Abstract
Myo-inositol is considered as an important osmoprotectant, which is directly involved in abiotic stress management in plants. We have biochemically and functionally characterized the inositol monophosphatase (CaIMP1) and IMP-like proteins (CaIMPL1 and CaIMPL2) from chickpea (Cicer arietinum). We had already reported the broad substrate specificity of CaIMP1 as determined through biochemical characterization. Our work also signifies the role of CaIMPL2 in the histidine pathway as it was able to catalyze the dephosphorylation of histidinol 1-P; however, IMPL1 was mostly involved in the hydrolysis of D-Ins 1-P and D Gal 1-P. As decoded by sequence similarity and phylogenetic study, CaIMP, CaIMPL1, and CaIMPL2 were found to be homologous enzymes, but we observed very contrasting differences in their substrate specificity, which may be the result of the divergent evolution of these enzymes.
1. Introduction
Inositol metabolism has been shown to play a crucial role in chickpeas under environmental stress. Increased myo-inositol content as well as a higher relative expression of myo-inositol 1-phosphate synthase (MIPS) has also been reported in chickpea []. In the myo-inositol biosynthesis pathway, the second step of the pathway, which is the conversion of inositol 1-phosphate to myo-inositol, is catalyzed by myo-inositol monophosphatase (IMP).
Chickpea being an important crop of the Asia-Pacific region endures many environmental stresses during its growth period, but it is considered as relatively tolerant towards abiotic stresses. Hence, chickpea is widely reported as an important crop for the genetic resources of tolerant genes against diverse abiotic stresses []. Much work has been done on the MIPS gene, while little is known about the detailed function and metabolic regulation of IMP in chickpea. We have isolated IMP (CaIMP) and two IMP-like protein genes (CaIMPL1 and CaIMPL2) from drought-tolerant legume chickpea (Cicer arietinum). We have already characterized the chickpea inositol monophosphatase (CaIMP1) both biochemically and functionally, and we also reported its peculiar broad substrate specificity []. Furthermore, we have overexpressed it in Arabidopsis thaliana and these overexpression lines of CaIMP1 were found to be tolerant to a variety of abiotic stresses. We have also shown improved seed germination under these abiotic stresses []. In this article, we explore the expression profiling and biochemical parameters of CaIMPL1 and CaIMPL2 recombinant proteins to obtain some information regarding the role of these IMP family enzymes in plant metabolism.
2. Materials and Methods
2.1. Isolation of Chickpea IMPL Genes along with Its cDNA and Sequence Study
Cicer arietinum cv. BGD72 was used and was procured from the Indian Agricultural Research Institute (IARI), New Delhi, India. Leaves from chickpea plant were used to extract total RNA using Tri reagent (Sigma, St. Louis, MO, USA) and cDNA was prepared using DNaseI treated RNA with random primers following the protocol of the cDNA synthesis kit (Applied Biosystems, Foster City, CA, USA). Amplification of full-length cDNA of CaIMPL1 and CaIMPL2 was performed using gene-specific primers. Sequence information was obtained from NCBI with Accession No. XM_004489614 (IMPL1) and XM_004494897 (IMPL2), followed by cloning into pJET 1.2 blunt vector (Thermo Scientific, Waltham, MA, USA). To obtain the information of genomic structure of genes (CaIMPL1 and CaIMPL2), PCR was performed using genomic DNA isolated from chickpea plant. Furthermore, the PCR product was eluted and cloned in pJET cloning vector (Thermo Scientific, Waltham, MA, USA) and it was followed by subsequent sequencing for sequence confirmation. All primer sequences are provided in Table A1 (Appendix A).
2.2. Expression Pattern of IMP Gene Family in Different Environmental Stresses and Different Tissues/Organs
Chickpea seeds were germinated, and 6-days-old seedlings were subjected to different abiotic stress treatments for 6 h (salt (150 mM), dehydration, cold (4 °C), heat (42 °C)), hormone treatment, 100 µM each (Abscisic acid, Salisylic acid, Auxin, Gibberellic acid) for 6 h, and the total chickpea RNA was isolated from the whole seedling using TRI reagent (Sigma, St Louis, MO, USA) from these seedlings. The method reported by Boominathan et al. (2004) was used for stress and hormone treatment []. An amount of 2 µg total RNA was used for cDNA preparation using ABI cDNA synthesis kit (Applied Biosystems, Foster City, CA, USA). With the help of Real Time PCR (qRT-PCR), expression analysis was performed for CaIMPL1 and CaIMPL2 along with CaIMP. Apart from this, to obtain an idea for the spatial expression of CaIMPL1 and CaIMPL2, genes in different organs of chickpea plant (root, stem, flower, seed) were used for total RNA isolation, which was followed by cDNA preparation, and then the expression analysis was performed. Real-time PCR reactions were set up on ABI Step one real-time PCR using real-time primers for CaIMPL1, CaIMPL2, and CaIMP1. Primer sequences are provided in Table A1 (Appendix A). The expression of CaIMPL1, CaIMPL2, and CaIMP1 mRNA was normalized to the expression of endogenous control 18S rRNA. For each real-time PCR reaction, 20 µL of a reaction mixture containing 2 µL of diluted RT product, 10 µL of SYBR Green PCR master mix (Applied Biosystems, Foster City, CA, USA), and 20 pico-molar of each forward and reverse primer was added. A negative control lacking RT enzyme was also included in each assay. The relative expression value of each transcript in all experiments was calculated using the ΔΔCT method.
2.3. Bacterial Overexpression, Purification, and Biochemical Characterization of CaIMPL1 and CaIMPL2 Gene(s) Product
For generating recombinant protein, the cDNA of CaIMPL1 and CaIMPL2 was subcloned into the expression vector pET-28a (Novagen, Madison, WI, USA) with His-tag. Then, the resulting plasmid was transferred to the E. coli BL-21 (DE3) cells []. Sequence was confirmed through sequencing and restriction digestion. Transformed E. coli cells were induced and culture was sonicated; the extracts were analyzed for expression on SDS-PAGE []. The solubilization and purification of the recombinant, which expressed as inclusion bodies, were then solubilized by the procedure reported by Majee et al. (2004) [] and the purification of protein of interest was performed using affinity chromatography (GE Healthcare, Sweden). The purified fractions of CaIMPL1 and CaIMPL2 proteins were used for biochemical characterization using malachite green dye-based inorganic phosphate estimation assay [].
3. Results
3.1. Molecular Cloning, Sequencing, and Characterization of CaIMPL1 and CaIMPL2 Genes and cDNA(s)
The amplification of full-length cDNA of CaIMPL1 and CaIMPL2 was performed using gene-specific primers followed by cloning into pJET 1.2 blunt vector (Thermo Scientific, Waltham, MA, USA) (Figure 1). The full-length sequence (CDS) of CaIMPL1 and CaIMPL2 was obtained from NCBI with Accession No. XM_004489614 (IMPL1) and XM_004494897 (IMPL2). The insert was confirmed through colony PCR and restriction digestion. The full-length sequence of CaIMPL1 including the 5′ and 3′ UTR was found to be 1329 bp, containing a coding region of 1044 bp, which encodes a polypeptide of 347 aa. The full-length sequence of CaIMPL2 is 1234 bp in length (including the 5′ and 3′ UTR), containing a coding region of 894 bp, which encodes a polypeptide of 297 aa (Figure 2).
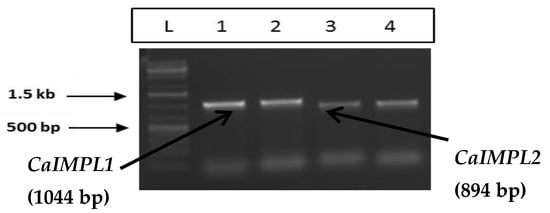
Figure 1.
PCR amplification of CaIMPL1, CaIMPL2 for bacterial expression and plant expression.
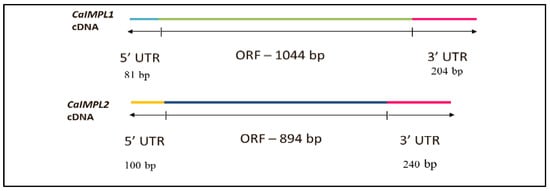
Figure 2.
Diagrammatic representation of CaIMPL1 and CaIMPL2 cDNA with UTR.
3.2. IMP Gene Family Transcript Differentially Expressed in Different Environmental Stresses, in Presence of Different Hormones, and in Different Tissues/Organs
The expression of both CaIMPL1 and CaIMPL2 along with CaIMP1 was found to be increased under all the abiotic stresses, while the maximum expression of all the three transcripts was reported in the case of dehydration stress. In comparison to CaIMPL1, CaIMPL2 showed lower expression. Among CaIMPL1, CaIMPL2, and CaIMP1, the highest transcript accumulation was observed in the case of CaIMPL1, under all the stresses (Figure 3A). Furthermore, the expression pattern of all three genes was investigated in the presence of exogenous hormones (Abscisic acid, Salicylic acid, Auxin, Gibberellic acid etc.), and the highest transcription induction of all these genes was observed in the case of ABA treatment. However, GA treatment could induce a slightly increased transcript accumulation in the case of all three genes (Figure 3B). Both CaIMPL1 and CaIMPL2 along with CaIMP1 were found to be differentially expressed in different organs of chickpea plant (Figure 3C).
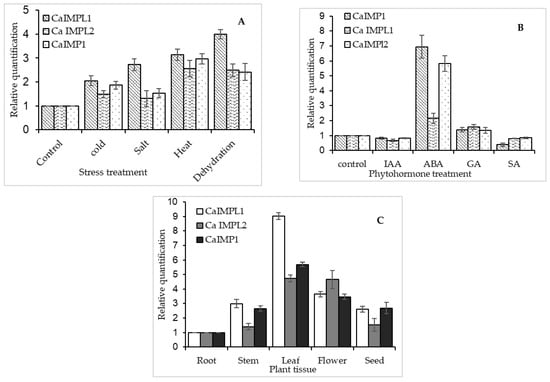
Figure 3.
Relative expression analysis of CaIMP1, CaIMPL1, and CaIMPL2 transcripts using real-time PCR (A) seedlings with different abiotic stress treatments, (B) under treatment of seedlings with exogenous hormones, and (C) in different organs of chickpea plant. We used 18S rRNA as an endogenous control for normalizing the relative expression value of each transcript in all experiments, and it was calculated using the ΔΔCT method (Applied Biosystems, Foster City, CA, USA). The data depicted in the charts were obtained from the triplicate analysis of three replicates. Error bars designate ± SD standard deviation.
3.3. CaIMPL1 and CaIMPL2 Proteins Exhibit Distinct Biochemical Properties
The culture of transformed cells (pET28a:CaIMPL1:BL21-DE3 and pET28a:CaIMPL2:BL21-DE3) was given and then induced by 0.5 mM IPTG as soon as the absorbance reached 0.6–0.8 at 600 nm. The cells were harvested using centrifugation, sonicated, and finally analyzed on 12% SDS-PAGE (Figure 4). CaIMPL1 and CaIMPL2 were expressed as a 32.7 and 38.1 kDa protein, respectively, mainly in the inclusion bodies, as evident by SDS-PAGE analysis. The solubilization of the recombinant protein was performed [] and the expressed protein was serially dialyzed, which was followed by purification using affinity chromatography (GE Healthcare, Sweden) and used for biochemical characterization study (Figure 5).
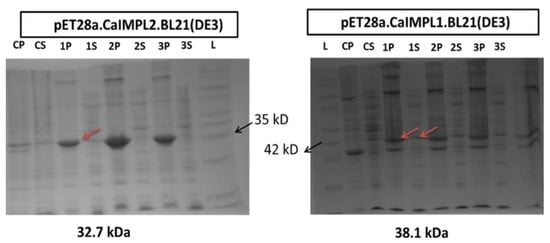
Figure 4.
SDS PAGE analysis of CaIMPL1 and CaIMPL2 overexpressed recombinant protein in E. coli BL21 (DE3). We used 12% SDS-PAGE gel. (CP, CS-Control having pET28a empty vector transformed induced cells; 1P-3S-CaIMPL1 and CaIMPL2 transformed cells; L, molecular weight ladder; P-pellet fractions; S-soluble fractions/supernatant fraction.)
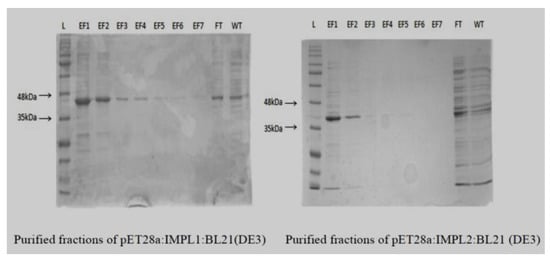
Figure 5.
Purified fractions of CaIMPL1 and CaIMPL2 proteins on 12% SDS PAGE.
The purified fractions of CaIMPL1 and CaIMPL2 proteins were used for biochemical characterization using malachite green dye-based inorganic phosphate estimation assay [] (Table 1).

Table 1.
Biochemical characterization of recombinant CaIMP1, CaIMPL1, and CaIMPL2.
Purified protein samples were initially used to test the substrate specificity using different sugar phosphates, including the general substrate myo-inositol 1-phosphate and D-Galactose 1-phosphate. Then, temperature optima, pH optima, and optimum cofactor concentration were estimated. Our data supports that CaIMPL2 protein chiefly catalyzed the hydrolysis of histidinol 1-phosphate (which is one step of histidine biosynthesis pathway) in contrast to the CaIMPL1 protein, which mainly showed the phosphatase activity with D-Ins 1-P in addition to other substrates such as the reported CaIMP1 (Figure 6). This diverse substrate specificity needs to be further explored to obtain more clarity about the role and regulation of these enzymes in plant metabolism. Various kinetic parameters have been summarized in Table 1.
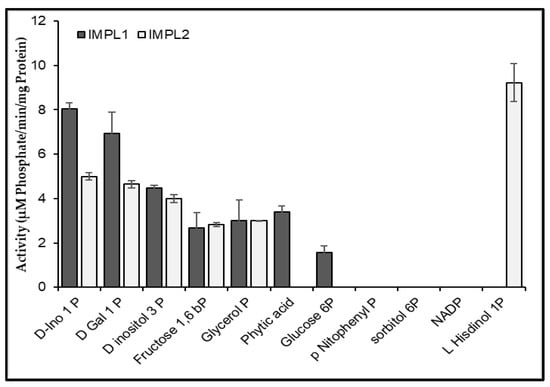
Figure 6.
Activity of recombinant CaIMPL1 and CaIMPL2 with different substrates. The data depicted in the charts were obtained from the triplicate analysis of three replicates. Error bars designate ± SD standard deviation.
4. Conclusions
Biochemical characterization of IMPL and activity comparisons of the CaIMPL1 and CaIMPL2 enzymes from Cicer arietinum were performed with various inositol and other phosphate substrates.
Our findings suggest that IMPL2 enzyme from chickpea could hydrolyze histidinol 1-phosphate, which indicates its role in the histidine biosynthesis pathway. While IMPL1 enzyme from chickpea could mainly hydrolyze D-Ins 1-P substrate, both CaIMPL1 and CaIMPL2, like CaIMP1, showed broad substate specificity.
IMPL1 and IMPL2 genes, whose functions are not yet completely known, were observed to be induced under various environmental stress conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/IECPS2021-12055/s1. Poster: Elucidating the Role of Inositol Monophosphatases Gene Family in Abiotic Stress Management.
Funding
This research was funded by the Science and Engineering Research Board (SERB), Government of India, under the scheme of Start Up Research Grant (Young Scientist)—Elucidating the Functional and Regulatory Aspects of inositol Monophosphatase like Proteins (IMPL1 and IMPL2) from drought-tolerant legume Chickpea (Cicer arietinum), grant number YSS/2014/001012/LS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A
Elucidating the role of inositol monophosphatases gene family in abiotic stress management.

Table A1.
Primer details.
Table A1.
Primer details.
| Primer No. | Primer Sequence (5′-3′) | Purpose |
|---|---|---|
| CaSS1F | ATGTTGTCACAGTGCCATCT | For cloning full-length CDS of CaIMPL2 sequence |
| CaSS2R | GCTCTAGATTCATTACAACGGTAA | |
| CaSS3F | ATGTCAATTGTATTCTCCGCAGC | For cloning full-length CDS of CaIMPL2 sequence |
| CaSS4R | GAGGACTACAGGGCAGACGTTTAA | |
| CaSS5F | GAATTCATGTTGTCACAGTGCCATCT | To clone full-length CaIMPL2 in bacterial expression vector |
| CaSS6R | CTCGAGCCGTTGTAATGAATCTAGAGC | |
| CaSS7F | GAATTCATGTCAATTGTATTCTCCGCAGC | To clone full-length CDS of CaIMPL1 in bacterial expression vector |
| CaSS8R | CTCGAGAACGTCTGCCCTGTAGTCCTC | |
| CaSS9F | GTGTACGCCAATCCTTGTGAAC | For real-time PCR in chickpea for CaIMPL2 |
| CaSS10R | CGACTACTCACGATTCCGCATA | |
| CaSS11F | CTCGGATGGATGGAGGAAAA | For real-time PCR in chickpea for CaIMPL1 |
| CaSS12R | GGAGGACGCCGTTTGAAA | |
| CaSS13F | AGCGTGTAGCTGCTTCAAACC | For real-time PCR in chickpea for CaIMP |
| CaSS14R | GTTTGGCGCAGAGCATCA | |
| CaSS15F | GCCCGCGACGTTGTGA | For real-time PCR for chickpea 18S (Endogenous control) |
| CaSS16R | CCTTGTTACGACTTCTCCTTCCTCTA |
References
- Boominathan, P.; Shukla, R.; Kumar, A.; Manna, D.; Negi, D.; Verma, P.K.; Chattopadhyay, D. Long Term Transcript Accumulation during the Development of Dehydration Adaptation in Cicer arietinum. Plant Physiol. 2004, 135, 1608–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.J.; Jauhar, P.P. Genetic Resources, Chromosome Engineering, and Crop Improvement; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar] [CrossRef]
- Saxena, S.C.; Salvi, P.; Kaur, H.; Verma, P.; Petla, B.P.; Rao, V.; Kamble, N.U.; Majee, M. Differentially expressed myo-inositol monophosphatase gene (CaIMP) in chickpea (Cicer arietinum L.) encodes a lithium-sensitive phosphatase enzyme with broad substrate specificity and improves seed germination and seedling growth under abiotic stresses. J. Exp. Bot. 2013, 64, 5623–5639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sambrook, J.; Russell, D.W. Expression of cloned genes in Escherichia coli. In Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001; Volume 3, pp. 15.23–15.24. [Google Scholar]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Majee, M.; Maitra, S.; Dastidar, K.G.; Pattnaik, S.; Chatterjee, A.; Hait, N.C.; Das, K.P.; Majumder, A.L. A Novel Salt-tolerant l-myo-Inositol-1-phosphate Synthase from Porteresia coarctata (Roxb.) Tateoka, a Halophytic Wild Rice. J. Biol. Chem. 2004, 279, 28539–28552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).