Gestational Age Is Positively Associated with Retinol and α-Tocopherol in Preterm Infants: The Mediating Role of Birth Weight
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Anthropometric Measurements
2.4. Biochemical Measurements
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Traber, M.G.; Head, B. Vitamin E: How much is enough, too much and why! Free Radic. Biol. Med. 2021, 177, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Didier, A.J.; Stiene, J.; Fang, L.; Watkins, D.; Dworkin, L.D.; Creeden, J.F. Antioxidant and Anti-Tumor Effects of Dietary Vitamins A, C, and E. Antioxidants 2023, 12, 632. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Gardner, E.M. The Function of Vitamin A in Cellular Growth and Differentiation, and Its Roles during Pregnancy and Lactation. In Nutrient Regulation during Pregnancy, Lactation, and Infant Growth; Allen, L., King, J., Lönnerdal, B., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1994; Volume 352, pp. 187–200. ISBN 978-1-4899-2577-0. [Google Scholar]
- Massaro, D.; Massaro, G.D. Lung Development, Lung Function, and Retinoids. N. Engl. J. Med. 2010, 362, 1829–1831. [Google Scholar] [CrossRef] [PubMed]
- Dowling, J.E. Vitamin A: Its many roles—From vision and synaptic plasticity to infant mortality. J. Comp. Physiol. A 2020, 206, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Blaner, W.S.; Shmarakov, I.O.; Traber, M.G. Vitamin A and Vitamin E: Will the Real Antioxidant Please Stand Up? Annu. Rev. Nutr. 2021, 41, 105–131. [Google Scholar] [CrossRef] [PubMed]
- Kamal-Eldin, A.; Appelqvist, L.-Å. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef]
- Ungurianu, A.; Zanfirescu, A.; Nițulescu, G.; Margină, D. Vitamin E beyond Its Antioxidant Label. Antioxidants 2021, 10, 634. [Google Scholar] [CrossRef]
- Assunção, D.G.F.; da Silva, L.T.P.; Camargo, J.D.d.A.S.; Cobucci, R.N.; Ribeiro, K.D.d.S. Vitamin E Levels in Preterm and Full-Term Infants: A Systematic Review. Nutrients 2022, 14, 2257. [Google Scholar] [CrossRef]
- Henriksen, C.; Helland, I.B.; Rønnestad, A.; Grønn, M.; Iversen, P.O.; Drevon, C.A. Fat-soluble vitamins in breast-fed preterm and term infants. Eur. J. Clin. Nutr. 2006, 60, 756–762. [Google Scholar] [CrossRef]
- Kositamongkol, S.; Suthutvoravut, U.; Chongviriyaphan, N.; Feungpean, B.; Nuntnarumit, P. Vitamin A and E status in very low birth weight infants. J. Perinatol. 2011, 31, 471–476. [Google Scholar] [CrossRef]
- Shenai, J.P.; Chytil, F.; Jhaveri, A.; Stahlman, M.T. Plasma vitamin A and retinol-binding protein in premature and term neonates. J. Pediatr. 1981, 99, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Hågå, P.; Lunde, G. SELENIUM AND VITAMIN E IN CORD BLOOD FROM PRETERM AND FULL TERM INFANTS. Acta Paediatr. 1978, 67, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Leonard, P.J.; Doyle, E.; Harrington, W. Levels of vitamin E in the plasma of newborn infants and of the mothers. Am. J. Clin. Nutr. 1972, 25, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Tsang, R.C.; Uauy, R.; Koletzko, B.; Zlotkin, S.H. (Eds.) Nutrition of the Preterm Infant: Scientific Basis and Practical Guidelines; Digital Educational Publishing: Cincinnati, OH, USA, 2005; pp. 141–173. [Google Scholar]
- Traber, M.; Cohn, W.; Muller, D. Absorption, Transport and Delivery to Tissues. In Vitamin E in Health and Disease; Packer, L., Fuchs, J., Eds.; Marcel Dekker: New York, NY, USA, 1992; pp. 35–53. [Google Scholar]
- Kono, N.; Arai, H. Platelet-activating factor acetylhydrolases: An overview and update. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2018, 1864, 922–931. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, S.M.; Blaner, W.S. Retinol and retinyl esters: Biochemistry and physiology. J. Lipid Res. 2013, 54, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Giacoia, G.P. Concentration of Serum Prealbumin and Retinol-Binding Proteins During Pregnancy. South. Med. J. 1984, 77, 1261–1263. [Google Scholar] [CrossRef] [PubMed]
- Jeanes, Y.M.; Hall, W.L.; Ellard, S.; Lee, E.; Lodge, J.K. The absorption of vitamin E is influenced by the amount of fat in a meal and the food matrix. Br. J. Nutr. 2004, 92, 575–579. [Google Scholar] [CrossRef]
- Marceau, G.; Gallot, D.; Lemery, D.; Sapin, V. Metabolism of Retinol During Mammalian Placental and Embryonic Development. In Vitamins & Hormones; Elsevier: Amsterdam, The Netherlands, 2007; Volume 75, pp. 97–115. ISBN 978-0-12-709875-3. [Google Scholar]
- Debier, C.; Larondelle, Y. Vitamins A and E: Metabolism, roles and transfer to offspring. Br. J. Nutr. 2005, 93, 153–174. [Google Scholar] [CrossRef]
- Gannon, B.M.; Rogers, L.M.; Tanumihardjo, S.A. Metabolism of Neonatal Vitamin A Supplementation: A Systematic Review. Adv. Nutr. Int. Rev. J. 2020, 12, 942–958. [Google Scholar] [CrossRef]
- Yang, W.; Jiao, M.; Xi, L.; Han, N.; Luo, S.; Xu, X.; Zhou, Q.; Wang, H. The association between maternal fat-soluble vitamin concentrations during pregnancy and infant birth weight in China. Br. J. Nutr. 2020, 125, 1058–1066. [Google Scholar] [CrossRef]
- Rao, N.A.; Wu, G.S. Oxygen free radicals and retinopathy of prematurity. Br. J. Ophthalmol. 1996, 80, 387. [Google Scholar] [CrossRef] [PubMed]
- Paiva, S.; Godoy, I.; Vannucchi, H.; Fávaro, R.M.; Geraldo, R.R.; Campana, A. Assessment of vitamin A status in chronic obstructive pulmonary disease patients and healthy smokers. Am. J. Clin. Nutr. 1996, 64, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Zachman, R.D.; Samuels, D.P.; Brand, J.M.; Winston, J.F.; Pi, J.T. Use of the intramuscular relative-dose-response test to predict bronchopulmonary dysplasia in premature infants. Am. J. Clin. Nutr. 1996, 63, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Clagett-Dame, M.; DeLuca, H.F. THEROLE OFVITAMINAINMAMMALIANREPRODUCTION ANDEMBRYONICDEVELOPMENT. Annu. Rev. Nutr. 2002, 22, 347–381. [Google Scholar] [CrossRef]
- Barekatain, B.; Saraeian, S.; Farghadani, M.; Armanian, A.M.; Shahsanaee, A.; Rouhani, E.; Safaei, A. Effect of vitamin E in prevention of intraventricular hemorrhage in preterm neonates. Int. J. Prev. Med. 2018, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.N.; Duggan, C. 50 Years Ago in The Journal of Pediatrics. J. Pediatr. 2017, 181, 162. [Google Scholar] [CrossRef]
- Cannavò, L.; Perrone, S.; Viola, V.; Marseglia, L.; Di Rosa, G.; Gitto, E. Oxidative Stress and Respiratory Diseases in Preterm Newborns. Int. J. Mol. Sci. 2021, 22, 12504. [Google Scholar] [CrossRef]
- Ogihara, T.; Mino, M. Vitamin E and preterm infants. Free Radic. Biol. Med. 2021, 180, 13–32. [Google Scholar] [CrossRef]
- Miyake, M.; Miki, M.; Yasuda, H.; Ogihara, T.; Mino, M. Vitamin E and the Peroxidizability of Erythrocyte Membranes in Neonates. Free Radic. Res. Commun. 1991, 15, 41–50. [Google Scholar] [CrossRef]
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2021, 209, 114477. [Google Scholar] [CrossRef]
- Matyas, M.; Hasmasanu, M.G.; Zaharie, G. Antioxidant Capacity of Preterm Neonates Assessed by Hydrogen Donor Value. Medicina 2019, 55, 720. [Google Scholar] [CrossRef] [PubMed]
- Dani, C.; Poggi, C.; Auten, R.L.; Farrow, K.N.; Collins, J.J.; Thébaud, B.; Wedgwood, S.; Steinhorn, R.H.; Berkelhamer, S.K.; Sdrimas, K.; et al. The Role of Genetic Polymorphisms in Antioxidant Enzymes and Potential Antioxidant Therapies in Neonatal Lung Disease. Antioxid. Redox Signal. 2014, 21, 1863–1880. [Google Scholar] [CrossRef] [PubMed]
- Duh-Leong, C.; Ghassabian, A.; Kannan, K.; Gross, R.S.; Ortiz, R.; Gaylord, A.; Afanasyeva, Y.; Lakuleswaran, M.; Spadacini, L.; Trasande, L. Prenatal oxidative stress and rapid infant weight gain. Int. J. Obes. 2023, 47, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Rakshasbhuvankar, A.A.; Pillow, J.J.; Simmer, K.N.; Patole, S.K. Vitamin A supplementation in very-preterm or very-low-birth-weight infants to prevent morbidity and mortality: A systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 2021, 114, 2084–2096. [Google Scholar] [CrossRef] [PubMed]
- Tateno, M.; Oshima, A. The relation between serum-vitamin E levels in the perinatal period and the birth weight of the neonate. Acta Obstet. Gynaecol. Jpn. 1973, 20, 177–181. [Google Scholar] [PubMed]
- Chen, H.-J.; Hsu, C.-H.; Chiang, B.-L. Serum retinol levels and neonatal outcomes in preterm infants. J. Formos. Med Assoc. 2017, 116, 626–633. [Google Scholar] [CrossRef]
- Skouroliakou, M.; Konstantinou, D.; Koutri, K.; Kakavelaki, C.; Stathopoulou, M.; Antoniadi, M.; Xemelidis, N.; Kona, V.; Markantonis, S. A double-blind, randomized clinical trial of the effect of ω-3 fatty acids on the oxidative stress of preterm neonates fed through parenteral nutrition. Eur. J. Clin. Nutr. 2010, 64, 940–947. [Google Scholar] [CrossRef]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Battaglia, F.C.; Lubchenco, L.O. A practical classification of newborn infants by weight and gestational age. J. Pediatr. 1967, 71, 159–163. [Google Scholar] [CrossRef]
- Deger, I.; Ertuğrul, S.; Yılmaz, S.T.; Özbey, Z.K.; Yolbaş, I.; Kaplan, I. The relationship between Vitamin A and Vitamin E levels and neonatal morbidities. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1963–1969. [Google Scholar] [CrossRef]
- Tao, E.; Chen, C.; Chen, Y.; Cai, L.; Yuan, T. The relationship between umbilical cord blood vitamin A levels and late preterm infant morbidities: A prospective cohort study. Eur. J. Pediatr. 2020, 180, 791–797. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, K.; Wei, X.-P.; Qu, P.; Liu, Y.-X.; Chen, J.; Li, T.-Y. Perinatal Vitamin A Status in Relation to Neurodevelopmental Outcome at two Years of Age. Int. J. Vitam. Nutr. Res. 2009, 79, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Kiatchoosakun, P.; Jirapradittha, J.; Panthongviriyakul, M.C.; Khampitak, T.; Yongvanit, P.; Boonsiri, P. Vitamin A supplementation for prevention of bronchopulmonary dysplasia in very-low-birth-weight premature Thai infants: A randomized trial. J. Med. Assoc. Thai. 2014, 97 (Suppl. 10), S82–S88. [Google Scholar] [PubMed]
- Giacomozzi, C.; Ghirri, P.; Lapolla, R.; Bartoli, A.; Scirè, G.; Serino, L.; Germani, D.; Boldrini, A.; Cianfarani, S. Retinol-binding protein 4 in neonates born small for gestational age. J. Endocrinol. Investig. 2010, 33, 217–221. [Google Scholar] [CrossRef]
- Smith, F.R.; Goodman, D.S.; Arroyave, G.; Viteri, F. Serum vitamin A, retinol-binding protein, and prealbumin concentrations in protein-calorie malnutrition. Am. J. Clin. Nutr. 1973, 26, 982–987. [Google Scholar] [CrossRef]
- Galinier, A.; Périquet, B.; Lambert, W.; Garcia, J.; Assouline, C.; Rolland, M.; Thouvenot, J.-P. Reference range for micronutrients and nutritional marker proteins in cord blood of neonates appropriated for gestational ages. Early Hum. Dev. 2005, 81, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Z.; Ren, W.-H.; Liao, W.-Q.; Zhang, G.-Y. Concentrations of Antioxidant Vitamins in Maternal and Cord Serum and Their Effect on Birth Outcomes. J. Nutr. Sci. Vitaminol. 2009, 55, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bell, E.F.; Hansen, N.I.; Brion, L.P.; Ehrenkranz, R.A.; Kennedy, K.A.; Walsh, M.C.; Shankaran, S.; Acarregui, M.J.; Johnson, K.J.; Hale, E.C.; et al. Serum Tocopherol Levels in Very Preterm Infants After a Single Dose of Vitamin E at Birth. Pediatrics 2013, 132, e1626–e1633. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.K.-L.; Lim, M.S.-F.; Choo, S.H.-T.; Tan, I.-K. Vitamin E status of infants at birth. J. Perinat. Med. 1999, 27, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; Decsi, T.; Domellöf, M.; Embleton, N.; Fewtrell, M.; et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Vitamins. Clin. Nutr. 2018, 37, 2366–2378. [Google Scholar] [CrossRef]
- Brion, L.P.; Bell, E.F.; Raghuveer, T.S. Vitamin E supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 2003, 2010, CD003665. [Google Scholar] [CrossRef]
- Tanaka, H.; Mino, M.; Takeuchi, T. A nutritional evaluation of vitamin E status in very low birth weight infants with respect to changes in plasma and red blood cell tocopherol levels. J. Nutr. Sci. Vitaminol. 1988, 34, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Sámano, R.; Martínez-Rojano, H.; Hernández, R.M.; Ramírez, C.; Quijano, M.E.F.; Espíndola-Polis, J.M.; Veruete, D. Retinol and α-Tocopherol in the Breast Milk of Women after a High-Risk Pregnancy. Nutrients 2017, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Bishara, R.; Dunn, M.S.; Merko, S.E.; Darling, P. Nutrient Composition of Hindmilk Produced by Mothers of Very Low Birth Weight Infants Born at Less Than 28 Weeks’ Gestation. J. Hum. Lact. 2008, 24, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Martysiak-Żurowska, D.; Szlagatys-Sidorkiewicz, A.; Zagierski, M. Concentrations of alpha- and gamma-tocopherols in human breast milk during the first months of lactation and in infant formulas. Matern. Child Nutr. 2012, 9, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Haug, M.; Laubach, C.; Burke, M.; Harzer, G. Vitamin E in Human Milk from Mothers of Preterm and Term Infants. J. Pediatr. Gastroenterol. Nutr. 1987, 6, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Fares, S.; Sethom, M.M.; Khouaja-Mokrani, C.; Jabnoun, S.; Feki, M.; Kaabachi, N. Vitamin A, E, and D Deficiencies in Tunisian Very Low Birth Weight Neonates: Prevalence and Risk Factors. Pediatr. Neonatol. 2014, 55, 196–201. [Google Scholar] [CrossRef] [PubMed]
- de Segura, A.G.; Escuder, D.; Montilla, A.; Bustos, G.; Pallás, C.; Fernández, L.; Corzo, N.; Rodríguez, J.M. Heating-induced Bacteriological and Biochemical Modifications in Human Donor Milk After Holder Pasteurisation. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 197–203. [Google Scholar] [CrossRef]
- Paulaviciene, I.J.; Liubsys, A.; Eidukaite, A.; Molyte, A.; Tamuliene, L.; Usonis, V. The Effect of Prolonged Freezing and Holder Pasteurization on the Macronutrient and Bioactive Protein Compositions of Human Milk. Breastfeed. Med. 2020, 15, 583–588. [Google Scholar] [CrossRef]
- Van Zoeren-Grobben, D.; Schrijver, J.; Berg, H.V.D.; Berger, H.M. Human milk vitamin content after pasteurisation, storage, or tube feeding. Arch. Dis. Child. 1987, 62, 161–165. [Google Scholar] [CrossRef]
- Ribeiro, K.D.S.; Melo, I.L.P.; Pristo, A.Z.O.; Dimenstein, R. The effect of processing on the Vitamin A content of human milk. J. Pediatr. 2005, 81, 61–64. [Google Scholar] [CrossRef]
- Chaves, J.O.; Fernandes, A.M.d.F.; Parreiras, P.M.; Breguez, G.S.; Passos, M.C.; da Cunha, L.R.; Menezes, C.C. Effect of storage on retinol content and total antioxidant capacity of human milk. Br. Food J. 2019, 122, 606–616. [Google Scholar] [CrossRef]
- Romeu-Nadal, M.; Castellote, A.; López-Sabater, M. Effect of cold storage on vitamins C and E and fatty acids in human milk. Food Chem. 2008, 106, 65–70. [Google Scholar] [CrossRef]
- Kaya, Ö.; Çınar, N. The effects of freezing and thawing on mature human milk’s contains: A systematic review. Midwifery 2023, 118, 103519. [Google Scholar] [CrossRef] [PubMed]
- Oliver, C.; Watson, C.; Crowley, E.; Gilroy, M.; Page, D.; Weber, K.; Messina, D.; Cormack, B. Vitamin and Mineral Supplementation Practices in Preterm Infants: A Survey of Australian and New Zealand Neonatal Intensive and Special Care Units. Nutrients 2019, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.F.D.S.; Andreto, L.M.; Vieira, C.S.D.S.; De Arruda, I.K.G.; Diniz, A.D.S. Serum Retinol Concentrations in Mothers and Newborns at Delivery in a Public Maternity Hospital in Recife, Northeast Brazil. J. Health Popul. Nutr. 2014, 32, 28–35. [Google Scholar] [PubMed]
- MacDonald, M.; Seshia, M. Avery’s Neonatology, Pathophysiology and Management of Newborn, 6th ed.; Wolters Kluwer: Gurgaon, India, 2005. [Google Scholar]
- Lapillonne, A.; Braillon, P.; Claris, O.; Chatelain, P.; Delmas, P.; Salle, B. Body composition in appropriate and in small for gestational age infants. Acta Paediatr. 1997, 86, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Kahn, S.; Platt, R.; Basso, O.; Evans, R.; Kramer, M.S. Small-for-gestational-age birth and maternal plasma antioxidant levels in mid-gestation: A nested case-control study. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.L.; Newton, R.B. Serum antioxidant activity in neonates. Arch. Dis. Child. 1988, 63, 748–750. [Google Scholar] [CrossRef]
- Shah, R.S.; Rajalakshmi, R.; Bhatt, R.V.; Hazra, M.N.; Patel, B.C.; Swamy, N.B.; Patel, T.V. Liver stores of vitamin A in human fetuses in relation to gestational age, fetal size and maternal nutritional status. Br. J. Nutr. 1987, 58, 181–189. [Google Scholar] [CrossRef]
- Kramer, M.S.; Kahn, S.R.; Platt, R.W.; Genest, J.; Rozen, R.; Chen, M.F.; Goulet, L.; Séguin, L.; Dassa, C.; Lydon, J.; et al. Antioxidant Vitamins, Long-Chain Fatty Acids, and Spontaneous Preterm Birth. Epidemiology 2009, 20, 707–713. [Google Scholar] [CrossRef]
- de Lira, L.Q.; Lima, M.S.R.; de Medeiros, J.M.S.; da Silva, I.F.; Dimenstein, R. Correlation of vitamin A nutritional status on alpha-tocopherol in the colostrum of lactating women. Matern. Child Nutr. 2011, 9, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Ametaj, B.N.; Nonnecke, B.J.; Franklin, S.T.; Horst, R.L.; Bidlack, W.R.; Stuart, R.L.; Beitz, D.C. Dietary Vitamin A Modulates the Concentrations of RRR-α-tocopherol in Plasma Lipoproteins from Calves Fed Milk Replacer. J. Nutr. 2000, 130, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Kiely, M.; Cogan, P.; Kearney, P.; Morrissey, P. Concentrations of tocopherols and carotenoids in maternal and cord blood plasma. Eur. J. Clin. Nutr. 1999, 53, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Rossholt, M.E.; Wendel, K.; Bratlie, M.; Aas, M.F.; Gunnarsdottir, G.; Fugelseth, D.; Pripp, A.H.; Domellöf, M.; Størdal, K.; Stiris, T.; et al. Vitamin A Status in Preterm Infants Is Associated with Inflammation and Dexamethasone Exposure. Nutrients 2023, 15, 441. [Google Scholar] [CrossRef] [PubMed]
- González-Corbella, M.; López-Sabater, M.; Castellote-Bargalló, A.; Campoy-Folgoso, C.; Rivero-Urgell, M. Influence of caesarean delivery and maternal factors on fat-soluble vitamins in blood from cord and neonates. Early Hum. Dev. 1998, 53, S121–S134. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.R.; Cavanaugh, J.L.; Norkus, E.P.; Plessinger, M.A.; Miller, R.K. The effect of labor on maternal and fetal vitamins C and E. Am. J. Obstet. Gynecol. 2002, 187, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Baydas, G.; Karatas, F.; Gursu, M.; Bozkurt, H.; Ilhan, N.; Yasar, A.; Canatan, H. Antioxidant Vitamin Levels in Term and Preterm Infants and Their Relation to Maternal Vitamin Status. Arch. Med Res. 2002, 33, 276–280. [Google Scholar] [CrossRef]
- Embleton, N.D.M.; Moltu, S.J.; Lapillonne, A.; Akker, C.H.v.D.; Carnielli, V.; Fusch, C.; Gerasimidis, K.; van Goudoever, J.B.; Haiden, N.M.; Iacobelli, S.; et al. Enteral Nutrition in Preterm Infants (2022): A Position Paper From the ESPGHAN Committee on Nutrition and Invited Experts. J. Pediatr. Gastroenterol. Nutr. 2022, 76, 248–268. [Google Scholar] [CrossRef]
- Tanumihardjo, S.A.; Russell, R.M.; Stephensen, C.B.; Gannon, B.M.; Craft, N.E.; Haskell, M.J.; Lietz, G.; Schulze, K.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)—Vitamin A Review. J. Nutr. 2016, 146, 1816S–1848S. [Google Scholar] [CrossRef]
| Total Sample (n = 30) | Males (n = 14) | Females (n = 16) | p-Value | |
|---|---|---|---|---|
| Gestational age (weeks) | 30.4 ± 2.55 | 30.6 ± 2.2 | 30.1 ± 2.8 | 0.628 |
| Birth weight (g) † | 1185 (1017–1535) | 1185 (1017–1637) | 1210 (1010–1502) | 0.881 |
| Low birth weight (%) * | 100 | 100 | 100 | ΝA |
| Very low birth weight (%) ** | 73.3 | 71.4 | 75.0 | 0.574 |
| Small for gestational age (%) | 23.3 | 42.8 | 6 | 0.018 |
| Retinol (μg/L) | 392.0 ± 162.9 | 405.7 ± 148.7 | 380.0 ± 178.3 | 0.669 |
| Retinol deficiency (n, %) § | 3 (10%) | 1 (7.1%) | 2 (12.5%) | 0.552 |
| α-tocopherol (mg/L) | 6.83 ± 3.02 | 6.63 ± 3.30 | 7.01 ± 2.85 | 0.742 |
| α-tocopherol deficiency (n, %) ∫ | 6 (20%) | 3 (21.4%) | 3 (18.8%) | 0.605 |
| Pearson’s Correlation | Weight-Adjusted Correlation | |||
|---|---|---|---|---|
| Retinol (ng/L) | α-Tocopherol (ng/L) | Retinol (ng/L) | α-Tocopherol (ng/L) | |
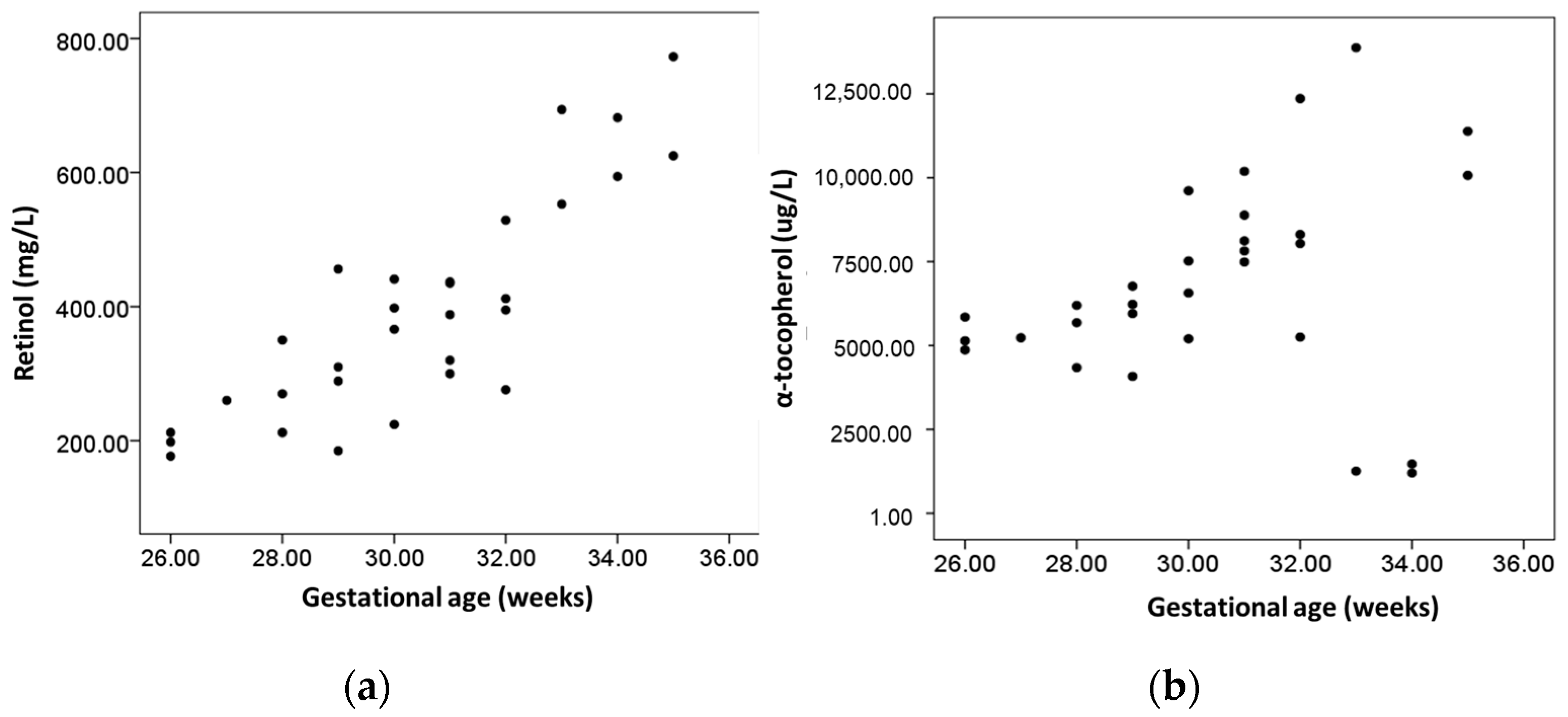
| Gestational age (weeks) | 0.845 (p < 0.001) | 0.255 (p = 0.173) | 0.193 (p= 0.316) | 0.180 (p = 0.351) ‡ |
| Birth weight (g) † | 0.884 (p < 0.001) | 0.201 (p = 0.286) ‡ | NA | NA |
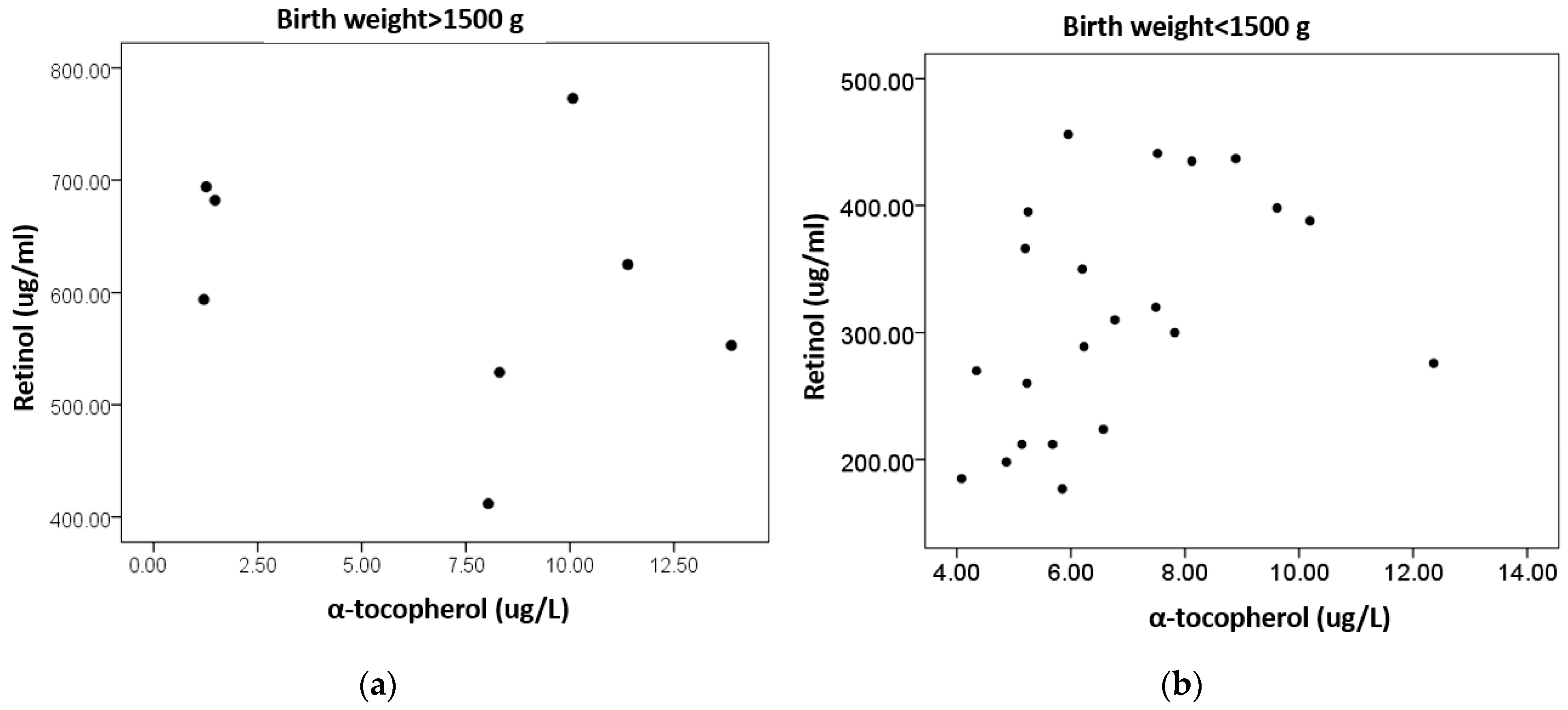
| Retinol (μg/L) | ΝA | 0.273 (p = 0.144) | ΝA | −0.238 (p = 0.215) |
| α-tocopherol (mg/L) | 0.070 (p = 0.715) | ΝA | −0.238 (p= 0.215) | ΝA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papandreou, P.; Detopoulou, P.; Skouroliakou, M. Gestational Age Is Positively Associated with Retinol and α-Tocopherol in Preterm Infants: The Mediating Role of Birth Weight. Dietetics 2023, 2, 366-376. https://doi.org/10.3390/dietetics2040027
Papandreou P, Detopoulou P, Skouroliakou M. Gestational Age Is Positively Associated with Retinol and α-Tocopherol in Preterm Infants: The Mediating Role of Birth Weight. Dietetics. 2023; 2(4):366-376. https://doi.org/10.3390/dietetics2040027
Chicago/Turabian StylePapandreou, Panos, Paraskevi Detopoulou, and Maria Skouroliakou. 2023. "Gestational Age Is Positively Associated with Retinol and α-Tocopherol in Preterm Infants: The Mediating Role of Birth Weight" Dietetics 2, no. 4: 366-376. https://doi.org/10.3390/dietetics2040027
APA StylePapandreou, P., Detopoulou, P., & Skouroliakou, M. (2023). Gestational Age Is Positively Associated with Retinol and α-Tocopherol in Preterm Infants: The Mediating Role of Birth Weight. Dietetics, 2(4), 366-376. https://doi.org/10.3390/dietetics2040027







