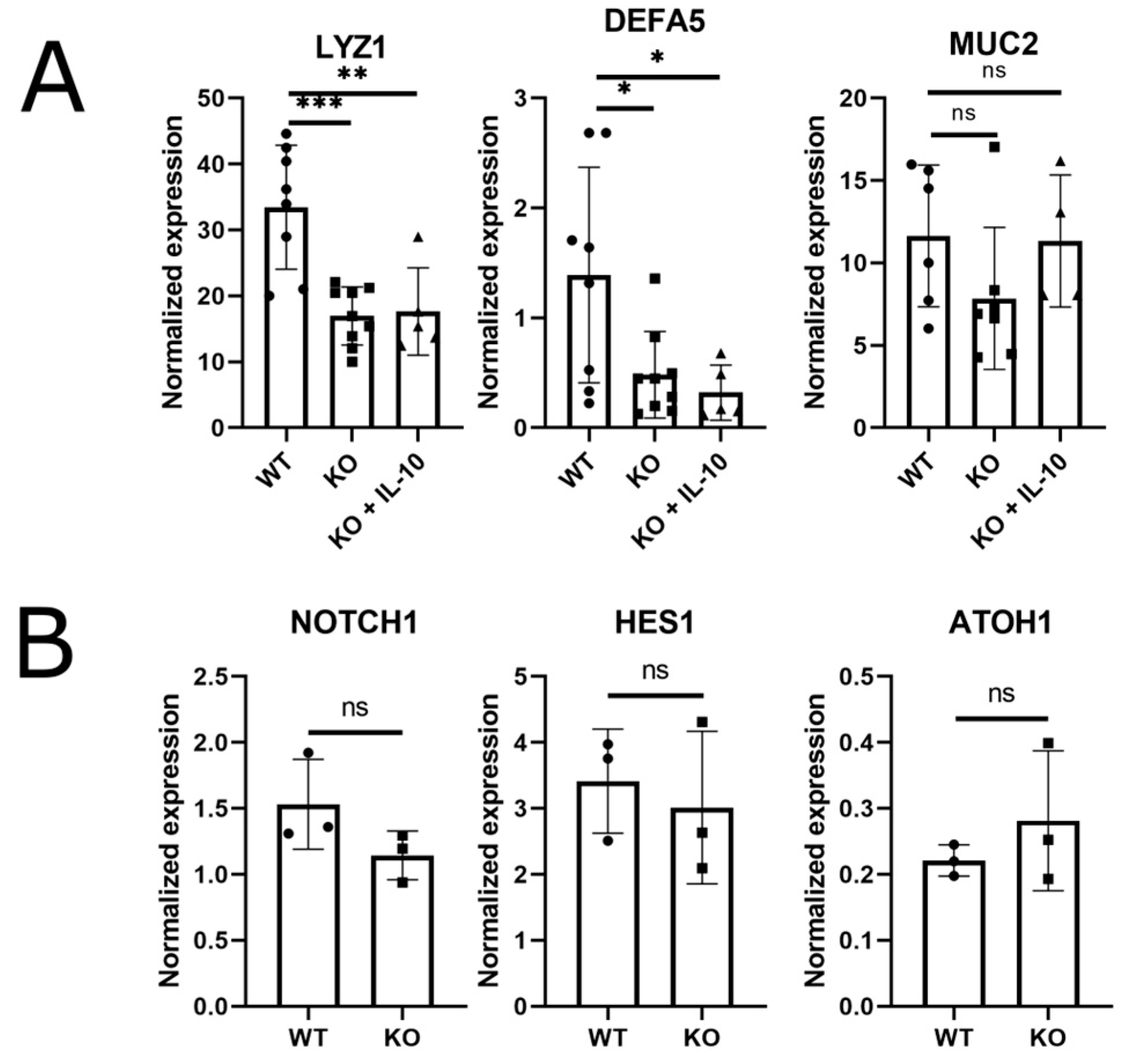
3.3. Paneth Cell Markers Are Reduced in the Absence of Epithelial IL-10
3.4. STAT3-Dependent IL-10 Signaling Occurs upon Stimulation of Apical IL-10RA Receptors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kopp, Z.A.; Jain, U.; Van Limbergen, J.; Stadnyk, A.W. Do antimicrobial peptides and complement collaborate in the intestinal mucosa? Front. Immunol. 2015, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y. Epithelial cells as a transmitter of signals from commensal bacteria and host immune cells. Front. Immunol. 2019, 10, 2057. [Google Scholar] [CrossRef] [PubMed]
- Stadnyk, A.W. Intestinal epithelial cells as a source of inflammatory cytokines and chemokines. Can. J. Gastroenterol. 2002, 16, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, K.R.; Shah, N.; Faizura-Yeop, I.; Kocacik Uygun, D.F.; Frede, N.; Muise, A.M.; Shteyer, E.; Filiz, S.; Chee, R.; Elawad, M.; et al. Clinical outcome in IL-10- and IL-10 receptor-deficient patients with or without hematopoietic stem cell transplantation. J. Allergy Clin. Immunol. 2013, 131, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.; Balschun, T.; Karlsen, T.H.; Sventoraityte, J.; Nikolaus, S.; Mayr, G.; Domingues, F.S.; Albrecht, M.; Nothnagel, M.; Ellinghaus, D.; et al. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat. Genet. 2008, 40, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Kühn, R.; Löhler, J.; Rennick, D.; Rajewsky, K.; Müller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993, 75, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, D.F.; Bond, M.W.; Mosmann, T.R. Two types of mouse t helper cell: IV. Th2 clones secrete a factor that inhibits cytokine production by Thl clones. J. Exp. Med. 1989, 170, 2081–2095. [Google Scholar] [CrossRef]
- Morhardt, T.L.; Hayashi, A.; Ochi, T.; Quirós, M.; Kitamoto, S.; Nagao-Kitamoto, H.; Kuffa, P.; Atarashi, K.; Honda, K.; Kao, J.Y.; et al. IL-10 produced by macrophages regulates epithelial integrity in the small intestine. Sci. Rep. 2019, 9, 1223. [Google Scholar] [CrossRef]
- Hayashi, A.; Sato, T.; Kamada, N.; Mikami, Y.; Matsuoka, K.; Hisamatsu, T.; Hibi, T.; Roers, A.; Yagita, H.; Ohteki, T.; et al. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe 2013, 13, 711–722. [Google Scholar] [CrossRef]
- Ina, K.; Kusugami, K.; Kawano, Y.; Nishiwaki, T.; Wen, Z.; Musso, A.; West, G.A.; Ohta, M.; Goto, H.; Fiocchi, C. Intestinal fibroblast-derived IL-10 increases survival of mucosal T cells by inhibiting growth factor deprivation- and Fas-mediated apoptosis. J. Immunol. 2005, 175, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.D.; Aljamaei, H.M.; Stadnyk, A.W. The production and function of endogenous interleukin-10 in intestinal epithelial cells and gut homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1343–1352. [Google Scholar] [CrossRef]
- Biton, M.; Haber, A.L.; Rogel, N.; Burgin, G.; Beyaz, S.; Schnell, A.; Ashenberg, O.; Su, C.-W.; Smillie, C.; Shekhar, K.; et al. T helper cell cytokines modulate intestinal stem cell renewal and differentiation. Cell 2018, 175, 1307–1320.e22. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, L.; Pardo-Roa, C.; Ramírez, G.; Vallejos, O.P.; Sebastián, V.P.; Riedel, C.A.; Álvarez-Lobos, M.; Bueno, S.M. The absence of interleukin 10 affects the morphology, differentiation, granule content and the production of cryptidin-4 in Paneth cells in mice. PLoS ONE 2019, 14, e0221618. [Google Scholar] [CrossRef] [PubMed]
- Schwerbrock, N.M.; Makkink, M.K.; van der Sluis, M.; Büller, H.A.; Einerhand, A.W.; Sartor, R.B.; Dekker, J. Interleukin 10-deficient mice exhibit defective colonic Muc2 synthesis before and after induction of colitis by commensal bacteria. Inflamm. Bowel Dis. 2004, 10, 811–823. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- George, M.M.; Rahman, M.; Connors, J.; Stadnyk, A.W. Opinion: Are organoids the end of model evolution for studying host intestinal epithelium/microbe interactions? Microorganisms 2019, 7, 406. [Google Scholar] [CrossRef] [PubMed]
- Kamanaka, M.; Kim, S.T.; Wan, Y.Y.; Sutterwala, F.S.; Lara-Tejero, M.; Galán, J.E.; Harhaj, E.; Flavell, R.A. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity 2006, 25, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Moore-Connors, J.M.; Kim, H.S.; Marshall, J.S.; Stadnyk, A.W.; Halperin, S.A.; Wang, J. CD43-, but not CD43+, IL-10-producing CD1dhi CD5+ B cells suppress type 1 immune responses during Chlamydia muridarum genital tract infection. Mucosal Immunol. 2015, 8, 94–106. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; Liu, D.; Su, Y. Normalizing genes for real-time polymerase chain reaction in epithelial and nonepithelial cells of mouse small intestine. Anal. Biochem. 2010, 399, 211–217. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, K.; Dow, L.; Lowe, S. Immunofluorescent staining of mouse intestinal stem cells. Bio-Protocol 2016, 6, e1732. [Google Scholar] [CrossRef] [PubMed]
- Altay, G.; Larrañaga, E.; Tosi, S.; Barriga, F.M.; Batlle, E.; Fernández-Majada, V.; Martínez, E. Self-organized intestinal epithelial monolayers in crypt and villus-like domains show effective barrier function. Sci. Rep. 2019, 9, 10140. [Google Scholar] [CrossRef]
- Haber, A.L.; Biton, M.; Rogel, N.; Herbst, R.H.; Shekhar, K.; Smillie, C.; Burgin, G.; Delorey, T.M.; Howitt, M.R.; Katz, Y.; et al. A single-cell survey of the small intestinal epithelium. Nature 2017, 551, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Tamagawa, Y.; Ishimura, N.; Uno, G.; Yuki, T.; Kazumori, H.; Ishihara, S.; Amano, Y.; Kinoshita, Y. Notch signaling pathway and Cdx2 expression in the development of Barrett’s esophagus. Lab. Investig. 2012, 92, 896–909. [Google Scholar] [CrossRef]
- Shouval, D.S.; Ouahed, J.; Biswas, A.; Goettel, J.A.; Horwitz, B.H.; Klein, C.; Muise, A.M.; Snapper, S.B. Interleukin 10 receptor signaling: Master regulator of intestinal mucosal homeostasis in mice and humans. Adv. Immunol. 2014, 122, 177–210. [Google Scholar] [CrossRef] [PubMed]
- Weber-Nordt, R.M.; Riley, J.K.; Greenlund, A.C.; Moore, K.W.; Darnell, J.E.; Schreiber, R.D. Stat3 recruitment by two distinct ligand-induced, tyrosine- phosphorylated docking sites in the interleukin-10 receptor intracellular domain. J. Biol. Chem. 1996, 271, 27954–27961. [Google Scholar] [CrossRef]
- Lueschow, S.R.; McElroy, S.J. The Paneth cell: The curator and defender of the immature small intestine. Front. Immunol. 2020, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Ashida, T.; Ito, T.; Ishikawa, C.; Tanabe, H.; Maemoto, A.; Watari, J.; Ayabe, T.; Mizukami, Y.; Fujiya, M.; et al. Expression of the antimicrobial peptide α-defensin/cryptdins in intestinal crypts decreases at the initial phase of intestinal inflammation in a model of inflammatory bowel disease, IL-10-deficient mice. Inflamm. Bowel Dis. 2010, 16, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, H.; Sun, X.; Zhu, M.J. Metformin improves ileal epithelial barrier function in interleukin-10 deficient mice. PLoS ONE 2016, 11, e0168670. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Hu, J.; Yang, X.; Wang, Y.; Lin, Z.; Sun, Q.; Liu, K. Interleukin-10 expands transit-amplifying cells while depleting Lgr5+ stem cells via inhibition of Wnt and notch signaling. Biochem. Biophys. Res. Commun. 2020, 533, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Papoutsopoulou, S.; Pollock, L.; Walker, C.; Tench, W.; Samad, S.S.; Bergey, F.; Lenzi, L.; Sheibani-Tezerji, R.; Rosenstiel, P.; Alam, M.T.; et al. Impact of interleukin 10 deficiency on intestinal epithelium responses to inflammatory signals. Front. Immunol. 2021, 12, 690817. [Google Scholar] [CrossRef] [PubMed]
- Brischetto, C.; Krieger, K.; Klotz, C.; Krahn, I.; Kunz, S.; Kolesnichenko, M.; Mucka, P.; Heuberger, J.; Scheidereit, C.; Schmidt-Ullrich, R. NF-κB determines Paneth versus goblet cell fate decision in the small intestine. Development 2021, 148, dev199683. [Google Scholar] [CrossRef] [PubMed]
- Mori-Akiyama, Y.; van den Born, M.; van Es, J.H.; Hamilton, S.R.; Adams, H.P.; Zhang, J.; Clevers, H.; de Crombrugghe, B. SOX9 Is required for the differentiation of Paneth cells in the intestinal epithelium. Gastroenterology 2007, 133, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Onyiah, J.C.; Colgan, S.P. Cytokine responses and epithelial function in the intestinal mucosa. Cell. Mol. Life Sci. 2016, 73, 4203–4212. [Google Scholar] [CrossRef]
- Kuwada, S.K.; Lund, K.A.; Li, X.F.; Cliften, P.; Amsler, K.; Opresko, L.K.; Wiley, H.S. Differential signaling and regulation of apical vs. basolateral EGFR in polarized epithelial cells. Am. J. Physiol. 1998, 275, C1419–C1428. [Google Scholar] [CrossRef] [PubMed]
- Kominsky, D.J.; Campbell, E.L.; Ehrentraut, S.F.; Wilson, K.E.; Kelly, C.J.; Glover, L.E.; Collins, C.B.; Bayless, A.J.; Saeedi, B.; Dobrinskikh, E.; et al. IFN-γ–mediated induction of an apical IL-10 receptor on polarized intestinal epithelia. J. Immunol. 2014, 192, 1267–1276. [Google Scholar] [CrossRef]
- Mizoguchi, A. Healing of intestinal inflammation by IL-22. Inflamm. Bowel Dis. 2012, 18, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, S.Z.; Tauro, S.; Das, I.; Tong, H.; Chen, A.C.; Jeffery, P.L.; McDonald, V.; Florin, T.H.; McGuckin, M.A. IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology 2023, 144, 357–368. [Google Scholar] [CrossRef]
- Jenkins, B.R.; Blaseg, N.A.; Grifka-Walk, H.M.; Deuling, B.; Swain, S.D.; Campbell, E.L.; Walk, S.T.; Kominsky, D.J. Loss of interleukin-10 receptor disrupts intestinal epithelial cell proliferation and skews differentiation towards the goblet cell fate. FASEB J. 2021, 35, e21551. [Google Scholar] [CrossRef] [PubMed]
- Morales, R.A.; Rabahi, S.; Diaz, O.E.; Salloum, Y.; Kern, B.C.; Westling, M.; Luo, X.; Parigi, S.M.; Monasterio, G.; Das, S.; et al. Interleukin-10 regulates goblet cell numbers through Notch signaling in the developing zebrafish intestine. Mucosal Immunol. 2022, 15, 940–951. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.; Di Cara, F.; Wang, J.; Stadnyk, A.W. Paneth Cells Are a Constitutive Source of IL-10 in Mouse Small Intestinal Organoids. Organoids 2025, 4, 4. https://doi.org/10.3390/organoids4010004
Nguyen H, Di Cara F, Wang J, Stadnyk AW. Paneth Cells Are a Constitutive Source of IL-10 in Mouse Small Intestinal Organoids. Organoids. 2025; 4(1):4. https://doi.org/10.3390/organoids4010004
Chicago/Turabian StyleNguyen, Huong, Francesca Di Cara, Jun Wang, and Andrew W. Stadnyk. 2025. "Paneth Cells Are a Constitutive Source of IL-10 in Mouse Small Intestinal Organoids" Organoids 4, no. 1: 4. https://doi.org/10.3390/organoids4010004
APA StyleNguyen, H., Di Cara, F., Wang, J., & Stadnyk, A. W. (2025). Paneth Cells Are a Constitutive Source of IL-10 in Mouse Small Intestinal Organoids. Organoids, 4(1), 4. https://doi.org/10.3390/organoids4010004





