Identification of New Potential Cyclooxygenase-2 Inhibitors Using Structure-Based Virtual Screening, Molecular Dynamics and Pharmacokinetic Modelling †
Abstract
1. Introduction
2. Materials and Methods
2.1. Library Selection, Pre-Evaluation/Filtration, and Preparation
2.2. Protein Retrieval, Preparation, and Grid Generation
2.3. Structure-Based Virtual Screening
2.4. Binding Free Energy Calculation Using the MMGBSA Method
2.5. In Silico Prediction of Pharmacokinetics, Toxicological, and Drug-like Properties
2.6. Molecular Dynamics (MD) Simulation
3. Results and Discussion
3.1. Molecular Docking and Binding Free Energy Calculations
3.2. ADME/T Profiles of the Best Hits
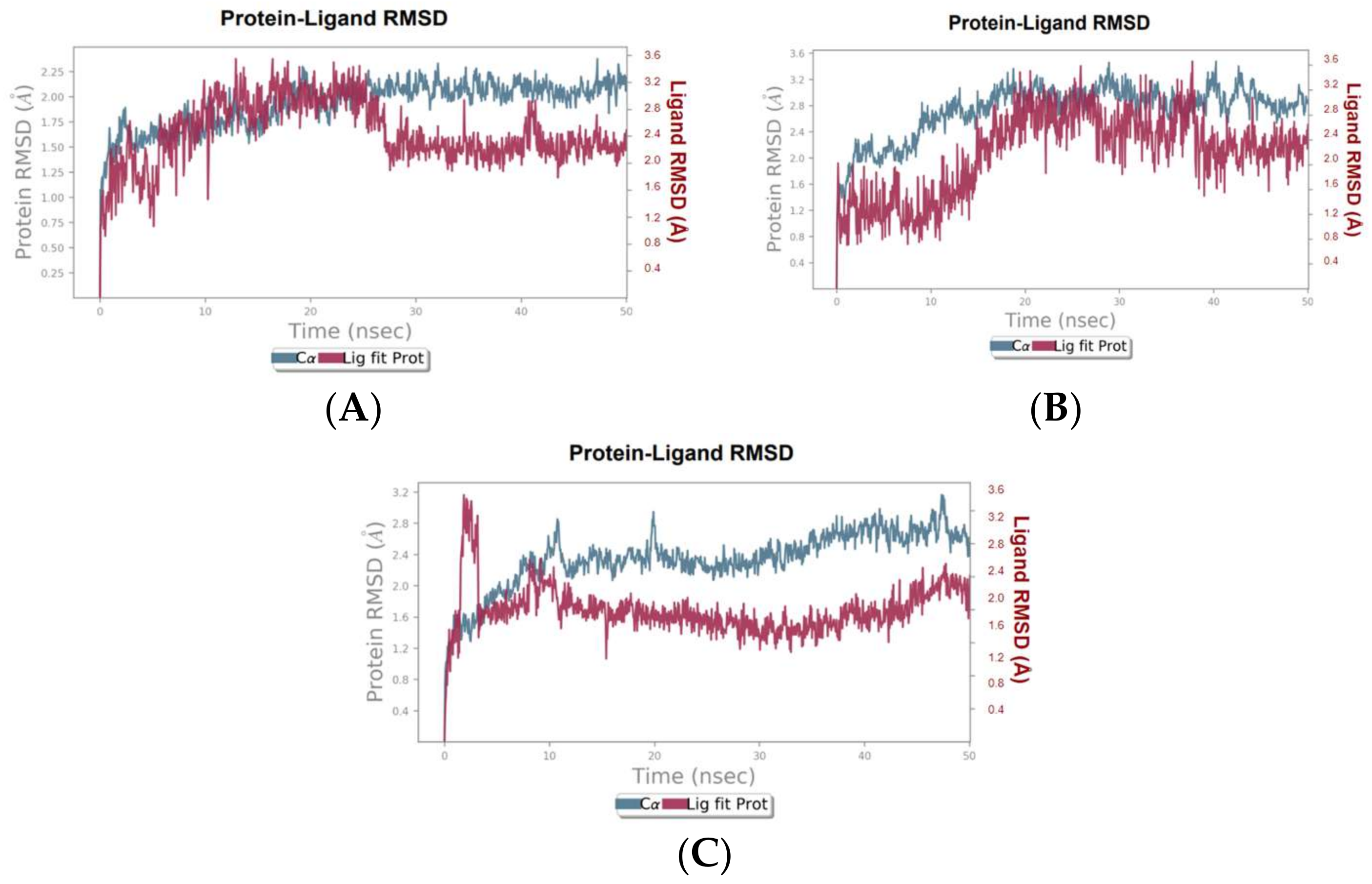
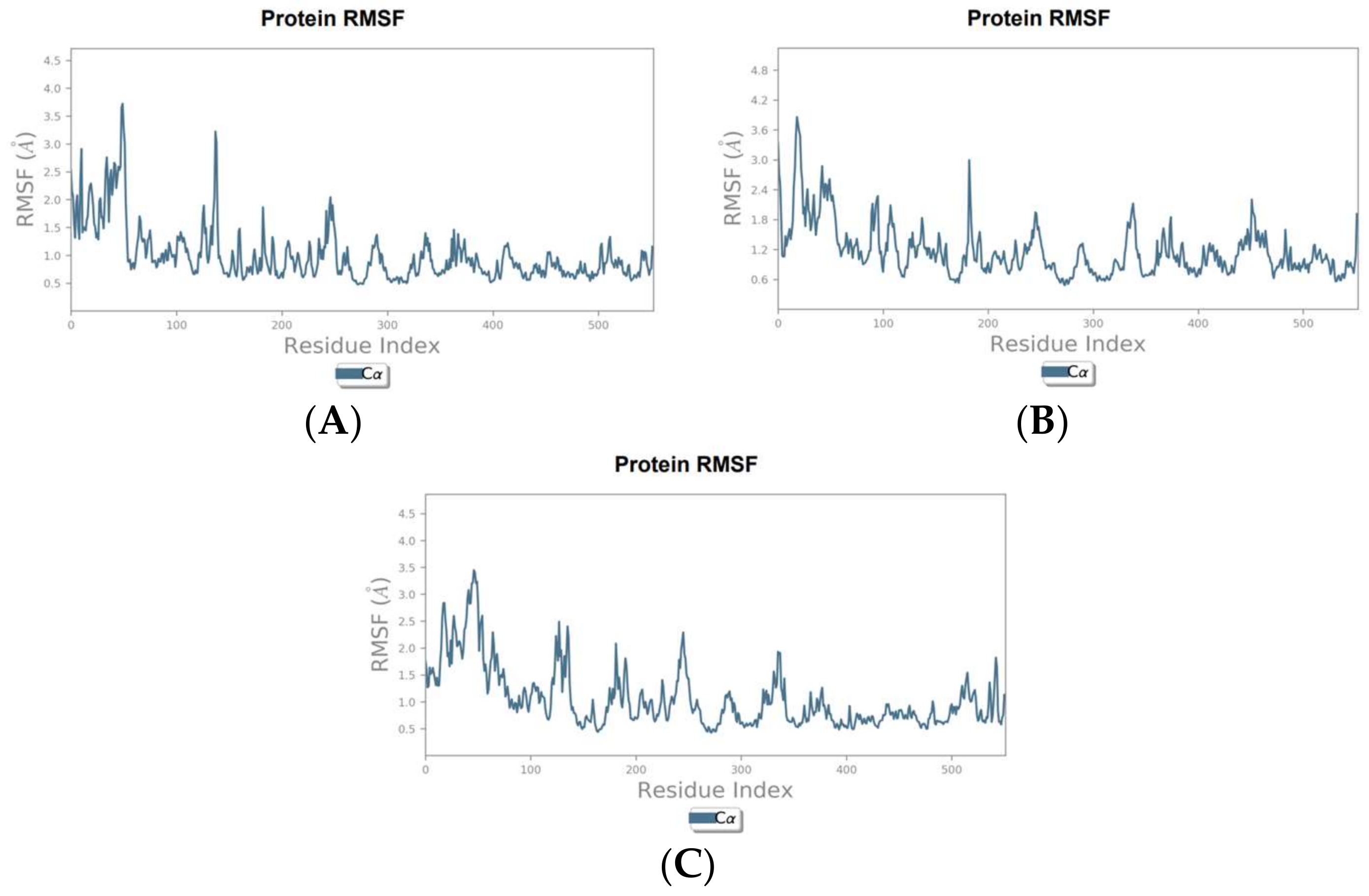
3.3. Molecular Dynamics Simulation Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martín-Sanz, P.; Hortelano, S.; Bosca, L.; Casado, M. Cyclooxygenase 2: Understanding the pathophysiological role through genetically altered mouse models. Front. Biosci. 2006, 11, 2876–2888. [Google Scholar] [CrossRef]
- Kharwar, A.; Mishra, A.; Singh, V.K.; Tiwari, A.K. In silico approach to design new cyclooxygenase-2 (COX-2) inhibitors based on MM/QM and ADMET analysis. Chem. Phys. Impact 2024, 8, 100509. [Google Scholar] [CrossRef]
- Zarghi, A.; Arfaei, S. Selective COX-2 inhibitors: A review of their structure-activity relationships. Iran. J. Pharm. Res. 2011, 10, 655. [Google Scholar] [PubMed]
- Smyth, E.M.; Grosser, T.; Wang, M.; Yu, Y.; FitzGerald, G.A. Prostanoids in health and disease. J. Lipid Res. 2009, 50, S423–S428. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghulikah, H.A.; El-Sebaey, S.A.; Bass, A.K.; El-Zoghbi, M.S. New pyrimidine-5-carbonitriles as COX-2 inhibitors: Design, synthesis, anticancer screening, molecular docking, and in silico ADME profile studies. Molecules 2022, 21, 7485. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.Y.; Abou-Amra, E.S.; El-Sebaey, S.A. Design and synthesis of new series of chiral pyrimidine and purine analogs as COX-2 inhibitors: Anticancer screening, molecular modeling, and in silico studies. J. Mol. Struct. 2023, 1278, 134930. [Google Scholar] [CrossRef]
- Ju, Z.; Li, M.; Xu, J.; Howell, D.C.; Li, Z.; Chen, F.E. Recent development on COX-2 inhibitors as promising anti-inflammatory agents: The past 10 years. Acta Pharm. Sin. B 2022, 6, 2790–2807. [Google Scholar] [CrossRef]
- Al-Turki, D.A.; Al-Omar, M.A.; Abou-Zeid, L.A.; Shehata, I.A.; Al-Awady, M.S. Design, synthesis, molecular modeling and biological evaluation of novel diaryl heterocyclic analogs as potential selective cyclooxygenase-2 (COX-2) inhibitors. Saudi Pharm. J. 2017, 25, 59–69. [Google Scholar] [CrossRef]
- Sharma, J.N.; Jawad, N.M. Adverse effects of COX-2 inhibitors. Sci. World J. 2005, 5, 629–645. [Google Scholar] [CrossRef]
- Rayar, A.M.; Lagarde, N.; Ferroud, C.; Zagury, J.F.; Montes, M.; Veitia, M.S.I. Update on COX-2 Selective Inhibitors: Chemical Classification, Side Effects and their Use in Cancers and Neuronal Diseases. Curr. Top. Med. Chem. 2017, 17, 2935–2956. [Google Scholar] [CrossRef]
- Murugesan, D.K.; Rajagopal, K.; Vijayakumar, A.R.; Sundararajan, G.; Raman, K.; Byran, G.; Emran, T.B. Design and Synthesis of Pyrazole-Substituted 9-Anilinoacridine Derivatives and Evaluation against Breast Cancer. J. Biol. Regul. Homeost. Agents 2024, 4, 2845–2859. [Google Scholar]
- Pan, T.; He, M.; Deng, L.; Li, J.; Fan, Y.; Hao, X.; Mu, S. Design, synthesis, and evaluation of the COX-2 inhibitory activities of new 1, 3-dihydro-2H-indolin-2-one derivatives. Molecules 2023, 12, 4668. [Google Scholar] [CrossRef] [PubMed]
- Rebai, R.; Carmena-Bargueño, M.; Toumi, M.E.; Derardja, I.; Jasmin, L.; Pérez-Sánchez, H.; Boudah, A. Identification of potent inhibitors of kynurenine-3-monooxygenase from natural products: In silico and in vitro approaches. Heliyon 2024, 10, e30287. [Google Scholar] [CrossRef] [PubMed]
- Raman, K.; Kalirajan, R.; Islam, F.; Zehravi, M.; Pratap Singh, L.; Rana, R.; Barua, R. Potential Inhibitors from Natural Compounds against SARS-CoV-2 Main Protease: A Systematic Molecular Modelling Approach. ChemistrySelect 2024, 7, e202303729. [Google Scholar] [CrossRef]
- Kassab, S.E.; Khedr M., A.; Ali H., I.; Abdalla M., M. Discovery of new indomethacin-based analogs with potentially selective cyclooxygenase-2 inhibition and observed diminishing to PGE2 activities. Eur. J. Med. Chem. 2017, 141, 306–321. [Google Scholar] [CrossRef]
- Faki, Y.; Er, A. Different chemical structures and physiological/pathological roles of cyclooxygenases. Rambam Maimonides Med. J. 2021, 12, e0003. [Google Scholar] [CrossRef]
- Dwivedi, A.K.; Gurjar, V.; Kumar, S.; Singh, N. Molecular basis for nonspecificity of nonsteroidal anti-inflammatory drugs (NSAIDs). Drug Discov. Today 2015, 20, 863–873. [Google Scholar] [CrossRef]
- Blobaum, A.L.; Marnett, L.J. Structural and functional basis of cyclooxygenase inhibition. J. Med. Chem. 2007, 50, 1425–1441. [Google Scholar] [CrossRef]
- Dershaby, N.H.; El-Hawash, S.A.; Kassab, S.E.; Daabees, H.G.; Abdel Moneim, A.E.; El-Miligy, M.M. Rational design and synthesis of new selective COX-2 inhibitors with In Vivo PGE2-lowering activity by tethering benzenesulfonamide and 1, 2, 3-triazole pharmacophores to some NSAIDs. Pharmaceuticals 2022, 15, 1165. [Google Scholar] [CrossRef]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Stallings, W.C. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef]
- El-Miligy, M.M.; Al-Kubeisi, A.K.; Bekhit, M.G.; El-Zemity, S.R.; Nassra, R.A.; Hazzaa, A.A. Towards safer anti-inflammatory therapy: Synthesis of new thymol–pyrazole hybrids as dual COX-2/5-LOX inhibitors. J. Enzym. Inhib. Med. Chem. 2023, 38, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Allegrone, G.; Pollastro, F.; Magagnini, G.; Taglialatela-Scafati, O.; Seegers, J.; Koeberle, A.; Appendino, G. The bibenzyl canniprene inhibits the production of pro-inflammatory eicosanoids and selectively accumulates in some Cannabis sativa strains. J. Nat. Prod. 2017, 80, 731–734. [Google Scholar] [CrossRef]
- Shin, J.; Choi, S.; Park, A.Y.; Ju, S.; Kweon, B.; Kim, D.U.; Kim, S. In Vitro and In Vivo Anti-Inflammatory and Antidepressant-like Effects of Cannabis sativa L. Extracts. Plants 2024, 13, 1619. [Google Scholar] [CrossRef]
- Zaiachuk, M.; Suryavanshi, S.V.; Pryimak, N.; Kovalchuk, I.; Kovalchuk, O. The anti-inflammatory effects of Cannabis sativa extracts on LPS-induced cytokines release in human macrophages. Molecules 2023, 28, 4991. [Google Scholar] [CrossRef] [PubMed]
- Frusciante, L.; Geminiani, M.; Olmastroni, T.; Mastroeni, P.; Trezza, A.; Salvini, L.; Santucci, A. Repurposing Castanea sativa Spiny Burr By-Products Extract as a Potentially Effective Anti-Inflammatory Agent for Novel Future Biotechnological Applications. Life 2024, 14, 763. [Google Scholar] [CrossRef] [PubMed]
- Gera, A.; Yadav, L.; Patil, C.R.; Posa, M.K.; Chandrakanth, B.; Kumar, S. Oroxylin A: Nature’s Arsenal Against Liver Fibrosis, Cancer, and Inflammatory Diseases. Health Sci. Rev. 2023, 10, 100143. [Google Scholar] [CrossRef]
- Ji, Y.; Han, J.; Lee, N.; Yoon, J.H.; Youn, K.; Ha, H.J.; Jun, M. Neuroprotective effects of baicalein, wogonin, and oroxylin A on amyloid beta-induced toxicity via NF-κB/MAPK pathway modulation. Molecules 2020, 25, 5087. [Google Scholar] [CrossRef]
- Yao, J.; Hu, R.; Sun, J.; Lin, B.; Zhao, L.; Sha, Y.; Guo, Q.L. Oroxylin a prevents inflammation-related tumor through down-regulation of inflammatory gene expression by inhibiting NF-κB signaling. Mol. Carcinog. 2014, 53, 145–158. [Google Scholar] [CrossRef]
- Aziz, N.; Kim, M.Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a modulator of skin aging and inflammation. Biofactors 2021, 47, 170–180. [Google Scholar] [CrossRef]
- Li, Y.; Frenz, C.M.; Chen, M.; Wang, Y.; Li, F.; Luo, C.; Liang, N.; Yang, H.; Bohlin, L.; Wang, C. Primary virtual and in vitro bioassay screening of natural inhibitors from flavonoids against COX-2. Chin. J. Nat. Med. 2011, 9, 156–160. [Google Scholar]
- Dong, R.; Huang, R.; Shi, X.; Xu, Z.; Mang, J. Exploration of the mechanism of luteolin against ischemic stroke based on network pharmacology, molecular docking and experimental verification. Bioengineered 2021, 12, 12274–12293. [Google Scholar] [CrossRef]
- Shahzadi, A.; Tariq, N.; Sonmez, H.; Waquar, S.; Zahid, A.; Javed, M.A.; Ozturk, M. Potential effect of luteolin, epiafzelechin, and albigenin on rats under cadmium-induced inflammatory insult: In silico and in vivo approach. Front. Chem. 2023, 11, 1036478. [Google Scholar] [CrossRef] [PubMed]

| Compound | PubChem CID | Docking Score (Kcal/mol) | MMGBSA (Kcal/mol) |
|---|---|---|---|
| Celecoxib (reference drug) | 2662 | −12.882 | −79.21 |
| Canniprene | 53439651 | −10.587 | −52.36 |
| Oroxylin A | 5320315 | −10.254 | −32.17 |
| Luteolin | 5280445 | −9.494 | −43.41 |
| Rofecoxib (reference drug) | 5090 | −9.357 | −64.67 |
| Compound | 2D Ligand Interactions | Types of Interactions |
|---|---|---|
| Celecoxib (reference inhibitor) | 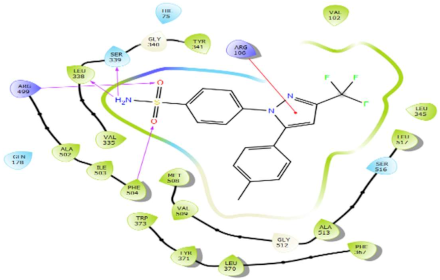 | H-bond: Phe504, Arg499, Leu338, Ser339 Charged (+): Arg499, Arg106. Polar: Ser516, Ser339, Gln178, His75 Hydrophobic: Val335, Leu338, Tyr341, Met508, Val509, Ala513, Leu517, Leu345, Ala502, Ile503, Phe504, Trp373, Leu370, Phe367, Val102 Pi-cation: Arg106 |
| Canniprene | 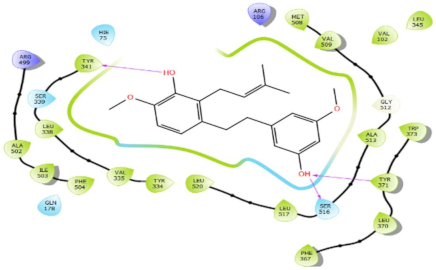 | H-bond: Tyr341, Ser516, Tyr371 Charged (+): Arg499, Arg106 Polar: His75, Ser339, Gln178, Ser516 Hydrophobic: Leu345, Val102, Met508, Val509, Ala513, Leu517, Leu520, Tyr334, Val335, Leu338, Tyr341, Trp373, Tyr371, Leu370, Phe367, Phe504, Ile503, Ala502 |
| Oroxylin A | 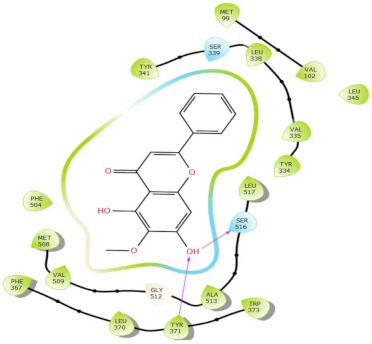 | H-bond: Tyr371, Ser516 Polar: Ser339, Ser516 Hydrophobic: Met99, Val102, Tyr341, Leu338, Val335, Tyr334, Leu345, Leu517, Ala513, Val509, Met508, Phe504, Phe367, Leu370, Tyr371, Trp373 |
| Luteolin |  | H-bond: Leu338, Ser516 Charged (+): Arg499, Arg106 Polar: His75, Gln178, Ser339, Ser516 Hydrophobic: Tyr371, Trp373, Val335, Leu338, Tyr341, Phe504, Ile503, Ala502, Val509, Ala513, Leu517 |
| Rofecoxib (reference inhibitor) |  | H-bond: Phe504, Arg499 Charged (+): Arg106, Arg499 Polar: His75, Gln178, Thr79, Ser516, Ser339 Hydrophobic: Ala502, Ile503, Phe504, Met508, Val509, Ala513, Leu517, Tyr334, Val335, Leu338, Tyr341, Leu345, Phe367, Leu370, Tyr371, Trp373 |
| Compound | MW | AccptHB | DonorHB | TPSA | Log Po/w | Water Solubility | Lipinski Rule | Veber Rule | Bioavailability Score |
|---|---|---|---|---|---|---|---|---|---|
| Canniprene | 342.43 | 4 | 2 | 58.92 | 4.32 | Moderate | Yes | Yes | 0.55 |
| Oroxylin A | 284.26 | 5 | 2 | 79.90 | 2.56 | Moderate | Yes | Yes | 0.55 |
| Luteolin | 286.24 | 6 | 4 | 111.13 | 1.73 | Soluble | Yes | Yes | 0.55 |
| Compound | GI Absorption | BBB Permeability | P-gp Substrate | Caco-2 Permeability | CNS Activity | CYP1A2 Inhibitor | CYP2C19 Inhibitor | CYP2C9 Inhibitor |
|---|---|---|---|---|---|---|---|---|
| Canniprene | High | Yes | No | 1362 | –2.198 | Yes | Yes | No |
| Oroxylin A | High | No | No | 633 | –2.21 | Yes | No | Yes |
| Luteolin | High | No | No | 45 | –2.251 | Yes | No | No |
| Hit N° | Hepatotoxicity | Carcinogenicity | Immunotoxicity | Mutagenicity | Cytotoxicity | Predicted LD50 | Class | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pr. | Pb. | Pr. | Pb. | Pr. | Pb. | Pr. | Pb. | Pr. | Pb. | |||
| 1 | Inact. | 0.74 | Inact. | 0.63 | Act. | 0.89 | Inact. | 0.76 | Inact. | 0.88 | 1930 | IV |
| 2 | Inact. | 0.72 | Inact. | 0.68 | Inact. | 0.81 | Inact. | 0.94 | Inact. | 0.95 | 4000 | V |
| 3 | Inact. | 0.69 | Act. | 0.68 | Inact. | 0.97 | Act. | 0.51 | Inact. | 0.99 | 3919 | V |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derardja, I.; Rebai, R.; Toumi, M.E.; Kebaili, F.F.; Boudah, A. Identification of New Potential Cyclooxygenase-2 Inhibitors Using Structure-Based Virtual Screening, Molecular Dynamics and Pharmacokinetic Modelling. Biol. Life Sci. Forum 2024, 35, 6. https://doi.org/10.3390/blsf2024035006
Derardja I, Rebai R, Toumi ME, Kebaili FF, Boudah A. Identification of New Potential Cyclooxygenase-2 Inhibitors Using Structure-Based Virtual Screening, Molecular Dynamics and Pharmacokinetic Modelling. Biology and Life Sciences Forum. 2024; 35(1):6. https://doi.org/10.3390/blsf2024035006
Chicago/Turabian StyleDerardja, Imene, Redouane Rebai, Mohamed Esseddik Toumi, Farouk Fethi Kebaili, and Abdennacer Boudah. 2024. "Identification of New Potential Cyclooxygenase-2 Inhibitors Using Structure-Based Virtual Screening, Molecular Dynamics and Pharmacokinetic Modelling" Biology and Life Sciences Forum 35, no. 1: 6. https://doi.org/10.3390/blsf2024035006
APA StyleDerardja, I., Rebai, R., Toumi, M. E., Kebaili, F. F., & Boudah, A. (2024). Identification of New Potential Cyclooxygenase-2 Inhibitors Using Structure-Based Virtual Screening, Molecular Dynamics and Pharmacokinetic Modelling. Biology and Life Sciences Forum, 35(1), 6. https://doi.org/10.3390/blsf2024035006






