Abstract
Phenolic compounds from propolis extract (PE) have antioxidant and antimicrobial properties; however, extracts from this raw material are not water soluble. This study aimed to stabilize the phenolic compounds from green propolis extract in cassava and potato starch nanoparticles produced by the anti-solvent precipitation method. The obtained materials displayed a crystalline structure related to starch nanomaterials with a V6h-type crystalline structure. The starch nanoparticles interacted with the phenolic compounds by means of hydrogen bonds and increased the hydrophobicity in the nanomaterials. The developed starch nanomaterials loaded with the phenolic compounds from PE could be potentially used as a novel ingredient in food packaging.
1. Introduction
Propolis is a resinous and heterogeneous material collected by Apis mellifera bees from different parts of plants, including the buds and exudates [1]. This natural compound has high amounts of flavonoids and phenolic acids, with it being used worldwide in traditional medicine due to its antioxidant and antimicrobial properties [2]. However, extracts from propolis have limited water solubility, reducing their application in the pharmacology and food industries [3].
Recently, Alves et al. [3] stabilized phenolic compounds from brown propolis extract using starch nanoparticles and observed that the obtained nanomaterials have high antioxidant activity. The authors concluded that the starch nanomaterials loaded with the phenolic compounds from brown propolis extract could be used as active ingredients in food packaging materials. Green propolis is another type of propolis abundant in Brazil, which has phenolic compounds with antioxidant and antimicrobial properties [4]. However, the stabilization of phenolic compounds from green propolis extracts using biopolymeric nanoparticles has not been investigated. Hence, this research aimed to produce and characterize cassava and potato starch nanoparticles loaded with the phenolic compounds from green propolis extract.
2. Materials and Methods
2.1. Materials
In the current research, starches isolated from cassava and potato were used as macromolecules. Native starches were purchased from Juréia and Shambala Naturais Food Industries (Florianópolis, Brazil). Green propolis was purchased from Breyer® (Formigas, Brazil). Distilled water, ethanol (≥99.6%, Êxodo Científica, São Paulo, Brazil), and hydrochloric acid (37 wt%, Neon, São Paulo, Brazil) were used as solvents. Potassium chloride and sodium carbonate were purchased from Dinâmica (São Paulo, Brazil). Folin–Ciocalteu reagent was acquired directly from Sigma-Aldrich (São Paulo, Brazil). All reagents used were of analytical grade, and they were used as received.
2.2. The Production of Starch Nanoparticles Loaded with the Phenolic Compounds from the Propolis Extract
Firstly, propolis extract (PE) was produced according to the methodology and best conditions described by Alves et al. [3]. In sequence, PE was acidified with hydrochloric acid (100:1 v/v, hydroethanolic solution: HCl 37 wt%, pH = 1). In parallel, starch nanoparticles (SNPs) were produced by the anti-solvent precipitation method [3,5]. Dispersions (5% w/w) of cassava starch and potato starch were prepared in distilled water at 25 °C followed by gelatinization at 90 °C for 30 min. The gelatinized starch solutions were cooled to 30 °C and then the acidified PE was dripped using a peristaltic pump (flow of 0.7 mL/min) in a 1:1 (% v/v) ratio.
The resulting slurry (starch dispersion + acidified PE) was stirred at 25 °C for 12 h and then centrifugated at 4000 rpm for 15 min using a centrifuge (Kasvi, São Paulo, Brazil). The SNPs were centrifuged three times with hydroethanolic solution (80% v/v) and finally washed with absolute ethanol (99.6%). The SNPs were separated by centrifugation and the ethanol was evaporated using a forced-air convection oven (Solidsteel, São Paulo, Brazil) at 60 °C for 10 min. Finally, the SNPs were frozen at −18 °C for 48 h and then lyophilized (Liotop L 101). The resulting nanomaterials loading the phenolic compounds from PE were named cassava (CSNPs-PE) and potato (PSNPs-PE) starch loading the phenolic compounds from PE, and cassava (CSNPs) and potato (PSNPs) starch nanoparticles without PE.
2.3. Characterization of the Starch Nanoparticles Loaded with the Phenolic Compounds from Green Propolis Extract
The loading efficiency (LE) of the total phenolic compounds (TPC) from the acidified PE stabilized with the SNPs was calculated using Equation (1) [6]:
where is the TPC of the PE and is the TPC of supernatant collected after the first centrifugation. The quantification of TPC was carried out using the method described by Alves et al. [3].
Diffraction analysis was performed with an X-ray diffractometer (Rigaku MiniFlex 600 DRX, Tokyo, Japan) equipped with Cu-Kα radiation (λ = 0.154056 nm). XRD diffractograms were obtained 2θ = 3° and 60° (rate of 10 °/min). Equation (2) (Bragg’s law) was used to calculate the interplanar spacing d (nm) from the X-ray patterns.
where n is the reflection order (n = 1), λ is the wavelength of CuKα radiation, and θ is the reflection angle [7].
Chemical bonds were studied using a Fourier transform infrared spectrometer (FTIR, Cary 600, Agilent, Santa Clara, CA, USA) in the wavenumber range of 4000 and 400 cm−1 (4 cm−1 resolution). In each analysis, 32 scans were performed [7].
The water contact angle (WCA) of all starch nanoparticles was investigated using the methodology reported by Amirabadi et al. [8]. The samples were compressed into tablets using a hydraulic machine with two heated plates at 25 °C and controlled by PID controllers. A cylindrical piston was used as a mold. Approximately 0.2 g of each sample was deposited in the mold and 1 ton of force was applied. The pellets were approximately 1.5 mm thick and 1 cm in diameter. The water contact angle of the compressed samples was analyzed in an optical tensiometer (Ramé-Hart 250), with 5 μL of water being dropped over each compressed sample. The WCA was defined as the average of 10 measurements taken over a 5 s interval.
3. Results
Characterization of the Starch Nanoparticles Loaded with the Phenolic Compounds from Green Propolis Extract
The green propolis extract had a TPC of 763.36 mg GAE/g. After anti-solvent precipitation, the LE oscillated between 65.45 and 73.32% in PSNPs-PE and CSNPs-PE, respectively.
The samples revealed X-ray diffractograms of starch nanomaterials (Figure 1a). In particular, the X-ray diffractograms were typical of a V6h-type crystalline structure, exhibiting diffraction peaks at 2θ = 13.0° (d = 0.68 nm) and 20.0° (d = 0.44 nm).
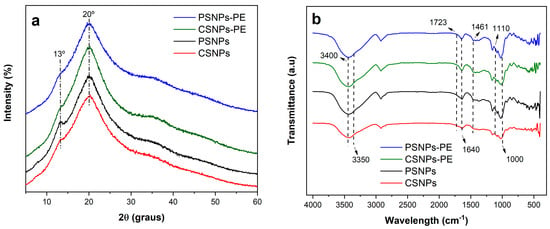
Figure 1.
(a) X-ray diffractograms and (b) FTIR spectra of starch nanoparticles with (PSNPs-PE and CSNPs-PE) and without (PSNPs and CSNPs) PE.
The FTIR spectra of the samples show a peak centered at 3400 cm−1, associated with the vibration of hydroxyl groups (O–H stretching) of the starch chains. A band at 3350 cm−1 was correlated with the O–H stretching vibration of the phenolic groups (Figure 1b) [9]. Furthermore, a slight band at 1723 cm−1 suggests the C=O stretching of the carboxylic group, indicating the presence of polyphenols from PE. Vibration of the phenol groups was also observed in the band centered at 1640 cm−1, assigned to aromatic ring C=C stretching, as well as aromatic C–H deformation vibration at 1110 cm−1 [9]. C–H deformations and aromatic stretching at 1461 cm−1 was correlated with the presence of flavonoids (hydrocarbons CH3 and CH2’s vibrations were overlapping) [9]. In the region around 1000 cm−1, a new band was observed in starch nanoparticles loaded with the phenolic compounds from green PE.
The WCA of the CSNPs and PSNPs remained constant at 41.05° ± 0.17 (Figure 2). With the incorporation of the phenolic compounds from PE, an increase in the WCA was observed (Figure 2); hence, the starch nanoparticles loaded with the phenolic compounds from green PE had a WCA ranging between 66.80° ± 1.21 (CSNPs-PE) and 75.70° ± 0.75 (PSNPs-PE).
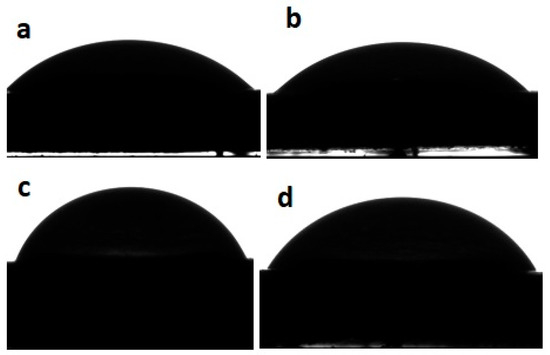
Figure 2.
The WCA of potato (a) and cassava (b) starch nanoparticles and potato (c) and cassava (d) starch nanoparticles loaded with the phenolic compounds from green PE.
4. Discussion
In the current research, starch nanoparticles based on cassava and potato starches had similar LE values when compared with starch nanoparticles loaded with the phenolic compounds from brown propolis extract [3]. These results suggest that the LE could be independent of the type of propolis used in the PE.
Regarding the crystalline and chemical bond results, it is possible to conclude that the nanoparticles are composed of six glucose units per helical turn [10], with it being the case that the phenolic compounds altered this crystalline structure since a reduction in the peak intensity at 13° was observed in the X-ray diffractograms. Furthermore, the displacement observed at 1000 cm−1 in the FTIR spectra of the starch nanoparticles loaded with the phenolic compounds from PE suggests that structural modification resulted in spatial displacement of and an increase in the CO group band, probably caused by hydrogen bonds between the phenolic compound from PE and amylose/amylopectin chains [11].
Finally, the increase in the WCA values confirms the presence of phenolic compounds from PE in the starch nanoparticles. These phenolic compounds have hydrophobic properties and then increased the WCA values. The increase in the WCA could be important in packaging materials that will be used in contact with food.
5. Conclusions
In the current research, cassava and potato starch nanoparticles loaded with the phenolic compounds from propolis extract (PE) were produced and characterized. The developed nanomaterials displayed a V6h-type crystalline structure, typical of starch nanoparticles. This crystalline structure was modified by the incorporation of phenolic compounds from PE. The FTIR results revealed that the starch chains interacted with the phenolic compounds from PE by means of hydrogen bonds. Finally, the starch nanoparticles had hydrophilic surfaces with a water contact angle (WCA) of 41.05°. With the incorporation of phenolic compounds from PE, the WCA in the starch nanomaterials increased between 60 and 80%, indicating that the phenolic compounds reduce the hydrophilicity of the nanoparticles. Based on these results, it can be considered that starch nanoparticles loaded with the phenolic compounds from PE can serve as promising ingredients to manufacture food packaging materials.
Author Contributions
M.J.d.S.A.: methodology, investigation, validation, formal analysis, and writing—original draft preparation; W.D.C.C.: investigation; A.R.M.: supervision and writing—review and editing; G.A.V.: conceptualization, formal analysis, resources, data curation, writing—original draft preparation, writing—review and editing, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was (partially) supported by Programa Iberomaricano de Ciencia y Tecnologia para el Desarrollo (CYTED) (through Red 121RT0108) and the Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC) (grants 2021TR000418 and 2021TR001887).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge CAPES (Coordination for the Improvement of Higher Education Personnel) for the doctoral fellowship awarded to the first author and the Central Chemical Analysis of Chemical Engineering and Food Engineering for the analyses. G.A. Valencia would like to thank the CNPq (National Council for Scientific and Technological Development) for the research fellowship (302434/2022-4).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bertotto, C.; Bilck, A.P.; Yamashita, F.; Anjos, O.; Bakar Siddique, M.A.; Harrison, S.M.; Brunton, N.P.; Carpes, S.T. Development of a biodegradable plastic film extruded with the addition of a Brazilian propolis by-product. LWT 2022, 157, 113124. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Vitchayakitti, W. Improving functional properties of chitosan films as active food packaging by incorporating with propolis. Food Hydrocoll. 2016, 61, 695–702. [Google Scholar] [CrossRef]
- Jaízia dos Santos Alves, M.; Rodrigues Monteiro, A.; Ayala Valencia, G. Antioxidant Nanoparticles Based on Starch and the Phenolic Compounds from Propolis Extract: Production and Physicochemical Properties. Starch/Stärke 2022, 74, 2100289. [Google Scholar] [CrossRef]
- Quintino, R.L.; Reis, A.C.; Fernandes, C.C.; Martins, C.H.G.; Colli, A.C.; Crotti, A.E.M.; Squarisi, I.S.; Ribeiro, A.B.; Tavares, D.C.; Miranda, M.L.D. Brazilian Green Propolis: Chemical Composition of Essential Oil and Their In Vitro Antioxidant, Antibacterial and Antiproliferative Activities. Braz. Arch. Biol. Technol. 2020, 63, e20190408. [Google Scholar] [CrossRef]
- dos Santos Alves, M.J.; Calvo Torres de Freitas, P.M.; Monteiro, A.R.; Ayala Valencia, G. Impact of the Acidified Hydroethanolic Solution on the Physicochemical Properties of Starch Nanoparticles Produced by Anti-Solvent Precipitation. Starch-Stärke 2021, 73, 2100034. [Google Scholar] [CrossRef]
- Quiroz, J.Q.; Velazquez, V.; Corrales-Garcia, L.L.; Torres, J.D.; Delgado, E.; Ciro, G.; Rojas, J. Use of plant proteins as microencapsulating agents of bioactive compounds extracted from annatto seeds (Bixa orellana L.). Antioxidants 2020, 9, 310. [Google Scholar] [CrossRef]
- Capello, C.; Leandro, G.C.; Campos, C.E.M.; Hotza, D.; Carciofi, B.A.M.; Valencia, G.A. Adsorption and desorption of eggplant peel anthocyanins on a synthetic layered silicate. J. Food Eng. 2019, 262, 162–169. [Google Scholar] [CrossRef]
- Amirabadi, S.; Milani, J.M.; Sohbatzadeh, F. Application of dielectric barrier discharge plasma to hydrophobically modification of gum arabic with enhanced surface properties. Food Hydrocoll. 2020, 104, 105724. [Google Scholar] [CrossRef]
- Svečnjak, L.; Marijanović, Z.; Okińczyc, P.; Marek Kuś, P.; Jerković, I. Mediterranean Propolis from the Adriatic Sea Islands as a Source of Natural Antioxidants: Comprehensive Chemical Biodiversity Determined by GC-MS, FTIR-ATR, UHPLC-DAD-QqTOF-MS, DPPH and FRAP Assay. Antioxidants 2020, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Hopfer, H.; Ziegler, G.R.; Kong, L. Starch-menthol inclusion complex: Structure and release kinetics. Food Hydrocoll. 2019, 97, 105183. [Google Scholar] [CrossRef]
- Pérez-Vergara, L.D.; Cifuentes, M.T.; Franco, A.P.; Pérez-Cervera, C.E.; Andrade-Pizarro, R.D. Development and characterization of edible films based on native cassava starch, beeswax, and propolis. NFS J. 2020, 21, 39–49. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).