Abstract
Aflatoxin B1 (AFB1), the mainly Aspergillus fungi derived mycotoxin, is well known for its carcinogenic effects on liver, and frequently occurs in food supplies, leading to fatal consequences in both farm animals and humans. Poultry, one of the most important segments of agro-industry, has been demonstrated to be extremely sensitive to AFB1 intake, which results in chickens’ low performance, decreased quality of both eggs and meat and a negative economic feedback. Oxidative stress caused by AFB1 plays a crucial role in chickens’ kidney damage by generating lipid peroxidation accompanied by a concomitant increase in the antioxidant enzymes involved in ROS metabolism (NADPH oxidase isoform 4 (NOX4) and its regulatory subunit p47-phox). The aim of the present work was to investigate the benefits of dietary supplementation, in chickens affected by AFB1 mycotoxicosis, using a new Feed additive (FA) containing a mixture of a tri-octahedral Na-smectite with a ligno-cellulose-based material an antioxidant adjuvant. Exposure of AFB1-treated chickens to the feed additive induced a significant down-regulation of both NOX4 and p47-phox genes expression levels. This trend was confirmed by their protein expression, demonstrating the great potential of the FA to counteract oxidative stress. To conclude, these results could open new perspectives in the methods of feeding chickens, using eco-friendly dietary supplements able to reduce AFB1-induced mycotoxicosis and to ameliorate poultry performances.
1. Introduction
Foodstuffs, grains and feed for animals are the ideal substrates for the growth of fungi and molds producing mycotoxins. The buildup of mycotoxins, the secondary metabolites produced during fungal replication, causes an accumulation in these sources of nourishment, which lead to economic losses as well as to problems for livestock, poultry and human health [1]. Owing to climate change, mycotoxigenic Aspergillus (A.) species have spread, putting the feed and food production chain at risk [2], also shifting in Mediterranean zones due to the average temperature rise and increase in CO2 levels and rainfall, promoting a worldwide contamination [3].
Aspergillus-derived mycotoxins are named Aflatoxins (AFs), and among them, AFB1, produced by A. flavus, is well known for its carcinogenic effects; in fact it is counted in group I of human carcinogenic compounds [4], and may cause hepatotoxicity [5], kidney and heart damage [6], immunotoxicity [7] and could also lead to fatal consequences in both farm animals and humans [8].Therefore, AF intake is legislated by the European Community, which has established a maximum quantity of it in foodstuffs, by placing the safe limit in a range between 2 μg/kg and 4 μg/kg [9].
Farm animals, especially poultry, one of the most important segments of agro-industry, have been demonstrated to be extremely sensitive to AFB1 intake, with consequences on the quality of both eggs and meat, and with impact on the food chain and its economic side [10,11].
AFB1 plays a crucial role in kidney damage in chickens due to the oxidative stress it induces in this organ [12]. So far, there are many detoxification methods described in the literature, but none is able to completely remove mycotoxins in foodstuffs [13]. In the last few years, many studies have engaged in the search for eco-friendly dietary supplements, which could prevent or reduce the oxidative stress, e.g., supplementation of Vitamins A, E and C have showed antioxidative effects in poultry birds [14].
Oxidative stress in the kidneys is correlated to NOX4, the most abundant NOX isoform at renal level. In fact, NOX4 has been demonstrated to be the most important contributor to ROS generation in the kidney in several pathological conditions [15]. Physiologically, NOXs are implicated in homeostasis because of their antioxidant defense, but in pathological states, their levels increase, inducing ROS accumulation [16]. For this reason, the inhibition of NOX4, together with its p47-phox subunit, could lead to a promising new nutraceutical strategy in feeding not only poultry, but also other farm animals [17].
In the present work, we investigate the benefits of dietary supplementation with a feed additive (FA) in chickens affected by AFB1 mycotoxicosis. In particular, we evaluate the role of this additive as antioxidant binder against kidney oxidative stress that affects kidneys of chickens poisoned by AFB1.
2. Experiments
2.1. Ethics Statement
The use and care of animals in this work were approved by the Bioethic Commettee of the University of Turin (Italy) (Approval number: 319508/2017-PR).
2.2. Animals and Diet
Twenty-four female broiler (ROSS 308) chickens, at the age of 21 days and measuring 860.25 ± 25.2 g in weight, were housed in a cage (according to Directive 2007/43) and received a standard basal diet (190–210 g/kg of crude protein; 12.6–13.6 MJ/kg of Metabolizable Energy; Aviagen) ad libitum. After 4 days of adaptation period, they were randomly divided into 4 experimental groups: CONTROL group (n = 6, basal diet); AFB1 group (n = 6, AFB1 = 0.02 mg/kg feed)); FA group (n = 6, FA = 5 g/kg feed) and AFB1 plus FA group (n = 6, AFB1 = 0.02 mg/kg feed, FA = 5 g/kg feed). The treatment lasted from 25 to 35 days of age, after which animals were sacrificed and the kidneys removed to perform the following experiments.
2.3. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.3.1. RNA Extraction and Complementary DNA (cDNA) Synthesis
Three replicate chicken kidney tissues for each animal group (CONTROL, AFB1, FA, AFB1 + FA) were used for RNA extraction. Tissues were homogenized in 0.5 mL of TRIZOL Reagent (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) using the Tissue lyser (MM300, Retsch, Conquer Scientific, Poway, CA, USA) and Tungsten Carbide Beads (3 mm) (Qiagen) for 5 min at 20.1 Hz until all samples were completely homogenized (as in Lauritano et al., 2013). After centrifuging at 12,000 rpm for 10 min at 4 °C to remove debris, the supernatant was passed through a 0.1 mm syringe-needle about 5 times (as in Asai et al., 2014). Total RNA was extracted by following the Trizol manufacturer’s protocol, and treated with DNase I (Merck KGaA, Darmstadt, Germany). RNA quantity was assessed by Nano-Drop (ND-1000 UV–Vis spectrophotometer; NanoDrop Technologies, Wilmington, DE, USA) monitoring the absorbance at 260 nm, while purity by monitoring the 260/280 nm and 260/230 nm ratios (Both ratios were approximately 2.0). For RT-qPCR, 1 µg for each sample was retrotranscribed into complementary DNA (cDNA) with the iScriptTM cDNA Synthesis Kit (BIORAD, Hercules, CA, USA), following the manufacturer’s instructions using the GeneAmp PCR System 9700 (Perkin Elmer, Waltham, MA, USA).
2.3.2. Selection of Gene of Interest and RT-qPCR
Five genes of interest (GOI) were selected: the anti-apoptotic protein BCL-2, NOX4 and regulatory subunitp47-phox. 18S was used as reference gene. In order to analyse the selected GOI, the primers in Table 1 were used (Table 1).

Table 1.
Gene names, primer forward (F) and reverse (R), amplicon size, oligo efficiencies (E) and correlation factors (R2), and GenBank accession numbers.
RT-qPCR experiments were carried out in a Viia7 real-time PCR system (Applied Biosystem, Thermo Fisher Scientific, Waltham, MA, USA). PCR reaction total volume was 10 µL, including 5 µL of Fast Start SYBR Green Master Mix (Roche, Basilea, Switzerland), 0.7 pmol/µL for each oligo, and 1 µL of the cDNA template (dilution of 1:10). The thermal profile used was: 95 °C for 10 min, 40 cycles of 95 °C for 1 s, and 60 °C for 20 s. To normalize GOI expression levels, 18S was used as reference gene. The Excel-applet qGene from Muller et al., (2002) was used for the expression levels analysis.
2.4. Western Blot Analysis
Kidney tissues of chickens were homogenized in a lysis buffer (RIPA buffer) with a protease inhibitor mix (cOmplete™, Mini, EDTA-free Protease Inhibitor Cocktail Tablets, Roche), employing Tissue Lyser system to promote lysis. In this phase, the cold chain was maintained. The BCA Protein Assay Kit (Bio-Rad, Milan, Italy) was used to measure total protein content of each sample.
NOX4, p47-phox and BCL-2 proteins expression were analyzed by Western Blot assay. Mini-PROTEAN® precast gel 4–12% (Bio-Rad) and Opti-Protein XL (abm) as a molecular weight marker were used. Trans-Blot® Turbo Nitrocellulose membrane (Bio-Rad) was used to transfer proteins. The membranes were probed with primary antibodies: NOX4 (rabbit monoclonal antibody, Abcam, dilution 1:1000), p47-phox (rabbit polyclonal antibody, Elabscience, dilution 1:500), BCL-2 (rabbit polyclonal antibody, Cell Signaling, dilution 1:1000) and GADPH (rabbit monoclonal antibody, Genetex, dilution 1:20,000), as housekeeping expression proteins. Blots were incubated with HRP conjugates secondary antibodies (Santa Cruz Biotechnology), according to the species of primary antibodies and developed using ECL substrate (Immobilon, Millipore). Signal intensity was quantified by ChemiDoc™ Imaging System (Bio-Rad) with the Bio-Rad Quantity One® software version 4.6.3. The results were expressed as arbitrary units.
2.5. Statistical Analysis
The GraphPad Prism Version 8.00 (GraphPad Software, San Diego, CA) was used for statistical analysis. Statistically significant differences were evaluated by one-way analysis of Variance 55 (ANOVA), followed by Turkey’s post-test. The experiments were performed at least in triplicate. * p < 0.05; ** p < 0.01; *** p < 0.001 was considered statistically significant.
3. Results
3.1. Gene Expression Results
NOX4, p47-phox and BCL-2 Genes Expression Results
Expression levels of NOX4 and its regulatory subunit p47-phox were investigated. Results, expressed as mean normalized expression, show that expression levels of NOX4 and p47-phox significantly increased in the AFB1 group, compared to the control (* p < 0.05, Figure 1a,b). Exposure of the AFB1 group to feed additive induces a decreased expression of NOX4 (# p < 0.05) (Figure 1a) and p47-phox (* p < 0.05) (Figure 1b) compared to the control. Regarding genes involved in apoptosis regulation, the results show that anti-apoptotic protein BCL-2 increased in AFB1 group respect to control, but feed additive (Figure 1c) was not able to restore these values.
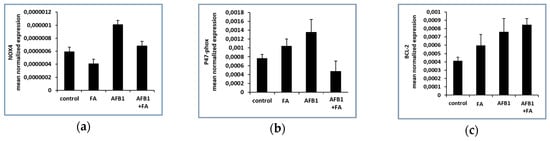
Figure 1.
NOX4, p47-phox and BCL-2 genes expression in CONTROL (n = 3), FA (n = 3), AFB1 (n = 3) and AFB1+ FA (n = 5) treated groups: (a) mRNA levels of NOX4; (b) mRNA levels of p47-phox; (c) mRNA levels of BCL-2. Values are presented as mean normalized expression (MNE) normalized towards 18S expression (mean ± standard error).
3.2. Protein Expression Results
NOX4, p47-phox and BCL-2 Proteins Expression Results
The trend shown in gene expression is also reflected in protein expression. Western blot analysis confirmed that NOX4 (Figure 2a) and p47-phox (Figure 2b) proteins were significantly up-regulated in AFB1 with respect to the control animals (* p < 0.05 vs. control). FA treatment restored the NOX4 values (* p < 0.05 AFB1 vs. AFB1+FA Figure 2a) and a similar trend is about p47-phox (Figure 2b). Western blot analysis for BCL-2 (Figure 2c) protein showed no significant increase in AFB1 with respect to the control animals (Figure 2c).
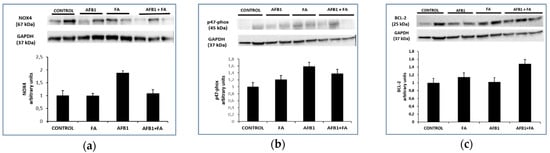
Figure 2.
NOX4, p47-phox and BCL-2 proteins expression in CONTROL (n = 3), FA (n = 3), AFB1 (n = 3) and AFB1+FA (n = 5) treated groups: (a) protein levels of NOX4; (b) protein levels of p47-phox; (c) protein levels of BCL-2. Values are presented as arbitrary units, normalized towards GAPDH.
4. Discussion
The continued presence of mycotoxins in feed is disadvantageous for poultry performance, representing a critical risk to chicken farming. Nutraceuticals are progressively evaluated as valid tools in veterinary medicine because of their capacity to counteract the presence of mycotoxins in animal feedings [17].
The FA has been tested and has demonstrated to be a valid binder for feed decontamination from AFB1. As a matter of fact, FA is able to down-regulate both the transcription and the expression of NOX4 (considered one of the crucial factors for oxidative stress) in chicken kidneys, together with its p47-phox subunit, suggesting its capacity to bind AFB1 according to the mechanism with which it acts, reducing bioavailability of this kind of mycotoxin. In particular, FA treatment shows an improvement in renal alterations by reverting the increased levels of ROS and activating antioxidant enzymes.
As regards anti-apoptotic action, BCL-2 is over-expressed in the AFB1 plus FA-treated group, demonstrating a lack of involvement of the apoptotic process in Aflatoxicosis. This data is still incomplete because it is necessary to also investigate the role of some pro-apoptotic proteins, e.g., BAX, in order to evaluate the BCL-2/BAX ratio.
In any case, the management of the environmental risk of chickens, by adding FA as an adsorbent supplement in animal diets, could prevent the deleterious effects of poultry mycotoxicosis.
5. Conclusions
The experiments performed in this work highlight the capacity of a new feed additive to revert nephrotoxicity induced by AFB1 in poultry.
Author Contributions
S.D., S.F., R.C., A.S. and G.A. conceived and designed the experiments; C.L. (Consiglia Longobardi), E.A., C.L. (Chiara Lauritano), V.R., S.D. and W.J. performed the experiments; S.D., C.L. (Consiglia Longobardi), E.A. and C.L. (Chiara Lauritano) analyzed the data; C.L. (Consiglia Longobardi), S.D. and R.C. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the European Union’s Horizon2020 Research and innovation programme under Grant Agreement No.678781 (MycoKey).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author.
Acknowledgments
The authors are grateful to Angela Petruccelli for her technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | linear dichroism |
| AFB1 | Aflatoxin B1 |
| ROS | Reactive oxygen species |
| NOX4 | NADPH oxidase 4 |
| FA | Feed Additive |
References
- Omotayo, O.P.; Omotayo, A.O.; Mwanza, M.; Babalola, O.O. Prevalence of Mycotoxins and Their Consequences on Human Health. Toxicol. Res. 2019, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Raduly, Z.; Szabo, L.; Madar, A.; Pocsi, I.; Csernoch, L. Toxicological and Medical Aspects of Aspergillus-Derived Mycotoxins Entering the Feed and Food Chain. Front. Microbiol. 2019, 10, 2908. [Google Scholar] [CrossRef] [PubMed]
- Marasas, W.F.O.; Gelderblom, W.; Shephard, G.; Vismer, H. Mycotoxins: A global problem. Mycotoxins Detect. Methods Manag. Public Health Agric. Trade 2008, 29–39. [Google Scholar] [CrossRef]
- A review of human carcinogens: Chemical agents and related occupations. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 2012; IARC Working Group, Ed.; IARC: Lyon, France, 2012; Volume 100F, pp. 225–244. [Google Scholar]
- Rotimi, O.A.; Rotimi, S.O.; Duru, C.U.; Ebebeinwe, O.J.; Abiodun, A.O.; Oyeniyi, B.O.; Faduyile, F.A. Acute aflatoxin B1—Induced hepatotoxicity alters gene expression and disrupts lipid and lipoprotein metabolism in rats. Toxicol. Rep. 2017, 4, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.; Kaya, E.; Karaca, A.; Karatas, O. Aflatoxin B1 induced renal and cardiac damage in rats: Protective effect of lycopene. Res. Vet. Sci. 2018, 119, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Meissonnier, G.; Pinton, P.; Laffitte, J.; Cossalter, A.-M.; Gong, Y.Y.; Wild, C.; Bertin, G.; Galtier, P.; Oswald, I. Immunotoxicity of aflatoxin B1: Impairment of the cell-mediated response to vaccine antigen and modulation of cytokine expression. Toxicol. Appl. Pharmacol. 2008, 231, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Guindon, K.A.; Bedard, L.L.; Massey, T.E. Elevation of 8-Hydroxydeoxyguanosine in DNA from Isolated Mouse Lung Cells Following In Vivo Treatment with Aflatoxin B1. Toxicol. Sci. 2007, 98, 57–62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- European Commission. Commission Regulation (EC) No 165/2010 of 26 February 2010 amending Regulation (EC) No 1881/2006 setting maximun levels of certain contaminants in foodstuffs as regards alatoxins. Off. J. Eur. Union L 2021, 50, 8–12. [Google Scholar]
- Bintvihok, A.; Thiengnin, S.; Doi, K.; Kumagai, S. Residues of aflatoxins in the liver, muscle and eggs of domestic fowls. J. Vet. Med. Sci. 2002, 64, 1037–1039. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.; Kim, J.E.; Coulombe, R. Aflatoxin B1 in poultry: Toxicology, metabolism and prevention. Res. Vet. Sci. 2010, 89, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Glahn, R.P.; Beers, K.W.; Bottje, W.G.; Wideman, R.F., Jr.; Huff, W.E. Altered renal function in broilers during aflatoxicosis. Poult. Sci. 1990, 69, 1796–1799. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Gonçalves, B.L.; de Neeff, D.V.; Ponzilacqua, B.; Coppa, C.F.S.C.; Hintzsche, H.; Sajid, M.; Cruz, A.G.; Corassin, C.H.; Oliveira, C.A.F. Aflatoxin in foodstuffs: Occurrence and recent advances in decontamination. Food Res. Int. 2018, 113, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Alpsoy, L.; Yildirim, A.; Agar, G. The antioxidant effects of vitamin A, C, and E on aflatoxin B1-induced oxidative stress in human lymphocytes. Toxicol. Ind. Health 2009, 25, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.S.; Wilcox, C.S. NADPH Oxidases in the Kidney. Antioxid. Redox Signal. 2006, 8, 1597–1607. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Srivastava, A.; Lall, R. Nutraceuticals in Veterinary Medicine; Springer International Publishing: Cham, Switzerland, 2019; Available online: https://link.springer.com/10.1007/978-3-030-04624-8 (accessed on 30 November 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).