Abstract
Studying human placental development and function presents significant challenges due to the inherent difficulties in obtaining and maintaining placental tissue throughout the course of an ongoing pregnancy. Here, we provide a detailed protocol for generating trophoblast organoids from chorionic villi obtained during ongoing pregnancy. Our method results in efficient generation of trophoblast organoids from chorionic villus sampling, does not require preselection of chorionic villi, and controls contamination of decidual gland organoids. The resulting trophoblast organoids spontaneously form syncytiotrophoblasts that start secreting hCG hormone amongst other placenta-specific factors. Our approach facilitates the generation of trophoblast organoids from a variety of genetic backgrounds, including trisomies and gene mutations, and can be aligned with prenatal diagnostic routines. The protocol requires up to 14 days and can be carried out by users with expertise in cell culture.
1. Introduction
Studying the human placenta has historically been fraught with challenges due to ethical considerations surrounding human sample usage, the complexities of obtaining and sustaining placental tissue during pregnancy, and the limited translational relevance of animal models due to species-specific variations in placental structure and function. Trophoblast cells that constitute the outer layer of the developing human embryo give rise to cell types that are responsible for many of the placenta’s transport, endocrine, and immunogenic functions. Isolation and maintenance of primary trophoblasts in culture is a difficult task due to their limited lifespan and fragile nature. The emergence of trophoblast organoids presents a promising platform to study primary trophoblasts in settings that more closely resemble trophoblast layers within the villous placenta. These three-dimensional structures, derived from placental tissue, faithfully replicate crucial aspects of trophoblast biology and hold the potential to surmount ethical, logistical, and biological hurdles, offering a transformative avenue for advancing human placenta research.
Over the last years, several methods have been established to generate trophoblast organoids from different sources of tissue, including early pregnancy terminations [1,2], naive pluripotent stem cells [3], and term placental tissue [4]. Recently, we have published the first method to generate trophoblast organoids during ongoing pregnancy using chorionic villus sampling (CVS) tissue [5]. CVS is performed at 10–14 weeks of gestation as a means of prenatal testing for fetal genetic abnormalities. Although all proposed methods of generating trophoblast organoids show high similarity and include identical steps, subtle differences, particularly in relation to the starting material, significantly influence organoid derivation rate and culture efficiency.
Here, we provide a detailed protocol for the generation of trophoblast organoids from CVS tissue. Since our original publication, we have slightly modified the culture conditions, resulting in increased derivation efficiency and obviating preselection of chorionic villi. Also, we found a way to deal effectively with contamination of decidual cells. Making use of surplus CVS tissue, this method can be readily integrated with prenatal diagnostic CVS pipelines. As such, we could derive trophoblast organoids from pregnancies diagnosed with fetal trisomies and other genetic disorders, providing an experimental model to study fetal genetic conditions in the placenta. In general, our method is easy to adopt, does not require specialized equipment and should be widely applicable to other clinical centers or laboratories that have access to CVS tissue.
2. Experimental Design
The workflow for the generation of trophoblast organoids from CVS tissue is outlined in Figure 1. Briefly, chorionic villi are enzymatically digested to enrich for trophoblast suspensions. Trophoblast suspensions, mainly consisting of cytotrophoblasts, are subsequently filtered and embedded in hydrogel drops as single cells. The drops are overlayed with trophoblast organoid medium (TOM), specifically tailored to support proliferation and fusion of primary trophoblasts isolated from chorionic villi. Small spheroids appear within 3–7 days and grow out to form villous organoid structures after 10–14 days of culture. Validation of trophoblast organoid origin and structure can be performed in a matter of minutes to days using a range of techniques and assays.
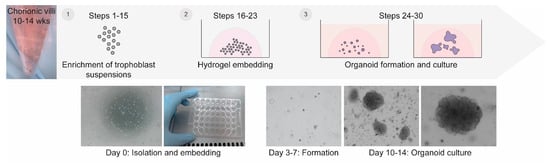
Figure 1.
Overview of the method for generation of trophoblast organoids from CVS tissue. Residual CVS tissue is dissociated to enrich for cytotrophoblast suspensions. Subsequently, these trophoblast cells are embedded as single cells within hydrogel droplets and cultured in trophoblast organoid medium. Small spheroids appear within 3–7 days of culture. Organoid cultures with a villous-like morphology establish after 10–14 days and can be maintained for at least two months.
2.1. Starting Material
To follow this protocol, it is imperative to organize CVS tissue collection and usage in alignment with national and institutional guidelines, including appropriate consent procedures. For the development of the initial method, we made use of chorionic villi that were picked from residual CVS material based on their morphology. In this adapted protocol, picking of chorionic villi is redundant as the modified culture conditions favor trophoblast proliferation and confine potential contamination of decidual cells. Material obtained by CVS can be collected and stored in PBS for up to 3 h. For longer-term storage, CVS material can be saved in serum-enriched culture medium (see Section 3.4—Washing medium) in a humidified incubator at 37 °C and 5% CO2 for up to 3 nights. We have successfully derived trophoblast organoids from only 5 mg of chorionic villi.
2.2. Dissociation
We optimized the dissociation of chorionic villi to enrich for viable trophoblast suspensions. Chorionic villi consist of mesenchymal cores with villous protrusions that in turn are lined with trophoblast layers. Using enzymatic digestion of the villi, we can efficiently detach these trophoblasts, resulting in suspensions that consist mainly (~90%) of cytotrophoblasts based on canonical markers (KRT7, CDH1, GATA3, ITGA6) (Figure 2). We observed some contamination of the obtained cell suspensions with small populations of stromal cells (VIM+ DCN+ COL1A1+), Hofbauer cells (CD53+ CD14+ CCL3+), and blood cells (ALAS+ SNCA+ AHSP+). However, these cells do not influence trophoblast organoid derivation or culture efficiency. Enzymatic digestion followed by removal of large tissue fragments and cell aggregates using cell strainers is sufficient for enrichment of viable cytotrophoblast suspensions, whereas additional separation techniques, including immunomagnetic cell sorting and density gradient centrifugation, did not result in improved cytotrophoblast yield or viability. After enzymatic digestion, mesenchymal cores of the chorionic villi remain intact and could serve as a source of fetal stromal cells, including fibroblasts or endothelial cells.
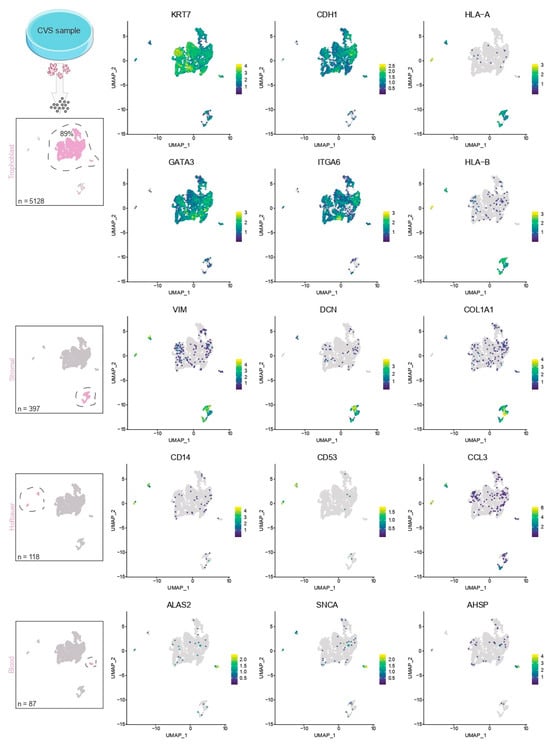
Figure 2.
Single-cell profiling of cell suspensions obtained after dissociation of residual CVS tissue. Villi were pooled and dissociated. Cells were processed on the 10× Genomics Chromium Platform. A total of 6481 transcriptomes were used for further analysis after quality-based filtering. Isolated cell suspensions mostly consist of cytotrophoblasts, with small populations of stromal, Hofbauer and blood cells.
2.3. Culture
Isolated trophoblasts are seeded as single cells in hydrogel drops and cultured for at least 10 to 14 days, until villous structures appear with an average diameter of 250–500 µm. After initial seeding of the trophoblasts, small spheroids with an average diameter of 100 µm appear after 3–7 days of culture. If these structures do not appear after 7 days, trophoblast organoid cultures will likely not develop. During culture, TOM needs to be refreshed every 2 to 3 days. Trophoblast organoid cultures can be established by passaging trophoblast organoids every 5 to 10 days depending on their size, density, and growth rate, and can be maintained for at least 2 months. Passaging is a critical step in establishing long-term cultures, as trophoblast cells within organoids start to differentiate and lose their proliferative capacity over time. Trophoblast organoids mainly consist of cytotrophoblasts that spontaneously fuse into syncytiotrophoblasts in standard culture conditions. With our adapted culture conditions, we could derive trophoblast organoids from a wide variety of CVS tissues (10–14 weeks gestational age) with an efficiency of 91% (20 out of 22 samples), compared to 72% efficiency using our original protocol. Trophoblast organoids can also be cryopreserved and re-cultured, making them suitable for biobanking.
2.4. Contamination of Decidual Cells
A common risk with trophoblast organoid cultures is contamination by maternal decidual cells. In our original protocol, trophoblast organoid cultures were occasionally overgrown by glandular epithelial cells that form large lumenized spheroids (up to 2 mm in diameter), resulting in loss of trophoblast organoids cultures. Decidual contamination is difficult to exclude completely. However, it can be diminished by sorting out decidual tissue from the CVS sample under the microscope and extensive washing of chorionic villi. We noticed that using a stiffer hydrogel (Cultrex UltiMatrix BME, R&D Systems, Minneapolis, MN, USA) impairs the growth, size, and shape of glandular spheroids without affecting trophoblast organoid cultures (Figure 3A). This can prevent glandular epithelial cells from dominating the culture, leaving room for trophoblast organoids to grow out and even for co-cultures to exist. Allowing simultaneous derivation of both trophoblast organoids and decidual gland organoids, our method might also facilitate future research that is focused on trophoblast-decidual interactions at the maternal–fetal interface.
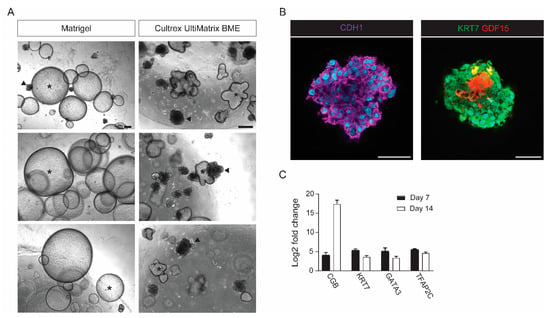
Figure 3.
Validation of CVS-derived trophoblast organoids. (A) Brightfield images of trophoblast organoid cultures (dense structures; marked with arrowheads) contaminated with decidual glandular epithelial cells (big lumenized spheroids; marked with asterisk). Trophoblast cells were cultured in either Matrigel or Cultrex UltiMatrix BME. Scale bar, 200 µm. (B) Representative whole-mount staining of trophoblast organoids at day 14 showing CDH1 (left), and KRT7 and GDF15 (right). Nuclei are stained with Hoechst. Scale bar, 100 µm. (C), Quantitative RT-PCR showing mRNA expression of trophoblast markers in Day 7 and Day 14 trophoblast organoids. Mean values (±SD) are normalized to CVS-derived fibroblasts (n = 3 for each group).
2.5. Validation
After successful establishment of trophoblast organoid cultures, a number of validation experiments can be performed to confirm trophoblast organoid origin and structure. Trophoblast organoids have a villous morphology without any lumen formation that can be observed using brightfield microscopy. Additionally, trophoblast organoid cultures start to secrete hCG hormones in the culture medium. This can easily be detected by dripping some of the culture medium on an over-the-counter pregnancy test following the manufacturer’s instructions. Whole mount immunohistochemical staining can be used to assess trophoblast organoid structure using cytotrophoblast (CDH1 and KRT7) and syncytiotrophoblast (GDF15) markers (Figure 3B). In CVS-derived trophoblast organoids, syncytiotrophoblasts are formed within the organoid cavity, lined by cytotrophoblasts. The expression of trophoblast markers (i.e., CGB, KRT7, GATA3, and TFAP2C) can be determined by quantitative RT-PCR (Figure 3C). Furthermore, genotyping and detailed genetic analysis can be performed upon DNA extraction. Trophoblast organoid cultures can be dissociated for single cell analyses, including single-cell RNA sequencing [5].
2.6. EVT Differentiation
In standard TOM conditions, cytotrophoblast in trophoblast organoids fuse and form syncytiotrophoblasts. In an alternative pathway, cytotrophoblast can also differentiate into extravillous trophoblast (EVT), cells with high migratory capacity that grow out of the placenta and invade the uterus. In CVS-derived trophoblast organoids, differentiation and outgrowth of extravillous trophoblast populations can be induced by removal of Wnt stimulation (CHIR99021 and R-spondin-1) from the TOM (Figure 4). After 3–5 days of culture, EVT-like cells exit the organoid structures and form cell columns. These columns act as conduits for invading cells that show a mesenchymal phenotype and are positive for the EVT marker HLA-G. These invading EVT can be observed after 7–10 days of culture and further migrate as single cells into the hydrogel.
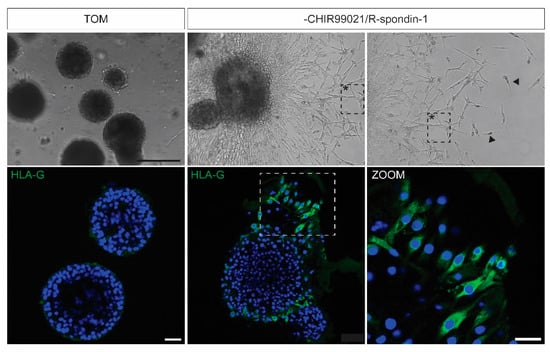
Figure 4.
Induction of EVT differentiation in CVS-derived trophoblast organoids. Brightfield and immunofluorescence images of trophoblast organoids cultured in standard TOM or in TOM without CHIR99021 and R-spondin-1. Removal of CHIR99021 and R-spondin-1 induces outgrowth of HLA-G+ cells that further migrate into the hydrogel. Cell columns (marked with asterisk) form conduits for migrating cells (marked with arrowheads). Scale bars, 300 µm (brightfield) and 100 µm (immunofluorescence).
2.7. Comparison to Stem Cell-Derived Trophoblast Organoids
Besides being derived from placental tissues, trophoblast organoids have also been derived from human trophoblast stem cells (TSC) [3]. Stem cell-derived trophoblast organoids show similar features compared to tissue-derived trophoblast organoids; however, it is not clear to what extent they differ from CVS-derived trophoblast organoids. At a transcriptional level, integrative analysis of previously published single-cell RNA sequencing data shows enrichment of syncytiotrophoblast-specific transcripts in CVS-derived trophoblast organoids compared to TSC-derived trophoblast organoids (Figure 5A,B; Supplementary Table S1). Whether syncytiotrophoblasts are more matured in CVS-derived organoids compared to TSC-derived trophoblast organoids requires additional functional analyses. Conversely, TSC-derived trophoblast organoids show enrichment of transcripts related to translation and ATP synthesis.
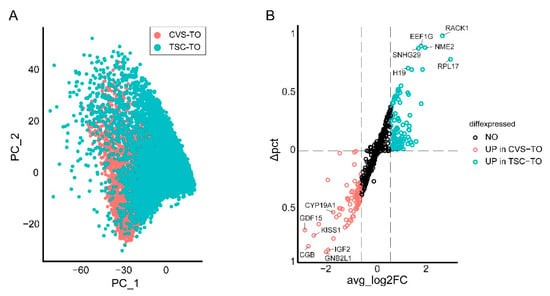
Figure 5.
Transcriptomic comparison of CVS-derived and TSC-derived trophoblast organoids. (A) PCA plot showing integrative analysis of single-cell RNA sequencing data of CVS-derived trophoblast organoids (CVS-TO, n = 16,440 cells) and TSC-derived trophoblast organoids (TSC-TO, n = 31,701 cells). Data are retrieved from previous studies [3,5] and are publicly available in the NCBI GEO repository (CVS-derived trophoblast organoids, GSE211429; TSC-derived trophoblast organoids, GSE172241). Integration of the datasets was performed according to the Seurat alignment workflow using the Leiden algorithm. (B) Volcano plot showing differentially expressed genes (avg_log2FC > 0.6 and adjusted p-value < 0.05) between CVS-TO and TSC-TO. Differences between the percentage of cells expressing the gene in each group (Δpct) are displayed on the y-axis. The complete list of differentially expressed genes is documented in Supplementary Table S1. All analyses were performed using the Seurat package (Version 5.0.1, Satija lab, New York, NY, USA) [6].
3. Materials
3.1. Biological Materials
- -
- Samples of chorionic villi from 10–14 weeks gestation obtained by CVS following standard procedures. Samples can be collected in PBS or culture medium (DMEM/RPMI 1640) without additives. Tissue should be recognized as a potential risk for bloodborne pathogens and should be handled with appropriate personal protective equipment. Samples obtained by transvaginal approaches have a higher likelihood to contain yeast infections. CVS tissues that are a potential hazard for emerging viruses should only be used according to specific biosafety guidelines (BSL-3/4) and require specialized equipment.
3.2. Reagents
- -
- DMEM (Gibco, Waltham, MA, USA; cat.no. 31966021)
- -
- Fetal bovine serum (Capricorn Scientific, Ebsdorfergrund, Germany; cat.no. FBS-12A)
- -
- Penicillin-Streptomycin (Sigma-Aldrich, St. Louis, MO, USA; cat.no. P0781)
- -
- Trypsin-EDTA (Sigma-Aldrich; cat.no. T3924)
- -
- DPBS (Sigma-Aldrich; cat.no. D8537)
- -
- Bovine serum albumin (Sigma-Aldrich; cat.no. A3294)
- -
- ACK lysing buffer (Gibco; cat.no. A1049201)
- -
- Cultrex UltiMatrix RGF BME (R&D Systems, Minneapolis, MN, USA; cat.no. BME00110)
- -
- Advanced DMEM/F12 (Gibco; cat.no. 12634028)
- -
- Knockout serum replacement (Gibco; cat.no. 10828010)
- -
- GlutaMAX (Gibco; cat.no. 35050038)
- -
- N-Acetyl-L-cysteine (Sigma-Aldrich; cat.no. A9165)
- -
- Nicotinamide (Sigma-Aldrich; cat.no. N0636)
- -
- N-2 supplement (Gibco; cat.no. 17502048)
- -
- B-27 supplement, minus vitamin A (Gibco; cat.no. 12587010)
- -
- Primocin (Invivogen, San Diego, CA, USA; cat.no. ant-pm-1)
- -
- Recombinant human EGF (Peprotech, London, UK; cat.no. AF-100-15)
- -
- GSK inhibitor CHIR99021 (Tocris, Bristol, UK; cat.no. 4423)
- -
- Recombinant human R-spondin-1 (Peprotech; cat.no. 120-38)
- -
- Recombinant human HGF (Peprotech; cat.no. 100-39)
- -
- Recombinant human FGF (Peprotech; cat.no. 100-18B)
- -
- Rock inhibitor Y-27632 (Millipore, Burlington, MA, USA; cat.no. 688000)
- -
- Prostaglandin E2 (Sigma-Aldrich; cat.no. P0409)
- -
- A83-01 (Tocris; cat.no. 2939)
- -
- Cultrex organoid harvesting solution (R&D Systems; cat.no. 3700-100-01)
- -
- Cellbanker 2 cryopreservation medium (Amsbio, Abingdon, UK; cat.no. 11914)
- -
- Paraformaldehyde (Sigma-Aldrich; cat.no. 158127)
- -
- Sucrose (Sigma-Aldrich; cat.no. S9378)
- -
- Anti-CDH1 antibody (Cell Signaling Technology, Danvers, MA, USA; cat.no. 3195, dilution 1:200)
- -
- Anti-KRT7 antibody (Agilent Technologies, Santa Clara, CA, USA; cat.no. M7018, dilution 1:100)
- -
- Anti-GDF15 antibody (Sigma-Aldrich; cat.no. HPA011191, dilution 1:150)
- -
- Anti-HLA-G antibody (Exbio, Vestec, Czechia; cat.no. 11-431-C100, dilution 1:100)
- -
- Alexa Fluor 488 antibody (Invitrogen, Waltham, MA, USA; cat.no. A32723, dilution 1:500)
- -
- Alexa Fluor 647 antibody (Invitrogen; cat.no. A32733, dilution 1:500)
3.3. Equipment
- -
- Microbiological class II safety cabinet (Scala Scientific, Ede, The Netherlands; cat.no. BIO 130 A2)
- -
- Incubator (PHCbi, Etten-Leur, The Netherlands; cat.no. MCO-170 AIC-UVD PE)
- -
- Centrifuge (ThermoFisher Scientific, Waltham, MA, USA; cat.no. 75004210)
- -
- Benchtop centrifuge (ThermoFisher Scientific; cat.no. 75002421)
- -
- Digital microscope (ThermoFisher Scientifc; cat.no. AMF5000)
- -
- Nitrile gloves (Ansell, Richmond, Australia; cat.no. 6034153)
- -
- Serological pipette controller (DLAB Scientific Co., Beijing, China; cat.no. 861051B)
- -
- Serological pipettes (Greiner Bio-One, Kremsmünster, Austria; cat.no. 607180)
- -
- Single-channel pipettes (Mettler Toledo, Hong Kong, China; cat.no. 17014391/17014382)
- -
- Pipette tips (Mettler Toledo; cat.no. 30389276/30389272)
- -
- Cell culture 48 well plate (Greiner Bio-One; cat.no. 677180)
- -
- Conical 15mL tubes (Greiner Bio-One; cat.no. 188271-N)
- -
- Microcentrifuge 1.5 mL tubes (Greiner Bio-One; cat.no. 616201)
- -
- Cell culture cryogenic tubes (ThermoFisher Scientific; cat.no. 366656)
- -
- Cell strainer 30 µm (Miltenyi Biotec, Bergisch Gladbach, Germany; cat.no. 130-098-458)
3.4. Reagent Setup
Washing medium
DMEM supplemented with 1× penicillin/streptomycin and 10% FBS (v/v). Store washing medium at 4 °C for up to 1 month.
Coating solution
Dissolve 1 g of BSA in 100 mL of PBS. Store at 4 °C for up to 1 month.
Cultrex UltiMatrix BME stocks
Thaw vial of Cultrex UltiMatrix GFR BME overnight on ice at 4 °C. Prepare aliquots of 100–200 µL in 1.5 mL microcentrifuge tubes and store at −80 °C. Avoid freeze–thaw cycles.
Trophoblast organoid medium (TOM)
Aliquot and store all TOM supplements according to the manufacturer’s instructions. For this protocol, we have slightly adjusted previously published TOM formulations [1]. TOM consists of Advanced DMEM/F12 supplemented with 1X B27 minus vitamin A, 1X N2, 10% Knockout serum replacement (vol/vol), 100 µg/mL primocin, 2 mM L-glutamine, 1.25 mM N-Acetyl-L-cysteine, 10 mM nicotinamide, 500 nM A83-01, 1.5 µM CHIR99021, 50 ng/mL recombinant human EGF, 80 ng/mL recombinant human R-spondin-1, 100 ng/mL recombinant human FGF2, 50 ng/mL recombinant human HGF, 5 µM Y—27632, and 2.5 µM prostaglandin E2 (Sigma).
4. Method
4.1. Preparation
Timing: 0.5–1 h
Before start of the protocol:
- -
- Thaw the desired number of Cultrex UltiMatrix RGF BME aliquots on ice for at least 1 h. Keep on ice at all times.
- -
- Thaw and/or warm Trypsin/EDTA.
- -
- Prepare and/or warm washing medium.
- -
- Prepare TOM.
- -
- Prepare coating solution.
- -
- Start cooling the benchtop centrifuge to 4 °C.
- -
- Warm a 48-wells plate in the incubator (37 °C and 5% CO2).
4.2. Enzymatic Digestion and Preparation of Cell Suspension
Timing: 1–1.5 h
- Collect surplus CVS tissue.
- Villi can be stored for up to 2 or 3 nights in washing medium in the incubator (37 °C and 5% CO2).
- Prewash pipettes with coating solution to prevent sticking of CVS tissue.
- Transfer all CVS tissue to a 15 mL conical tube.
- Let the villi settle down or centrifuge at 300× g for 5 min at room temperature.
- Repeat until you have collected all the tissue.
- Aspirate supernatant.
- Incubate villi in 2 mL Trypsin-EDTA for 30 min in the incubator (37 °C and 5% CO2) or water bath (37 °C). Longer incubation will result in lower cell viability.
- Prewash a 30 µm cell strainer with 5 mL washing medium (collect in 15 mL tube).
- Prewash pipettes with coating solution to prevent sticking of tissue.
- Pipette villi up and down (~50/100 times) with a coated P1000 pipette and apply to cell strainer.
- Repeat until all tissue is processed.
- Wash the cell strainer with 5 mL washing medium.
- Centrifuge at 300× g for 5 min at room temperature.
- If the pellet contains large amounts of blood, follow steps 12a–c. Otherwise, proceed with step 13.
- Resuspend pellet in 1–2 mL ACK lysing buffer (1:5 ratio of cell pellet: ACK lysing buffer).
- Incubate for 5–10 min at room temperature while gently flicking the tube every 2 min. Duration of the incubation depends on the severity of red blood cell contamination; lysis should be visually assessed.
- Centrifuge at 300× g for 5 min at room temperature.
- Aspirate supernatant.
- Resuspend the pellet in 1 mL ice-cold Advanced DMEM/F12 and transfer to a 1.5 mL microcentrifuge tube.
- Count the cells.
4.3. Initiation of Trophoblast Organoid Cultures
Timing: 0.5 h
- 16.
- Centrifuge the isolated cells at 300× g for 5 min at 4 °C.
- 17.
- Carefully aspirate as much medium as possible without disturbing the pellet and gently flick the tube to loosen the pellet.
- 18.
- Put cells on ice.
- 19.
- Add Cultrex UltiMatrix RGF BME to establish a concentration of 1 × 104–1 × 105 cells/25 µL of hydrogel and carefully mix by pipetting. Prevent the formation of air bubbles.
- 20.
- Put cells on ice.
- 21.
- Pipette small hydrogel domes (20–25 µL) in the middle of every well. Prevent the formation of air bubbles.
- 22.
- Let the hydrogel domes settle for 1–2 min at room temperature and carefully transfer the plate to the incubator (37 °C and 5% CO2).
- 23.
- After 2 min, invert the plate upside down in one movement and incubate for 10–13 min. Inverting the plate facilitates spreading of the cells within the hydrogel domes. In the meantime, warm TOM medium.
- 24.
- Add 250 µL pre-warmed TOM medium to each well.
4.4. Maintenance of Trophoblast Organoids Cultures
Timing: 2–3 weeks
- 25.
- Refresh TOM every 2–4 days. Organoid structures should appear after 5 to 7 days.
- 26.
- Induction of EVT differentiation:
- Culture trophoblast organoids in TOM for 7 to 10 days (small structures should be developed).
- Aspirate TOM.
- Replace TOM for TOM without CHIR99021 and R-spondin-1 (TOM-EVT). Refresh TOM-EVT every 2–4 days.
- Outgrowth of EVT-like cells can be observed after 3–5 days. Invasive cells can be observed after 7–10 days.
- 27.
- Passaging: In order to establish long-term cultures, trophoblast organoids need to be passaged every 5 to 10 days depending on their size, density, and growth rate. Organoid cultures should be passaged when at least half of the structures reach a diameter of 200 µm.
- Break down the hydrogel domes by scraping using a pipette tip.
- Transfer the content (TOM with broken hydrogel) of the well to a 1.5 mL microcentrifuge tube. Collect up to 4 wells per tube. Prewash pipettes with coating solution to prevent sticking of organoids.
- Wash every well with 250 µL of ice-cold Advanced DMEM/F12 and add to the tube. Prewash pipettes with coating solution to prevent sticking of organoids.
- Centrifuge at 300× g for 5 min at room temperature.
- Aspirate supernatant and add 200 µL of ice-cold Advanced DMEM/F12.
- Mechanically dissociate the organoids by extensive pipetting (200–500 times) using small pipette tips until organoids are broken into hardly visible particles. Prewash pipettes with coating solution to prevent sticking of organoids.
- Add 1 mL Advanced DMEM/F12 and centrifuge at 300× g for 5 min at room temperature.
- Proceed with step 16. Residual hydrogel should be taken into account when adding new Cultrex UltiMatrix RGF BME. Organoid cultures should be passaged at a 1:2 ratio. For cryopreservation, proceed with Step 29.
- 28.
- Harvesting:
- Aspirate TOM.
- Dissolve the organoid-containing drops in 500 µL ice-cold Cell Recovery solution for 30 min at 4 °C.
- Break down any residual hydrogel domes by scraping using a pipette tip.
- Collect the content of the well in a 1.5 mL microcentrifuge tube. Prewash pipettes with coating solution to prevent sticking of organoids.
- Wash every well with 500 µL of ice-cold Advanced DMEM/F12 and add to the tube. Prewash pipettes with coating solution to prevent sticking of organoids.
- 29.
- Cryopreservation:
- Perform Step 27: Passaging.
- Aspirate supernatant.
- Resuspend in 1 mL of Cellbanker-2 cryopreservation medium and transfer to labeled cryovials.
- Store cryovials at 80 °C for 24–48 h.
- Transfer cryovials to liquid nitrogen storage. Organoid cultures can be stored for at least 3 years.
- 30.
- Thawing:
- Transfer cryovial to a water bath (37 °C) and thaw as quickly as possible.
- Add 10 mL of Advanced DMEM/F12 and transfer to a 15 mL conical tube.
- Centrifuge at 300× g for 5 min at room temperature.
- Aspirate supernatant and proceed with Step 15.
4.5. Single-Cell Isolation
Timing: 1h
- 31.
- Pool organoids for dissociation into single cells.
- 32.
- Perform Step 28: Harvesting.
- 33.
- Centrifuge at 300× g for 5 min at room temperature.
- 34.
- Aspirate supernatant and resuspend in 1 mL Trypsin-EDTA. Prewash pipettes with coating solution to prevent sticking of CVS tissue.
- 35.
- Incubate for 15 min at 37 °C.
- 36.
- Mechanically dissociate the organoids by extensive pipetting (200–500 times) using small pipette tips.
- 37.
- Prewash a 30 µm cell strainer with 1 mL Advanced DMEM/F12 (collect in 15 mL tube).
- 38.
- Filter the cell suspension of the cell strainer and wash the cell strainer with 4 mL of Advanced DMEM/F12.
- 39.
- Centrifuge at 300× g for 5 min at room temperature.
- 40.
- Count cells and check viability. If proceeding with single-cell RNA sequencing using the 10× Chromium platform, resuspend cells in Advanced DMEM/F12 to a final concentration of 700–1000 cells/µL. We pooled roughly 50 Day-14 organoids to obtain 10,000–15,000 high-quality cells for downstream analysis.
5. Limitations
While our method for generating trophoblast organoids from chorionic villus sampling represents a significant step forward in modeling placental biology, it is essential to acknowledge the limitations inherent to our approach. Understanding these limitations is crucial for interpreting the results and optimizing the utility of trophoblast organoids for studying placental development and function. One of the main limitations is the selective presence of trophoblast cells in CVS-derived trophoblast organoids. Similar to most trophoblast organoid models, CVS-derived trophoblast organoids lack the diversity of cell types present in the in vivo placental villi, including stromal cells, endothelial cells and Hofbauer cells. In the placenta, these cell types play crucial roles in vascularization and immunomodulation. The absence of these cells within the trophoblast organoids limits our ability to fully replicate the complex microenvironment of placental villi and may affect the interpretation of certain physiological and functional aspects of placental biology. Further development of CVS-derived trophoblast organoid models might include co-culturing with (matched) populations of stromal, endothelial or immune cells, all of which can be potentially isolated from residual CVS tissue. Additionally, integration with microfluidic cell culture devices might increase the complexity and fidelity of CVS-derived trophoblast organoids, emulating physiologically relevant interactions at higher levels of tissue organization.
A second limitation of our CVS-derived trophoblast organoids is the reversed polarity of the organoid structures. While the cultivation of cells within hydrogels offers the advantage of creating a tissue-like environment by providing a basement membrane, it introduces a bias in the orientation of the cells. In this setup, CVS-derived cytotrophoblast tend to align themselves with their basal surface in contact with the hydrogel, resulting in differentiation of these cells towards the inside of the organoid. As such, syncytiotrophoblasts are formed within the organoid cavity, opposite to the in vivo architecture where syncytiotrophoblast line the outside layers of placental villi. Consequently, this limitation could affect the extent to which our model accurately represents certain aspects of placental functionality, especially those related to pathogen defense and exchange of nutrients and drugs. A recent study reported a methodology that successfully addressed the issue of trophoblast organoid polarity, developing organoids that contain syncytiotrophoblast on the outer surface [7]. For this, trophoblast organoids were transferred to suspension cultures, a method previously shown to control epithelial polarity [8]. We anticipate that CVS-derived trophoblast organoids are amenable to this methodology. However, future research should examine potential effects on organoid derivation efficiency and long-term expansion.
6. Summary
In conclusion, our method provides efficient generation of trophoblast organoids from CVS and opens the door to a range of exciting opportunities for studying placental development and function during ongoing pregnancy. This protocol outlines the handling of CVS tissue, tailored dissociation of CVS tissue to enrich for cytotrophoblast populations, and cultivation of trophoblast organoids. More specifically, our method facilitates simultaneous derivation of both trophoblast and decidual gland structures. This protocol takes ~2.5 h to complete from tissue collection to initiation of trophoblast organoids cultures, with an additional 2–3 weeks of cultivation.
Supplementary Materials
The following information can be downloaded at: https://www.mdpi.com/article/10.3390/organoids3010005/s1, Table S1: Differential gene expression analysis between CVS-derived and hTSC-derived trophoblast organoids.
Author Contributions
Conceptualization, B.v.R. and O.S.; methodology, B.v.R., N.v.K., D.V.O., M.K. and O.S.; investigation, O.S., writing—original draft preparation, O.S.; writing—review and editing, B.v.R., J.G. and O.S.; visualization, O.S.; supervision, B.v.R., J.G. and O.S.; funding acquisition, B.v.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by personal grant (funding number: CAM20-13) from the Erasmus MC Sophia Foundation (B.v.R.).
Institutional Review Board Statement
Utilization of tissues and all experimental procedures were performed according the regulatory framework provided by the Erasmus University Medical Center Ethics Board (study number: OZBS71.19172) and are in accordance with the guidelines in the The Declaration of Helsinki 2000.
Informed Consent Statement
Patient consent was waived in line with the existing Dutch ethical regulations for the use of surplus human tissue, and within the appropriate regulatory framework for anonymized use of surplus human tissue provided by the Erasmus University Medical Center Ethics Board.
Data Availability Statement
All data associated with this study are present in the paper of in the Supplementary Information. Raw sequencing data are publicly available in the NCBI repository (GSE211429; GSE172241). Code used for analysis is available upon request.
Acknowledgments
We acknowledge support from the Department of Clinical Genetics with collection of the CVS tissue.
Conflicts of Interest
The authors declare no competing interests.
References
- Turco, M.Y.; Gardner, L.; Kay, R.G.; Hamilton, R.S.; Prater, M.; Hollinshead, M.S.; McWhinnie, A.; Esposito, L.; Fernando, R.; Skelton, H.; et al. Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature 2018, 564, 263–267. [Google Scholar] [CrossRef]
- Haider, S.; Meinhardt, G.; Saleh, L.; Kunihs, V.; Gamperl, M.; Kaindl, U.; Ellinger, A.; Burkard, T.R.; Fiala, C.; Pollheimer, J.; et al. Self-Renewing Trophoblast Organoids Recapitulate the Developmental Program of the Early Human Placenta. Stem Cell Rep. 2018, 11, 537–551. [Google Scholar] [CrossRef]
- Karvas, R.M.; Khan, S.A.; Verma, S.; Yin, Y.; Kulkarni, D.; Dong, C.; Park, K.M.; Chew, B.; Sane, E.; Fischer, L.A.; et al. Stem-cell-derived trophoblast organoids model human placental development and susceptibility to emerging pathogens. Cell Stem Cell 2022, 29, 810–825.e8. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Semmes, E.C.; Ovies, C.; Megli, C.; Permar, S.; Gilner, J.B.; Coyne, C.B. Innate immune signaling in trophoblast and decidua organoids defines differential antiviral defenses at the maternal-fetal interface. Elife 2022, 11, e79794. [Google Scholar] [CrossRef] [PubMed]
- Schäffers, O.J.M.; Dupont, C.; Bindels, E.M.; Van Opstal, D.; Dekkers, D.H.W.; Demmers, J.A.A.; Gribnau, J.; van Rijn, B.B. Single-Cell Atlas of Patient-Derived Trophoblast Organoids in Ongoing Pregnancies. Organoids 2022, 1, 106–115. [Google Scholar] [CrossRef]
- Hao, Y.; Stuart, T.; Kowalski, M.H.; Choudhary, S.; Hoffman, P.; Hartman, A.; Srivastava, A.; Molla, G.; Madad, S.; Fernandez-Granda, C.; et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat. Biotechnol. 2023, 42, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liang, P.; Yang, H.; Coyne, C.B. Trophoblast organoids with physiological polarity model placental structure and function. J. Cell Sci. 2023, 137, jcs261528. [Google Scholar] [CrossRef] [PubMed]
- Co, J.Y.; Margalef-Català, M.; Monack, D.M.; Amieva, M.R. Controlling the polarity of human gastrointestinal organoids to investigate epithelial biology and infectious diseases. Nat. Protoc. 2021, 16, 5171–5192. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).