Abstract
Mabroom dates (Phoenix dactylifera L.) are recognized as one of the most important crops in Qatar. Fresh fruit dates are susceptible to mould and post-harvest spoilage, resulting in a significant financial loss. Octanoic fatty acid (OFA) has been shown to regulate the growth of mould-causing organisms such as fungi and bacteria. It is known to have antibacterial properties. The objective of the current study was to evaluate the in vitro effect of OFA on the post-harvest pathogens of Mabroom fruits. Fresh, apparently healthy, and fully ripe Mabroom dates were obtained from the National Agriculture and Food Corporation (NAFCO). The chosen fruits were packed in sterile, well-ventilated plastic boxes and transported to the lab under controlled conditions. The fruits were distributed into five groups (G1 to G5). The groups G1, G2, and G3 received 1%, 2%, and 3.5% OFA, respectively, while G4 was left untreated and G5 was washed only with tap water as a positive control treatment. Each group contained 200 g of fresh and healthy semi-soft dates. The samples were then dried and incubated in a humidity chamber at 25 °C ± 2 for seven days. The signs and symptoms of decay were monitored and recorded. The presence of pathogens was confirmed via phenotypic and microscopic-based methods. The results showed a significant difference (p ≤ 0.05) among the groups. OFA at 3.5% had the strongest inhibitory action against post-harvest pathogens, followed by OFA2%. However, there were no differences (p ≤ 0.05) between OFA1% and the control groups. Aspergillus spp., Penicillium spp., Rhizopus spp., and Botrytis spp. were most abundant in the control group, followed by OFA2% and OFA1%, respectively. In conclusion, octanoic fatty acid at 3.5% may improve the quality of date fruits through its high antimicrobial activity, reduce the effect of post-harvest decay, minimize the loss of date fruits during storage, and improve the sustainability of date fruits. Further experiments are necessary to confirm the effectiveness of OFA as a green solution for sustainable date fruit production.
1. Introduction
Qatar is one of the world’s major producers of dates, with a total yield of 21,491 metric tonnes. Over 581 thousand date trees were grown on 2469 hectares in 2010. Approximately 7% of all crops produced in Qatar were dates [1]. Mabroom date fruits (Phoenix dactylifera L.), a member of the Arecaceae palm family, are rich in important minerals and high in nutrients. North Africa, the Middle East, and the Arabian Peninsula are their origins. Worldwide, fruit production and consumption have been rising steadily [2]. According to research, the fibre content of dates varies from 6.4% to 11.5%, depending on their type, making them important sources of dietary fibre [3,4]. Date fruits also contain substantial quantities of potassium, magnesium, phosphorus, calcium, zinc, and iron [5,6] and are a significant source of vitamins B1, B2, and C [7,8].
The quality of date fruits as they are being stored has been negatively impacted by post-harvest illnesses and mould-causing microorganisms. Numerous studies have demonstrated that even fruits that are thought to be stable, such as semi-dry and dry dates, may be contaminated by mould and bacteria under circumstances that are not optimal, resulting in an overall decrease in quality and financial losses [9]. Fungi such as Alternaria, Aspergillus, and Penicillium can grow on soft fruit, especially dates, when harvested after rainy and humid conditions [10,11,12,13]. Yeast, which belongs to the Zygosaccharomyces species are almost highly tolerant of the sugar content of date fruits [13,14]. During pathogenesis, some fungi-causing moulds can produce different secondary metabolites, such as mycotoxins, which are highly toxic to the consumers of the contaminated products. Aflatoxins and ochratoxins are the most important mycotoxins, which are produced mainly by Aspergillus spp. [15]. Many studies discussed the aflatoxin contamination in date fruits [16,17,18,19].
Over the past few decades, there has been a great deal of interest in the antimicrobial effects of various plant-based pesticides for controlling pathogens. These plant-based pesticides have been reported as effective inhibitors of pathogens. Additionally, they prevent or lessen the negative impacts that synthetic pesticides have on the environment or ecosystem [20,21]. Octanoic fatty acid (OFA) is a 100% natural pesticide, also known as caprylic acid (chemical formula: C8H16O2). It is a naturally occurring medium-chain fatty acid found in various plant and animal sources, such as coconut palm, human milk, vegetables, and fruits. Commercial octanoic acid used in formulations (e.g., for antimicrobial or antifungal purposes) is often derived from the fractionation of coconut or palm kernel oil. It is generally recognized as a safe component by the Food and Drug Administration, US Department of Health and Human Services [22]. Its food preservative properties were approved on different vegetables and fruit juices [23], meat products [24], and dairy products [25]. It works through a process of cell wall intrusion and disruption of protein functioning. Through these actions, octanoic acid can adversely affect cellular organisms (bacteria), non-cellular organisms (viruses), and multicellular fungi and moulds. Medium-chain fatty acids such as octanoic acid (C8:0) exhibit a wide range of anti-microbial actions against pathogenic microbes [25,26,27]. However, few studies have discussed the preservative role of the OFA pathogens of stored fruits, especially Mabroom date fruits. Therefore, the current study was conducted to investigate the impact of various octanoic acid concentrations on the presence of post-harvest moulds in the quality of Mabroom date fruits under storage conditions.
2. Materials and Methods
2.1. Preparation of Octanoic Acid Supplemented Dates
Fresh Mabroom dates (Phoenix dactylifera L.), at their full ripeness stage, were obtained from the National Agriculture and Food Corporation (NAFCO) (Arusha, Tanzania). Five experimental groups (G1–G5) were set up. Each group contained 200 g of fresh, seemingly healthy dates.
Four groups were thoroughly washed with tap water, surface-sterilized with 1% NaOCl, and rinsed in 3 changes of sterilized distilled water; the excess water was removed by placing them onto sterile filter paper. Then groups G1, G2, and G3 were dipped in 500 mL of 1%, 2%, and 3.5% of OFA solution (Aussan Laboratories, Victoria, Australia), respectively. As the control groups, group G4 remained untreated while G5 was washed with tap water only. The samples were then air-dried, set into four replicates, and incubated for one week at 25 °C ± 2 in a moist chamber under dark conditions to encourage the growth of post-harvest pathogens.
2.2. Microbial Investigations
After one week of incubation, the decaying symptoms and signs developing on the fruits were visually examined, and microbial analysis was carried out. After removing all the seeds, 25 g of the treated date fruits were thoroughly mixed with 225 mL of 0.10 peptone water in a stomacher (Bagmixer 400 p, Grosseron, Herblain, France) for 1 min. Yeast and mould enumerations were made by spreading the samples on the surface of a Yeast Extract Glucose Chloramphenicol Agar (Merck Chimie, Fontenay Sous Bois, France) and incubated at 25 °C for 3 to 5 days (NF ISO 21527-2, Geneva, Switzerland, 2008 [28]. Each treatment was replicated 4 times. The cultures were repeatedly sub-cultured for purification. Single-spore cultures of the obtained fungal microorganisms were prepared following Goh’s [29]. The obtained cultures were subjected to microscopic examinations under a light microscope (400×). The pathogens were identified based on their morphological characters and microscopic structures (conidiophores, conidia, sporangia, and hyphae).
2.3. Statistical Analysis
Data obtained from the experiment were subjected to a one-way ANOVA using the SPSS software version 24 (SPSS® Inc., Chicago, IL, USA). The differences between the means were tested at the 0.05 probability level. The data presented in this manuscript are expressed in the form of (mean ± standard deviation).
3. Results and Discussion
Figure 1 and Figure 2 display the effects of various octanoic acid concentrations. The dates from three groups, including the dates that were supplemented with octanoic fatty acid (OFA1%), untreated, and only washed, were completely infected (100 ± 00%) with different mould contaminants. No significant effects were recorded between OFA1% and the control groups, while in all fruits supplemented with OFA2%, the presence of moulds was 40 ± 51.6%. On the other hand, all Mabroom date fruits supplemented with OFA3.5% were completely healthy, demonstrating the highest inhibitory effect against fungi. Moreover, 3.5% of the OA-supplemented fruits yielded a significant improvement (p ≤ 0.05) in stored fruits compared to OFA1% and OFA2%. According to the preliminary identification based on morphological characteristics, following Seifert et al. [30], the genera of Aspergillus, Rhizopus, and Botrytis have been isolated and identified. Aspergillus niger is recognized by its distinctive black colonies, conidial heads, vesicles, and phialides. Rhizopus sp. is identified by its fast-growing, cottony colonies and sporangia with sporangiospores. Penicillium spp. is identified by its brush-like conidiophores. Botrytis spp. is recognized by its unique grey, dense cottony colonies with characteristic conidiophores and branching. These were detected at high levels in the control groups, followed by OA1% and OA2%, respectively.
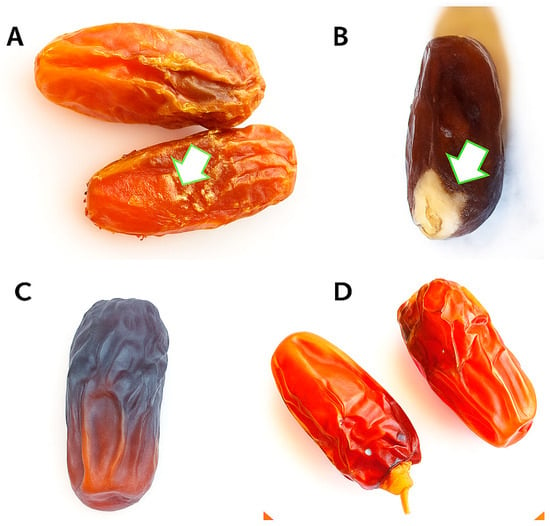
Figure 1.
Shows the effects of different concentrations of OFA solution on stored date fruits. (A) Date fruits washed with water only showed symptoms of moulds (white arrow). (B) Untreated date fruits showed a high level of moulds and bacteria (white arrows). (C) Date fruits supplemented with OFA2% showed no symptoms of moulds and bacteria. (D) Date fruits treated with OFA3.5% were completely healthy. OFA: Octanoic fatty acid.
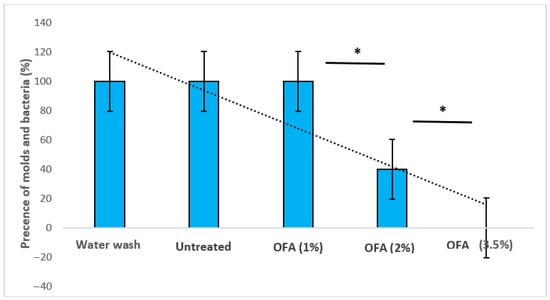
Figure 2.
Shows the effects of different concentrations (1%, 2%, and 3.5%) of OFA compared to control groups (water wash only and untreated) on stored date fruits. Groups with a star (*) are significantly different at p ≤ 0.05. OFA: Octanoic fatty acid solution.
The current results are partially in line with the findings of López-Velázquez et al. [31], who noticed that the OFA derived from the Vitex mollis fruits had a high inhibitory action against Colletotrichum gloeosporioides with a minimal inhibitory concentration (MIC) of 0.5 milligrams/mL. Our results also show some agreement with those of Výrostková et al. [32], who found that OA has a strong inhibitory action against Salmonella enterica, Escherichia coli CCM 3988, Enteritidis CCM 4420, Listeria monocytogenes, Staphylococcus aureus, and a wide range of foodborne bacterial strains. It is also known for its highly antibacterial activities against a wide range of Gram-negative and Gram-positive bacterial pathogens [33] such as Salmonella spp. and Escherichia coli O157:H7 [34]. Furthermore, elevated OFA levels have the potential to enhance the antibacterial activity against specific microbes [35,36]. The implementation of OFA is legally permitted and safe in the food industry [28]. The way in which the OFA could interact with the pathogen is not clear, but recently López-Velázquez et al. [31] suggested that this fatty acid could interact with lipid compounds in fungal membranes and cause a disruption in its components; this disruption was much higher in spores.
Author Contributions
Conceptualization, A.S.H.A. and E.A.H.M.; Data curation, E.A.H.M. and K.P.; Investigation, A.S.H.A.; Methodology, A.S.H.A.; Resources, A.S.H.A.; Supervision, A.S.H.A.; Writing—original draft, E.A.H.M.; Writing—review and editing, K.P. and A.S.H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Author Elshafia Ali Hamid Mohammed was employed by the Agricultural Research Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Muhammed, N.H.; Ahmed, O.E.; Ahmed, T.A.; Al-Yafai, M.S. Date Palm Status and Perspective in Qatar. In Date Palm Genetic Resources and Utilization; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 323–351. ISBN 978-94-017-9706-1. [Google Scholar]
- Botes, A.; Zaid, A. Date Production Support Program; FAO Plant Production and Protection Paper; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2002. [Google Scholar]
- Alqahtan, N.; Makki, H.M.; Mohamed, H.; Ali, D.; Al-Senaien, W. Processing and Quality Evaluations of a Date-Strawberry Mixed Jam for Young Children. Pak. J. Nutr. 2022, 21, 47–52. [Google Scholar] [CrossRef]
- Elghazali, M.N.; Tawfeuk, H.Z.; Gomaa, R.A.; Abbas, A.A.; Tantawy, A.A. Effect of Dehydration Methods on Physicochemical Properties of Aswan Dry Dates. Assiut J. Agric. Sci. 2020, 51, 50–64. [Google Scholar] [CrossRef]
- Kuras, M.J.; Zielińska-Pisklak, M.; Duszyńska, J.; Jabłońska, J. Determination of the Elemental Composition and Antioxidant Properties of Dates (Phoenix dactyliferia) Originated from Different Regions. J. Food Sci. Technol. 2020, 57, 2828–2839. [Google Scholar] [CrossRef] [PubMed]
- Aldhafiri, F.K. Evaluation of Biochemical Parameters, Phenolic Compounds and Antioxidant Capacity of Some Varieties of Phoenix dactylifera L. (Date Fruits) to Determine the Nutritional Impact Values. Mediterr. J. Nutr. Metab. 2017, 10, 153–164. [Google Scholar] [CrossRef]
- Aljaloud, S.; Colleran, H.L.; Ibrahim, S.A. Nutritional Value of Date Fruits and Potential Use in Nutritional Bars for Athletes. Food Nutr. Sci. 2020, 11, 463–480. [Google Scholar] [CrossRef]
- Al-Alawi, R.A.; Al-Mashiqri, J.H.; Al-Nadabi, J.S.M.; Al-Shihi, B.I.; Baqi, Y. Date Palm Tree (Phoenix dactylifera L.): Natural Products and Therapeutic Options. Front. Plant Sci. 2017, 8, 845. [Google Scholar] [CrossRef]
- Dehghan-Shoar, Z.; Hamidi-Esfahani, Z.; Abbasi, S. Effect of temperature and modified atmosphere on quality preservation of sayer date fruits (Phoenix dactylifera L.). J. Food Process Preserv. 2010, 34, 323–334. [Google Scholar] [CrossRef]
- Shenasi, M.; Aidoo, K.E.; Candlish, A.A.G. Microflora of Date Fruits and Production of Aflatoxins at Various Stages of Maturation. Int. J. Food Microbiol. 2002, 79, 113–119. [Google Scholar] [CrossRef]
- Gherbawy, Y.A.; Elhariry, H.M.; Bahobial, A.A.S. Mycobiota and Mycotoxins (Aflatoxins and Ochratoxin) Associated with Some Saudi Date Palm Fruits. Foodborne Pathog. Dis. 2012, 9, 561–567. [Google Scholar] [CrossRef]
- Ibrahim, S.; Rahma, M. Isolation and Indentification of Fungi Associated with Date Fruits (Phoenix dactylifera, Linn) Sold at Bayero University, Kano, Nigeria. Bayero J. Pure Appl. Sci. 2011, 2, 127–130. [Google Scholar] [CrossRef]
- Hamad, S.H. Microbial Spoilage of Date Rutab Collected from the Markets of Al-Hofuf City in the Kingdom of Saudi Arabia. J. Food Prot. 2008, 71, 1406–1411. [Google Scholar] [CrossRef]
- Martorell, P.; Stratford, M.; Steels, H.; Fernández-Espinar, M.T.; Querol, A. Physiological Characterization of Spoilage Strains of Zygosaccharomyces bailii and Zygosaccharomyces rouxii Isolated from High Sugar Environments. Int. J. Food Microbiol. 2007, 114, 234–242. [Google Scholar] [CrossRef]
- Roehuck, B.D.; Maxuitenko, Y.Y. Biochemical Mechanisms and Biological Implication of the Toxicity of Aflatoxins as Related to Aflatoxin Carcinogenesis. In The Toxicology of Aflatoxins: Human Health, Veterinary, and Agricultural Significance; Academic Press: San Diego, CA, USA, 1994. [Google Scholar]
- Almaghrabi, M.; Morgan, M. Susceptibility of Ajwa Dates (Phoenix dactylifera) to Aflatoxin Contamination Based on Liquid Chromatography Combined with Electrospray Ionisation-Triple Quadrupole Tandem-Mass Spectrometry (LC-ESI-MS/MS). Int. Food Res. J. 2023, 30, 324–333. [Google Scholar] [CrossRef]
- Almaghrabi, M.A. The Occurrence of Aflatoxins in Date Palm (Phoenix dactylifera L.) Worldwide. J. Food Qual. 2022, 2022, 1–9. [Google Scholar] [CrossRef]
- Alghalibi, S.M.S.; Shater, A.-R.M. Mycoflora and Mycotoxin Contamination of Some Dried Fruits in Yemen Republic. Assiut Univ. Bull. Environ. Res. 2004, 7.2, 19–27. [Google Scholar] [CrossRef]
- Naeem, I.; Ismail, A.; Rehman, A.; Ismail, Z.; Saima, S.; Naz, A.; Faraz, A.; De Oliveira, C.; Benkerroum, N.; Aslam, M.; et al. Prevalence of Aflatoxins in Selected Dry Fruits, Impact of Storage Conditions on Contamination Levels and Associated Health Risks on Pakistani Consumers. Int. J. Environ. Res. Public Health 2022, 19, 3404. [Google Scholar] [CrossRef]
- Mahajan, A.; Das, S. Plants and Microbes—Potential Source of Pesticide for Future Use. Pestic. Inf. 2003, 28, 33–38. [Google Scholar]
- Hamid, E.A.; Elhassan, S.M.; Abubaker, M.Y.A. Use of Botanicals Against Citrus Canker (Xanthomonas Axonopodis Pv. Citri) Affecting Lime (Citrus Aurantifolia Swingle) in Sudan. J. Hortic. Sci. Technol. 2020, 3, 93–97. [Google Scholar] [CrossRef]
- Direct Food Substances Affirmed as Generally Recognized as Safe: 21 CFR §184.1025. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1025 (accessed on 4 April 2019).
- Kim, S.A.; Rhee, M.S. Highly Enhanced Bactericidal Effects of Medium Chain Fatty Acids (Caprylic, Capric, and Lauric Acid) Combined with Edible Plant Essential Oils (Carvacrol, Eugenol, β-Resorcylic Acid, Trans-Cinnamaldehyde, Thymol, and Vanillin) against Escherichia coli O157:H7. Food Control 2016, 60, 447–454. [Google Scholar] [CrossRef]
- Mohan, A.; Pohlman, F.W.; McDaniel, J.A.; Hunt, M.C. Role of Peroxyacetic Acid, Octanoic Acid, Malic Acid, and Potassium Lactate on the Microbiological and Instrumental Color Characteristics of Ground Beef. J. Food Sci. 2012, 77, M188–M193. [Google Scholar] [CrossRef]
- Nair, M.K.M.; Vasudevan, P.; Hoagland, T.; Venkitanarayanan, K. Inactivation of Escherichia coli O157:H7 and Listeria monocytogenes in Milk by Caprylic Acid and Monocaprylin. Food Microbiol. 2004, 21, 611–616. [Google Scholar] [CrossRef]
- Hilgren, J.D.; Salverda, J.A. Antimicrobial Efficacy of a Peroxyacetic/Octanoic Acid Mixture in Fresh-Cut-Vegetable Process Waters. J. Food Sci. 2000, 65, 1376–1379. [Google Scholar] [CrossRef]
- Kim, S.A.; Rhee, M.S. Synergistic Antimicrobial Activity of Caprylic Acid in Combination with Citric Acid against Both Escherichia coli O157:H7 and Indigenous Microflora in Carrot Juice. Food Microbiol. 2015, 49, 166–172. [Google Scholar] [CrossRef]
- Aussan L44 and L42 Disinfectants. Available online: https://aussan.com.au/l44-l42 (accessed on 27 April 2024).
- Goh, T.K. Single-spore isolation using a hand-made glass needle. J. Fungal Divers. 1999, 2, 47–63. [Google Scholar]
- Seifert, K.; Morgan-Jones, G.; Gams, W.; Kendrick, B. The Genera of Hyphomycetes; CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2011; p. 997. [Google Scholar]
- López-Velázquez, J.G.; Ayón-Reyna, L.E.; Vega-García, M.O.; López-Angulo, G.; López-López, M.E.; López-Zazueta, B.A.; Delgado-Vargas, F. Caprylic Acid in Vitex mollis Fruit and Its Inhibitory Activity against a Thiabendazole-resistant Colletotrichum gloeosporioides Strain. Pest. Manag. Sci. 2022, 78, 5271–5280. [Google Scholar] [CrossRef]
- Výrostková, J.; Pipová, M.; Bujňák, L.; Bujňáková, D.; Krahulec, J. Antimicrobial Effect of Hydrogen Peroxide and Octanoic Acid on Bacterial Strains Isolated from Food and Water. Pol. J. Vet. Sci. 2020, 23, 451–457. [Google Scholar] [CrossRef]
- Hulankova, R.; Borilova, G.; Steinhauserova, I. Combined Antimicrobial Effect of Oregano Essential Oil and Caprylic Acid in Minced Beef. Meat Sci. 2013, 95, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Redondo-Solano, M.; Thippareddi, H. Inactivation of Escherichia coli O157:H7 and Salmonella spp. on Alfalfa Seeds by Caprylic Acid and Monocaprylin. Int. J. Food Microbiol. 2010, 144, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Baky, N. Differential Antimicrobial Effectiveness of Camel Lactoferrin-Oleic Acid and Bovine Lactoferrin-Oleic Acid Complexes against Several Pathogens. SOJ Biochem. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Huang, C.B.; George, B.; Ebersole, J.L. Antimicrobial Activity of N-6, n-7 and n-9 Fatty Acids and Their Esters for Oral Microorganisms. Arch. Oral. Biol. 2010, 55, 555–560. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).