Abstract
Spent coffee grounds (SCGs) are currently considered abandoned landfill waste despite retaining valuable organic compounds, especially with a high protein content of 16.64 ± 0.13 g/g dried SCGs and a high oil content of 15.48 ± 0.17 g/g dried SCGs. As a result, SCGs could serve as a potential source of valuable ingredients. However, utilizing a single technical strategy of alternative green extractions was insufficient for extracting the target compounds and hydrolyzing proteins. This work aimed to optimize the operating parameters of enzyme-assisted extraction (EAE), microwave-assisted extraction (MAE), and subcritical water extraction (SWE) using a response surface methodology. The results showed that EAE, at a papain-to-substrate ratio (E/S) of 0.5 and a duration of 15 min, generated a %DH of 93.39% and provided a water soluble protein concentration (WSPC) in the range of 400 to 800 µg/mL. Moreover, MAE provided a maximum %DH of 9.72% at 600 watts and 10 min, while SWE produced a maximum %DH of 13.41% at 160 °C in 17.5 min. However, the WSPCs of MAE and SWE extracts were comparable at ~250 µg/mL. The combination MAE−SWE enhanced the %DH of hydrolysates by more than the combined SWE−MAE, SWE, and MAE. However, the effects of differential hydrolysis on bioactivity are not directly correlated with %DH. In this study, the highest antioxidant activity was found at an E/S of 0.5 and in 15 min for EAE, at 350 W and in 10 min for MAE, and at 160 °C and in 30 min for SWE. This work demonstrated that the valorization of SCGs not only reduces the amount of waste but also yields functional cosmeceutical and nutraceutical ingredients.
1. Introduction
Spent coffee grounds (SCGs) are considered waste generated by the coffee industry despite still retaining various bioactive compounds associated with potential health advantages. Consequently, SCGs could act as a viable source of high-value ingredients for various applications.
Typically, enzymatic hydrolysis is performed via proteases with high specificity, operating under mild conditions. In this study, papain was used as a plant-derived proteolytic enzyme sourced from papaya (Carica papaya L.), which is used to facilitate the breakdown of peptides into lower molecular weights [1]. Nonetheless, challenges such as the elevated cost of enzymes, their allergenic properties, the requirement of additional downstream processes, and the need for various chemicals to maintain the extraction condition remain points of concern.
Extraction techniques such as subcritical water hydrolysis (SWH) and microwave-assisted hydrolysis (MAH) represent non-conventional proteolysis techniques that align with sustainable principles. In practice, SWH is employed at 110–300 °C, a pressure of 20–100 bar, and within a short reaction time (5–60 min) in a liquid state to release bioactive peptides [2]. Protein hydrolysis occurs using a biocatalyst with a high ionization reaction: namely, H+ combines with the N-terminal of the protein chain, resulting in peptide bond cleavage; then, at that position, OH− combines with the carbon cation of the C-terminal. In contrast, MAH using electromagnetic waves not only increases the extraction yield within a shorter time due to superheating water molecules but also improves the %DH and antioxidant capacity. Presently, MAH is considered a simple, rapid, and highly efficient extraction method.
This work aimed to optimize the operating parameters of enzyme-assisted extraction (EAE), microwave-assisted extraction (MAE), and subcritical water extraction (SWE) using a response surface methodology. The combination of these techniques is investigated as well.
2. Materials and Methods
2.1. Preparation of Spent Coffee Ground (SCG) Samples
SCGs (Arabica coffee variety) were donated by Starbucks, Bangkok, Thailand, and used as the starting material. Initially, it was dried by natural convection for 24 h at room temperature (35 ± 5 °C) and then dried by vacuum hot air oven at 60 °C for 6 h. The proximate analysis was determined according to the official AOAC method (2000). SCGs consisted of 2.58 ± 0.12% (w/w) moisture, 16.64 ± 0.13% (w/w) crude protein, 15.48 ± 0.17% (w/w) lipids, 28.14 ± 1.00% (w/w) water insoluble fiber, 31.18 ± 0.82% (w/w) carbohydrate, and 1.98 ± 0.02% (w/w) ash.
2.2. Protein Hydrolysate Production
EAE was performed as described in [3] with modifications. Briefly, samples at a solid/solvent ratio of 1:20 (w/v) were hydrolyzed by papain at an enzyme-to-substrate (E/S) ratio of 0.1–0.5 in phosphate buffer (PBS) for 15–180 min with constant rotational shaking at 150 rpm and 50 °C. The hydrolysis reactions were terminated by heating at 85 °C for 20 min with occasional agitations. Microwave hydrolysis was carried out by Mars 6 synthesis (CEM Co., Matthews, NC, USA) at 100–600 watts for 2–10 min. Subcritical assisted hydrolysis was performed by using a laboratory-scale extractor [4]. Briefly, the sample was loaded into the extraction vessel (Parr Instruments Company, Moline, IL, USA). The investigated temperatures were in the ranges of 110 ± 5–160 ± 5 °C for 5–30 min at an initial pressure of 20 bar. In all experiments, the insoluble portion of the solution was removed by filtration and centrifugation at 10,000× g for 30 min at 4 °C, and the supernatant was collected and stored at −20 °C until further preliminary investigation.
2.3. Assays
2.3.1. Water Soluble Protein Content (WSPC) and Degree of Hydrolysis (%DH)
The soluble protein concentrations were determined by using Bradford’s assay [5]. The absorbance was read at 595 nm using a standard curve of bovine serum albumin (BSA). The %DH was determined using the o-phthalaldehyde (OPA) method in which a change in solution color was detected at 340 nm.
2.3.2. Total Phenolic Content, Antioxidant Capacity, and Total Polysaccharide Content
The total phenolic content (TPC) was assessed using the modified Folin–Ciocalteu assay [6]. It was measured in a 96-well flat bottom plate using a spectrophotometer (Multiskan GO, Thermo Fisher Scientific Inc., Waltham, MA, USA) at 725 nm and expressed in mg gallic acid equivalent (mg GAE) per dry weight sample. For antioxidant activities, DPPH radical scavenging ability was determined following the approach described in [7]. The change in solution color was measured at 517 nm and expressed in mg of L-ascorbic acid equivalent (mg AAE/g SCG) and a half maximal inhibitory concentration (IC50). Total polysaccharide (TPS) was determined using the phenol sulfuric acid method described elsewhere [8] with D-glucose as the reference standard, and the absorbance was measured at 490 nm and reported in g GLU/100 g SCGs.
2.4. Experimental Design and Data Analysis
The optimal conditions were established through a response surface methodology employing a face-centered composite design, which involved two factors at three levels each. Design Expert® software (Stat-Ease, Inc., Minneapolis, MN, USA) was utilized to create an experimental design consisting of 11 treatments, as detailed in Table 1.

Table 1.
Experimental units and response variables of protein hydrolysate.
3. Results and Discussion
3.1. Preliminary Study of Protein Extraction Conditions
The parameters under investigation included a papain-to-substrate ratio ranging from 0.1 to 0.5 and from 15 to 180 min for EAE. The parameters for MAE consisted of power levels that varied from 100 to 600 watts and durations of 2–10 min. The temperature was in the range of 110–160 °C and 5–30 min for SWE.
As shown in Figure 1(a1), the rate of hydrolysis at 0.5 E/S was complete at 93.39 ± 0.29% within 15 min and remained constant around 84.65−93.39% over 180 min. Moreover, %DH increased with E/S ratios from 0.1 to 0.5. The highest antioxidant activity was also found at an E/S of 0.5 and 15 min (See Figure 1(b1)). Generally, SCGs were processed through a roasting process and espresso machine with a rough water pressure of 15 bars, leading to partial hydrolysis into smaller polypeptides. Moreover, MAE and SWE hydrolyze proteins through the use of thermal energy and microwave energy, respectively. These methods are assisted by hydronium ions. MAE showed that %DH linearly increased with extraction time (See Figure 1(a2)). The highest %DH was observed at 600 W and 10 min. However, at a microwave power of 600 W, boiling of the solution was observed at 10 min and the DPPH activities showed a downward trend (See Figure 1(b2)). Although MAE exhibited relatively high hydrolysis at 600 watts for 10 min, it demonstrated a diminishing trend for DPPH activity. Consequently, the optimal condition was 350 watts for 10 min. For SWE, the extraction at 160 °C for 17.5−30 min showed the highest %DH (See Figure 1(a3)). The highest DPPH activities were detected at a temperature of 160 °C as well (See Figure 1(b3)). Notably, while %DH plays a significant role in peptide bond cleavage into shorter peptides, chain length and its associated DPPH activities emerged as crucial factors.
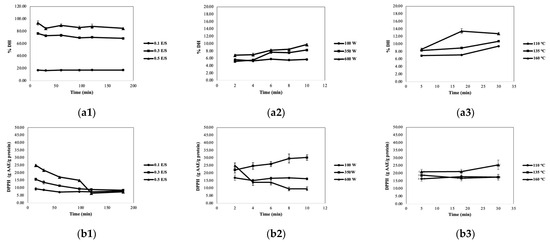
Figure 1.
Influences of papain-to-substrate ratio (E/S) on %DH (a1) and DPPH activity (b1), microwave power level on %DH (a2) and DPPH activity (b2), and extraction temperature on %DH (a3) and DPPH activity (b3).
3.2. Process Optimization by RSM
In the context of RSM analysis, the optimization results are depicted in Figure 2.
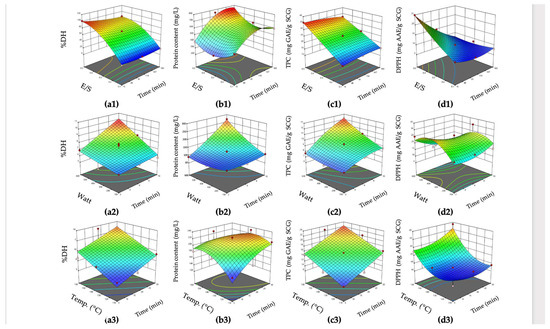
Figure 2.
Response surface plots of (a) %DH, (b) protein content, (c) total phenolic content (TPC), and (d) DPPH for EAE (a1–d1), MAE (a2–d2), and SWE (a3–d3).
3.3. Optimal Conditions and Combination Technique
In Table 2, for the EAE technique, the highest %DH was observed at an E/S ratio of 0.5 for a duration of 15–60 min. Although %DH shows a significant efficiency in peptide bond cleavage, DPPH activities still emerged as a crucial factor. It was found that EAE exhibited lower DPPH radical scavenging activities compared with the MAE and SWE techniques. Moreover, TPS reached 30.02–33.66 g GLU/100 g SCGs due to terminating its enzyme activity at 85 °C for 20 min, which is a suitable condition for polysaccharide extraction. Among the studied treatments, SWE emerged as the most prominent and effective method in terms of antioxidant activity, followed by MAE, displaying an appropriate %DH, elevated protein content, and minimal TPS. Based on RSM optimization, the conditions of MAE and SWE were selected to study the effects of the combined techniques. The hydrolysis efficiency achieved through the MAE-SWE combination exceeded that of SWE-MAE, as well as individual SWE and MAE. However, it is noteworthy that the combination resulted in an increase in %DH, which potentially led to a reduction in bioactivity.

Table 2.
Optimal conditions and combination technique.
4. Conclusions
The protein hydrolysates from SCGs were successfully extracted via EAE, MAE, SWE, and their combination. The results revealed that papain exhibited the highest proteolytic activity. The optimal conditions for maximum DPPH activities were identified as follows: an E/S ratio of 0.5 for 15 min in EAE, 350 watts for 10 min in MAE, and 160 °C for 30 min in SWE. Efficient hydrolysis was achieved through the combined MAE-SWE process compared with individual MAE and SWE procedures. The effects of differential hydrolysis on bioactivity are not directly correlated with %DH. This study illustrates that repurposing SCGs simultaneously diminishes waste generation and facilitates their utilization as valuable ingredients in the realms of functional cosmeceuticals and nutraceuticals.
Author Contributions
P.H.: Investigation, Writing—original draft preparation, Methodology, Data analysis. S.N.: Methodology, Project administration, and Supervision. N.P.: Resource and Writing—original draft preparation. W.S.: Conceptualization, Resource, Validation, and Writing—review and editing. A.K.: Methodology, Writing—review and editing, and Supervision. R.S.: Conceptualization, Validation, Writing (reviewing and editing), Visualization, and Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Chulalongkorn University Second Century Fund (C2F).
Institutional Review Board Statement
Research Unit in Bioconversion/Bioseparation for Value-Added Chemical Production, the Institute of Biotechnology and Genetic Engineering, Chulalongkorn University.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Acknowledgments
The authors are grateful to Starbucks, Bangkok, Thailand for supplying the spent coffee grounds used for the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sosalagere, C.; Adesegun Kehinde, B.; Sharma, P. Isolation and functionalities of bioactive peptides from fruits and vegetables: A reviews. Food Chem. 2022, 366, 130494. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Chun, B.-S. Subcritical water hydrolysis for the production of bioactive peptides from tuna skin collagen. J. Supercrit. Fluids 2018, 141, 88–96. [Google Scholar] [CrossRef]
- Cotabarren, J.; Rosso, A.M.; Tellechea, M.; Garcia-Pardo, J.; Rivera, J.L.; Obregon, W.D.; Parisi, M.G. Adding value to the chia (Salvia hispanica L.) expeller: Production of bioactive peptides with antioxidant properties by enzymatic hydrolysis with Papain. Food Chem. 2019, 274, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Sakdasri, W.; Arnutpongchai, P.; Phonsavat, S.; Bumrungthaichaichan, E.; Sawangkeaw, R. Pressurized hot water extraction of crude polysaccharides, β-glucan, and phenolic compounds from dried gray oyster mushroom. LWT 2022, 168, 113895. [Google Scholar] [CrossRef]
- Kheeree, N.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Choowongkomon, K.; Karnchanatat, A. ACE inhibitory peptides derived from de-fatted lemon basil seeds: Optimization, purification, identification, structure-activity relationship and molecular docking analysis. Food Funct. 2020, 11, 8161–8178. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Dwiecki, K.; Siger, A.; Tomaszewska-Gras, J.; Michalak, M.; Polewski, K. Contribution of phenolic acids isolated from green and roasted boiled-type coffee brews to total coffee antioxidant capacity. Eur. Food Res. Technol. 2016, 242, 641–653. [Google Scholar] [CrossRef]
- Sangtitanu, T.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Karnchanatat, A. Peptides obtained from edible mushrooms: Hericium erinaceus offers the ability to scavenge free radicals and induce apoptosis in lung cancer cells in humans. Food Funct. 2020, 11, 4927–4939. [Google Scholar] [CrossRef] [PubMed]
- Srimongkol, P.; Songserm, P.; Kuptawach, K.; Puthong, S.; Sangtanoo, P.; Thitiprasert, S.; Thongchul, N.; Phunpruch, S.; Karnchanatat, A. Sulfated polysaccharides derived from marine microalgae, Synechococcus sp. VDW, inhibit the human colon cancer cell line Caco-2 by promoting cell apoptosis via the JNK and p38 MAPK signaling pathway. Algal Res. 2023, 69, 102919. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).