Abstract
The generally accepted approach for increasing plant resistance to heavy metals is the treatment by phytohormones of different natures. In particular, brassinosteroids are highly effective in decreasing the level of toxic ions damage effects on plant growth. ABA and wheat germ agglutinin (WGA) contents were measured in the same plant with the help of enzyme immunoassay method. The localization of lignin and suberin on cross sections of wheat roots stained with berberine hemisulfate/toluidine blue was performed. The mitotic index was determined as percentage of mitotic cells of the total number of the cells. It was found that 0.4 μM 24-epibrassinolide (EBR) seed pretreatment under the influence of 1 mM cadmium acetate in the presence of fluridone (Fl) inhibited the stress-induced accumulation of ABA, while the WGA content in plants stayed higher than in stressed EBR-untreated wheat roots. Moreover, EBR-pretreated wheat plants under cadmium stress formed in the root cell walls’ Casparian bands and suberin lamellae, which are the critical locations in the apoplastic barriers of endodermis and exodermis. Pre-sowing EBR-treatment promoted acceleration of Casparian bands and suberin lamellae formation without inhibiting growth processes. We have demonstrated the involvement of wheat lectin in the realization of EBR-induced protective effect on plants under cadmium stress. An important contribution to EBR-induced strengthening the barrier properties of the cell walls of the studied tissues is the ability of EBR to induce ABA-independent accumulation of WGA, which further promotes the increasing lignin and suberin biopolymers deposition in the cell walls of wheat roots.
1. Introduction
Wheat germ agglutinin (WGA) is a classical wheat lectin belonging to the RAB protein family [1]. WGA is an active participant in ABA-controlled reactions in response to biotic and abiotic stresses [2], and some portion of this wheat lectin is excreted in the area of root apical meristem, apparently becoming an exogenous agent for the plants [3]. Lectins are carbohydrate-binding proteins known for their role in the regulation of innate immune responses in various organisms [4]. Their role in signaling pathways involved in adaptation to abiotic stress is also recently well understood.
The influence of cadmium ions has a toxic effect on plants, causes osmotic stress and induces the accumulation of ABA in plants [5]. In this regard, an actual question ariseswith respect to WGA involvement as an excreted protein in the process of wheat protection from toxic cadmium effect. Some authors mention possible increased lectin genes expression in response to cadmium stress in the roots of plant species hypertolerant to such stress [6]. The key role of ABA in the induction of WGA synthesis and accumulation under the 1 mM cadmium acetate treatment was demonstrated. In turn, this protein contributed to the maintenance of root cell mitotic activity under stress at higher levels and indicated lectin participation in the ABA-triggered defense reactions of wheat plants during cadmium stress [3].
The generally accepted approach for increasing the plant resistance relative to heavy metals is the treatment by phytohormones of different nature. In particular, brassinosteroids are highly effective in decreasing the level of toxic ions damage effects on plant growth [7]. Previously, it was found that 24-epibrassinolide (EBR) treatment causes the significant WGA gene transcription activation and causes an increase in WGA content in wheat seedlings, as well as the absence of changing in the endogenous ABA concentration [8]. These data, along with the previously obtained results on the WGA treatment possibility to enhance cell division of EBR-pretreated seedlings against the background of endogenous lectin deficiency, indicate the interaction of the wheat lectin system and EBR in the regulation of growth processes [9].
In this regard it was important to elucidate the WGA participation in EBR-induced wheat defense reactions in response to cadmium stress and to evaluate the endogenous ABA contribution to the regulation of WGA level in EBR-pre-sowing treated plants under cadmium acetate treatment.
2. Experiments
Experiments were performed with 7dayold wheat seedlings of Triticum aestivum L., cultivar Bashkirskaya 26. Wheat seeds were sterilized in 96% ethanol, and then they were soaked in a solution of 0.4 μM of 24-epibrassinolide or distilled water for 3 h. The 7dayseedlings were preliminarily isolated from the endosperm and grown in glasses with 0.1 strength Hoagland-Arnon nutrient solution or 1 mM cadmium acetate (Cd (CH3COO)2·2H2O) for 7 h. Plants incubated in a Hoagland-Arnon solution served as control. To elucidate the ABA participation in the regulation of WGA content in wheat roots, the 5 mg/L inhibitor of ABA biosynthesis fluridone (Fl) was used.
ABA and WGA contents were measured in the same plant with the help of enzyme immunoassay method [10] using specific polyclonal rabbit antibodies against ABA and WGA and anti-rabbit antibodies labeled with peroxidase.
The mitotic index was determined in the root apical meristems of the seedlings as a percentage of mitotic cells of total number of the cells.
Lignin and suberin were identified using an aqueous solution of berberine hemisulfate (0.1% w/v) [11]. In order to enhance the fluorescence intensity, the sections were additionally stained for 15 min with toluidine blue (0.05% w/v) in 0.1 M phosphate buffer, pH 5.6. Stained sections were embedded in a 0.1% FeCl3/50% glycerol mixture and viewed with a confocal microscope LSM 510 (Carl Zeiss, Jena, Germany) using a combination of filters BP 450-490/FT 510/LP 520.
3. Results and Discussion
Pre-sowing EBR-treatmentof 0.4 µM twice reduced the level of cadmium-induced accumulation of ABA, as well as WGA in wheat roots and in the root environment (Figure 1). These data indicate the less damaging effect of cadmium on EBR-pretreated plants. Fl pretreatment, together with EBR-treatment, resulted in the inhibition of stress-induced ABA accumulation. However, the WGA content in these seedlings was maintained at a level close to that in the variant with only EBR exposure and that was also reflected in the WGA content in the external environment. This indicates the ABA-independent EBR’s ability to regulate the quantitative level of WGA in plant roots under cadmium stress and, therefore, indicates the presence of alternative pathways for hormonal regulation of lectin concentration, which is involved in the development of wheat resistance to cadmium. The EBR-protective effect is also indicated by prevention of the growth-inhibiting effect of cadmium on wheat plants and the maintenance of the mitotic index of EBR-pretreated plants at the control level. This protective effect of EBR on cell division persisted in the presence of Fl, which confirms the independent nature of EBR action on wheat plants. Thus, we have demonstrated the involvement of lectin, which plays an important role in the formation of nonspecific wheat resistance, in the realization of EBR-protective effects on wheat plants under cadmium stress.
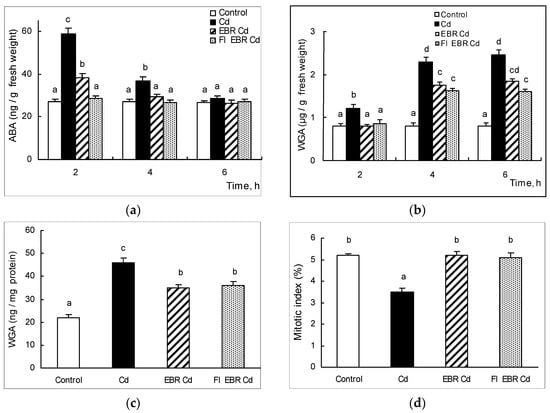
Figure 1.
Effect of cadmium acetate stress on (a) ABA (ng/g fresh weight); (b) WGA (µg/g fresh weight); (c) WGA (ng/mg protein) contents; and (d) Mitotic index (%). Reliably different values (n = 20) at the level p < 0.05 are indicated with different letters, t-test.
The ability of EBR-pretreatment to activate WGA synthesis and accumulation in wheat seedlings, their interaction in the growth processes regulation and the ability of this lectin to be excreted into the incubation medium obviously can make an important contribution to the realization of their protective effect on plants under cadmium acetate stress.
Since cadmium ions are mainly adsorbed at roots, the anatomies and molecular structures of the root cell walls are, therefore, critical. Endodermal and exodermal apoplastic barrier reinforcement plays an important role in general in protecting roots and plants from toxic ion uptake [12]. Depending on the lignin and suberin biopolymers content as well as their localization, the apoplastic barriers’ transport properties are realized. It was found that roots of various plant species with higher suberin and lignin content might be less permeable to cadmium ions and, therefore, more resistant to its uptake and translocation [13]. The identified EBR ability in enhancing hydrogen peroxide production and peroxidase activity [14] probably contributes to phytohormone participation in lignin and suberin synthesis, which is involved in the reinforcement of the barrier properties of the cell walls and is an effective barrier against the cadmium ions’ penetration [15] and limits the diffusion of heavy metal throughout the plant.
It was revealed that an increase in the fluorescence of Caspari belts in the exodermis and endodermis of cross sections of the root basal part under cadmium stress indicates an additional deposition of lignin and suberin, which limits apoplastic transport (Figure 2). The radial exodermis walls were especially thickened. In the endodermis, there was an uneven thickening of the radial and significant reinforcement of the internal tangential cell walls near the pericycle border, the pericycle itself, the stelar parenchyma and, in some degree, the cortex. The EBR-pretreatment induced the plant preadaptive effects and led not only to additional lignin and suberin deposition in the endodermis and the parenchyma of the root cylinder compared with the control but also in the exodermis; this is likely due to the acceleration of the plants’ development. The formation of Casparian bands and suberin lamellae is observed in both the endodermal and exodermal apoplastic barriers of phytohormone-pretreated roots under stress, while the thickening of the cortex and stele cell walls was not as significant as when under stress. At the same time, EBR-pre-sowing treatment of seeds promoted the acceleration of Casparian bands and suberin lamellae formation in the root cell walls at the critical places in the apoplastic barriers of the endodermis and exodermis without inhibiting growth processes.
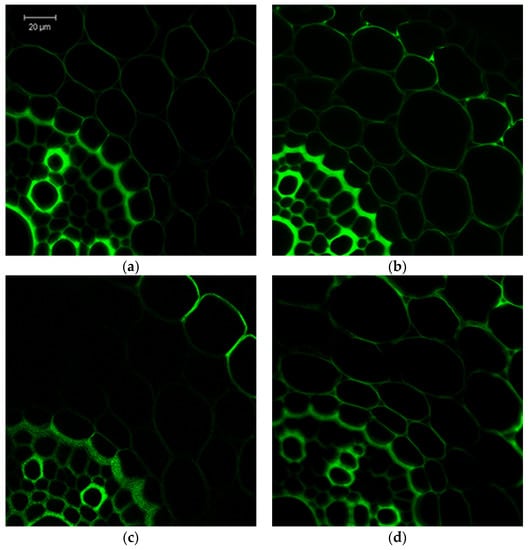
Figure 2.
The lignin and suberin localization and detection of Casparian bands in root cross sections of the 7day wheat seedlings stained with berberine sulfate/toluidine blue. (a) Control; (b) 7 h 1 mM Cd; (c) 0.4 µM EBR seed pretreatment; (d) 0.4 µM EBR + 7 h Cd. Bars = 20 µm.
Thus, the presence of lignin and suberin depositions in the root cell walls in the critical places of the endodermal and exodermal apoplastic barriers was revealed under EBR treatment and stress, which was reflected in the decrease in the negative effect degree of stress on growth processes.
4. Conclusions
An important contribution to the EBR-induced reinforcement of the cell walls’ barrier properties of the studied tissues was made. The EBR’s ability to induce ABA-independent accumulation of WGA is revealed. This in turn accelerates the phenolic biopolymers deposition, limiting toxic ion uptake to the inner tissues of the root and shoot.
Author Contributions
M.B., A.L. and F.S. conceived and designed the experiments; M.B. and A.L. performed the experiments; M.B., A.L. and F.S. analyzed the data; M.B. and A.L. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This work was supported by the Russian Foundation for Basic Research (grant No. 20-04-00904 a), partly within the framework of the state assignment (theme No. AAAA-A16-116020350029-1).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Skriver, K.; Mundy, J. Gene expression in response to abscisic acid and osmotic stress. Plant Cell 1990, 2, 503–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, D.; Shekha, S.; Bisht, S.; Kumar, V.; Varma, A. Ectopic overexpression of lectin in transgenic Brassica juncea plants exhibit resistance to fungal phytopathogen and showed alleviation to salt and drought stress. J. Bioeng. Biomed. Sci. 2015, 5, 147. [Google Scholar] [CrossRef] [Green Version]
- Bezrukova, M.V.; Fatkhutdinova, R.A.; Lubyanova, A.R.; Mursabaev, A.R.; Fedyaev, V.V.; Shakirova, F.M. Lectin involvement in the development of wheat tolerance to cadmium toxicity. Russ. J. Plant Physiol. 2011, 58, 1048–1054. [Google Scholar] [CrossRef]
- Tsaneva, M.; Van Damme, E.J.M. 130 years of plant lectin research. Glycoconj. J. 2020, 37, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Rucińska-Sobkowiak, R. Water relations in plants subjected to heavy metal stresses. Acta Physiol. Plant 2016, 38, 257. [Google Scholar] [CrossRef] [Green Version]
- Weber, M.; Trampczynska, A.; Clemens, S. Comparative transcriptome analysis of toxic metal responses in Arabidopsis thaliana and the Cd2+-hypertolerant facultative metallophyte Arabidopsis halleri. Plant Cell Environ. 2006, 29, 950–963. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A.; Hayat, S. Effect of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shakirova, F.M.; Bezrukova, M.V.; Aval’baev, A.M.; Gimalov, F.R. Stimulation of wheat germ agglutinin gene expression in root seedlings by 24-epibrassinolide. Russ. J. Plant Physiol. 2002, 49, 225–228. [Google Scholar] [CrossRef]
- Bezrukova, M.V.; Aval’baev, A.M.; Kil’dibekova, A.R.; Fatkhutdinova, R.A.; Shakirova, F.M. Interaction of wheat lectin with 24-epibrassinolide in the regulation of cell division in wheat roots. Dokl. Biol. Sci. 2002, 387, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Shakirova, F.M.; Bezrukova, M.V. Effect of 24-epibrassinolide and salinity on the level of ABA and lectin in wheat seedling roots. Russ. J. Plant Physiol. 1998, 45, 388–391. [Google Scholar]
- Junghans, U.; Langenfeld-Heyser, R.; Polle, A.; Teichmann, T. Effect of auxin transport inhibitors and ethylene on the wood anatomy of poplar. Plant Biol. 2004, 6, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Ranathunge, K.; Kim, Y.X.; Wassmann, F.; Kreszies, T.; Zeisler, V.; Schreiber, L. The composite water and solute transport of barley (Hordeum vulgare) roots: Effect of suberized barriers. Ann. Bot. 2017, 119, 629–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lux, A.; Martinka, M.; Vaculík, M.; White, P.J. Root responses to cadmium in the rhizosphere: A review. J. Exp. Bot. 2011, 62, 21–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakirova, F.M.; Avalbaev, A.M.; Bezrukova, M.V.; Fatkhutdinova, R.A.; Maslennikova, D.R.; Yuldashev, R.A.; Allagulova, C.R.; Lastochkina, O.V. Hormonal intermediates in the protective action of exogenous phytohormones in wheat plants under salinity. In Phytohormones and Abiotic Stress Tolerance in Plants; Khan, N.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 185–228. [Google Scholar] [CrossRef]
- Parrotta, L.; Guerriero, G.; Sergeant, K.; Cai, G.; Hausman, J.F. Target or barrier? The cell wall of early-and later-diverging plants vs. cadmium toxicity: Differences in the response mechanisms. Front. Plant Sci. 2015, 6, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).