Abstract
Elevated cholesterol levels, or hypercholesterolemia, have been recognized as the underlying cause of various diseases, most notably cardiovascular diseases. Unfortunately, most cholesterol-lowering (or anti-hypercholesterolemic) drugs are associated with several adverse effects, emphasizing the need to identify new cholesterol-lowering strategies. Natural products, particularly bioactive phytochemicals, have gained significant attention for their safer profile, fewer side effects, and potential health benefits, including cholesterol-lowering properties. The citrus fruit bergamot (Citrus bergamia) is renowned for its diverse array of bioactive phytochemicals. In this study, an in silico approach was utilized to assess the cholesterol-lowering potential of phytochemicals derived from C. bergamia. Molecular docking using AutoDock Vina of the selected phytochemicals was performed against the HMG-CoA reductase (HMGR), an enzyme targeted for hypercholesterolemia. Our results indicated that among the selected 20 phytochemicals, 8, namely eriocitrin, narirutin, scolymoside, neodiosmin, brutieridin, neohesperidin, rhoifolin, and naringin, exhibited better binding affinities than the conventional HMGR inhibitor, atorvastatin (−9.2 kcal/mol). Notably, among these top eight phytochemicals, eriocitrin displayed the most favorable binding affinity of −10.0 kcal/mol. These findings strongly imply that C. bergamia possesses potential HMGR-inhibitory activity and anti-hypercholesterolemic activity, primarily due to the high binding affinities exhibited by its phytochemical constituents. Therefore, further studies must be considered to comprehensively explore the cholesterol-lowering properties of C. bergamia phytochemicals.
1. Introduction
Hypercholesterolemia, a metabolic condition characterized by high levels of cholesterol in the blood, is known to be a risk factor for atherosclerosis, heart attack, and coronary heart diseases [1]. HMG-CoA reductase (HMGR) is the rate-limiting enzyme that catalyzes the conversion of HMG-CoA to mevalonate, a precursor that is needed for cholesterol biosynthesis. The inhibition of HMGR at this committed step has proven effective in treating hypercholesterolemia [2].
Statins are a class of cholesterol-lowering drugs that act as competitive inhibitors of the HMGR enzyme. However, concerns have been expressed regarding the adverse effects of long-term statin use, highlighting the need to identify new cholesterol-lowering strategies with safer profiles and fewer side effects [3,4].
Citrus bergamia, commonly known as bergamot, is native to southern Italy and is a citrus fruit that is rich in bioactive phytochemicals, specifically flavonoids and polyphenolic compounds [5,6]. Previous studies have reported its cholesterol-lowering properties, with evidence showing that administration of C. bergamia can reduce total cholesterol and low-density lipoprotein cholesterol levels in both animal and human models [7,8,9]. However, to date, limited information is available regarding the activity of its phytochemicals in targeting the key enzymes that regulate cholesterol levels in the body. Moreover, there is a lack of in silico molecular docking studies that thoroughly explore the potential of its various phytochemicals to inhibit HMGR. To address this gap, we conducted molecular docking using AutoDock Vina to evaluate 20 selected polyphenolic compounds from C. bergamia as potential cholesterol-lowering agents.
2. Materials and Methods
2.1. Preparation of Protein
The 3D crystal structure of human HMG-CoA reductase (HMGR) in a complex with the inhibitor atorvastatin (see Figure 1) (PDB ID: 1HWK), with a resolution of 2.22 Å, was downloaded in the PDB file format from the Research Collaboratory for Structural Bioinformatics Protein Data Bank (RCSB PDB) (www.rcsb.org, accessed on 23 January 2024). The PDB file was prepared using Discovery Studio Visualizer (DSV) software (version 21.1.0.20298). HMGR forms a tightly associated tetramer with four active sites: two located between polypeptide chains A and B, and the other two between chains C and D, symmetrically [10]. For the docking study, only chains A and B were used. All bound substances, including co-crystallized ligands, coenzymes, water molecules, and protein chains C and D, were removed from the HMGR 3D structure using DSV.
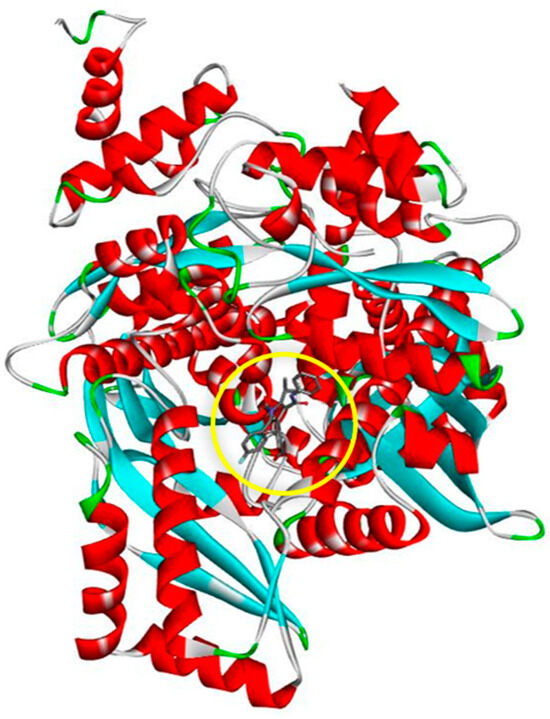
Figure 1.
Three-dimensional crystal structure of HMG-CoA reductase (HMGR) with co-crystallized atorvastatin (encircled in yellow) bound at the active site.
2.2. Preparation of Ligands
Ligands targeting HMGR were selected from a library comprising 20 polyphenolic phytochemicals, identified in studies by Russo et al. and Baron et al. [5,6]. This library specifically consisted of 18 flavonoids (12 flavones and 6 flavanones) and 2 coumarins. The 3D structures of the ligands were downloaded from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/, accessed on 23 January 2024). For the ligands that were not available in the PubChem database, their 2D structures were manually drawn in BIOVIA Draw software (version 21.1) and subsequently converted to 3D using DSV. We performed geometry optimization and energy minimization of the ligands using Avogadro software (version 1.2.0) using the universal force field and the steepest descent algorithm.
2.3. Molecular Docking
Prior to docking, the AutoDockTools (ADT) software (version 1.5.6) was used to further prepare the protein and the 20 ligands. Using ADT, polar hydrogens and Kollman charges were added to the protein structure, while the number of torsions for the ligands was kept at default. Both the protein and ligand structures were then saved in PDBQT file formats. The AutoDock Vina software (version 1.1.2) was used for the molecular docking simulations. In these simulations, the protein was kept rigid, while the ligands were flexible. The docking grid box (size: x = 30, y = 30, z = 30 at 1 Å spacing) was positioned on the coordinates of the co-crystallized atorvastatin (center: x = 2.894171, y = −9.553439, z = −12.241561) and set to cover its binding site, which is surrounded by the amino acid residues Glu 559A, Cys 561A, Ala 564A, Ser 565A, Lys 735A, Asn 755A, Leu 853A, Ala 856A, Arg 590B, Val 683B, Ser 684B, Asp 690B, Lys 691B, and Lys 692B, based on the crystal structure analysis in DSV. AutoDock Vina was run and executed using the Command Prompt program on a Windows computer. AutoDock Vina generated binding orientations for each ligand with corresponding binding affinity values. The binding affinities of all 20 ligands were recorded and compared to that of the reference inhibitor, atorvastatin. The molecular interactions of each protein–ligand complex were then visualized and analyzed using DSV.
3. Results
3.1. Validation of the Docking Protocol
To evaluate the accuracy of AutoDock Vina as an appropriate docking tool for this study, the co-crystallized ligand (atorvastatin) was redocked into the active site of HMG-CoA reductase (HMGR), and the docked orientation was compared to the crystal structure orientation by calculating the root-mean-square deviation (RMSD)—a measure of the average distance between the atoms of superimposed structures. Generally, RMSD values ≤ 2.0 Å indicate that the docking protocol can accurately reproduce the binding orientation of the co-crystallized ligand [11]. In this study, the superimposed structures (see Figure 2) resulted in an RMSD value of 0.85 Å, indicating that our docking results are good and valid and that AutoDock Vina is reliable for docking the 20 phytochemicals into the active site of HMGR.
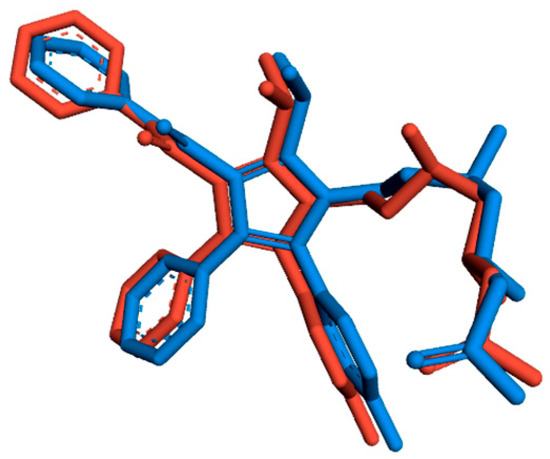
Figure 2.
Superimposed 3D structures of the co-crystallized (blue) and docked (red) orientations of atorvastatin.
3.2. Binding Affinities of the Phytochemicals
Table 1 summarizes the binding affinities of the 20 selected Citrus bergamia phytochemicals after docking into the active site of HMGR. The results reveal that eight phytochemicals, namely naringin, rhoifolin, neohesperidin, brutieridin, neodiosmin, scolymoside, narirutin, and eriocitrin, exhibited stronger (or more negative) binding affinities (−9.5 to −10.0 kcal/mol) compared to the conventional HMGR inhibitor, atorvastatin (−9.2 kcal/mol).

Table 1.
Details and binding affinity values of the 20 studied ligands.
3.3. Protein–Ligand Interactions of the Top Ligands
Table 2 provides a summary of the interactions between the top eight ligands and the amino acid residues within the active site of HMGR. Notably, eriocitrin demonstrated the best binding affinity among these phytochemicals, suggesting a strong and favorable interaction with HMGR.

Table 2.
Molecular interactions of the top 8 ligands with HMGR amino acid residues.
As seen in Figure 3, eriocitrin forms hydrogen bonds with amino acid residues, including Gly 560A, Asn 755A, Arg 590B, Met 655B, Lys 691B, Lys 692B, Asp 690B, and Ala 751A, within the active site of HMGR. It also forms hydrophobic interactions with Met 655B and Gly 806B and electrostatic interactions with Glu 559A. Atorvastatin, shown in Figure 4, forms hydrogen bonds with Ser 565A and Ser 661B via its bulky hydrophobic moiety, while its HMG-like moiety forms hydrogen bonds with Lys 735A, His 752A, Asn 755A, Arg 590B, Lys 692B, and Asp 690B. Additionally, atorvastatin forms hydrophobic interactions with Ala 856A, Leu 853A, Val 683B, Cys 561A, and Ala 564A. Notably, both eriocitrin and atorvastatin share common hydrogen bond interactions with Asn 755A, Arg 590B, Lys 692B, and Asp 690B, possibly indicating a similar binding pattern at the active site of HMGR.
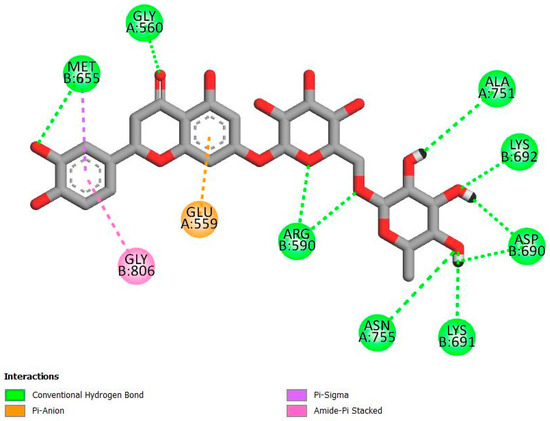
Figure 3.
Two-dimensional interactions of the top-ranked ligand, eriocitrin, with HMGR amino acid residues.
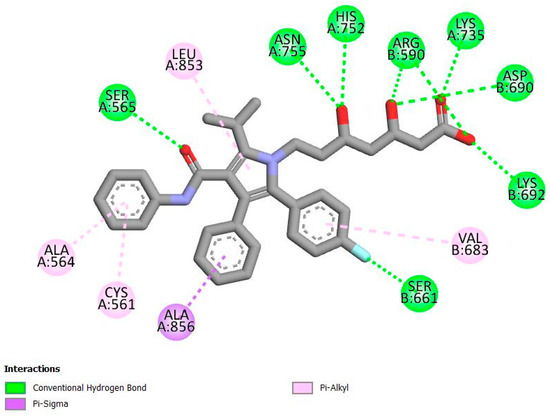
Figure 4.
Two-dimensional interactions of atorvastatin with HMGR amino acid residues.
4. Discussion
The binding affinity measures the strength of interaction between a ligand and a target protein or enzyme [12]. More negative values indicate stronger binding affinities and greater stability [13,14]. In Table 1, the more negative binding affinities of the flavanones eriocitrin, narirutin, brutieridin, and neohesperidin, as well as the flavones scolymoside, neodiosmin, rhoifolin, and naringin, compared to atorvastatin—a known HMG-CoA reductase (HMGR) inhibitor—suggest that these bergamot phytochemicals may have greater potential for HMGR inhibition and could serve as more effective cholesterol-lowering agents than atorvastatin.
The finding that eriocitrin exhibited the most negative binding affinity among the top eight phytochemicals suggests a potentially superior inhibitory effect on HMGR compared to the other seven phytochemicals. Essentially, eriocitrin forms the most stable complex with HMGR among the studied phytochemicals, suggesting its potential as a potent inhibitor of HMGR activity.
The HMG-like moiety of atorvastatin mimics the natural substrate (HMG-CoA) of the enzyme, enabling it to competitively inhibit HMG-CoA reductase (HMGR) by binding to its active site with high affinity. This prevents the enzyme from catalyzing the conversion of HMG-CoA to mevalonate, a crucial step in cholesterol biosynthesis. By inhibiting HMG-CoA reductase, statins reduce low-density lipoprotein cholesterol (LDLc) levels, which in turn decreases the hepatic cholesterol content. This reduction triggers an upregulation of hepatic LDL receptors, enhancing the clearance of LDLc from the bloodstream [15]. As seen in the results, eriocitrin and the HMG-like moiety of atorvastatin share common hydrogen bond interactions with Asn 755A, Arg 590B, Lys 692B, and Asp 690B. This similarity in hydrogen bond interactions between them may suggest a similar competitive inhibition mechanism.
Furthermore, previous studies have reported the beneficial effects of orally administered bergamot juice in reducing total cholesterol (TC), low-density lipoprotein cholesterol (LDLc), and triglycerides (TGs), while increasing high-density lipoprotein cholesterol (HDLc) levels. This effect has been observed both in animal models and in dyslipidemic patients, with reductions of up to 40% in TC, LDLc, and TG [7,8,9,16]. These findings support the potential of bergamot phytochemicals in contributing to cholesterol-lowering effects and underscore their relevance as effective agents for managing cholesterol levels.
While the current molecular docking results highlight the promising inhibitory potential of these phytochemicals, the clinical relevance of these findings must be considered in the context of pharmacokinetics. ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicity) properties play a critical role in determining the drug-like behavior of these compounds. Although detailed ADMET data for the studied phytochemicals are not available in the current study, future research should explore these parameters to assess their bioavailability, metabolism, and potential toxicity. For example, compounds with strong binding affinities may still face limitations due to poor absorption or rapid metabolism, which could reduce their effectiveness as therapeutic agents. Eriocitrin, despite showing the strongest binding affinity, would require an in-depth ADMET analysis to determine if it has favorable pharmacokinetic properties for clinical use, such as sufficient oral bioavailability and minimal toxicity.
Another important factor to consider is the typical amount of these phytochemicals that is consumed in a regular diet compared to the dose of atorvastatin administered therapeutically. Eriocitrin and the other flavonoids are naturally present in citrus fruits, but the concentration of these compounds in a typical diet is likely much lower than the pharmacologically active doses of atorvastatin. This raises questions about whether dietary intake alone would be sufficient to achieve comparable cholesterol-lowering effects, or if higher, more concentrated forms (e.g., supplements) would be necessary. In a previous study, Gliozzi et al. (2013) reported that the bergamot polyphenol fraction effectively reduced total cholesterol, LDL, and urinary mevalonate and was safe when used alone or in combination with rosuvastatin (another type of statin) over a 30-day period [17]. While these results suggest that bergamot can be safely combined with statins, it is essential to consider the potential for food–drug interactions between the individual flavonoids and statins. Consuming these phytochemicals alongside atorvastatin or other statins might either enhance or interfere with the drug’s effects due to competition for the same target enzyme, HMG-CoA reductase.
5. Conclusions
Citrus bergamia, commonly known as bergamot, shows potential as an HMG-CoA reductase inhibitor and an anti-hypercholesterolemic agent based on molecular docking analysis. This could be due to the strong binding affinities of its phytochemical constituents observed in silico. While the molecular docking results provide strong evidence for the cholesterol-lowering potential of eriocitrin and related phytochemicals, future studies should incorporate ADMET analysis to evaluate their pharmacokinetic properties. Additionally, the typical dietary intake of these compounds and the potential for food–drug interactions should be further explored to assess their clinical relevance and applicability as natural cholesterol-lowering agents. Therefore, further in vitro and in vivo studies are needed to confirm these in silico findings and to thoroughly investigate the cholesterol-lowering effects of C. bergamia phytochemicals.
Author Contributions
Conceptualization, investigation, and writing—original draft preparation, N.J.A., P.B.A., J.B., J.C. and B.D.C.; methodology, N.J.A. and B.D.C.; software and validation, B.D.C.; formal analysis, writing—review and editing, visualization, and supervision, N.J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to concerns regarding data privacy.
Acknowledgments
The authors would like to acknowledge colleagues and friends from the Department of Chemistry and the University of Science and Technology of Southern Philippines for their support and encouragement in carrying out this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jalaja, R.; Leela, S.G.; Mohan, S.; Nair, M.S.; Gopalan, R.K.; Somappa, S.B. Anti-hyperlipidemic potential of natural product based labdane-pyrroles via inhibition of cholesterol and triglycerides synthesis. Bioorg. Chem. 2021, 108, 104664. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Baek, A.; Sakkiah, S.; Park, C.; John, S.; Lee, K.W. Exploration of virtual candidates for human HMG-CoA reductase inhibitors using pharmacophore modeling and molecular dynamics simulations. PLoS ONE 2013, 8, e83496. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, S.; Raghunath, A.; Raghunath, S. Statin therapy: Review of safety and potential side effects. Acta Cardiol. Sin. 2016, 32, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tocmo, R.; Nauman, M.C.; Haughan, M.A.; Johnson, J.J. defining the cholesterol lowering mechanism of bergamot (Citrus Bergamia) extract in HepG2 and Caco-2 Cells. Nutrients 2021, 13, 3156. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Arigò, A.; Calabrò, M.L.; Farnetti, S.; Mondello, L.; Dugo, P. Bergamot (Citrus bergamia Risso) as a source of nutraceuticals: Limonoids and flavonoids. J. Funct. Foods 2016, 20, 10–19. [Google Scholar] [CrossRef]
- Baron, G.; Altomare, A.; Mol, M.; Garcia, J.L.; Correa, C.; Raucci, A.; Mancinelli, L.; Mazzotta, S.; Fumagalli, L.; Trunfio, G.; et al. Analytical profile and antioxidant and anti-inflammatory activities of the enriched polyphenol fractions isolated from bergamot fruit and leave. Antioxidants 2021, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Miceli, N.; Mondello, M.R.; Monforte, M.T.; Sdrafkakis, V.; Dugo, P.; Crupi, M.L.; Taviano, M.F.; De Pasquale, R.; Trovato, A. Hypolipidemic effects of Citrus Bergamia Risso et Poiteau juice in rats fed a hypercholesterolemic diet. J. Agric. Food Chem. 2007, 55, 10671–10677. [Google Scholar] [CrossRef] [PubMed]
- Lamiquiz-Moneo, I.; Giné-González, J.; Alisente, S.; Bea, A.M.; Pérez-Calahorra, S.; Marco-Benedí, V.; Baila-Rueda, L.; Jarauta, E.; Cenarro, A.; Civeira, F.; et al. Effect of bergamot on lipid profile in humans: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 60, 3133–3143. [Google Scholar] [CrossRef] [PubMed]
- Mollace, V.; Scicchitano, M.; Paone, S.; Casale, F.; Calandruccio, C.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Nucera, S.; et al. hypoglycemic and hypolipemic effects of a new lecithin formulation of bergamot polyphenolic fraction: A double blind, randomized, placebo-controlled study. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Gesto, D.S.; Pereira, C.M.S.; Cerqueira, N.M.F.S.; Sousa, S.F. An atomic-level perspective of HMG-CoA-reductase: The targe enzyme to treat hypercholesterolemia. Molecules 2020, 25, 3891. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, D.; Caballero, J. Is it reliable to take the molecular docking top scoring position as the best solution without considering available structural data? Molecules 2018, 23, 1038. [Google Scholar] [CrossRef] [PubMed]
- Kastritis, P.L.; Bonvin, A.M.J.J. On the binding affinity of macromolecular interactions: Daring to ask why proteins interact. J. R. Soc. Interface 2013, 10, 20120835. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Liu, X.; Russell, P.; Li, J.; Pan, W.; Fu, J.; Zhang, A. Evaluation of the binding performance of flavonoids to estrogen receptor alpha by Autodock, Autodock Vina and Surflex-Dock. Ecotoxicol. Environ. Saf. 2022, 233, 113323. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, Y.; Xia, Y.; Ai, S.; Liang, J.; Sang, P.; Ji, X.; Liu, S. Insights into protein-ligand interactions: Mechanisms, models, and methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. Cholesterol Lowering Drugs. In Endotext [Internet]; P MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK395573/ (accessed on 27 August 2024).
- Ferrarese, I.; Lupo, M.G.; Rossi, I.; Sut, S.; Loschi, F.; Allegrini, P.; Riva, A.; Ferri, N.; Dall’Acqua, S. Bergamot (Citrus bergamia) peel extract as new hypocholesterolemic agent modulating PCSK9 expression. J. Funct. Foods 2023, 108, 105724. [Google Scholar] [CrossRef]
- Gliozzi, M.; Walker, R.; Muscoli, S.; Vitale, C.; Gratteri, S.; Carresi, C.; Musolino, V.; Russo, V.; Janda, E.; Ragusa, S.; et al. Bergamot polyphenolic fraction enhances rosuvastatin-induced effect on LDL-cholesterol, LOX-1 expression and protein kinase B phosphorylation in patients with hyperlipidemia. Int. J. Cardiol. 2013, 170, 140–145. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).