Production of Protein Hydrolysates from Spent Coffee Grounds via Microwave, Enzymatic, and Subcritical Water Extractions and Their Combination †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Spent Coffee Ground (SCG) Samples
2.2. Protein Hydrolysate Production
2.3. Assays
2.3.1. Water Soluble Protein Content (WSPC) and Degree of Hydrolysis (%DH)
2.3.2. Total Phenolic Content, Antioxidant Capacity, and Total Polysaccharide Content
2.4. Experimental Design and Data Analysis
3. Results and Discussion
3.1. Preliminary Study of Protein Extraction Conditions
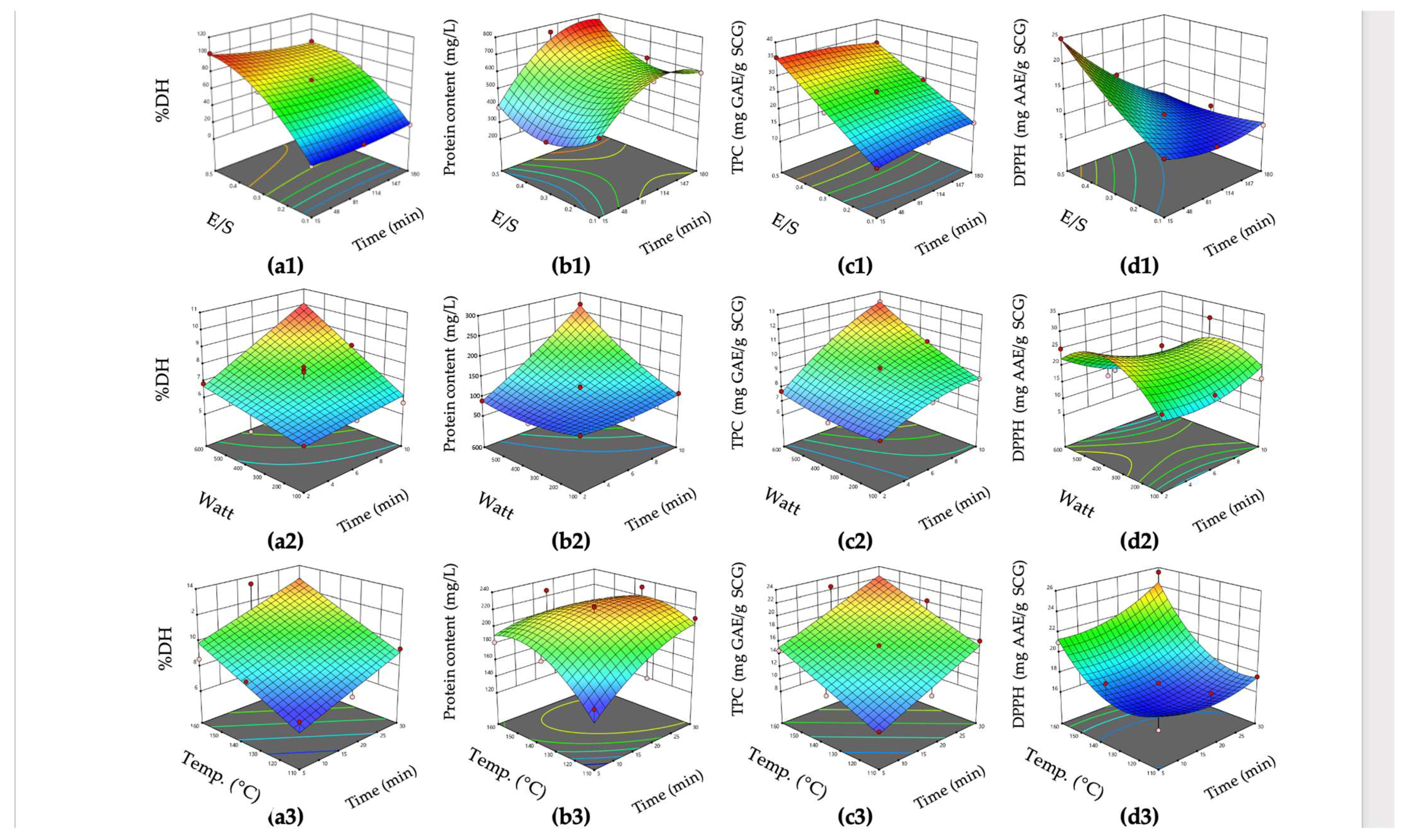
3.2. Process Optimization by RSM
3.3. Optimal Conditions and Combination Technique
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sosalagere, C.; Adesegun Kehinde, B.; Sharma, P. Isolation and functionalities of bioactive peptides from fruits and vegetables: A reviews. Food Chem. 2022, 366, 130494. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Chun, B.-S. Subcritical water hydrolysis for the production of bioactive peptides from tuna skin collagen. J. Supercrit. Fluids 2018, 141, 88–96. [Google Scholar] [CrossRef]
- Cotabarren, J.; Rosso, A.M.; Tellechea, M.; Garcia-Pardo, J.; Rivera, J.L.; Obregon, W.D.; Parisi, M.G. Adding value to the chia (Salvia hispanica L.) expeller: Production of bioactive peptides with antioxidant properties by enzymatic hydrolysis with Papain. Food Chem. 2019, 274, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Sakdasri, W.; Arnutpongchai, P.; Phonsavat, S.; Bumrungthaichaichan, E.; Sawangkeaw, R. Pressurized hot water extraction of crude polysaccharides, β-glucan, and phenolic compounds from dried gray oyster mushroom. LWT 2022, 168, 113895. [Google Scholar] [CrossRef]
- Kheeree, N.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Choowongkomon, K.; Karnchanatat, A. ACE inhibitory peptides derived from de-fatted lemon basil seeds: Optimization, purification, identification, structure-activity relationship and molecular docking analysis. Food Funct. 2020, 11, 8161–8178. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Dwiecki, K.; Siger, A.; Tomaszewska-Gras, J.; Michalak, M.; Polewski, K. Contribution of phenolic acids isolated from green and roasted boiled-type coffee brews to total coffee antioxidant capacity. Eur. Food Res. Technol. 2016, 242, 641–653. [Google Scholar] [CrossRef]
- Sangtitanu, T.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Karnchanatat, A. Peptides obtained from edible mushrooms: Hericium erinaceus offers the ability to scavenge free radicals and induce apoptosis in lung cancer cells in humans. Food Funct. 2020, 11, 4927–4939. [Google Scholar] [CrossRef] [PubMed]
- Srimongkol, P.; Songserm, P.; Kuptawach, K.; Puthong, S.; Sangtanoo, P.; Thitiprasert, S.; Thongchul, N.; Phunpruch, S.; Karnchanatat, A. Sulfated polysaccharides derived from marine microalgae, Synechococcus sp. VDW, inhibit the human colon cancer cell line Caco-2 by promoting cell apoptosis via the JNK and p38 MAPK signaling pathway. Algal Res. 2023, 69, 102919. [Google Scholar] [CrossRef]

| Methods | Factors | |
|---|---|---|
| X1 | X2 | |
| Microwave-assisted extraction | 100−600 watt (power) | 2−10 min (time) |
| Subcritical-assisted extraction | 110−160 °C (temperature) | 5−30 min (time) |
| Enzymatic-assisted extraction | 0.1−0.5 (E/S) | 15−180 min (time) |
| Methods | % DH | DPPH (IC50, µg/mL) | WSPC (µg/mL) | TPS (g GLU/100 g SCGs) | pH |
|---|---|---|---|---|---|
| EAE (0.5 E/S) | 7.00 ± 0.05 a | ||||
| 15 min | 93.39 ± 0.29 a | 34.17 ± 1.04 a | 474.46 ± 1.77 a | 30.49 ± 0.19 a | |
| 30 min | 86.34 ± 0.05 b | 36.59 ± 0.40 b | 515.60 ± 10.98 b | 33.66 ± 0.75 b | |
| 60 min | 89.64 ± 1.35 c | 38.92 ± 0.54 c | 628.57 ± 8.97 c | 30.02 ± 0.07 d | |
| MAE (350 W, 10 min) | 9.36 ± 0.13 d | 16.54 ± 0.99 d | 341.33 ± 5.66 d | 17.26 ± 0.78 e | 5.98 ± 0.03 b |
| SWE (160 °C, 30 min) | 12.73 ± 0.33 e | 15.37 ± 0.38 e | 158.00 ± 3.00 e | 18.26 ± 0.17 e | 4.97 ± 0.02 c |
| MAE−SWE | 14.39 ± 0.25 f | 25.93 ± 1.57 f | 320.12 ± 0.71 f | 27.76 ± 0.06 f | 4.92 ± 0.01 d |
| SWE−MAE | 13.60 ± 0.20 ef | 24.18 ± 1.19 g | 279.10 ± 6.65 g | 24.09 ± 0.28 g | 4.92 ± 0.02 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunsub, P.; Ngamprasertsith, S.; Prichapan, N.; Sakdasri, W.; Karnchanatat, A.; Sawangkeaw, R. Production of Protein Hydrolysates from Spent Coffee Grounds via Microwave, Enzymatic, and Subcritical Water Extractions and Their Combination. Biol. Life Sci. Forum 2023, 26, 43. https://doi.org/10.3390/Foods2023-15029
Hunsub P, Ngamprasertsith S, Prichapan N, Sakdasri W, Karnchanatat A, Sawangkeaw R. Production of Protein Hydrolysates from Spent Coffee Grounds via Microwave, Enzymatic, and Subcritical Water Extractions and Their Combination. Biology and Life Sciences Forum. 2023; 26(1):43. https://doi.org/10.3390/Foods2023-15029
Chicago/Turabian StyleHunsub, Panusorn, Somkiat Ngamprasertsith, Nattapong Prichapan, Winatta Sakdasri, Aphichart Karnchanatat, and Ruengwit Sawangkeaw. 2023. "Production of Protein Hydrolysates from Spent Coffee Grounds via Microwave, Enzymatic, and Subcritical Water Extractions and Their Combination" Biology and Life Sciences Forum 26, no. 1: 43. https://doi.org/10.3390/Foods2023-15029
APA StyleHunsub, P., Ngamprasertsith, S., Prichapan, N., Sakdasri, W., Karnchanatat, A., & Sawangkeaw, R. (2023). Production of Protein Hydrolysates from Spent Coffee Grounds via Microwave, Enzymatic, and Subcritical Water Extractions and Their Combination. Biology and Life Sciences Forum, 26(1), 43. https://doi.org/10.3390/Foods2023-15029








