Elucidation of the Radiosensitivity Level of Amorphophallus paeoniifolius (Dennst.) Nicolson Embryogenic Callus Induced by Gamma Ray Irradiation †
Abstract
1. Introduction
2. Experiments
3. Results and Discussion
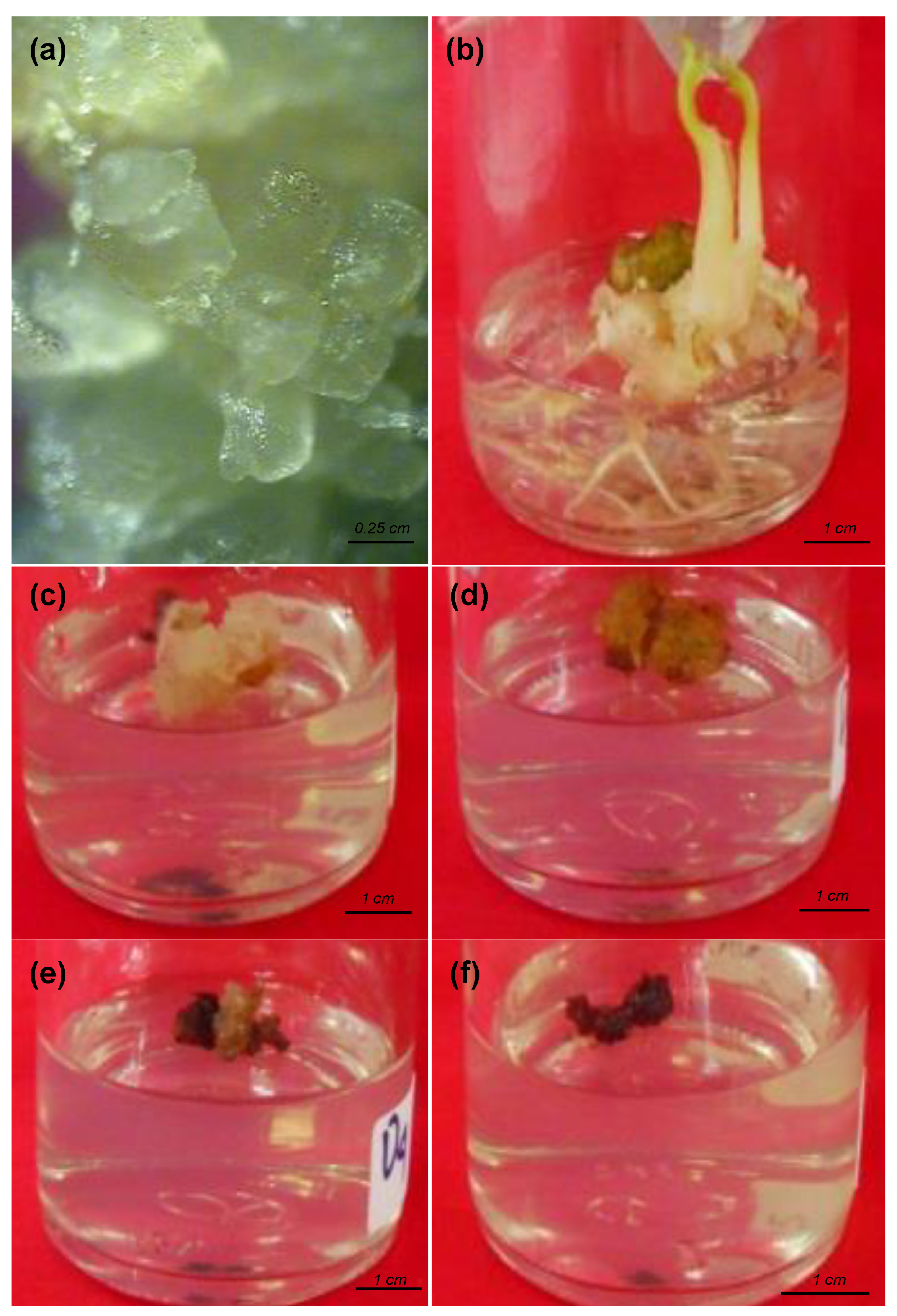
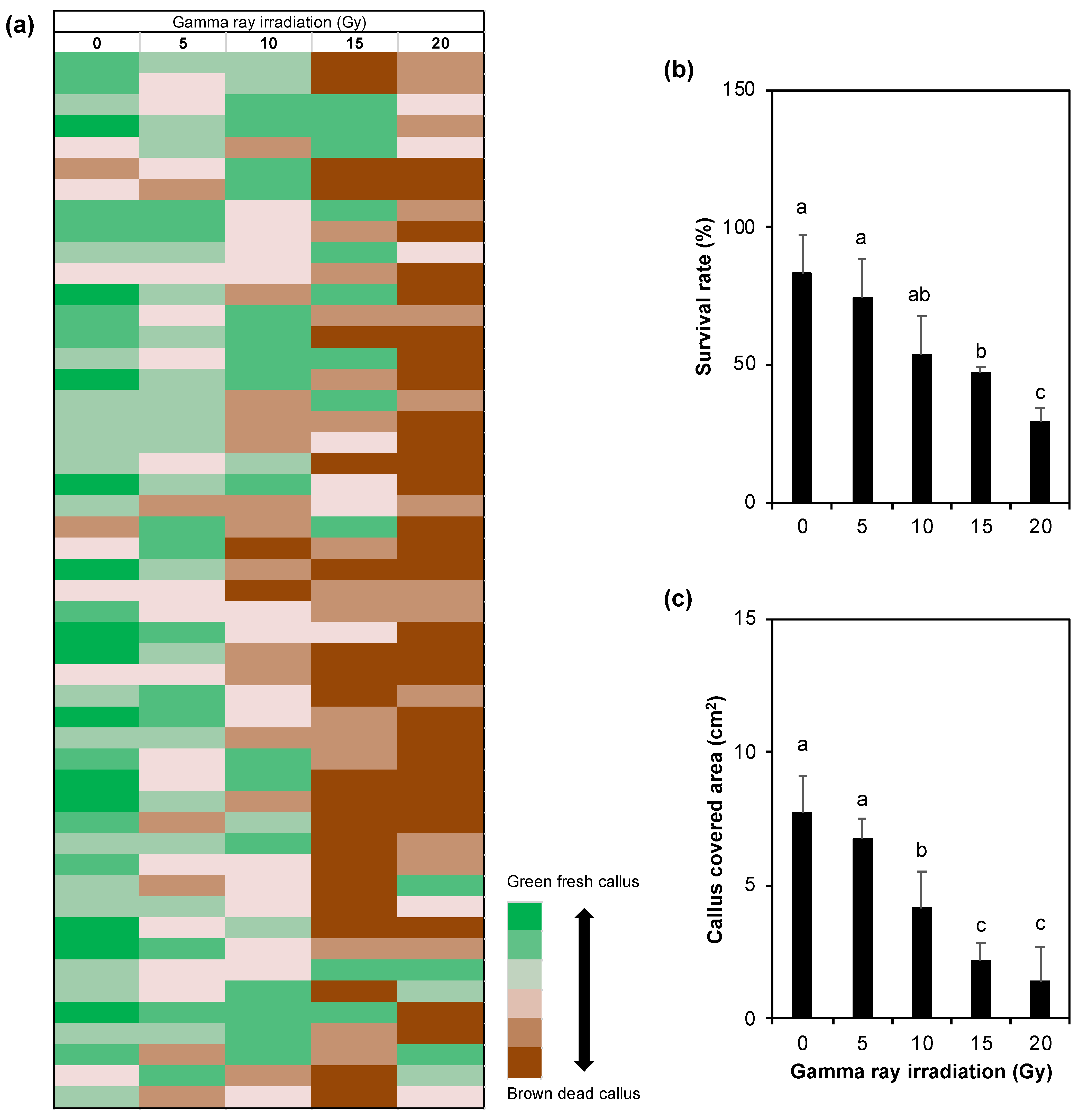
3.1. Embryogenic Callus Survival Rate
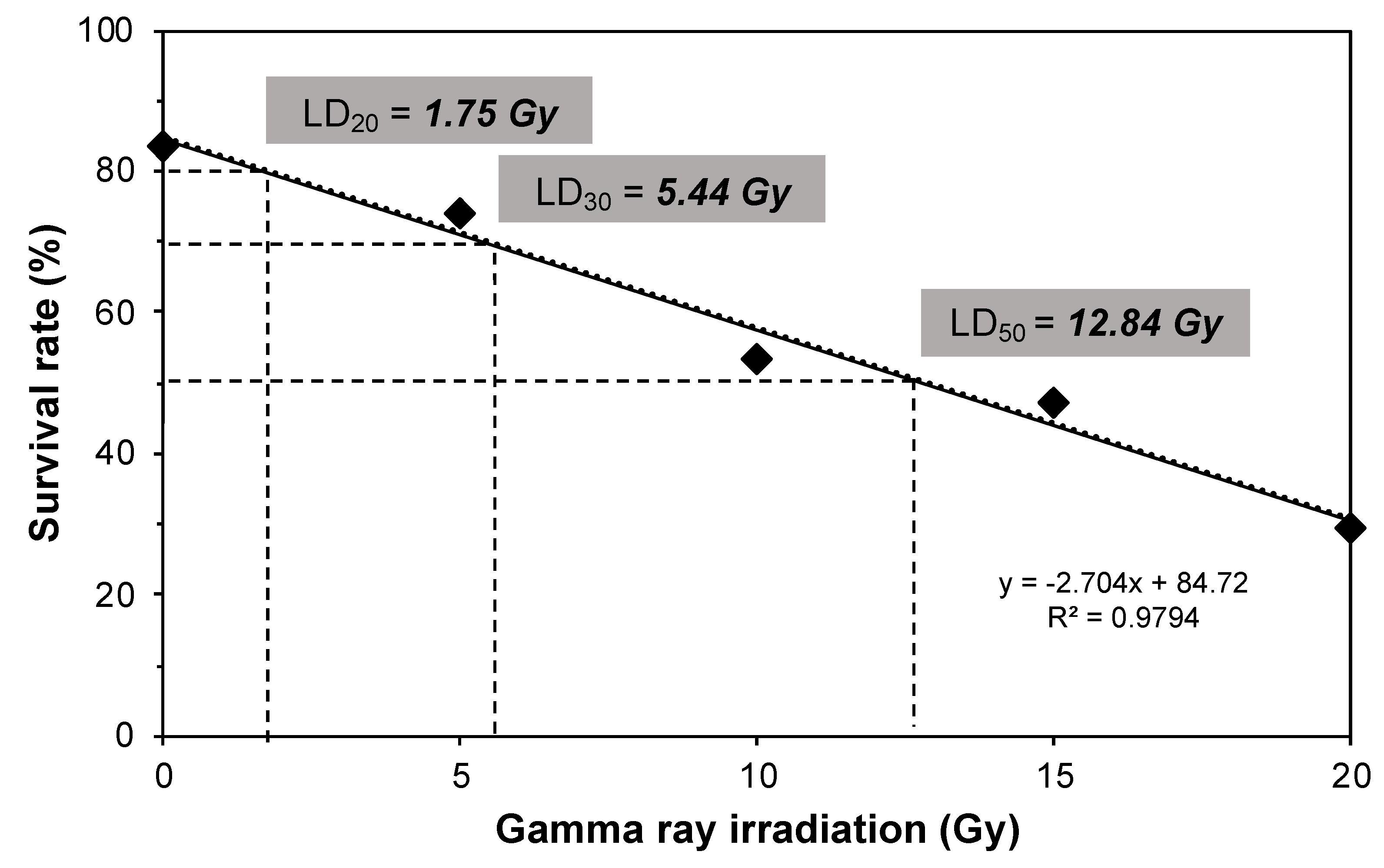
3.2. Radiosensitivity Level of the Embryogenic Callus
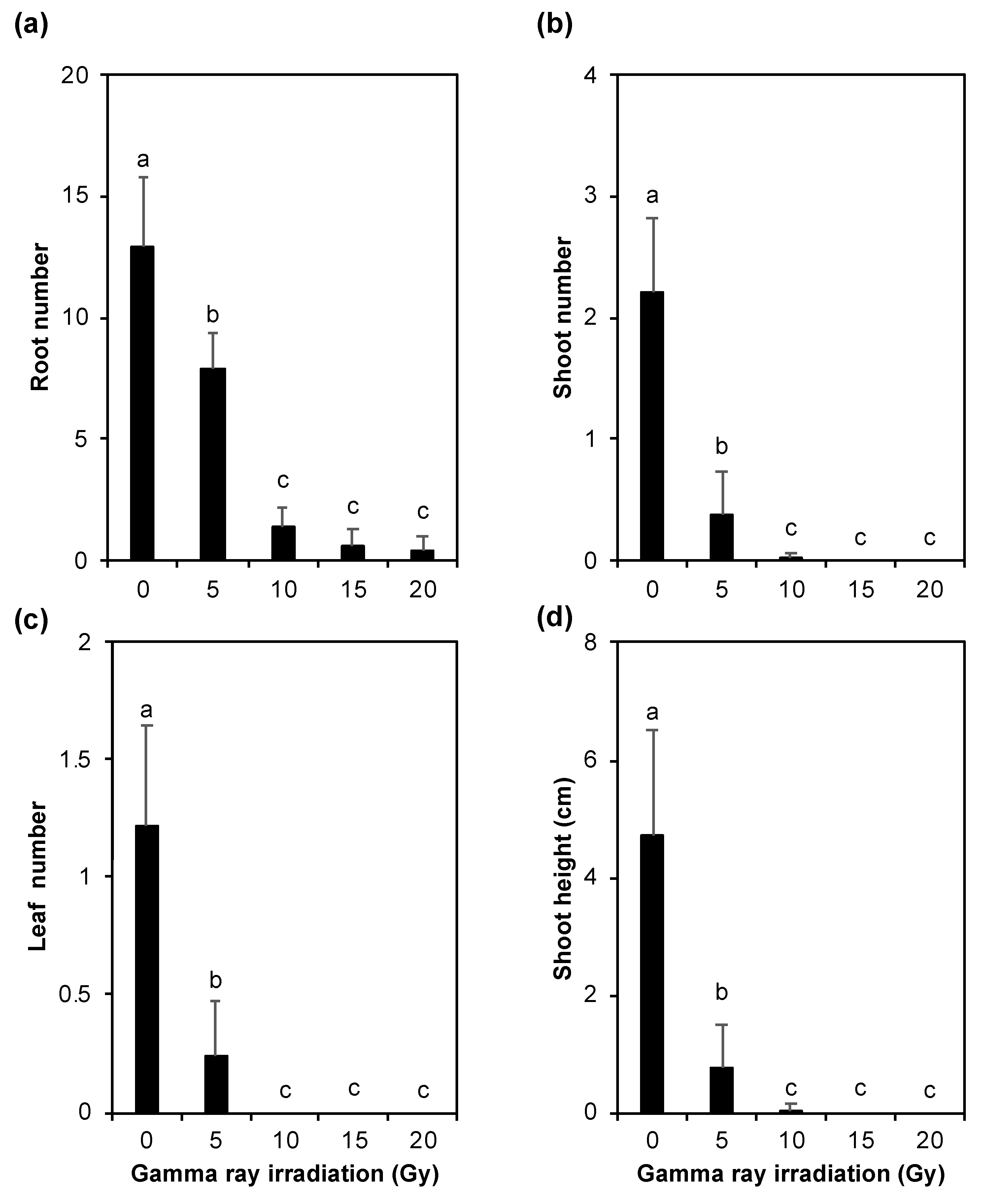
3.3. Growth of Plantlets
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santosa, E.; Lian, C.L.; Sugiyama, N.; Misra, R.S.; Boonkorkaew, P.; Thanomchit, K. Population structure of elephant foot yams (Amorphophallus paeoniifolius (Dennst.) Nicolson) in Asia. PLoS ONE 2017, 12, e0180000. [Google Scholar] [CrossRef] [PubMed]
- Angayarkanni, J.; Ramkumar, K.M.; Priyadharshini, U.; Ravendran, P. Antioxidant potential of Amorphophallus paeoniifolius in relation to their phenolic content. Pharm. Biol. 2010, 48, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Hurkadale, P.J.; Shelar, P.A.; Palled, S.G.; Mandavkar, Y.D.; Khedkar, A.S. Hepatoprotective activity of Amorphophallus paeoniifolius tubers against paracetamol-induced liver damage in rats. Asian. Pac. J. Trop. Biomed. 2012, 2, S238–S242. [Google Scholar] [CrossRef]
- Morvin, Y.J.E.; Prabhu, S.; Vijayakumar, S. An ethnobotanical study of medicinal plants used by traditional healers in silent valley of Kerala, India. J. Ethnopharmacol. 2014, 154, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Dey, Y.N.; Wanjari, M.M.; Kumar, D.; Lomash, V.; Jadhav, A.D. Curative effect of Amorphophallus paeoniifolius tuber on experimental hemorrhoids in rats. J. Ethnopharmacol. 2016, 192, 183–191. [Google Scholar] [CrossRef]
- Reddy, C.K.; Suriya, M.; Vidya, P.V.; Vijina, K.; Haripriya, S. Effect of γ-irradiation on structure and physico-chemical properties of Amorphophallus paeoniifolius starch. Int. J. Biol. Macromol. 2015, 79, 309–315. [Google Scholar] [CrossRef]
- Sukhija, S.; Singh, S.; Riar, C.S. Effect of oxidation, cross-linking and dual modification on physicochemical, crystallinity, morphological, pasting and thermal characteristics of elephant foot yam (Amorphophallus paeoniifolius) starch. Food Hydrocoll. 2016, 55, 56–64. [Google Scholar] [CrossRef]
- Suriya, M.; Rajput, R.; Reddy, C.K.; Haripriya, S.; Bashir, M. Functional and physicochemical characteristics of cookies prepared from Amorphophallus paeoniifolius flour. J. Food Sci. Technol. 2017, 54, 2156–2165. [Google Scholar] [CrossRef]
- Suriya, M.; Reddy, C.K.; Haripriya, S.; Harsha, N. Influence of debranching and retrogradation time on behavior changes of Amorphophallus paeoniifolius nanostarch. Int. J. Biol. Macromol. 2018, 120, 230–236. [Google Scholar] [CrossRef]
- Yuzammi. The diversity of aroids (Araceae) in Bogor Botanic Gardens, Indonesia: Collection, conservation and utilization. Biodiversitas 2018, 19, 140–152. [Google Scholar] [CrossRef]
- Nagar, M.; Sharanagat, V.S.; Kumar, Y.; Singh, L.; Mani, S. Influence of xanthan and agar-agar on thermo-functional, morphological, pasting and rheological properties of elephant foot yam (Amorphophallus paeoniifolius) starch. Int. J. Biol. Macromol. 2019, 136, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Mutaqin, A.Z.; Kurniadie, D.; Iskandar, J.; Nurzaman, M.; Partasasmita, R. Ethnobotany of suweg, Amorphophallus paeoniifolius: Utilization and cultivation in West Java, Indonesia. Biodiversitas 2020, 21, 1635–1644. [Google Scholar]
- Ravi, V.; Ravindran, C.; Suja, G. Growth and productivity of elephant foot yam (Amorphophallus paeoniifolius (Dennst.) Nicolson): An overview. J. Root Crop 2009, 35, 131–142. [Google Scholar]
- Bradbury, J.H.; Nixon, R.W. The acridity of raphides from the edible aroids. J. Sci. Food Agric. 1998, 76, 608–616. [Google Scholar] [CrossRef]
- Singh, A.K.; Chaurasiya, A.K.; Mitra, S. Oxalate content in elephant foot yam (Amorphophallus paeoniifolius Dennst.-Nicolson) dry and fry cubes. J. Pharma. Phytochem. 2018, 7, 2905–2909. [Google Scholar]
- Abraham, L.N.; Kamala, S.; Sreekumar, J.; Makeshkumar, T. Optimization of parameters to improve transformation efficiency of elephant foot yam (Amorphophallus paeoniifolius (Dennst.) Nicolson. 3 Biotech 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Ahloowalia, B.S.; Maluszynski, M.; Nichterlein, K. Global impact of mutation-derived varieties. Euphytica 2004, 135, 187–204. [Google Scholar] [CrossRef]
- Cheng, L.; Yang, H.; Lin, B.; Wang, Y.; Li, W.; Wang, D.; Zhang, F. Effect of gamma-ray radiation on physiological, morphological characters and chromosome aberrations of minitubers in Solanum tuberosum L. Int. J. Radiat. Biol. 2010, 86, 791–799. [Google Scholar] [CrossRef]
- Khumaida, N.; Maharani, S.; Ardie, S.W. The leaf color performance on several lines of cassava and its relation with tuber yield as early reference. Procedia Environ. Sci. 2015, 24, 39–46. [Google Scholar] [CrossRef][Green Version]
- Fadli, N.; Syarif, Z.; Satria, B.; Akhir, N. The effect of gamma Cobalt-60 ray irradiation on cultivar growth in taro white (Xhanthosoma sagittifolium L.). Int. J. Environ. Agric. Biotechnol. 2018, 3, 2020–2025. [Google Scholar] [CrossRef]
- Nurilmala, F.; Hutagaol, R.P.; Widhyastini, I.M.; Widyastuti, U.; Suharsono, S. Somaclonal variation induction of bogor taro (Colocasia esculenta) by gamma irradiation. Biodiversitas 2017, 18, 28–33. [Google Scholar] [CrossRef]
- Sianipar, N.F.; Maarisit, W. Detection of gamma-irradiated mutant of rodent tuber (Typhonium flagelliforme Lodd.) in vitro culture by RAPD molecular marker. Procedia Chem. 2015, 14, 285–294. [Google Scholar] [CrossRef]
- Kuranouchi, T.; Kumazaki, T.; Kumagai, T.; Nakatani, M. Breeding erect plant type sweetpotato lines using cross breeding and gamma-ray irradiation. Breed Sci. 2016, 66, 456–461. [Google Scholar] [CrossRef]
- Poerba, Y.S.; Imelda, M.; Wulansari, A.; Martanti, D. Induksi mutasi kultur in vitro Amorphophallus muelleri Blume dengan irradiasi gamma (in Indonesian). J. Tek. Ling. 2009, 10, 355–364. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Karp, A. Mechanisms of somaclonal variation. Biotechnol. Biotechnol. Equip. 1993, 7, 20–25. [Google Scholar] [CrossRef]
- Hutami, S.; Mariska, I.; Supriati, Y. Peningkatan keragaman genetik tanaman melalui keragaman somaklonal (in Indonesian). J. AgroBiogen 2016, 2, 81. [Google Scholar] [CrossRef]
- Krishna, H.; Alizadeh, M.; Singh, D.; Singh, U.; Chauhan, N.; Eftekhari, M.; Sadh, R.K. Somaclonal variations and their applications in horticultural crops improvement. 3 Biotech 2016, 6, 54. [Google Scholar] [CrossRef]
- Sparrow, A.H.; Schwemmer, S.S.; Bottino, P.J. Effects of external gamma radiation from radioactive fallout on plants with special reference to crop production. Radiat. Bot. 1971, 11, 85–118. [Google Scholar] [CrossRef]
- Hayati, D.; Aisyah, S.I.; Krisantini. Radiosensitivity levels of in vitro cultured Celosia cristata planlets by γ—ray irradiation. J. Trop. Crop Sci. 2016, 3, 61–65. [Google Scholar] [CrossRef]
- Isnaini, Y.; Novitasari, Y. Penentuan kisaran dosis iradiasi gamma optimal dalam pemuliaan mutasi Nepenthes ampullaria Jack. secara in vitro (in Indonesian). J. Ilm. Apl. Isot. dan Radiasi 2020, 16, 15–22. [Google Scholar]
- Astuti, D.; Sulistyowati, Y.; Nugroho, S. Uji radiosensitivitas sinar gamma untuk menginduksi keragaman genetik sorgum berkadar lignin tinggi (in Indonesian). J. Ilm. Apl. Isot. dan Radiasi 2019, 15, 1–6. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivai, R.R.; Isnaini, Y.; Yuzammi. Elucidation of the Radiosensitivity Level of Amorphophallus paeoniifolius (Dennst.) Nicolson Embryogenic Callus Induced by Gamma Ray Irradiation. Biol. Life Sci. Forum 2022, 11, 93. https://doi.org/10.3390/IECPS2021-11951
Rivai RR, Isnaini Y, Yuzammi. Elucidation of the Radiosensitivity Level of Amorphophallus paeoniifolius (Dennst.) Nicolson Embryogenic Callus Induced by Gamma Ray Irradiation. Biology and Life Sciences Forum. 2022; 11(1):93. https://doi.org/10.3390/IECPS2021-11951
Chicago/Turabian StyleRivai, Reza Ramdan, Yupi Isnaini, and Yuzammi. 2022. "Elucidation of the Radiosensitivity Level of Amorphophallus paeoniifolius (Dennst.) Nicolson Embryogenic Callus Induced by Gamma Ray Irradiation" Biology and Life Sciences Forum 11, no. 1: 93. https://doi.org/10.3390/IECPS2021-11951
APA StyleRivai, R. R., Isnaini, Y., & Yuzammi. (2022). Elucidation of the Radiosensitivity Level of Amorphophallus paeoniifolius (Dennst.) Nicolson Embryogenic Callus Induced by Gamma Ray Irradiation. Biology and Life Sciences Forum, 11(1), 93. https://doi.org/10.3390/IECPS2021-11951





