Abstract
Leveraging advancements in metabolomics and other cutting-edge technologies, precision neuronutrition aims to identify personalized nutrient requirements to optimize brain health outcomes and prevent neurological disorders. The main pathological mechanisms of brain health disruption include neuroinflammation, oxidative stress, gut–brain disturbances and nutrient deficiencies. Recent studies have identified biological markers for all those mechanisms. Precision interventions for maintaining brain health and optimizing outcomes include omega-3 fatty acids, vitamin B12, vitamin D, magnesium, coenzyme q10, polyphenols, l-carnitine, prebiotics and probiotics. Precision neuronutrition offers a promising approach to optimizing brain health through personalized nutrient interventions. Continued research in this field holds great potential for improving brain health outcomes.
1. Introduction
According to WHO experts, brain health is the state of proper cognitive, sensory, socio-emotional, behavioral and motor functioning, allowing a person to reach their full potential throughout time, regardless of presence or absence of disorders [1]. In the context of brain health optimization, a number of researchers [2,3] call for attention to the developing field of neuroscience–nutritional cognitive neuroscience. This scientific branch aims to investigate how nutrition impacts the brain’s development, overall well-being, and the aging process [4,5]. Recently, the term neuronutrition has been used actively [6,7]. Neuronutrition is an interdisciplinary field studying the influence of various aspects of nutrition on brain health, neurological disease prevention and treatment throughout life. The brain requires specific nutrients to maintain its structural integrity, support cognitive processes, and protect against neurodegenerative diseases [8]. Assessing brain health objectively is a crucial challenge in neuroscience, particularly in detecting and diagnosing early neurocognitive changes, including those caused by nutritional deficiencies [9]. Precision neuronutrition aims to identify personalized nutrient requirements to optimize brain health outcomes and prevent neurological disorders [10,11].
2. Brain Health Status
Biomarkers indicative of brain health status reflect neuroinflammation, oxidative stress, gut–brain disturbances and nutrient deficiencies.
2.1. Neuroinflammation
The main biomarkers of neuroinflammation are C-reactive protein, interleukin-6 and tumor necrosis factor-alpha. Levels of these biomarkers are altered in a number of neurological diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), epilepsy and others [12,13,14]. However, for a more accurate assessment of neuroinflammation, taking into account other molecular markers, such as chemokines, microglial cytokines [15,16] and angiogenesis factors [17], is necessary. However, assessing the degree of neuroinflammation poses a number of difficulties, due to the complex biology of the process, and the need for new strategies to collect and analyze relevant data [18,19].
2.2. Oxidative Stress
Oxidative stress has been proven to play a crucial part in pathogenesis of many neurodegenerative diseases, including AD, PD, amyotrophic lateral sclerosis and Huntington’s disease [20,21,22]. The brain is particularly vulnerable to oxidative damage, and the excessive formation of reactive oxygen species (ROS) can lead to neuronal cell death [23]. Antioxidant enzymes catalase and glutathione peroxidase-1 have been quantified in plasma as indicators of oxidative stress [24]. F2-isoprostanes are stereoisomers of prostaglandin F2 and are considered the most reliable markers for monitoring oxidative stress [25,26]. 8-isoprostane is considered a marker of oxidative stress. It can be measured in various biological fluids, including urine, plasma [27], saliva [28] and exhaled air condensate [29]. Advanced oxidation protein products are the end products of the reaction between plasma albumin and chlorinated oxidants [30], and can be measured in blood plasma [24]. Protein carbonylation is an oxidative transformation induced by ROS, reactive nitrogen species, reactive halogen species and reactive aldehydes [31]. They are considered markers of oxidative stress [25] that can be measured in blood plasma. 8-hydroxy-2′-deoxyguanosine, 8-oxo-7,8-dihydroguanosine and malondialdehyde are also indicative of oxidative stress [32,33,34]. Many biomarkers associated with oxidative stress can be measured in biological samples using standard assays [35,36]. The ability to accurately detect free radical formation in cells and tissues is critical for the development of appropriate therapeutic antioxidant approaches to brain health [37].
2.3. Gut–Brain Disturbances
Gut microbiota and its metabolites have been shown to play a role in pathogenesis and progression of a number of neurological diseases through gut-brain axis regulation [38]. Short-chain fatty acids are metabolites that may affect brain function and are associated with some neurological disorders [38]. Indoles are involved in various neurological functions and are associated with several neurological disorders [39]. Secondary bile acids can serve as activators of bile acid receptors in the brain, and their affinity for individual receptors varies [40]. The gut-brain axis is a potential target for the development of new treatments for neurological disorders, and the role of secondary bile acids in this axis is an area of active research [41]. Serotonin, dopamine, 5-aminovaleric acid and taurine are neurotransmitters produced by intestinal bacteria that regulate neurotransmission in the brain as well as gut itself [42]. Liposaccharide binding protein, zonulin and claudin-3 are biomarkers reflecting damage to the epithelial blood-gut barrier [43,44,45].
2.4. Nutrient Deficiencies
Nutrient deficiencies can lead to the manifestation of neuroinflammation, oxidative stress and a wide range of neurological problems, including encephalopathy, cognitive impairment and psychiatric disorders [42,46,47]. Deficiency is most often caused by poor nutrition, including not eating enough calories, a lack of certain foods such as fruits and vegetables in diets and eating disorders, and alters vitamin and mineral absorption [48,49]. Vitamin B12 deficiency is associated with cognitive impairment, polyneuropathy and psychiatric manifestations [46]. Thiamine deficiency can cause Wernicke–Korsakoff syndrome [50]. Vitamin D deficiency can lead to neurological manifestations such as depression, cognitive impairment and multiple sclerosis [51]. Magnesium deficiency has been associated with many neurological disorders such as AD, stroke, migraine, depression and cerebellar syndrome [52,53,54]. Coenzyme Q10 may have neuroprotective effects in neurological diseases, including AD, PD, Huntington’s disease, amyotrophic lateral sclerosis and stroke [55,56]. Neurological manifestations of carnitine deficiency include hypotension, burning pain, decreased endurance, sensory impairment, developmental delay, rigidity and myopathy [57,58]. These biomarkers could provide real-time feedback on the effectiveness of nutrient interventions.
3. Precision Nutrient Interventions
There are other crucial substances that play a significant role in brain health [59]. These substances can specifically target neuroinflammation, oxidative stress, and gut–brain disturbances. Recent research has shown that dietary polyphenols may have beneficial effects on neurological diseases by attenuating oxidative stress and reducing the risk of developing neurodegenerative diseases such as AD, stroke, multiple sclerosis, PD and Huntington’s disease [60]. Polyphenols have great potential to address brain aging by simultaneously modulating the gut–brain axis [60]. Probiotics can have beneficial effects on patients with neurological diseases by reducing oxidative stress and reducing the risk of developing neurodegenerative diseases such as AD, stroke, multiple sclerosis, PD, etc. [61]. Non-digestible oligosaccharides have neuroprotection effects by modulating the gut–brain axis [62]. Consuming omega-3 fatty acids has been shown to improve learning, memory, cognitive well-being and blood flow in the brain [63]. Omega-3 supplementation may also target neuroinflammation [64], oxidative stress [65] and gut-brain disturbances [66]. A deficiency in omega-3 fatty acids increases the risk of neurodegenerative disorders [67] and accelerates brain aging [68]. Overall, omega-3 fatty acids are essential for maintaining optimal brain health (Figure 1).
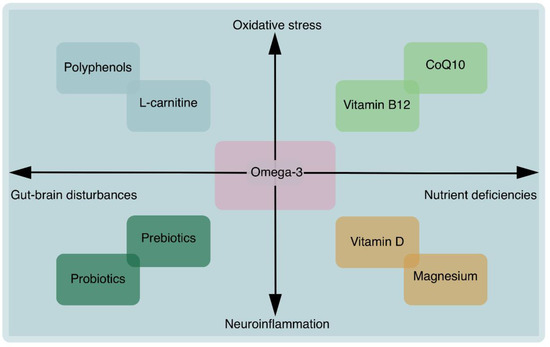
Figure 1.
Overview of precision nutritional interventions for brain health.
4. Conclusions
The field of precision neuronutrition holds great promise in optimizing brain health through targeted nutrient interventions based on an individual’s brain health status. In order to accurately assess brain health, a personalized approach is necessary, taking into account an individual’s nutrient, biochemical and metabolic characteristics. By adapting scientific findings to each person’s unique profile, brain health outcomes can effectively be optimized. Continued research in this area has the potential to revolutionize approaches to nutrition for the brain and to improve overall brain health.
Author Contributions
Conceptualization, A.B.D. and A.V.B.; methodology, Y.D.V.; software, V.N.N.; validation, A.B.D., Y.D.V. and V.N.N.; formal analysis, V.N.N.; investigation, V.N.N.; resources, V.N.N.; data curation, A.V.B.; writing—original draft preparation, V.N.N.; writing—review and editing, Y.D.V.; visualization, V.N.N.; supervision, A.V.B.; project administration, A.B.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Optimizing Brain Health across the Life Course: WHO Position Paper; World Health Organization: Geneva, Switzerland, 2022; Available online: https://apps.who.int/iris/handle/10665/361251 (accessed on 12 September 2023).
- Dominguez, L.J.; Veronese, N.; Vernuccio, L.; Catanese, G.; Inzerillo, F.; Salemi, G.; Barbagallo, M. Nutrition, Physical Activity, and Other Lifestyle Factors in the Prevention of Cognitive Decline and Dementia. Nutrients 2021, 13, 4080. [Google Scholar] [CrossRef] [PubMed]
- Poulose, S.M.; Miller, M.G.; Scott, T.; Shukitt-Hale, B. Nutritional Factors Affecting Adult Neurogenesis and Cognitive Function. Adv. Nutr. Int. Rev. J. 2017, 8, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Zamroziewicz, M.K.; Barbey, A.K. Nutritional Cognitive Neuroscience: Innovations for Healthy Brain Aging. Front. Neurosci. 2016, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Cusick, S.E.; Georgieff, M.K. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days”. J. Pediatr. 2016, 175, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Jiraungkoorskul, W. Review of Neuro-nutrition Used as Anti-Alzheimer Plant, Spinach, Spinacia oleracea. Pharmacogn Rev. 2016, 10, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, B.N.; Rao, T.S.; Prakasam, A.; Sambamurti, K.; Rao, K.J. Neuronutrition and Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 19, 1123–1139. [Google Scholar] [CrossRef] [PubMed]
- Badaeva, A.V.; Danilov, A.B.; Clayton, P.; Moskalev, A.A.; Karasev, A.V.; Tarasevich, A.F.; Vorobyeva, Y.D.; Novikov, V.N. Perspectives on Neuronutrition in Prevention and Treatment of Neurological Disorders. Nutrients 2023, 15, 2505. [Google Scholar] [CrossRef]
- Possin, K.L.; Moskowitz, T.; Erlhoff, S.J.; Rogers, K.M.; Johnson, E.T.; Steele, N.Z.R.; Higgins, J.J.; Stiver, J.; Alioto, A.G.; Farias, S.T.; et al. The Brain Health Assessment for Detecting and Diagnosing Neurocognitive Disorders. J. Am. Geriatr. Soc. 2018, 66, 150–156. [Google Scholar] [CrossRef]
- Brennan, L.; de Roos, B. Role of metabolomics in the delivery of precision nutrition. Redox Biol. 2023, 65, 102808. [Google Scholar] [CrossRef]
- Tebani, A.; Bekri, S. Paving the Way to Precision Nutrition Through Metabolomics. Front. Nutr. 2019, 6, 41. [Google Scholar] [CrossRef]
- Gorji, A. Neuroinflammation: The Pathogenic Mechanism of Neurological Disorders. Int. J. Mol. Sci. 2022, 23, 5744. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E.; Deuschl, G. Neuroinflammation—A common thread in neurological disorders. Nat. Rev. Neurol. 2019, 15, 429–430. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Barclay, W.; Shinohara, M.L. Inflammasome activation in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE). Brain Pathol. 2017, 27, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Mattsson, N.; Stomrud, E.; Lindberg, O.; Palmqvist, S.; Zetterberg, H.; Blennow, K.; Hansson, O. CSF biomarkers of neuroinflammation and cerebrovascular dysfunction in early Alzheimer disease. Neurology 2018, 91, e867–e877. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Forum on Neuroscience and Nervous System Disorders. Biomarkers of Neuroinflammation: Proceedings of a Workshop; The National Academies Press: Washington, DC, USA, 2017. [Google Scholar] [CrossRef]
- Cao, M.C.; Cawston, E.E.; Chen, G.; Brooks, C.; Douwes, J.; McLean, D.; Graham, E.S.; Dragunow, M.; Scotter, E.L. Serum biomarkers of neuroinflammation and blood-brain barrier leakage in amyotrophic lateral sclerosis. BMC Neurol. 2022, 22, 216. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Díaz-Hung, M.; Fraguela, M.G. Oxidative stress in neurological diseases: Cause or effect? Neurologia 2014, 29, 451–452. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxidative Med. Cell. Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef]
- Ho, E.; Karimi Galougahi, K.; Liu, C.-C.; Bhindi, R.; Figtree, G.A. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013, 1, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Katerji, M.; Filippova, M.; Duerksen-Hughes, P. Approaches and Methods to Measure Oxidative Stress in Clinical Samples: Research Applications in the Cancer Field. Oxidative Med. Cell. Longev. 2019, 2019, 1279250. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sun, D.; Xiu, G.; Lazarus, P.; Vachani, A.; Penning, T.M.; Whitehead, A.S.; Muscat, J.E. Quantification of Plasma 8-Isoprostane by High-Performance Liquid Chromatography with Tandem Mass Spectrometry in a Case-Control Study of Lung Cancer. Int. J. Environ. Res. Public Health 2022, 19, 12488. [Google Scholar] [CrossRef] [PubMed]
- Meera, S.; Sarangarajan, R.; Rajkumar, K. 8-Isoprostane: A salivary oxidative stress biomarker for oral submucous fibrosis and oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2020, 24, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Shoman, Y.; Wild, P.; Hemmendinger, M.; Graille, M.; Sauvain, J.-J.; Hopf, N.B.; Canu, I.G. Reference Ranges of 8-Isoprostane Concentrations in Exhaled Breath Condensate (EBC): A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 3822. [Google Scholar] [CrossRef]
- Bagyura, Z.; Takács, A.; Kiss, L.; Dósa, E.; Vadas, R.; Nguyen, T.D.; Dinya, E.; Soós, P.; Szelid, Z.; Láng, O.; et al. Level of advanced oxidation protein products is associated with subclinical atherosclerosis. BMC Cardiovasc. Disord. 2022, 22, 5. [Google Scholar] [CrossRef]
- Gonos, E.S.; Kapetanou, M.; Sereikaite, J.; Bartosz, G.; Naparło, K.; Grzesik, M.; Sadowska-Bartosz, I. Origin and pathophysiology of protein carbonylation, nitration and chlorination in age-related brain diseases and aging. Aging 2018, 10, 868–901. [Google Scholar] [CrossRef]
- Di Minno, A.; Aveta, A.; Gelzo, M.; Tripodi, L.; Pandolfo, S.D.; Crocetto, F.; Imbimbo, C.; Castaldo, G. 8-Hydroxy-2-Deoxyguanosine and 8-Iso-Prostaglandin F2α: Putative Biomarkers to assess Oxidative Stress Damage Following Robot-Assisted Radical Prostatectomy (RARP). J. Clin. Med. 2022, 11, 6102. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R. Protein oxidation and aging. Free. Radic. Res. 2006, 40, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Katrenčíková, B.; Vaváková, M.; Paduchová, Z.; Nagyová, Z.; Garaiova, I.; Muchová, J.; Ďuračková, Z.; Trebatická, J. Oxidative Stress Markers and Antioxidant Enzymes in Children and Adolescents with Depressive Disorder and Impact of Omega-3 Fatty Acids in Randomised Clinical Trial. Antioxidants 2021, 10, 1256. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, Bryan; et al. “Measurement of Oxidative Stress Markers In Vitro Using Commercially Available Kits. In Measuring Oxidants and Oxidative Stress in Biological Systems; Berliner, L., Parinandi, N., Eds.; Springer: Cham, Switzerland, 2020; pp. 39–60. [Google Scholar] [CrossRef]
- Swer, N.M.; Venkidesh, B.S.; Murali, T.S.; Mumbrekar, K.D. Gut microbiota-derived metabolites and their importance in neurological disorders. Mol. Biol. Rep. 2023, 50, 1663–1675. [Google Scholar] [CrossRef] [PubMed]
- Zengil, S.; Laloğlu, E. Evaluation of Serum Zonulin and Occludin Levels in Bipolar Disorder. Psychiatry Investig. 2023, 20, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.M.; DeMorrow, S. Bile Acid Signaling in Neurodegenerative and Neurological Disorders. Int. J. Mol. Sci. 2020, 21, 5982. [Google Scholar] [CrossRef] [PubMed]
- Hurley, M.J.; Bates, R.; Macnaughtan, J.; Schapira, A.H. Bile acids and neurological disease. Pharmacol. Ther. 2022, 240, 108311. [Google Scholar] [CrossRef]
- Park, J.; Kim, C.H. Regulation of Common Neurological Disorders by Gut Microbial Metabolites. Exp. Mol. Med. 2021, 53, 1821–1833. [Google Scholar] [CrossRef]
- Varanoske, A.N.; McClung, H.L.; Sepowitz, J.J.; Halagarda, C.J.; Farina, E.K.; Berryman, C.E.; Lieberman, H.R.; McClung, J.P.; Pasiakos, S.M.; Karl, J.P. Stress and the gut-brain axis: Cognitive performance, mood state, and biomarkers of blood-brain barrier and intestinal permeability following severe physical and psychological stress. Brain Behav. Immun. 2022, 101, 383–393. [Google Scholar] [CrossRef]
- Marizzoni, M.; Mirabelli, P.; Mombelli, E.; Coppola, L.; Festari, C.; Lopizzo, N.; Luongo, D.; Mazzelli, M.; Naviglio, D.; Blouin, J.-L.; et al. A peripheral signature of Alzheimer’s disease featuring microbiota-gut-brain axis markers. Alzheimer’s Res. Ther. 2023, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef] [PubMed]
- Hammond, N.; Wang, Y.; Dimachkie, M.M.; Barohn, R.J. Nutritional Neuropathies. Neurol. Clin. 2013, 31, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.A.; Balcer, L.J.; Galetta, S.L. The Neurological Complications of Nutritional Deficiency following Bariatric Surgery. J. Obes. 2012, 2012, 608534. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.K.; Dhuli, K.; Donato, K.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Connelly, S.T.; Bellinato, F.; Gisondi, P.; et al. Main nutritional deficiencies. J. Prev. Med. Hyg. 2022, 63 (Suppl. 3), E93–E101. [Google Scholar] [CrossRef] [PubMed]
- Sankararaman, S.; Hendrix, S.J.; Schindler, T. Update on the management of vitamins and minerals in cystic fibrosis. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2022, 37, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Hrubša, M.; Siatka, T.; Nejmanová, I.; Vopršalová, M.; Krčmová, L.K.; Matoušová, K.; Javorská, L.; Macáková, K.; Mercolini, L.; Remião, F.; et al. Biological Properties of Vitamins of the B-Complex, Part 1: Vitamins B1, B2, B3, and B5. Nutrients 2022, 14, 484. [Google Scholar] [CrossRef]
- Plantone, D.; Primiano, G.; Manco, C.; Locci, S.; Servidei, S.; De Stefano, N. Vitamin D in Neurological Diseases. Int. J. Mol. Sci. 2022, 24, 87. [Google Scholar] [CrossRef]
- Xue, W.; You, J.; Su, Y.; Wang, Q. The Effect of Magnesium Deficiency on Neurological Disorders: A Narrative Review Article. Iran. J. Public Health 2019, 48, 379–387. [Google Scholar] [CrossRef]
- Kirkland, A.E.; Sarlo, G.L.; Holton, K.F. The Role of Magnesium in Neurological Disorders. Nutrients 2018, 10, 730. [Google Scholar] [CrossRef]
- Alateeq, K.; Walsh, E.I.; Cherbuin, N. Dietary magnesium intake is related to larger brain volumes and lower white matter lesions with notable sex differences. Eur. J. Nutr. 2023, 62, 2039–2051. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Haddadi, R.; Saki, S.; Kourosh-Arami, M.; Rashno, M.; Mojaver, A.; Komaki, A. Neuroprotective effects of coenzyme Q10 on neurological diseases: A review article. Front. Neurosci. 2023, 17, 1188839. [Google Scholar] [CrossRef] [PubMed]
- Aaseth, J.; Alexander, J.; Alehagen, U. Coenzyme Q10 supplementation—In ageing and disease. Mech. Ageing Dev. 2021, 197, 111521. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.L.; Simmons, P.A.; Vehige, J.; Willcox, M.D.; Garrett, Q. Role of carnitine in disease. Nutr. Metab. 2010, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Crefcoeur, L.L.; Visser, G.; Ferdinandusse, S.; Wijburg, F.A.; Langeveld, M.; Sjouke, B. Clinical characteristics of primary carnitine deficiency: A structured review using a case-by-case approach. J. Inherit. Metab. Dis. 2022, 45, 386–405. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.R.; Kirby, T.O.; Marshall, T.M.; Church, D.D.; Jajtner, A.R.; Esposito, R. Foundational Nutrition: Implications for Human Health. Nutrients 2023, 15, 2837. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D. Dietary polyphenols as modulators of brain functions: Biological actions and molecular mechanisms underpinning their beneficial effects. Oxidative Med. Cell. Longev. 2012, 2012, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.F.; Ahmad, S.R.; Lim, Y.C.; Zulkipli, I.N. The Use of Probiotic Therapy in Metabolic and Neurological Diseases. Front. Nutr. 2022, 9, 887019. [Google Scholar] [CrossRef]
- Divyashri, G.; Sadanandan, B.; Chidambara Murthy, K.N.; Shetty, K.; Mamta, K. Neuroprotective Potential of Non-Digestible Oligosaccharides: An Overview of Experimental Evidence. Front. Pharmacol. 2021, 12, 712531. [Google Scholar] [CrossRef]
- Dighriri, I.M.; Alsubaie, A.M.; Hakami, F.M.; Hamithi, D.M.; Alshekh, M.M.; A Khobrani, F.; E Dalak, F.; A Hakami, A.; Alsueaadi, E.H.; Alsaawi, L.S.; et al. Effects of Omega-3 Polyunsaturated Fatty Acids on Brain Functions: A Systematic Review. Cureus 2022, 14, e30091. [Google Scholar] [CrossRef]
- Mora, I.; Arola, L.; Caimari, A.; Escoté, X.; Puiggròs, F. Structured Long-Chain Omega-3 Fatty Acids for Improvement of Cognitive Function during Aging. Int. J. Mol. Sci. 2022, 23, 3472. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejhad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
- Kousparou, C.; Fyrilla, M.; Stephanou, A.; Patrikios, I. DHA/EPA (Omega-3) and LA/GLA (Omega-6) as Bioactive Molecules in Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 10717. [Google Scholar] [CrossRef]
- Chappus-McCendie, H.; Chevalier, L.; Roberge, C.; Plourde, M. Omega-3 PUFA metabolism and brain modifications during aging. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 94, 109662. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).