Abstract
Red radish (Raphanus sativus L.) attracts interest, not only for its taste, but also for its health-promoting properties. Most of these properties are due to the presence of anthocyanins, glucosinolates and flavonols, whose benefits have been widely reported. However, little is known about how abiotic stress could affect the presence of these biomolecules in an adult plant of red radish. In the Region of Murcia, Spain, one of the main issues of concern, due to edaphoclimatic and economic factors, is salinity stress. This work aims to analyse the effect of salinity in red radish physiology and metabolism. A study based on hydroponic culture was designed to evaluate the effects of salinity (0, 40, 80, and 120 mM) on plant size, discerning between the bulb and the aerial part. Furthermore, RP-HPLC-DAD analysis was performed in order to determine secondary metabolites of red radish. Preliminary results suggest that salinity stress at high concentrations compromises the plant vegetative development. However, stresses are widely reported to stimulate the secondary metabolism, hindering the processes of finding a balance between nutritional value and production.
1. Introduction
Red radish (Raphanus sativus L.) is a root vegetable crop of the Brassicaceae family. Its root is consumed raw, in salad or pickled, and appreciated for its pungent flavour and crisp texture. In addition, it has been reported that this vegetable has plenty of phytomolecules that contribute to enhance human health, such as anthocyanins, vitamin C, and glucosinolates, as well as diverse micronutrients [1]. Specifically, glucosinolates not only regulate its final flavour (increasing its pungency from mild to strong), but also have been widely studied for their anti-tumorigenic, antioxidant and microbiome-regulating properties [2,3].
One of the main problems of soil degradation in culture areas under a semiarid Mediterranean climate is salinization. This process has been progressively increasing in the South-East of Spain during the last decades, leading to a decrease in soil quality, yield and production, and even to land abandonment [4]. Plant physiology and metabolite production could be affected by this changes in soil conditions, and the final flavour could even be influenced if the glucosinolate production is altered [5]. Research performed in Brassica species has demonstrated that salinity mainly increased glucosinolates production, with few impact in its yield [6]. However, little to no information is available about the effect of soil salinity in red radish. Studies performed under drought stress reported a decrease in total glucosinolate concentration [5].
In brief, the main aim of this work is to elucidate the effect of salinity stress in red radish physiological development and secondary metabolism.
2. Experiments
2.1. Growing Conditions
Fifty red radish seeds (Raphanus sativus L.), from SAKATA Seed Iberica S.L.U., were prehydrated and airated in de-ionized water during 24 h. Then, seeds were transferred to vermiculite and kept in an incubator at 37 °C and darkness for 2 days. Seedlings were transferred to hydroponic culture with complete Hoagland Solution. When plants reached a 5–leaves stage (aprox. 3 weeks) they were harvested, and leaves and the edible part (root) were processed separately. Samples were kept at −80 °C for further freeze-drying.
2.2. Extraction and Determination of Glucosinolates
One hundred milligrams of each freeze-dried powder was extracted with 1 mL of methanol 70% (v/v) and heated in a bath at 70 °C for 30 min. Samples were centrifuged at 17,500× g for 15 min. Supernatants were collected, filtered through a 0.22 µm Millex-millipore filter (Millipore, Billerica, MA, USA), and kept in vials. The ESI-HPLC-DAD-MS2 fragmentation was analysed in order to identify the different glucosinolates present in the red radish. The equipment employed was a Luna C18 100A column (150 × 1.0 mm, 3 µm particle size; Phenomenex Macclesfield, UK). Experimental conditions are described in Garcia-Ibañez et al. [7]. For further quantification intact glucosinolates were identified according to their spectra and elution order, sinigrin and glucobrassicin were employed as external standards (Phytochem, Neu-Ulm, Germany).
2.3. Statistical Analysis
Statistical analysis was performed using a one-way ANOVA and a HSD Tukey as a post hoc test. All the analyses were performed in RStudio (version 4.0.2).
3. Results
3.1. Fresh Weight Determination
Figure 1 shows the influence of three different NaCl concentrations (40, 80, 120 mM) in the red radish fresh weight. According to the aerial part, a decrease by 2 times was observed when the treatments were compared with the control samples (p < 0.05). Nevertheless, no statistically significant differences were observed between the different saline treatments for the aerial part biomass (p > 0.05). Regarding to the roots, no statistically significant differences were observed between control, 40 and 80 mM NaCl (p > 0.05). However, when comparing the red radish roots from 120 mM NaCl treatment with the control, fresh weight decreased by a 38% (p < 0.05). Finally, whole plant (including the complete root, not only the edible part) fresh weight was measured. A statistically significant decrease was observed between control plants and each treatment (p < 0.05). Specifically, treatments with 40 and 80 mM NaCl showed a similar decrease in a 35% when compared with control samples (p < 0.05). A decrease by a 63% was observed when compared the whole plant fresh weight from 120 mM NaCl with the control (p < 0.05).
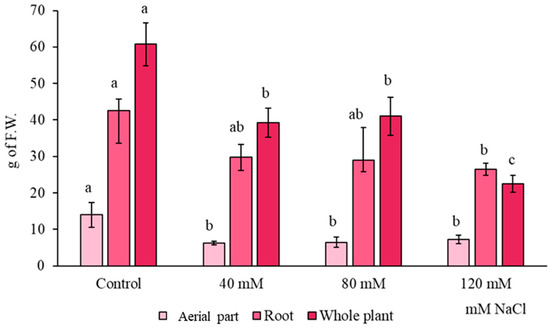
Figure 1.
Influence of salinity (40, 80, and 120 mM) on fresh weight (g) from aerial part, roots and whole plant of red radish (n = 5 ± SD). Different letters indicate statistically significant differences in the HSD Tukey test (p < 0.05).
3.2. Effect of Salinity in Glucosinolates Accumulation
Salinity effects in glucosinolate accumulation is showed in Figure 2. According to aliphatic glucosinolates accumulation (Figure 2A), concentrations were higher by 1.5 times in red radish roots under control conditions, when compared to the aerial part (p < 0.05). A 30% decrease was observed in the aerial part total aliphatic concentration when a treatment of 40 mM NaCl was applied (p < 0.05). Furthermore, under the same saline concentrations, a decrease of 55% was detected (p < 0.05). However, no remarkable statistically significant differences were present in the treatments with 80 and 120 mM NaCl when compared to 40 mM samples (p > 0.05). Moreover, no differences were observed between the aerial part and the roots when the three salinity treatments were applied (p > 0.05).
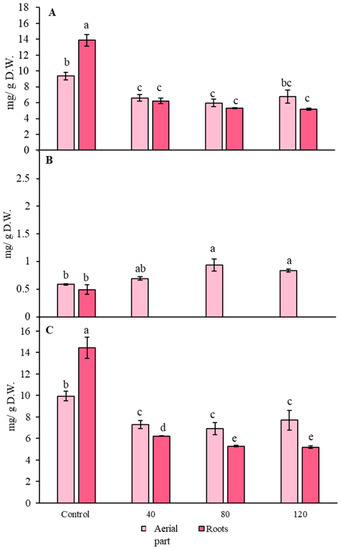
Figure 2.
Presence of glucosinolates (mg/g D.W.) in the aerial part and roots under salinity conditions (0, 40, 80 and 120 mM). Quantification is from (A) Aliphatic; (B) indolic and (C) total amount of glucosinolates (n = 5 ± SD). Different letters indicate statistically significant differences in the HSD Tukey test (p < 0.05).
Total indolic glucosinolate concentration is represented in Figure 2B. No statistically significant differences were found between the aerial part and roots in control plants (p > 0.05). Surprisingly, no indolic glucosinolates were detected in a quantifiable amount in roots from samples under salinity stress (40, 80 and 120 mM, p < 0.05). For the aerial part, an increase by 1.6 times was appreciated in red radish plans grown in 80- and 120-mM conditions (p < 0.05). However, no statistically significant differences were observed between 40 mM treatment and the control aerial parts (p > 0.05).
Finally, total glucosinolate concentration was analysed (Figure 2C). Highest total concentrations were found in the control aerial part and roots (p < 0.05). A decrease by 26% was perceived in the aerial part when a treatment of 40 mM NaCl was employed (p < 0.05). However, no differences were found for the aerial part under the three saline concentrations (40, 80 and 120 mM). A decrease by 2 times was observed when comparing 40 mM NaCl roots with control (p < 0.05). In addition, a decrease of 15% was produced in total glucosinolate accumulation in roots when NaCl concentrations were increased up to 80 and 120 mM (p < 0.05). Nevertheless, no statistically significant differences were observed between those concentrations neither in roots nor the aerial part (p > 0.05).
4. Discussion
Salinity stress is one of the main concerns in the Region of Murcia agronomic practices, not only due to its increase by edaphoclimatic conditions, but also for its economic impact. Nevertheless, little information is available about the effect of salinity in red radish (Rapahanus sativus L.) yield and production.
Previous works performed on broccoli plants showed a decrease by 60% in the fresh weight of the aerial part when 40 mM NaCl treatments were applied [8]. In contrast, the results obtained in this work showed no statistically significant differences in the aerial part until 120 mM NaCl was applied. This could be due to the different physiognomy of both plants, even belonging to the same family (Brassicaceae).
About the presence of glucosinolates, broccoli experiments performed under field conditions in a semiarid Mediterranean climate showed no statistically significant differences between control and salinity treatments in both leaves and roots [9]. However, in our experiments, main decrease in total aliphatic glucosinolates concentration was observed between control and 40 mM NaCl plants (Figure 2A).
However, salinity in broccoli favoured the accumulation of indolic glucosinolates in the aerial part [9], which is similar to the pattern found in red raddish leaves (Figure 2B). Nevertheless, broccoli plants also showed an increase in roots measurements, which contrast the dramatically decrease observed in red radish roots. This could be due to the thickened root (bulb) morphology that shows the red radish. Similar results were observed in total glucosinolates content in broccoli demonstrating that this vegetable is able to accumulate this biomolecules under high salinity conditions [6]. Differences showed in red raddish could suggest a remobilization of these glucosinolates, perhaps secreted to the media or soil under this stress condition. Since glucosinolates exudation has been described under biostimulation conditions, it might be possible that saline stress triggers a similar response [9]. In addition, it must be taken into account that the experiments carried out in broccoli were perfomed in field where other factors might interfere, such as soil characteristics and climatic conditions. Since our experiment was performed under controlled conditions, the results reflect only the effect of salinity in red radish metabolism.
5. Conclusions
In brief, our study has shown the negative the influence of high salinity concentrations in red radish biomass and yield. Furthermore, the type of glucosinolate which is synthetized under each salinity level, and in each organ, could be an important factor of the metabolic response that should be further studied.
Author Contributions
The experiments were designed by D.A.M. and M.C.; P.G.-I. carried out the experimental part. The formal data analysis was performed by P.G.-I. and D.A.M., P.G.-I. wrote the first draft of the manuscript and D.A.M. and M.C. revised it. Funding acquisition by M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Spanish Ministry of Economy, Industry and Competitiveness through the Strategic Programme “CIEN”, Ref-IDI-20170842 (BIOTAGUT). Co-author P. Garcia-Ibañez (P.G.-I.) was funded by a competitive predoctoral grant fom the Fundación Séneca-Regional Government of Murcia (CARM), Ref. 21273/FPI/19.
Data Availability Statement
The data are available on request to the corresponding author.
Acknowledgments
The authors acknowledge SAKATA Seed Iberica S.L.U. for gently providing the seeds used in this work.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DW | dry weight |
| SD | standard deviation |
References
- Mahmoud, S.H.; Salama, D.; El-Tanahy, A.M.; El-Samad, E.H.A. Utilization of seaweed (Sargassum vulgare) extract to enhance growth, yield and nutritional quality of red radish plants. Ann. Agric. Sci. 2019, 64, 167–175. [Google Scholar] [CrossRef]
- Miękus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Swiergiel, A.H. Health Benefits of Plant-Derived Sulfur Compounds, Glucosinolates, and Organosulfur Compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Hoeflinger, J.L.; Neme, B.P.; Jeffery, E.H.; Miller, M.J. Dietary Broccoli Alters Rat Cecal Microbiota to Improve Glucoraphanin Hydrolysis to Bioactive Isothiocyanates. Nutrients 2017, 9, 262. [Google Scholar] [CrossRef] [Green Version]
- Acosta Avilés, J.A.; Faz Cano, A.; Martínez-Martínez, S. El Impacto de la Ganadería y la Agricultura en los Ecosistemas Terrestres.—Efecto de la Actividad Agrícola en Los contenidos de Sales en Suelos de Murcia: Comparación con Otros Usos de Suelo. In Congreso Internacional Sobre Desertificación; Universidad de Murcia: Murcia, Spain, 2020. [Google Scholar]
- Schlering, C.; Dietrich, H.; Frisch, M.; Schreiner, M.; Schweiggert, R.; Will, F.; Zinkernagel, J. Chemical composition of field grown radish (Raphanus sativus L. Var. Sativus) as influenced by season and moderately reduced water supply. J. Appl. Bot. Food Qual. 2019, 92, 343–354. [Google Scholar] [CrossRef]
- Rios, J.J.; Agudelo, A.; Moreno, D.A.; Carvajal, M. Growing broccoli under salinity: The influence of cultivar and season on glucosinolates content. Sci. Agric. 2020, 77, 20190028. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Ibañez, P.; Moreno, D.A.; Nuñez-Gomez, V.; Agudelo, A.; Carvajal, M. Use of elicitation in the cultivation of Bimi® for food and ingredients. J. Sci. Food Agric. 2019, 100, 2099–2109. [Google Scholar] [CrossRef]
- López-Berenguer, C.; Ballesta, M.M.; Moreno-Fernández, D.Á.; Carvajal, M.; Garcia-Viguera, C. Growing Hardier Crops for Better Health: Salinity Tolerance and the Nutritional Value of Broccoli. J. Agric. Food Chem. 2009, 57, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.; Pascual, J.; Guillen, M.; Lopez-Martinez, A.; Carvajal, M. Influence of foliar Methyl-jasmonate biostimulation on exudation of glucosinolates and their effect on root pathogens of broccoli plants under salinity condition. Sci. Hortic. 2021, 282, 110027. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).