Abstract
Cancer survivors consider nutrition to be highly important and are motivated to seek information about lifestyle changes, including nutrition, to improve their long-term health. Despite this, suboptimal dietary intake is still reported. Understanding cancer-specific barriers and facilitators to healthy eating among this population could help develop targeted interventions for this group. This study explored the barriers and facilitators to following a healthy diet among cancer survivors using the Theoretical Domains Framework (TDF) and COM-B model. Semi-structured focus groups with 20 cancer survivors were conducted between February and August 2021, which were transcribed verbatim. Seven key TDF domains accounted for 87% of all coded responses. These were (a) environmental context and resources; (b) knowledge; (c) behavioural regulation; (d) social/professional role and identity; (e) belief about consequences; (f) social influences; and (g) skills. Key barriers were lack of knowledge, non-specific or irrelevant information, environmental aspects, and family. Facilitators were awareness of the importance of nutrition, the health benefits of a plant-based diet, confidence in cooking skills, organisation, balance, family, time, and viewing themselves as someone who eats healthily and access to fresh produce. Enablement was the most prominently linked intervention function. This work can inform future interventions in this area and ensure they are end-user-centred.
1. Introduction
The continual improvements in early detection and management of cancer has substantially increased the number of cancer survivors [1]. There are many long-term physiological and psychological effects of cancer [2]. Compared to age-matched populations, cancer survivors are more likely to experience co-morbidities [3] such as cardiovascular disease [4], diabetes [5], and osteoporosis [6]. Furthermore, cancer treatment can pose several issues impacting the ability of survivors to maintain a healthy lifestyle, such as changes in taste, fatigue, anxiety, and depression [7,8,9]. The increased risk may be associated with clinical or genetic factors and lifestyle factors [10]. Increasing physical activity and incorporating a healthy diet post-treatment is associated with reducing the risk of cancer recurrence and mortality while improving health-related quality of life [11].
Cancer survivors have little compliance with the World Cancer Research Foundation/American Institute for Cancer Research (WCRF/AICR) guidelines [10]. A study of 1196 survivors of colorectal cancer indicated only 12% adhered to 6 or more of the eight WCRF/AICR recommendations assessed [12]. Another study found that, compared to individuals without a history of breast cancer, breast cancer survivors reported poorer adherence to WCRF/AICR recommendations [13]. Despite this, in some studies, survivors report that post-treatment nutritional changes are in line with the World Cancer Research Fund cancer prevention recommendations, which focus on increasing intakes of whole grains, vegetables, fruit, and beans, and reducing intakes of red meat and processed food [14]. Similarly, a United Kingdom qualitative study on cancer survivors’ perceptions of diet and cancer in [15] described that post-treatment changes were mostly coherent with dietary recommendations. Yet, there was discussion of dietary supplements and other non-evidence-based practices, similar to Irish studies [14,16].
Being diagnosed with cancer may create a ‘teachable moment’ where individuals acknowledge the role of nutrition and are highly motivated to seek information and adopt risk-reducing healthy lifestyle changes [17]. Evidence-based nutrition information, dietary guidance and support must be available to capitalise on this. Preferably, survivors desire this advice to be from healthcare professionals [18]. Unfortunately, as of now, in Ireland, a lack of resources means that very few cancer survivors are referred for dietetic support [14,16]. Additionally, cancer survivors report barriers that highlight the difficulty in adopting a healthy diet. In an exploratory study, breast cancer survivors (n = 315) reported the main barriers to making healthy food choices as fatigue (72.1%) and stress (69.5%) followed by treatment-related impacts on eating habits (e.g., change in tastes) (31.4% to 48.6%). Further barriers reported were a lack of motivation, anxieties about pre-existing comorbidities, inadequate knowledge and lack of resources (e.g., time or financial restraints) [19,20,21,22].
There needs to be more literature on barriers to a healthy diet, especially compared to more developed barriers to exercise [23]. Cancer survivors deserve tailored interventions that consider the barriers and enablers to healthy eating that they report experiencing.
The Behaviour Change Wheel (BCW) is a framework for designing and evaluating interventions [24]. It delivers a systematic way of developing intervention and policy categories that stimulate change [24]. The Medical Research Council for developing interventions outlines that researchers need a theoretical understanding of the barriers preventing change [25]. This can be attained by using existing literature and, if required, supported with qualitative interviews with targeted cohorts [25].
The COM-B (Capability, Opportunity, Motivation—Behaviour) model is at the foundation of the BCW framework. The model determines that human behaviour depends on three aspects - capability, opportunity, and motivation [24]. This model does not prioritise any individual component; nevertheless, it recognises how modifying one or multiple components could prime behaviour change [24].
The COM-B model can be built upon the Theoretical Domains Framework (TDF) [26]. It comprises fourteen domains that include the spectrum of behavioural determinants. Each domain is further expanded by respective core components (e.g., capabilities) [26]. The TDF is descriptive [27] and can be mapped onto the COM-B components [26]. The TDF offers insights into which domains are most crucial when looking at a target behaviour, enabling a better understanding and eventually modifying of that dietary behaviour.
Researchers have recently started using TDF and COM-B to investigate barriers and enablers to changing dietary behaviour [28,29,30]. For example, studies have been undertaken to implement the Mediterranean- DASH intervention for Neurodegenerative Delay (MIND) diet among middle-aged adults in the United Kingdom, to improve dietary adherence in high-performance athletes and to decrease sugar intake in young adults [28,29,30]. Across these studies, the COM-B model, combined with the TDF, was influential in informing interventions and promoting behavioural changes. Additionally, further studies have used the COM-B model to design dietary interventions to encourage the Mediterranean diet in adults at risk of Cardiovascular disease [31] and enrich the eating habits of teenagers and young adults using an app [32].
This study aims to address a deficit in the literature by highlighting the perceived barriers and facilitators to healthy eating in cancer survivors who are at least six months post-cancer treatment using the TDF and COM-B model. Given the importance of a healthy diet for cancer survivors, it is necessary to identify the barriers and facilitators impacting adherence to this. Identifying these factors will help address cancer survivors’ concerns and inform strategies to ensure greater adoption.
2. Materials and Methods
The Standards for Reporting Qualitative Research (SRQR) reporting guidelines were used to structure the reporting of this study [33].
2.1. Ethical Approval
The Institute Research Ethics Committee at the Institute of Technology Sligo (now part of the Atlantic Technological University) granted ethical approval (reference, 2020023). Written informed consent was provided at the time of enrolment, with verbal informed consent being obtained at the time of the focus groups.
2.2. Design
This study utilised semi-structured focus groups with cancer survivors between February and August 2021.
2.3. Participant Recruitment
Participants were recruited from another research study looking at the nutrition practices of Irish cancer survivors [14]. After completing this cross-sectional survey, participants could indicate if they were interested in further discussing nutrition within a focus group. A participant information sheet, consent form, and a link to book into a focus group at their preferred time were emailed to those who expressed interest in taking part. Interested participants needed to be aged 18+ years, have a cancer diagnosis, have completed active treatment at least six months ago and not be in palliative care to be eligible.
2.4. Materials
The moderator’s guide (developed by LK and revised by all authors) was informed by the Theoretical Domains Framework [26] and mapped onto each of the components of the COM-B model (Table 1). The TDF consists of fourteen domains, which are outlined in Table 1.

Table 1.
Interview prompt questions based on the Theoretical Domains Framework.
2.5. Healthy Diet
For this study, the World Cancer Research Fund cancer prevention recommendations were used to define a healthy diet. These recommendations promote a dietary pattern high in whole grains, vegetables, fruit, and beans, while reducing consumption of red meat and processed food [10].
2.6. Researcher Characteristics and Reflexivity
The researchers include two academic dietitians (LK and PD) and one postgraduate researcher (NOC). We all have experience conducting research with those with a cancer diagnosis; in addition, LK and PD have worked in previous non-academic roles providing nutrition support in oncology settings. None of the researchers have had a personal diagnosis of cancer; however, all have had close family and friends who have received this diagnosis. The researchers have an array of skills in qualitative research having undertaken several studies utilising this methodology in recent years. LK has attended workshops on the COM-B framework and behaviour change interventions, while NOC has undertaken a module in qualitative research methodologies.
2.7. Procedure
Five focus groups were conducted online by NOC using Microsoft Teams. These could not occur in person due to the COVID-19 pandemic. In addition, two interviews were conducted with participants who were unable to attend the focus groups but who wished to take part. Focus groups lasted between 60–90 min while the interviews took 20–30 min. The COM-B framework promotes sourcing information from different sources as the best way to understand a target behaviour, therefore the inclusion of both focus groups and interviews was useful [24]. The same questions were used for both interviews and focus groups. All were audio-recorded with consent from each of the participants.
2.8. Data Analysis
Demographic data were analysed using descriptive statistic functions in SPSS version 26. Cross tabs were used to determine any differences between those who took part in the study and those who declined. The recorded focus groups and interviews were transcribed verbatim. Recordings were deleted once transcribed. Excel was used as specialised software was not required due to the sample size. No identifiable data e.g., names were collected or transcribed. Two researchers (NOC and LK) independently familiarised themselves with the transcripts, coded the data and assigned initial code names. Each was inductively identified as a barrier or facilitator to healthy eating, and there was an initial agreement of >90%, demonstrating an acceptable level of agreement [34]. Differences were settled through discussion. These codes were then deductively mapped to TDF domains and COM-B, giving consideration to the definitions of each domain. Deductive mapping was independently carried out by both NOC and LK, with any disagreements resolved through discussion with PD.
Summative content analysis was then undertaken. Here the frequency of each identified code was determined and a final count for each domain was determined, allowing each domain to be rank-ordered. This identified which components and domains of the theoretical models were the main barriers and facilitators to healthy eating in cancer survivors. The key domains (highest % of mentions) are presented in greater detail with supporting quotes in the results section.
2.9. Intervention Options
TDF domains were linked to their COM-B counterparts. Then utilising the BCW, COM-B components were mapped to intervention functions to determine the best approach to address the barriers identified and further support the facilitators. These intervention functions were then mapped to policy categories to determine which would be best suited to support that intervention type [24].
3. Results
3.1. Participant Demographics
Of 170 individuals who participated in the original quantitative study, contact details were provided by 84 individuals who were interested in participating in a further related study. When the cancer survivors (n = 84) were contacted re-participation in this study, 20 responded to confirm (response rate = 11.8%, 20/170). A significant difference (<0.001) was found in the number of years since completing treatment between those who ultimately participated and those who declined (both those who declined from the start and those who initially expressed interest but then declined).
The cohort was predominately female (n = 17, 85%), breast cancer survivors (n = 12, 60%) who were ≤5 years post-treatment, had a third-level education (n = 20, 100%) and a mean age of 51.3 ± 11.9 years (23–69 years) (Table 2).

Table 2.
Characteristics of the cancer survivors (n = 20) who participated in the study.
3.2. Barriers and Facilitators to a Healthy Diet
Across the dataset, 237 mentions were fitted to 11 of the 14 domains of the Theoretical Domain Framework (TDF) and mapped to the six sectors of the COM-B model. Table 3 summarises the frequency of responses mapped to each TDF domain, with 73% of all mentions reported as facilitators to healthy eating, compared with 27% of comments reported as barriers.

Table 3.
Frequency of responses mapped to each TDF domain.
Seven key domains accounted for 87% of all coded responses. These were (a) environmental context and resources; (b) knowledge; (c) behavioural regulation; (d) social/professional role and identity; (e) belief about consequences; (f) social influences; (g) skills. The barriers and enablers for these key domains are described below with example quotations.
The least-commonly reported domains were memory, attention, and decision processes (0.4%) and beliefs about capabilities (1.7%) (Figure 1). The three domains of optimism, reinforcement and goals were not mapped.
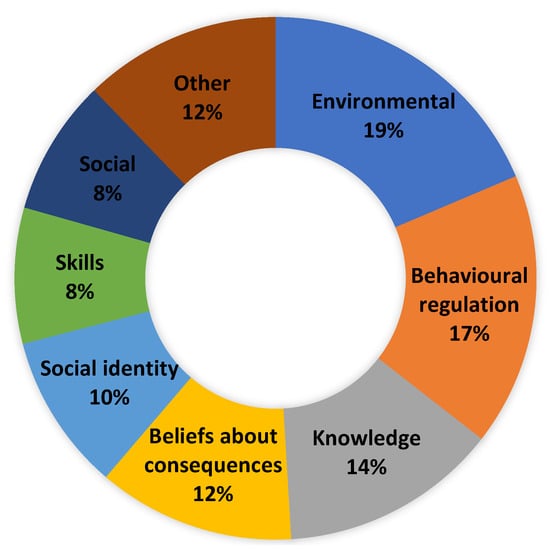
Figure 1.
Proportion of total barriers and facilitators by TDF domain.
3.3. Key Domains
3.3.1. Environmental Context and Resources (Physical Opportunity)
Environmental context and resources were the most identified domain, accounting for 18.6% of total mentions (Figure 1). As a facilitator, environmental context and resources accounted for 5.4% of all mentions (Table 3). They were focused on some individuals feeling that they now had more time to dedicate to eating healthily due to COVID-19 and not being able to leave the house as much and also sick leave. There was an acknowledgement that this would become much more difficult once they returned to work and more normal routines. Availability of fresh local produce was also a facilitator, with local butchers and access to freshly caught fish being mentioned and two participants attempting to grow some of their own food.
“I’m on sick leave now, so I find I have the time to prep.”(Female, aged 46, breast cancer, 1 year post-treatment)
“I get the fresh fish comes three or four days a week from Kilybegs.”(Male, aged 69, prostate cancer, 1 year post-treatment)
Environmental context and resources accounted for 54.7% of all mentioned barriers (Table 3). The impact of symptoms such as loss of appetite and early satiety and side effects of treatment, e.g., fatigue, were all mentioned as barriers to eating healthily.
“I wanted to eat properly, and then my gut was affected.”(Female, aged 67, colon, 1 year post-treatment)
“If I could get the vomiting sorted out.”(Female, aged 52, oesophagus cancer, 1 year post-treatment)
“I have no appetite at all.”(Female, aged 52, oesophagus cancer, 1 year post-treatment)
There was a consensus from participants that the focus on weight portrayed by healthcare professionals and resources, where provided, was not helpful and that a direction away from weight and towards health would be well received moving forward. Many individuals indicated that they had gained weight over the course of treatment, so the provision of information around preventing weight gain and consuming additional calories was not relevant to them and was quite unhelpful.
“The emphasis needs to come off weight and more on what’s your body going to be lacking after chemotherapy.”(Female, aged 53, non-Hodgkin’s Lymphoma, 1 year post-treatment)
All participants repeatedly mentioned the cost as a significant barrier to making healthy food choices. It was felt that healthy foods were more expensive than more processed or convenient alternatives.
“Definitely costs more to eat healthily.”(Female, aged 56, breast cancer, 10 years post-treatment)
“The cost of it can hinder you from eating healthily.”(Female, aged 67, colon cancer, 1 year post-treatment)
The time to plan for and prepare healthy meals was an additional barrier, with many indicating that it takes much more time to cook a healthy meal from scratch than relying on more convenient foods.
“it’s tough. You need a lot of discipline and much time as well to prepare and finish and plan.”(Male, aged 57, prostate cancer, 3 years post-treatment)
“Difficult timewise.”(Female, aged 46, breast cancer, 1 year post-treatment)
It was felt that eating healthily becomes more difficult once you leave the home, and participants felt that the food choices available in other settings such as the workplace made it more challenging to make healthy choices.
“In work, I find you slip, if you’re buying your lunches.”Female, aged 42, breast cancer, 2 years post-treatment)
“Moved into my parents’ house while I was having treatments…there are chocolates and there’s temptations here all the time so that would be my downfall.”(Female, aged 46, breast cancer, 1 year post-treatment)
3.3.2. Behavioural Regulation (Psychological Capability)
Behavioural regulation accounted for 16.9% of total mentions (Figure 1), and 23.1% of facilitator mentions and were not indicated as a barrier (Table 3).
All participants made it clear that the only way that they could implement healthy eating practices was to be very organised and to incorporate several strategies such as shopping lists, prepping vegetables at the start of the week ready to cook, batch cooking, freezing extra food and having a menu plan for the week. Others mentioned not buying “a lot of things that aren’t good for you”.
“Always have a fridge full of vegetables.”(Female, aged 61, breast cancer, 3 years post-treatment)
“Plan out some meals for the week.”(Female, aged 67, colon cancer, 1-year post-treatment)
There was a consensus that balance and not being too strict was important for maintaining a healthy diet, with an “80:20” approach being incorporated by many.
“I have to have the balance.”(Female, aged 49, breast cancer, 14 years post-treatment)
“I think it’s also not good to be hauling ourselves over the coals if we crumble at the odd meal.”(Female, aged 34, breast cancer, 1 year post-treatment)
“I try Monday to Friday at least I am pretty strict”(Female, aged 56, breast cancer, 10 years post-treatment)
3.3.3. Knowledge (Psychological Capability)
Knowledge accounted for 13.5% of total mentions (Figure 1) and 12.1% of facilitator mentions (Table 3).
There was an understanding of the importance of nutrition in recovery from cancer, providing energy, and preventing recurrence.
“It is as important, if not more, as all the drugs we received along the way.”(Female, aged 34, breast cancer, 1 year post-treatment)
“It’s very important….feed my body with the proper nutrients, that are going to aid my recovery.”(Female, aged 61, breast cancer, 2 years post-treatment)
The health benefits of a more plant-based diet were mentioned on several occasions throughout the focus groups, with many trying to make more plant-based choices, mainly when eating out.
“I feel like all the evidence is on the side of plant-based.”(Female, aged 62, breast cancer, 8 years post-treatment)
Knowledge accounted for 17.2% of barrier mentions. Not being sure what to eat as an alternative to foods that would be considered less healthy was a barrier to implementing healthy eating.
“There must be an alternative to that, and I haven’t got that information.”(Male, aged 51, lymphoma, 2 years post-treatment)
In addition, there was a feeling that there was a lack of information and that any information provided was non-specific and, in some cases, irrelevant, e.g., providing information on weight loss when weight gain was experienced. There was a desire for more individualised and specific advice.
“I know we’re completely left on our own.”(Female, aged 37, breast cancer, 18 months post-treatment)
“There was nothing there.”(Female, aged 61, breast cancer, 2 years post-treatment)
“Want to know more about nutrition.”(Female, aged 67, colon cancer, 1 year post-treatment)
“Nice to have personalised.”(Female, aged 67, lung/bowel cancer, 2 years post-treatment)
“We should know what we should eat to get good.”(Female, aged 49, breast cancer, 14 years post-treatment)
3.3.4. Beliefs about Consequences (Reflective Motivation)
Beliefs about consequences accounted for 12.2% of total mentions (Figure 1), and 16.8% of facilitator mentions were not indicated as a barrier (Table 3).
There was an apparent belief among all participants that eating healthily had led to improved energy and that it could contribute to helping the body heal after treatment, thereby speeding up recovery and assisting in a return to normality. There was also a belief that it would help to prevent cancer recurrence.
“Feed my body with the proper nutrients that are going to aid my recovery.”(Female, aged 61, breast cancer, 2 years post-treatment)
“My energy levels have improved greatly.”(Female, aged 62, breast cancer, 8 years post-treatment)
“Going to speed up your recovery.”(Female, aged 23, acute myeloid leukaemia, 9 years post-treatment)
“It’s what you want to get back to normality.”(Female, aged 55, breast cancer, 5 years post-treatment)
“To help me and fight cancer coming back.”(Female, aged 53, non-Hodgkin’s lymphoma, 1 year post-treatment)
3.3.5. Social/Professional Role or Identity (Social Opportunity)
Social identity accounted for 9.7% of all mentions (Figure 1) and 13.3% of facilitator mentions and was not indicated as a barrier (Table 3). All participants viewed themselves as someone who ate a healthy diet and made healthy food choices most of the time.
“Whatever I pick is geared towards being healthy.”(Female, aged 67, colon cancer, 1 year post-treatment)
“You want to be as healthy as you can.”(Female, aged 23, acute myeloid leukaemia, 9 years post-treatment)
3.3.6. Social Influences (Social Opportunity)
Social influences accounted for 8.4% of total mentions (Figure 1) and 7.5% of facilitator mentions (Table 3). Other family members believed that following a healthy diet or making similar food choices was a great aid in making healthy food choices.
“We all have that kind of try to eat well attitude, and I think that helps.”(Female, aged 42, breast cancer, 2 years post-treatment)
“We are on the same level, my partner, so there’s no problem there eating healthily.”(Female, aged 67, lung/bowel cancer, 2 years post-treatment)
“I think my family would be a help.”(Female, aged 62, breast cancer, 8 years post-treatment)
Social influences accounted for 11% of barrier mentions. Family members were not interested in following a similar dietary pattern or where several meals needed to be cooked to meet all the household preferences; this was cited as a very difficult barrier to overcome.
“I think it’s very difficult sometimes if other people and family aren’t following the same nutritional programme that you’re following.”(Female, aged 47, breast cancer, 2 years post-treatment)
“There are three teenagers in this house, so you know, it doesn’t work.”(Male, aged 51, lymphoma, 2 years post-treatment)
“If they’re not on board it’s very hard.”(Male, aged 57, prostate cancer, 3 years post-treatment)
3.3.7. Skills (Physical Capability)
Skills accounted for 8.4% of total mentions (Figure 1), and 11.5% of facilitator mentions were not indicated as a barrier (Table 3). The cohort stated they felt they had the cooking skills to eat healthily.
“I would call myself a reasonable cook.”(Female, aged 56, breast cancer, 10 years post-treatment)
“I do have the skills.”(Female, aged 42, breast cancer, 2 years post-treatment)
3.4. Identifying Intervention Options
The barriers most frequently mentioned by participants related to the environmental context, resources, social influences and knowledge. The most relevant intervention functions to address these specific barriers were determined using the BCW approach outlined in Figure 2. Potential interventions to facilitate healthy eating in Irish cancer survivors have been suggested.
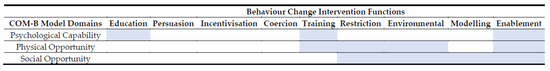
Figure 2.
Mapping of COM-B Model barriers and potential intervention functions. Where the COM-B domain aligns with potential intervention functions is highlighted in blue.
Therefore, from our results, ‘psychological capacity’, in particular, lack of knowledge or provision of non-specific information; ‘physical opportunity’, in that there are time and cost implications, that nutrition impact symptoms have an impact, there is a lack of control outside of home environment and too much focus on weight and finally ‘social opportunity’, more specifically family influence was substantial barriers to following a healthy diet. Based on our findings, the following intervention functions are suggested:
- (1)
- Enablement—increasing means and reducing barriers to increase capability or opportunity (e.g., behavioural support to change dietary behaviour; strategies to reduce the impact of symptoms).
- (2)
- Environmental restructuring—changing the physical environment (e.g., re-designing a work cafeteria to emphasise healthy foods, clear menu labelling, price incentives, point of purchase messaging)
- (3)
- Restrictions—designed to limit access to an unhealthy substance (e.g., sugar-sweetened beverage tax, food standards within hospitals)
- (4)
- Training—imparting skills (e.g., how to cook healthier meals, how to shop for healthy food on a budget)
Further linking indicated that any policy category (communication/marketing; guidelines; fiscal; regulation; legislation; environmental; service provision) could be significant when developing interventions.
4. Discussion
This is the first study that sought to elicit the factors influencing healthy eating in cancer survivors in Ireland. Our findings revealed 87% of barriers and facilitators to eating healthily were attributed to seven TDF domains: environmental context and resources; knowledge; behavioural regulation; social/professional role and identity; belief about consequences; social influences and skills. These domains were associated with five of the six COM-B elements without strong evidence for automatic motivation.
Using the COM-B model [24] was helpful in recognising the factors that acted as barriers and facilitators to healthy eating in Irish cancer survivors. Mapping the data to TDF [26] allowed for further understanding of the determinants of this behaviour. Ten of the TDF domains (knowledge, behavioural motivation, skills, social/professional role or identity, social influences, environmental context and resources, beliefs about capabilities, beliefs about consequences, intentions, and emotion) were highlighted as having a role, with limited to no evidence for optimism, goals, memory, or reinforcement.
Participants recognised the benefits of a plant-based diet, with many making efforts to adopt this. This is in line with the recommendations of the World Cancer Research Fund [10] to increase fruit, vegetables and whole grains and decrease red and processed meat intake. Higher adherence to these recommendations is linked with better global health status and functioning scores and reduced fatigue in colorectal cancer survivors [36]. Plant-based diets can decrease the risk of obesity, type 2 diabetes, coronary heart disease, and all-cause mortality [37]. The production of plant-based foods also inclines to be less environmentally destructive and resource-intensive than raising animals for human consumption [38,39].
The cost and availability of healthy food have been recognised as two important dimensions within the greater concept of food access [40]. Participants in this study highlighted both concepts. The cost of consuming a healthy diet was mentioned by all participants, with a strong view that this was more expensive than a more convenient or unhealthy diet. Previous work with colorectal cancer survivors found that the probability of adopting a healthy diet was lower when individuals believed it was too expensive [41]. The price of healthy food as a barrier to adopting a healthy diet has also been highlighted by other studies on breast cancer survivors and when interviewing the parents of children with cancer [22,42,43]. Access to healthy food is a central pillar in the ‘whole systems’ approach to improving nutrition and reducing chronic disease [44].
Family members were highlighted as facilitators and barriers to healthy eating depending on their practices and attitudes. Family members have been shown to positively influence diet quality [45] and the adoption of healthy behaviours in cancer survivors [46]. Where family members act as caregivers, their attempts to eat healthier can increase patient adoption of higher quality diets [41]. Some participants highlighted that it became challenging to adopt when children or spouses did not follow a similar dietary pattern or were not interested in a healthy diet. The majority of our cohort were women, and it has been shown that other family members can quite often affect the food decision-making processes of women and that male spouses and, secondarily, children tended to be prioritised when it came to food choices [47].
4.1. Implications for Practice
The findings in this study reveal various potential enablers and barriers to cancer survivors following a healthy diet thereby, identifying potential targets for future interventions designed to encourage healthy eating in cancer survivors.
The drivers of healthy eating behaviours in cancer survivors can be intrinsic (personal to the cancer survivors) or extrinsic (determined by the healthcare system or environment). Based on our findings, increasing means/reducing barriers that raise capability or opportunity may be most beneficial and effective. Applying the intervention functions of the COM-B model to the elements most frequently mentioned (Psychological Capability, Social and Physical Opportunity) indicated that enablement might be most effective in changing behaviours.
There was deemed to be too much focus on weight, in particular a focus on weight gain, which for many was not suitable guidance due to weight gain experienced during treatment. Nutrition screening tools also focus on parameters of undernutrition but do not consider of the implications of excess body weight [48]. Participants preferred a focus on health, increasing energy and addressing persistent symptoms. It is important to take this into consideration when developing interventions for cancer survivors. It should be framed in the context of leading international guidance from agencies such as Obesity Canada who have produced resources on obesity management that begins with asking for permission to discuss weight loss with patients.
4.2. Limitations
The main limitation of this study, as with most qualitative research, is that the sample size is relatively small. However, it is in line with other COM-B [28,49] and dietary studies [50,51,52], and greater than several [53,54,55]. This study was mainly conducted with female breast cancer survivors; at the same time, this is reflective of many studies on cancer survivors. Additional research from a diverse range of cancer types and increased representation of males is needed. The finding in our study may be situation-specific and, therefore, not generalisable to all cancer survivors. However, we did not aim for generalisability but rather to provide an in-depth analysis of the barriers and facilitators to healthy eating in cancer survivors, highlighting areas that can be targeted when designing interventions. Barriers and facilitators are apparent and, therefore, may not predict improvements in healthy eating.
Moving forward, we would like to conduct focused research particularly on subgroups of survivors and achieve ample sample sizes within those subgroups that allow for saturation of responses; however, this study still could be a helpful guide to target more specific points to develop strategies while advertising adherence to a healthy diet in cancer survivors.
5. Conclusions
This theoretically underpinned and cancer survivor-led research adopted both the COM-B model and the TDF to better understand barriers and facilitators to healthy eating in Irish cancer survivors. Furthermore, intervention functions and policy categories most likely effective have been identified. This work can inform future interventions in this area and ensure they are end-user-centred.
Author Contributions
Conceptualisation, L.K.; methodology, L.K., P.D. and N.O.; formal analysis, L.K. and N.O.; investigation, L.K. and N.O.; data curation, L.K. and N.O.; writing—original draft preparation, L.K.; writing—review and editing, P.D. and N.O.; visualisation, L.K.; supervision, L.K. and P.D.; project administration, L.K. and N.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Research Ethics Committee of the Institute of Technology Sligo (now the Atlantic Technological University) (reference, 2020023).
Informed Consent Statement
All participants involved in the study provided informed consent was obtained.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We wish to acknowledge all who took the time to participate in this study.
Conflicts of Interest
All authors declare no conflict of interest.
References
- Hegarty, J.; Murphy, A.H.T.; O’Mahony, M.; Landers, M.; McCarthy, B.; Lehane, E.; Noonan, B.; Fitzgerald, S.; Reidy, M.; Saab, M.M.; et al. National Cancer Survivorship Needs Assessment: Living with and Beyond Cancer in Ireland; National Cancer Control Programme: Dublin, Ireland, 2019. [Google Scholar]
- Robien, K.; Demark-Wahnefried, W.; Rock, C.L. Evidence-Based Nutrition Guidelines for Cancer Survivors: Current Guidelines, Knowledge Gaps, and Future Research Directions. J. Am. Diet. Assoc. 2011, 111, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Teepen, J.C.; Kremer, L.; Ronckers, C.M.; van Leeuwen, F.E.; Hauptmann, M.; Dulmen-Den Broeder, V.; Van Der Pal, H.J.; Jaspers, M.W.; Tissing, W.J.; Den Heuvel-Eibrink, V. Long-term risk of subsequent malignant neoplasms after treatment of childhood cancer in the DCOG LATER study cohort: Role of chemotherapy. J. Clin. Oncol. 2017, 35, 2288–2298. [Google Scholar] [CrossRef] [PubMed]
- Armenian, S.H.; Xu, L.; Ky, B.; Sun, C.; Farol, L.T.; Pal, S.K.; Douglas, P.S.; Bhatia, S.; Chao, C. Cardiovascular Disease Among Survivors of Adult-Onset Cancer: A Community-Based Retrospective Cohort Study. J. Clin. Oncol. 2016, 34, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Liu, Z.; Thong, M.S.Y.; Doege, D.; Arndt, V. Higher Incidence of Diabetes in Cancer Patients Compared to Cancer-Free Population Controls: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 1808. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W.; Suh, B.; Lim, H.; Suh, Y.-S.; Choi, Y.J.; Jeong, S.-M.; Yun, J.M.; Song, S.O.; Park, Y. Increased Risk of Osteoporotic Fracture in Postgastrectomy Gastric Cancer Survivors Compared with Matched Controls: A Nationwide Cohort Study in Korea. Am. J. Gastroenterol. 2019, 114, 1735–1743. [Google Scholar] [CrossRef]
- Bryant, A.L.; Walton, A.L.; Phillips, B. Cancer-related fatigue: Scientific progress has been made in 40 years. Clin. J. Oncol. Nurs. 2015, 19, 137–139. [Google Scholar] [CrossRef]
- Coa, K.I.; Epstein, J.B.; Ettinger, D.; Jatoi, A.; McManus, K.; Platek, M.E.; Price, W.; Stewart, M.; Teknos, T.N.; Moskowitz, B. The Impact of Cancer Treatment on the Diets and Food Preferences of Patients Receiving Outpatient Treatment. Nutr. Cancer 2015, 67, 339–353. [Google Scholar] [CrossRef]
- Boltong, A.; Keast, R. The influence of chemotherapy on taste perception and food hedonics: A systematic review. Cancer Treat. Rev. 2012, 38, 152–163. [Google Scholar] [CrossRef]
- WCRF; AIfCRD. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report 2. 2018. Available online: http://dietandcancerreport.org (accessed on 15 July 2022).
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012, 62, 242–274. [Google Scholar] [CrossRef]
- Winkels, R.M.; Lee, L.; Beijer, S.; Bours, M.J.; Duijnhoven, F.J.B.; Geelen, A.; Hoedjes, M.; Mols, F.; Vries, J.; Weijenberg, M.P.; et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research lifestyle recommendations in colorectal cancer survivors: Results of the PROFILES registry. Cancer Med. 2016, 5, 2587–2595. [Google Scholar] [CrossRef]
- Kałędkiewicz, E.; Szostak-Węgierek, D. Current and past adherence to the World Cancer Research Fund/American Institute for Cancer Research recommendations in survivors of breast cancer. Rocz. Panstw. Zakl. Hig. 2019, 70, 295–305. [Google Scholar] [CrossRef]
- O’Callaghan, N.; Douglas, P.; Keaver, L. Nutrition Practices among Adult Cancer Survivors Living on the Island of Ireland: A Cross-Sectional Study. Nutrients 2022, 14, 767. [Google Scholar] [CrossRef] [PubMed]
- Beeken, R.; Williams, K.; Wardle, J.; Croker, H. “What about diet?” A qualitative study of cancer survivors’ views on diet and cancer and their sources of information. Eur. J. Cancer Care 2016, 25, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.S.; Rice, N.; Kingston, E.; Kelly, A.; Reynolds, J.V.; Feighan, J.; Power, D.G.; Ryan, A.M. A national survey of oncology survivors examining nutrition attitudes, problems and behaviours, and access to dietetic care throughout the cancer journey. Clin. Nutr. ESPEN 2020, 41, 331–339. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Aziz, N.M.; Rowland, J.H.; Pinto, B.M. Riding the Crest of the Teachable Moment: Promoting Long-Term Health after the Diagnosis of Cancer. J. Clin. Oncol. 2005, 23, 5814–5830. [Google Scholar] [CrossRef]
- Shea–Budgell, M.; Kostaras, X.; Myhill, K.; Hagen, N. Information Needs and Sources of Information for Patients during Cancer Follow-Up. Curr. Oncol. 2014, 21, 165–173. [Google Scholar] [CrossRef]
- Jones, L.W.; Courneya, K.S.; Peddle-McIntyre, C.; Mackey, J.R. Oncologists’ opinions towards recommending exercise to patients with cancer: A Canadian national survey. Support. Care Cancer 2005, 13, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Ventura, E.E.; Ganz, P.A.; Bower, J.E.; Abascal, L.; Petersen, L.; Stanton, A.L.; Crespi, C. Barriers to physical activity and healthy eating in young breast cancer survivors: Modifiable risk factors and associations with body mass index. Breast Cancer Res. Treat. 2013, 142, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Mackenbach, J.D.; Brage, S.; Forouhi, N.G.; Griffin, S.J.; Wareham, N.J.; Monsivais, P. Does the importance of dietary costs for fruit and vegetable intake vary by socioeconomic position? Br. J. Nutr. 2015, 114, 1464–1470. [Google Scholar] [CrossRef]
- Cho, D.; Park, C. Barriers to physical activity and healthy diet among breast cancer survivors: A multilevel perspective. Eur. J. Cancer Care 2017, 27, e12772. [Google Scholar] [CrossRef] [PubMed]
- Ottenbacher, A.J.; Day, R.S.; Taylor, W.C.; Sharma, S.V.; Sloane, R.; Snyder, D.C.; Kraus, W.E.; Demark-Wahnefried, W. Exercise among breast and prostate cancer survivors—What are their barriers? J. Cancer Surviv. 2011, 5, 413–419. [Google Scholar] [CrossRef]
- Michie, S.; Van Stralen, M.M.; West, R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement. Sci. 2011, 6, 42. [Google Scholar] [CrossRef]
- Craig, P.; Dieppe, P.; Macintyre, S.; Michie, S.; Nazareth, I.; Petticrew, M. Developing and evaluating complex interventions: The new Medical Research Council guidance. BMJ 2008, 337, a1655. [Google Scholar] [CrossRef]
- Cane, J.; O’Connor, D.; Michie, S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement. Sci. 2012, 7, 37. [Google Scholar] [CrossRef]
- Francis, J.; Eccles, M.; Johnston, M.; Walker, A.E.; Grimshaw, J.M.; Foy, R.; Kaner, E.F.S.; Smith, L.; Bonetti, D. Constructing Questionnaires Based on the Theory of Planned Behaviour: A Manual for Health Services Researchers. Centre for Health Services Research. 2004. Available online: https://openaccess.city.ac.uk/id/eprint/1735/1/TPB%20Manual%20FINAL%20May2004.pdf (accessed on 15 July 2022).
- Timlin, D.; McCormack, J.M.; Simpson, E.E. Using the COM-B model to identify barriers and facilitators towards adoption of a diet associated with cognitive function (MIND diet). Public Health Nutr. 2020, 24, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Al Rawahi, S.H.; Asimakopoulou, K.; Newton, J.T. Factors related to reducing free sugar intake among white ethnic adults in the UK: A qualitative study. BDJ Open 2018, 4, 17024. [Google Scholar] [CrossRef]
- Bentley, M.R.; Mitchell, N.; Sutton, L.; Backhouse, S.H. Sports nutritionists’ perspectives on enablers and barriers to nutritional adherence in high performance sport: A qualitative analysis informed by the COM-B model and theoretical domains framework. J. Sports Sci. 2019, 37, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, C.T.; Moore, S.E.; Appleton, K.M.; Cupples, M.E.; Erwin, C.; Kee, F.; Prior, L.; Young, I.S.; McKinley, M.C.; Woodside, J.V. Development of a peer support intervention to encourage dietary behaviour change towards a Mediterranean diet in adults at high cardiovascular risk. BMC Public Health 2018, 18, 1194. [Google Scholar] [CrossRef] [PubMed]
- Rohde, A.; Duensing, A.; Dawczynski, C.; Godemann, J.; Lorkowski, S.; Brombach, C. An App to Improve Eating Habits of Adolescents and Young Adults (Challenge to Go): Systematic Development of a Theory-Based and Target Group–Adapted Mobile App Intervention. JMIR mHealth uHealth 2019, 7, e11575. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, B.C.; Harris, I.B.; Beckman, T.J.; Reed, D.A.; Cook, D.A. Standards for Reporting Qualitative Research: A Synthesis of Recommendations. Acad. Med. 2014, 89, 1245–1251. [Google Scholar] [CrossRef]
- Atkins, L.; Francis, J.; Islam, R.; O’Connor, D.; Patey, A.; Ivers, N.; Foy, R.; Duncan, E.M.; Colquhoun, H.; Grimshaw, J.M.; et al. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement. Sci. 2017, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, D.P. Considerations in the choice of interobserver reliability estimates. J. Appl. Behav. Anal. 1977, 10, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Van Veen, M.R.; Mols, F.; Bours, M.J.L.; Weijenberg, M.P.; Kampman, E.; Beijer, S. Adherence to the World Cancer Research Fund/American Institute for Cancer Research recommendations for cancer prevention is associated with better health–related quality of life among long-term colorectal cancer survivors: Results of the PROFILES registry. Support. Care Cancer 2019, 27, 4565–4574. [Google Scholar] [CrossRef]
- Fraser, G.E. Vegetarian diets: What do we know of their effects on common chronic diseases? Am. J. Clin. Nutr. 2009, 89, 1607S–1612S. [Google Scholar] [CrossRef]
- Pimentel, D.; Pimentel, M. Sustainability of meat-based and plant-based diets and the environment. Am. J. Clin. Nutr. 2003, 78, 660S–663S. [Google Scholar] [CrossRef] [PubMed]
- Baroni, L.; Cenci, L.; Tettamanti, M.; Berati, M. Evaluating the environmental impact of various dietary patterns combined with different food production systems. Eur. J. Clin. Nutr. 2006, 61, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Caspi, C.E.; Sorensen, G.; Subramanian, S.; Kawachi, I. The local food environment and diet: A systematic review. Health Place 2012, 18, 1172–1187. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Park, S.Y.; Choi, G. Facilitators and Barriers to Adoption of a Healthy Diet in Survivors of Colorectal Cancer. J. Nurs. Scholarsh. 2019, 51, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Arroyave, W.D.; Clipp, E.C.; Miller, P.E.; Jones, L.W.; Ward, D.S.; Bonner, M.J.; Rosoff, P.M.; Snyder, D.C.; Demark-Wahnefried, W. Childhood Cancer Survivors’ Perceived Barriers to Improving Exercise and Dietary Behaviors. Oncol. Nurs. Forum 2008, 35, 121–130. [Google Scholar] [CrossRef]
- Raber, M.; Crawford, K.; Baranowski, T.; Sharma, S.V.; Schick, V.; Markham, C.; Roth, M.; Wakefield, C.E.; Chandra, J. Meal planning values impacted by the cancer experience in families with school-aged survivors—A qualitative exploration and recommendations for intervention development. Support. Care Cancer 2019, 28, 1305–1313. [Google Scholar] [CrossRef]
- Bagnall, A.-M.; Radley, D.; Jones, R.; Gately, P.; Nobles, J.; Van Dijk, M.; Blackshaw, J.; Montel, S.; Sahota, P. Whole systems approaches to obesity and other complex public health challenges: A systematic review. BMC Public Health 2019, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Park, S.; Choi, G.-S. Association of support from family and friends with self-leadership for making long-term lifestyle changes in patients with colorectal cancer. Eur. J. Cancer Care 2018, 27, e12846. [Google Scholar] [CrossRef]
- Satia, J.A.; Walsh, J.F.; Pruthi, R.S. Health Behavior Changes in White and African American Prostate Cancer Survivors. Cancer Nurs. 2009, 32, 107–117. [Google Scholar] [CrossRef]
- Beagan, B.L.; Chapman, G.E. Family Influences on Food Choice: Context of Surviving Breast Cancer. J. Nutr. Educ. Behav. 2004, 36, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.S.; Martin, R.M.; Renehan, A.G.; Cade, J.; Copson, E.R.; Cross, A.J.; Grimmett, C.; Keaver, L.; King, A.; Riboli, E.; et al. Cancer survivorship, excess body fatness and weight-loss intervention—Where are we in 2020? Br. J. Cancer 2021, 124, 1057–1065. [Google Scholar] [CrossRef]
- Szinay, D.; Perski, O.; Jones, A.; Chadborn, T.; Brown, J.; Naughton, F. Perceptions of Factors Influencing Engagement with Health and Well-being Apps in the United Kingdom: Qualitative Interview Study. JMIR mHealth uHealth 2021, 9, e29098. [Google Scholar] [CrossRef]
- Swartz, J.J.; Dowray, S.; Braxton, D.; Mihas, P.; Viera, A.J. Simplifying healthful choices: A qualitative study of a physical activity based nutrition label format. Nutr. J. 2013, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Mete, R.; Shield, A.; Murray, K.; Bacon, R.; Kellett, J. What is healthy eating? A qualitative exploration. Public Health Nutr. 2019, 22, 2408–2418. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.; Drake, T.; Vollmer, R.L. A qualitative research study comparing nutrition advice communicated by registered Dietitian and non-Registered Dietitian bloggers. J. Commun. Health 2020, 13, 55–63. [Google Scholar] [CrossRef]
- De Leo, A.; Bayes, S.; Bloxsome, D.; Butt, J. Exploring the usability of the COM-B model and Theoretical Domains Framework (TDF) to define the helpers of and hindrances to evidence-based practice in midwifery. Implement. Sci. Commun. 2021, 2, 7. [Google Scholar] [CrossRef]
- Whittenbury, K.; Kroll, L.; Dubicka, B.; Bull, E.R. Exploring barriers and facilitators for mental health professionals delivering behavioural activation to young people with depression: Qualitative study using the Theoretical Domains Framework. BJPsych Open 2022, 8, e38. [Google Scholar] [CrossRef] [PubMed]
- Lucci, V.-E.M.; McKay, R.C.; McBride, C.B.; McGrath, M.S.; Willms, R.; Gainforth, H.L.; Claydon, V.E. Barriers and facilitators to changing bowel care practices after spinal cord injury: A Theoretical Domains Framework approach. Spinal Cord 2022, 60, 664–673. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).