Abstract
Cow milk oligosaccharides (CMOs) are complex carbohydrates found in cow milk that resemble the oligosaccharides in human milk and are essential for regulating the immune system and forming the gut flora of infants. As prebiotics, they promote the growth of specific beneficial gut bacteria, such as Lactobacilli and Bifidobacteria, thus promoting the creation of short-chain fatty acids for gut health. Furthermore, CMOs correlate with enhanced infant immune system development, offering safeguards against pathogens and anti-inflammatory benefits. The results of recent CMO research are revealed in this review, together with their biological importance and potential applications. Their relevance to infant nutrition is highlighted, as is their potential to be used as bioactive ingredients in novel functional foods and nutraceuticals. This study also describes upcoming obstacles and opportunities for CMO research, such as understanding their structures and functions, improving extraction methods, and expanding applications to different age groups.
1. Introduction
Oligosaccharides are crucial biological molecules found in various sources, including glycoproteins, bacteria, fungi, plants, and milk [1,2,3,4,5]. This review focuses on cow milk oligosaccharides (CMOs), complex carbohydrates present in cow milk that bear a striking structural resemblance to human milk oligosaccharides (HMOs) [6]. One prominent characteristic of cow milk oligosaccharides is their abundant incorporation of Neu5Ac (N-acetylneuraminic acid) [7]. These compounds play a pivotal role in shaping the composition of the infant gut microbiota [8] and modulating the immune system.
CMOs function as prebiotics [9,10,11], exhibiting a unique ability to selectively nurture the growth of beneficial gut bacteria, such as Bifidobacteria and Lactobacilli [12]. This fosters the production of short-chain fatty acids, which contribute to overall gut health. Furthermore, CMOs have been linked to the enhanced development and function of the infant immune system. They provide defenses against pathogens and exhibit anti-inflammatory properties. Recent CMO structure elucidation also provided deep insights [13].
2. Cow Milk Oligosaccharides in Ancient Literature and Ayurveda
In ancient literature and Ayurveda, cow’s milk was valued for its ability to support the growth of newborns’ immune, neurological, and skeletal systems, making it a respected alternative to mother’s milk [14]. Recent scientific research has revealed that cow milk oligosaccharides play a crucial role in brain development, immunomodulation, human growth stimulation, anti-inflammatory effects, antioxidant properties, and enhancing lactation in women [15,16]. Despite historical limitations, cow milk remains potent due to its complex structured oligosaccharides, which are central to numerous vital biological processes for human development.
3. Classification of Cow Milk Oligosaccharides
Cow milk oligosaccharides (CMOs) exhibit a diverse classification based on their structural characteristics. Notably, the majority of bovine milk oligosaccharides (BMOs) are characterized by their acidic nature, with approximately 70% being sialylated, while a smaller fraction, less than 1%, is fucosylated, as reported by Bruggencate et al. [17]. The documentation of neutral oligosaccharides in bovine milk or colostrum was initially published in 1984 by Saito et al. [18]. Notably, bovine milk contains fewer types of oligosaccharides compared to human milk, with a higher prevalence of sialylated oligosaccharides and a reduced presence of fucosylated oligosaccharides [19,20,21,22]. CMOs can be further categorized into two distinct types: normal and branched. This structural classification system provides a valuable framework for a comprehensive understanding and effective categorization of these significant compounds [16,23,24].
4. Oligosaccharides Abundance in Cow Milk:
A study conducted by Meng et al. [25] unveiled the presence of 19 different types of oligosaccharides in cow colostrum and 9 in buffalo colostrum. Notably, cow colostrum is rich in neutral disaccharides (m/z 385.15), neutral trisaccharides (m/z 547.21), and acidic oligosaccharides (m/z 635.23). In contrast, buffalo milk contains a higher proportion of neutral oligosaccharides, accounting for 88.88% of the total, compared to 63.16% in cow milk [25].
Figure 1a illustrates that among cow milk samples, the top five milk oligosaccharide components with the highest relative abundances are m/z 547.21, m/z 749.29, m/z 635.23, m/z 385.15, and m/z 426.176. These oligosaccharides constitute 52.22%, 9.96%, 9.85%, 9.11%, and 4.77%, respectively, of the total milk oligosaccharide content. Furthermore, the analysis of IgG oligosaccharides from 13 different animal species, as presented by Raju et al., sheds light on the critical role of cell line selection in producing recombinant IgGs for human therapy.
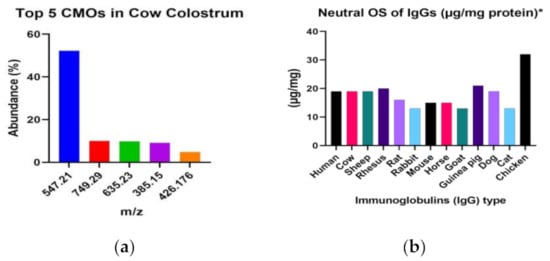
Figure 1.
(a) Top 5 CMOs’ abundance found in cow colostrum by Meng et al. [25]; (b) the quantitative analysis of neutral oligosaccharides was performed using the phenol-sulfuric acid method. The obtained values were determined by assuming an average molecular weight of 150 kDa for IgGs, by Raju et al. [26] (b).
Raju et al. [26], in their research, enhance our understanding of how glycosylation impacts protein therapeutics produced through transgenic technology. As interest grows in utilizing transgenic animals like goats, cows, and sheep for protein therapeutic expression, these data underscore the distinct glycosylation patterns found in IgGs from these species, potentially influencing their biological and pharmacological properties [26].
5. Improved Extraction Methods of CMOs and Other Milk Oligosaccharides
Choosing the right extraction method for CMOs depends on various factors. Solid-phase extraction (SPE) ensures precision and high purity, ideal for specific oligosaccharides. Graphitized carbon-solid phase extraction enhances BMO extraction without lactose hydrolysis [27]. Gel filtering chromatography is suitable for size-based separations. Enzyme digestion isolates lactose-related oligosaccharides effectively. Ultrafiltration is ideal for managing large sample volumes. Hydrophilic interaction-liquid chromatography (HILIC) offers a high resolution and sensitivity for hydrophilic oligosaccharides [28]. Bell et al. achieved 95% pure oligosaccharide recovery from fermented whey permeate via lactose hydrolysis and yeast fermentation through nano-filtration [29]. The choice depends on research objectives, sample characteristics, and available resources, with various techniques often combined.
6. Biological Importance of CMOs
CMOs help to protect against infectious agents by promoting beneficial bacteria growth (prebiotic) and by inhibiting pathogen binding to host cell ligands, preventing infections [30]. Research by Jakobsen et al. found that BMOs favor the growth of B. longum, ssp. longum and Parabacteroides distasonis while inhibiting Clostridium perfringens and Escherichia coli [31]. Milk oligosaccharides also reduce the attachment of enterotoxic Escherichia coli strains in calf intestines [32,33]. Perdijk et al. studied sialyllactose from bovine milk and found that it influenced microbiota composition, promoting Bacteroides and Bifidobacteria growth, leading to distinct changes in short-chain fatty acid profiles [34].
7. Oligosaccharides for Health
Oligosaccharides like cynatroside B and Stemmoside E-K show promise for preventing Alzheimer’s disease and anti-proliferative effects [35]. Spirostanol pentasaccharide from Allium macleanii inhibits tumor growth, while Neisseria meningitidis lipopolysaccharide affects host interactions. Prebiotic oligosaccharides impact immunity, brain development, and lipid metabolism. Mannose-rich glycoproteins alleviate asthma symptoms, and fucose derivatives hinder tumor growth. Sugar structure affects daunorubicin’s anticancer properties [36,37,38,39,40].
8. Cow and Human Milk Similarities in Supporting Bifidobacteria Growth
Certain cow milk oligosaccharides (CMOs) resemble HMOs, potentially sharing functions [41,42,43]. Enriched bovine milk supplements with oligosaccharides enhance gut development and colonization [44]. Both cow milk (CM) whey and human milk (HM) contain factors promoting intestinal bifidobacteria growth in infants, with a-LA, LF, and non-protein components playing a role. The specific CM whey factors are still unknown. Different bifidobacteria strains respond differently to CM growth promoters based on NAcGlu or protein reliance. NAcGlu and gastric mucin encourage certain strains’ growth, while whey proteins are less effective [45]. The study by Paul McJarrow et al. found that sialylated milk oligosaccharides (SMOs) in cow milk, including sialyl lactose and sialyl lactosamine, decrease significantly in concentration from the first to the fifth milking [46]. A similar study on the seasonal variation of CMOs was conducted by Zhiqian Liu et al. [47]. The variety and abundance of SMOs in cow’s milk are notably lower, ranging from 0.035 to 0.042 g per liter (g/L), when contrasted with human milk, where mature milk typically contains 2 to 3 g/L of SMOs. LoCascio et al. found that HMOs mimic complex HMO structures and can serve as selective prebiotics. Bifidobacterium infantis showed a fourfold increase in growth on purified HMOs, outperforming Bifidobacterium breve and Bifidobacterium longum bv. longum. B. infantis utilized 64% of the total HMOs, while B. breve and B. longum bv. longum mainly consumed lacto-N-tetraose, accounting for 35% and 24% of total HMO consumption, respectively [48].
9. Effects of Sialylated Milk Oligosaccharides
Cowardin et al. introduced gut bacteria from a malnourished infant into germ-free mice and provided them with a diet enriched with cow-derived SMOs. This led to increased cecal succinate levels, elevated tuft cell numbers in the small intestine, and activation of a succinate-induced tuft cell pathway associated with Th2 immune responses [49]. Sialic acid, present in breast milk glycoconjugates, is crucial for brain development. Human milk’s anti-inflammatory components inhibit certain immune responses, and SMOs may have potential in neoplastic disease treatment. Human milk contains carbohydrate antigens linked to cancers. Modest amounts of deoxyhexonic and arachidonic acids in breast milk aid immunological development. Studies suggest that nursing infants with milk oligosaccharides may offer protection against rheumatoid arthritis, diabetes, and multiple sclerosis [50].
10. Knowledge Gap
Further research, particularly during the first week of nursing (transition milk phase), is essential to understand CMOs as bioactive components in functional foods and nutraceuticals. This review focuses on early lactation studies related to CMOs, highlighting their bioactive functions and potential in innovative products. Investigating CMOs in cow milk to support infant gut health is a valuable research objective [51,52].
11. Conclusions
CMOs are emerging as noteworthy players in the realm of nutrition and health. While they may not match the complexity and abundance of their human milk counterparts, they exhibit promising health-promoting properties, particularly in infant nutrition. As research in this area continues to expand, CMOs hold the potential to become valuable components in various applications, benefitting not only infants but also individuals seeking enhanced health and well-being. It is clear that CMOs have a vast array of applications, and further studies are required to unveil their full potential and the extent of their impact on infant health. Overall, this review serves as a valuable resource for researchers, nutritionists, and healthcare professionals interested in CMOs and their implications for human health.
Author Contributions
Conceptualization, investigation, data curation, D.D.A.P.C.; writing—original draft preparation, D.D.; writing—review, editing and data curation, S.C.; supervision, editing and data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This review of research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors sincerely appreciate the support and encouragement from the Department of Chemistry, University of Lucknow, under the leadership of Anil Mishra, and the Department of Chemistry, Sri J.N.M.P.G. College, led by Ajai Kumar Mishra.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huang, C.; Sun, S.; Yan, J.; Wang, H.; Zhou, J.; Gao, H.; Xie, W.; Li, Y.; Chai, W. Identification of Carbohydrate Peripheral Epitopes Important for Recognition by Positive-Ion MALDI Multistage Mass Spectrometry. Carbohydr. Polym. 2020, 229, 115528. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, J.; Wang, Y.; Wang, R.; Hao, X.; Wang, R.; Zheng, Y.; An, X.; Qi, J. Feruloyl Oligosaccharides, Isolated from Bacterial Fermented Wheat Bran, Exhibit Antioxidant Effects in IPEC-J2 Cells and Zebrafish Model. Food Sci. Nutr. 2023, 11, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Ojwach, J.; Adetunji, A.I.; Mutanda, T.; Mukaratirwa, S. Oligosaccharides Production from Coprophilous Fungi: An Emerging Functional Food with Potential Health-Promoting Properties. Biotechnol. Rep. 2022, 33, e00702. [Google Scholar] [CrossRef] [PubMed]
- Orihara, K.; Yahagi, K.; Saito, Y.; Watanabe, Y.; Sasai, T.; Hara, T.; Tsukuda, N.; Oki, K.; Fujimoto, J.; Matsuki, T. Characterization of Bifidobacterium Kashiwanohense That Utilizes Both Milk- and Plant-Derived Oligosaccharides. Gut Microbes 2023, 15, 2207455. [Google Scholar] [CrossRef]
- Fukuda, K.; Yamamoto, A.; Ganzorig, K.; Khuukhenbaatar, J.; Senda, A.; Saito, T.; Urashima, T. Chemical Characterization of the Oligosaccharides in Bactrian Camel (Camelus Bactrianus) Milk and Colostrum. J. Dairy Sci. 2010, 93, 5572–5587. [Google Scholar] [CrossRef]
- Aldredge, D.L.; Geronimo, M.R.; Hua, S.; Nwosu, C.C.; Lebrilla, C.B.; Barile, D. Annotation and Structural Elucidation of Bovine Milk Oligosaccharides and Determination of Novel Fucosylated Structures. Glycobiology 2013, 23, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Durham, S.D.; Wei, Z.; Lemay, D.G.; Lange, M.C.; Barile, D. Creation of a Milk Oligosaccharide Database, MilkOligoDB, Reveals Common Structural Motifs and Extensive Diversity across Mammals. Sci. Rep. 2023, 13, 10345. [Google Scholar] [CrossRef]
- Quinn, E.M.; Joshi, L.; Hickey, R.M. Symposium Review: Dairy-Derived Oligosaccharides—Their Influence on Host–Microbe Interactions in the Gastrointestinal Tract of Infants. J. Dairy Sci. 2020, 103, 3816–3827. [Google Scholar] [CrossRef]
- Bode, L. Human Milk Oligosaccharides: Every Baby Needs a Sugar Mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef]
- Sischo, W.M.; Short, D.M.; Geissler, M.; Bunyatratchata, A.; Barile, D. Comparative Composition, Diversity, and Abundance of Oligosaccharides in Early Lactation Milk from Commercial Dairy and Beef Cows. J. Dairy Sci. 2017, 100, 3883–3892. [Google Scholar] [CrossRef]
- Vandenplas, Y. Oligosaccharides in Infant Formula. Br. J. Nutr. 2002, 87, S293–S296. [Google Scholar] [CrossRef]
- Akkerman, R.; Faas, M.M.; de Vos, P. Non-Digestible Carbohydrates in Infant Formula as Substitution for Human Milk Oligosaccharide Functions: Effects on Microbiota and Gut Maturation. Crit. Rev. Food Sci. Nutr. 2019, 59, 1486–1497. [Google Scholar] [CrossRef]
- Gunjan; Kumar, K.; Deepak, D. Structural Characterization of Novel Milk Oligosaccharide Aurose from Cow Colostrum. J. Mol. Struct. 2019, 1176, 394–401. [Google Scholar] [CrossRef]
- Gunjan; Yadav, S.; Shukla, M.; Deepak, D. Isolation and Structure Elucidation of a Novel Nonasaccharide ‘Tarose’ from Bos Primigenius Taurus (Jarsi Cow) Colostrum. J. Biol. Chem. Res. 2022, 39, 243–263. [Google Scholar]
- Roy, T.; Deepak, D. Antioxidant Properties of Milk Oligosaccharides from Various Ruminants. Int. J. Pharma Bio Sci. 2014, 5, B-400–B-408. [Google Scholar]
- Ranjan, A.K.; Sharma, M.; Shukla, M.; Deepak, D. Structure Elucidation of a Novel Pentasaccharide Ariesose from Ovies Aries Milk by 2-Dimensional NMR and Mass Spectrometry. Trends Carbohydr. Res. 2023, 15, 9–21. [Google Scholar]
- Ten Bruggencate, S.J.; Bovee-Oudenhoven, I.M.; Feitsma, A.L.; van Hoffen, E.; Schoterman, M.H. Functional Role and Mechanisms of Sialyllactose and Other Sialylated Milk Oligosaccharides. Nutr. Rev. 2014, 72, 377–389. [Google Scholar] [CrossRef]
- Saito, T.; Itoh, T.; Adachi, S. Presence of Two Neutral Disaccharides Containing N-Acetylhexosamine in Bovine Colostrum as Free Forms. Biochim. Biophys. Acta -Gen. Subj. 1984, 801, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Itoh, T.; Adachi, S.; Suzuki, T.; Usui, T. The Chemical Structure of Neutral and Acidic Sugar Chains Obtained from Bovine Colostrum κ-Casein. BBA -Gen. Subj. 1981, 678, 257–267. [Google Scholar] [CrossRef]
- Urashima, T.; Saito, T.; Ohmisya, K.; Shimazaki, K. Structural Determination of Three Neutral Oligosaccharides in Bovine (Holstein-Friesian) Colostrum, Including the Novel Trisaccharide; GalNAcαl-3Galβ1-4Glc. Biochim. Biophys. Acta -Gen. Subj. 1991, 1073, 225–229. [Google Scholar] [CrossRef]
- Tao, N.; Ochonicky, K.L.; German, J.B.; Donovan, S.M.; Lebrilla, C.B. Structural Determination and Daily Variations of Porcine Milk Oligosaccharides. J. Agric. Food Chem. 2010, 58, 4653–4659. [Google Scholar] [CrossRef]
- Nakajima, K.; Kinoshita, M.; Matsushita, N.; Urashima, T.; Suzuki, M.; Suzuki, A.; Kakehi, K. Capillary Affinity Electrophoresis Using Lectins for the Analysis of Milk Oligosaccharide Structure and Its Application to Bovine Colostrum Oligosaccharides. Anal. Biochem. 2006, 348, 105–114. [Google Scholar] [CrossRef]
- Singh, A.K.; Deepak, D. Isolation and Structure Elucidation of Novel Yak Milk Oligosaccharide “Nienose”. Trends Carbohydr. Res. 2020, 12, 15–26. [Google Scholar]
- Verma, P.; Sarkar, J.; Deepak, D. Isolation, Purification and Structure Elucidation of Novel Hexasaccharide Mesose from Camel Milk by NMR. Trends Carbohydr. Res. 2019, 11, 52–58. [Google Scholar]
- Meng, X.P.; Jiao, S.M.; Qin, S.Q.; Yang, X.B.; Li, J.S.; Wei, J.H.; Wang, Z.; Du, Y.G.; Xian-Pu, M.; Si-Ming, J.; et al. Comparative Study on Milk Oligosaccharides in Buffalo and Cow Colostrum Milk. Prog. Biochem. Biophys. 2017, 44, 942–948. [Google Scholar] [CrossRef]
- Raju, T.S.; Briggs, J.B.; Borge, S.M.; Jones, A.J.S. Species-Specific Variation in Glycosylation of Igc: Evidence for the Species-Specific Sialylation and Branch-Specific Galactosylation and Importance for Engineering Recombinant Glycoprotein Therapeutics. Glycobiology 2000, 10, 477–486. [Google Scholar] [CrossRef]
- Pyles, M.B.; Brock, K.; Schendel, R.R.; Lawrence, L.M. Improved Methods for Mare Milk Analysis: Extraction and Quantification of Mare Milk Carbohydrates and Assessment of FTIR-Based Macronutrient Quantification. Front. Nutr. 2023, 10, 1066463. [Google Scholar] [CrossRef]
- Mariño, K.; Lane, J.A.; Abrahams, J.L.; Struwe, W.B.; Harvey, D.J.; Marotta, M.; Hickey, R.M.; Rudd, P.M. Method for Milk Oligosaccharide Profiling by 2-Aminobenzamide Labeling and Hydrophilic Interaction Chromatography. Glycobiology 2011, 21, 1317–1330. [Google Scholar] [CrossRef]
- de Moura Bell, J.M.L.N.; Cohen, J.L.; de Aquino, L.F.M.C.; Lee, H.; de Melo Silva, V.L.; Liu, Y.; Domizio, P.; Barile, D. An Integrated Bioprocess to Recover Bovine Milk Oligosaccharides from Colostrum Whey Permeate. J. Food Eng. 2018, 216, 27–35. [Google Scholar] [CrossRef]
- Hakkarainen, J.; Toivanen, M.; Leinonen, A.; Frängsmyr, L.; Strömberg, N.; Lapinjoki, S.; Nassif, X.; Tikkanen-Kaukanen, C. Human and Bovine Milk Oligossaccharides Inhibit Neisseria Meningitidis Pili Attachment in Vitro. J. Nutr. 2005, 135, 2445–2448. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, L.M.A.; Sundekilde, U.K.; Andersen, H.J.; Nielsen, D.S.; Bertram, H.C. Lactose and Bovine Milk Oligosaccharides Synergistically Stimulate B. Longum Subsp. Longum Growth in a Simplified Model of the Infant Gut Microbiome. J. Proteome Res. 2019, 18, 3086–3098. [Google Scholar] [CrossRef]
- Johansson, P.; Nilsson, J.; Ångström, J.; Miller-Podraza, H. Interaction of Helicobacter Pylori with Sialylated Carbohydrates: The Dependence on Different Parts of the Binding Trisaccharide Neu5Acα 3Galβ4GlcNAc. Glycobiology 2005, 15, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.J.; Martín-Sosa, S.; Hueso, P. Binding of Milk Oligosaccharides by Several Enterotoxigenic Escherichia Coli Strains Isolated from Calves. Glycoconj. J. 2003, 19, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Perdijk, O.; Van Baarlen, P.; Fernandez-Gutierrez, M.M.; Van den Brink, E.; Schuren, F.H.J.; Brugman, S.; Savelkoul, H.F.J.; Kleerebezem, M.; Van Neerven, R.J.J. Sialyllactose and Galactooligosaccharides Promote Epithelial Barrier Functioning and Distinctly Modulate Microbiota Composition and Short Chain Fatty Acid Production in Vitro. Front. Immunol. 2019, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Yuca, H. Capsicum annuum L. In Novel Drug Targets with Traditional Herbal Medicines: Scientific and Clinical Evidence; Gürağaç Dereli, F.T., Ilhan, M., Belwal, T., Eds.; Springer: Cham, Switzerland, 2022; pp. 95–108. [Google Scholar]
- Mountzouris, K.C.; Balaskas, C.; Fava, F.; Tuohy, K.M.; Gibson, G.R.; Fegeros, K. Profiling of Composition and Metabolic Activities of the Colonic Microflora of Growing Pigs Fed Diets Supplemented with Prebiotic Oligosaccharides. Anaerobe 2006, 12, 178–185. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Lager, I.; Looger, L.L.; Hilpert, M.; Lalonde, S.; Frommer, W.B. Conversion of a Putative Agrobacterium Sugar-Binding Protein into a FRET Sensor with High Selectivity for Sucrose. J. Biol. Chem. 2006, 281, 30875–30883. [Google Scholar] [CrossRef]
- Ibrahim, O.O. Functional Oligosaccharides: Chemicals Structure, Manufacturing, Health Benefits, Applications and Regulations. J. Food Chem. Nanotechnol. 2018, 4, 65–76. [Google Scholar] [CrossRef]
- Zhu, L.; Cao, X.; Chen, W.; Zhang, G.; Sun, D.; Wang, P.G. Syntheses and Biological Activities of Daunorubicin Analogs with Uncommon Sugars. Bioorganic Med. Chem. 2005, 13, 6381–6387. [Google Scholar] [CrossRef]
- Barile, D.; Tao, N.; Lebrilla, C.B.; Coisson, J.D.; Arlorio, M.; German, J.B. Permeate from Cheese Whey Ultrafiltration Is a Source of Milk Oligosaccharides. Int. Dairy J. 2009, 19, 524–530. [Google Scholar] [CrossRef]
- Silanikove, N.; Leitner, G.; Merin, U.; Prosser, C.G. Recent Advances in Exploiting Goat’s Milk: Quality, Safety and Production Aspects. Small Rumin. Res. 2010, 89, 110–124. [Google Scholar] [CrossRef]
- Robinson, R.C. Structures and Metabolic Properties of Bovine Milk Oligosaccharides and Their Potential in the Development of Novel Therapeutics. Front. Nutr. 2019, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Obelitz-Ryom, K.; Rendboe, A.K.; Nguyen, D.N.; Rudloff, S.; Brandt, A.B.; Nielsen, D.S.; Heckmann, A.B.; Chichlowski, M.; Sangild, P.T.; Thymann, T.; et al. Bovine Milk Oligosaccharides with Sialyllactose for Preterm Piglets. Nutrients 2018, 10, 1489. [Google Scholar] [CrossRef]
- Petschow, B.W.; Talbott, R.D. Response of Bifidobacterium Species to Growth Promoters in Human and Cow Milk. Pediatr. Res. 1991, 29, 208–213. [Google Scholar] [CrossRef]
- McJarrow, P.; Van Amelsfort-Schoonbeek, J. Bovine Sialyl Oligosaccharides: Seasonal Variations in Their Concentrations in Milk, and a Comparison of the Colostrums of Jersey and Friesian Cows. Int. Dairy J. 2004, 14, 571–579. [Google Scholar] [CrossRef]
- Liu, Z.; Auldist, M.; Wright, M.; Cocks, B.; Rochfort, S. Bovine Milk Oligosaccharide Contents Show Remarkable Seasonal Variation and Intercow Variation. J. Agric. Food Chem. 2017, 65, 1307–1313. [Google Scholar] [CrossRef]
- LoCascio, R.G.; Ninonuevo, M.R.; Freeman, S.L.; Sela, D.A.; Grimm, R.; Lebrilla, C.B.; Mills, D.A.; German, J.B. Glycoprofiling of Bifidobacterial Consumption of Human Milk Oligosaccharides Demonstrates Strain Specific, Preferential Consumption of Small Chain Glycans Secreted in Early Human Lactation. J. Agric. Food Chem. 2007, 55, 8914–8919. [Google Scholar] [CrossRef]
- Cowardin, C.A.; Ahern, P.P.; Kung, V.L.; Hibberd, M.C.; Cheng, J.; Guruge, J.L.; Sundaresan, V.; Head, R.D.; Barile, D.; Mills, D.A.; et al. Mechanisms by Which Sialylated Milk Oligosaccharides Impact Bone Biology in a Gnotobiotic Mouse Model of Infant Undernutrition. Proc. Natl. Acad. Sci. USA 2019, 116, 11988–11996. [Google Scholar] [CrossRef]
- Danishefsky, S.J.; Allen, J.R. From the Laboratory to the Clinic: A Retrospective on Fully Synthetic Carbohydrate-Based Anticancer Vaccines. Angew. Chem. Int. Ed. 2000, 39, 836–863. [Google Scholar] [CrossRef]
- Tao, N.; DePeters, E.J.; Freeman, S.; German, J.B.; Grimm, R.; Lebrilla, C.B. Bovine Milk Glycome. J. Dairy Sci. 2008, 91, 3768–3778. [Google Scholar] [CrossRef] [PubMed]
- Tao, N.; DePeters, E.J.; German, J.B.; Grimm, R.; Lebrilla, C.B. Variations in Bovine Milk Oligosaccharides during Early and Middle Lactation Stages Analyzed by High-Performance Liquid Chromatography-Chip/Mass Spectrometry. J. Dairy Sci. 2009, 92, 2991–3001. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).