Bioinformatics-Assisted Proteomics of Metal(Loid) Tolerance in Arabidopsis †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Contaminated Soil, Biochar and Bacillus sp.
2.2. Pot Experiment and Soil Pore Water and Plant Analysis
2.3. Proteomics and Bioinformatics Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Soil Pore Water and Plant Analysis
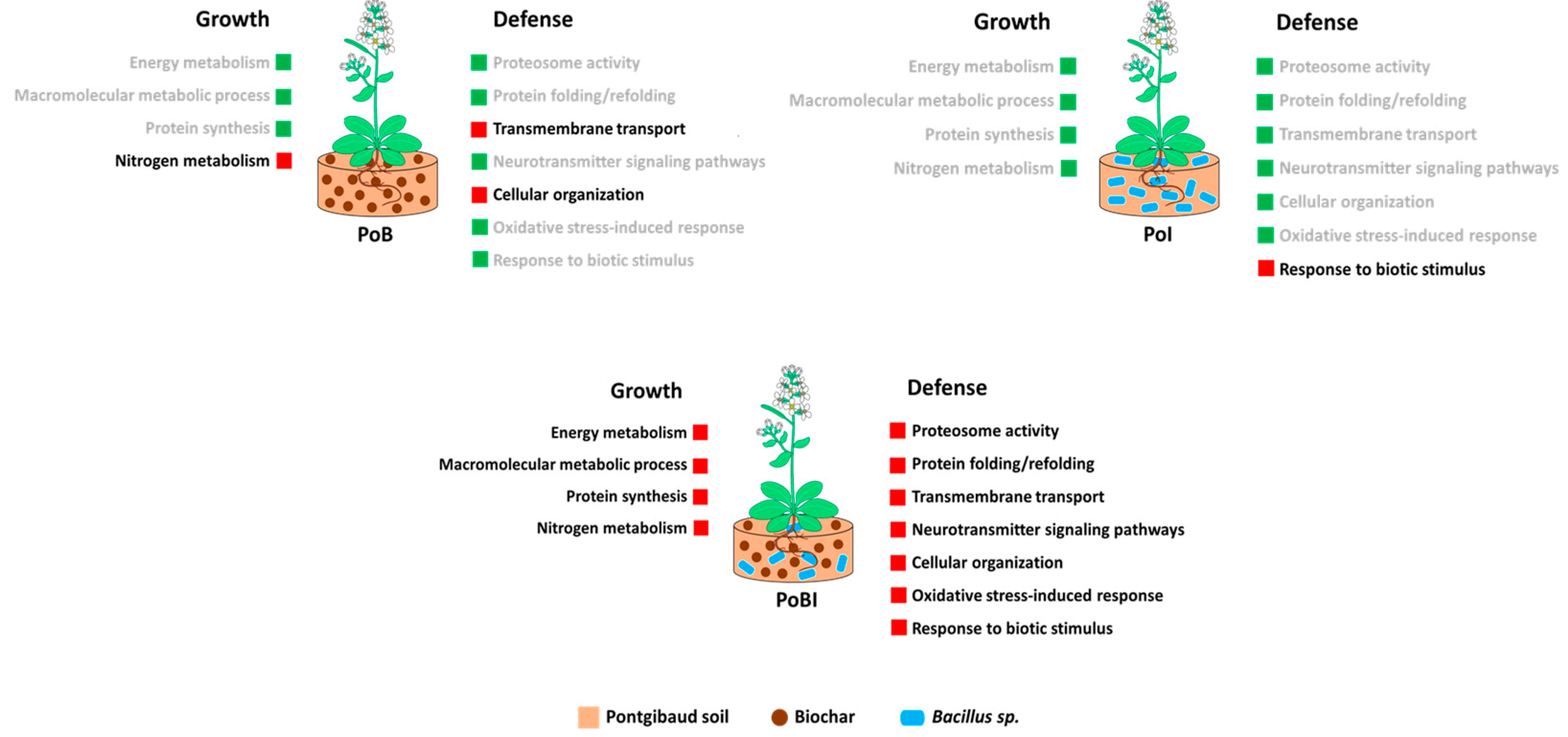
3.2. Bioinformatics-Assisted Proteomics Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, J.Y.; Zhang, J.C.; Ren, L.H.; Zhou, Y.Y.; Gao, J.; Luo, L.; Yanga, Y.; Penga, Q.; Huanga, H.; Chen, A. Diagnosis of soil contamination using microbiological indices: A review on heavy metal pollution. J. Environ. Manag. 2019, 242, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Li, Z.; Wang, Z.; Li, C.; Wei, H. Significance of soil microbe in microbial-assisted phytoremediation: An effective way to enhance phytoremediation of contaminated soil. Int. J. Environ. Sci. Technol. 2020, 17, 2477–2484. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular mechanisms of metalhyperaccumulation in plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef] [PubMed]
- Hossain, Z.; Nouri, M.Z.; Komatsu, S. Plant cell organelle proteomics in response to abiotic stress. J. Proteome Res. 2012, 11, 37–48. [Google Scholar] [CrossRef]
- Hossain, Z.; Komatsu, S. Contribution of proteomics studies towards understanding plant heavy metal stress response. Front. Plant Sci. 2013, 3, 310. [Google Scholar] [CrossRef] [Green Version]
- Fiorentino, N.; Mori, M.; Cenvinzo, V.; Duri, L.G.; Gioia, L.; Visconti, D.; Fagnano, M. Assisted phytoremediation for restoring soil fertility in contaminated and degraded land. Ital. J. Agron. 2018, 13, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Sarfraz, R.; Li, S.; Yang, W.; Zhou, B.; Xing, S. Assessment of physicochemical and nutritional characteristics of waste mushroom substrate biochar under various pyrolysis temperatures and times. Sustainability 2019, 11, 277. [Google Scholar] [CrossRef] [Green Version]
- Touceda-González, M.; Brader, G.; Antonielli, L.; Ravindran, V.B.; Waldner, G.; Friesl-Hanl, W.; Corretto, E.; Campisano, A.; Pancher, M.; Sessitsch, A. Combined amendment of immobilizers and the plant growth-promoting strain Burkholderia phytofirmans PsJN favours plant growth and reduces heavy metal uptake. Soil Biol. Biochem. 2015, 91, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wienkoop, S.; Baginsky, S.; Weckwerth, W. Arabidopsis thaliana as a model organism for plant proteome research. J. Proteom. 2010, 73, 2239–2248. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, J.M.; Chacón-Madrid, K.; Galazzi, R.M.; Campos, B.K.; Arruda, S.C.; Azevedo, R.A.; Arruda, M.A. Evaluation of silicon influence on the mitigation of cadmium-stress in the development of Arabidopsis thaliana through total metal content, proteomic and enzymatic approaches. J. Trace Elem. Med. Biol. 2017, 44, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Cottard, F. Résultats des Caractérisations Complémentaires Effectués sur Différents Milieux Dans le District Minier de Pontgibaud (63); BRGM/RP-58571-FR; Centre Scientifique et Technique Service Environnement Industriel et Procédés Innovants: Orléans, France, 2010; p. 78. [Google Scholar]
- Lebrun, M.; Miard, F.; Nandillon, R.; Hattab-Hambli, N.; Scippa, G.S.; Bourgerie, S.; Morabito, D. Eco-restoration of a mine technosol according to biochar particle size and dose application: Study of soil physico-chemical properties and phytostabilization capacities of Salix viminalis. J. Soil Sediment. 2018, 18, 2188–2202. [Google Scholar] [CrossRef]
- Nandillon, R.; Lebrun, M.; Miard, F.; Gaillard, M.; Sabatier, S.; Morabito, D.; Bourgerie, S. Contrasted tolerance of Agrostis capillaris metallicolous and nonmetallicolous ecotypes in the context of a mining technosol amended by biochar, compost and iron sulphate. Environ. Geochem. Health 2019, 43, 1457–1475. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, M.; Miard, F.; Bucci, A.; Trupiano, D.; Nandillon, R.; Naclerio, G.; Scippa, G.S.; Morabito, D.; Bourgerie, S. Evaluation of direct and biochar carrier-based inoculation of Bacillus sp. on As-and Pb-contaminated technosol: Effect on metal (loid) availability, Salix viminalis growth, and soil microbial diversity/activity. Environ. Sci. Pollut. Res. 2021, 28, 11195–11204. [Google Scholar] [CrossRef]
- Simiele, M.; Sferra, G.; Lebrun, M.; Renzone, G.; Bourgerie, S.; Scippa, G.S.; Morabito, D.; Scaloni, A.; Trupiano, D. In-depth study to decipher mechanisms underlying Arabidopsis thaliana tolerance to metal(loid) soil contamination in association with biochar and/or bacteria. Environ. Exp. Bot. 2021, 182, 104335. [Google Scholar] [CrossRef]
- Purakayastha, T.J.; Bera, T.; Bhaduri, D.; Sarkar, B.; Mandal, S.; Wade, P.; Kumari, S.; Biswas, S.; Menon, M.; Pathak, H.; et al. A review on biochar modulated soil condition improvements and nutrient dynamics concerning crop yields: Pathways to climate change mitigation and global food security. Chemosphere 2019, 227, 345–365. [Google Scholar] [CrossRef]
- Li, G.; Khan, S.; Ibrahim, M.; Sun, T.R.; Tang, J.F.; Cotner, J.B.; Xu, Y.Y. Biochars induced modification of dissolved organic matter (DOM) in soil and its impact on mobility and bioaccumulation of arsenic and cadmium. J. Hazard. Mater. 2018, 348, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.F.; Ge, H.G.; Li, C.; Zhao, Z.P.; Song, F.M.; Hu, S.B. Enhanced phytoextraction of heavy metals from contaminated soil by plant co-cropping associated with PGPR. Water Air Soil Pollut. 2015, 226, 29. [Google Scholar] [CrossRef]
- Rojjanateeranaj, P.; Sangthong, C.; Prapagdee, B. Enhanced cadmium phytoremediation of Glycine max L. Through bioaugmentation of cadmium-resistant bacteria assisted by biostimulation. Chemosphere 2017, 185, 764–771. [Google Scholar] [CrossRef]
- Lomaglio, T.; Hattab-Hambli, N.; Bret, A.; Miard, F.; Trupiano, D.; Scippa, G.S.; Motelica-Heino, M.; Bourgerie, S.; Morabito, D. Effect of biochar amendments on the mobility and (bio) availability of As, Sb and Pb in a contaminated mine technosol. J. Geochem. Explor. 2016, 182, 138–148. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Wang, Y.; Zhang, S.; Guo, Q.; Jin, Y.; Chen, J.; Gao, Y.; Ma, H. Transcriptomic and physiological analyses of Medicago sativa L. roots in response to lead stress. PLoS ONE 2017, 12, 0175307. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ji, J.; Harris-Shultz, K.R.; Wang, H.; Wang, H.; Abd-Allah, E.F.; Luo, Y.; Hu, X. The dynamic changes of the plasma membrane proteins and the protective roles of nitric oxide in rice subjected to heavy metal cadmium stress. Front. Plant Sci. 2016, 7, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamas, M.J.; Sharma, S.K.; Ibstedt, S.; Jacobson, T.; Christen, P. Heavy metals and metalloids as a cause for protein misfolding and aggregation. Biomolecules 2014, 4, 252–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viger, M.; Hancock, R.D.; Miglietta, F.; Taylor, G. More plant growth but less plant defence? First global gene expression data for plants grown in soil amended with biochar. GCB Bioenergy 2016, 7, 658–672. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simiele, M.; Sferra, G.; Lebrun, M.; Renzone, G.; Bourgerie, S.; Scippa, G.S.; Morabito, D.; Scaloni, A.; Trupiano, D. Bioinformatics-Assisted Proteomics of Metal(Loid) Tolerance in Arabidopsis. Biol. Life Sci. Forum 2022, 11, 65. https://doi.org/10.3390/IECPS2021-12050
Simiele M, Sferra G, Lebrun M, Renzone G, Bourgerie S, Scippa GS, Morabito D, Scaloni A, Trupiano D. Bioinformatics-Assisted Proteomics of Metal(Loid) Tolerance in Arabidopsis. Biology and Life Sciences Forum. 2022; 11(1):65. https://doi.org/10.3390/IECPS2021-12050
Chicago/Turabian StyleSimiele, Melissa, Gabriella Sferra, Manhattan Lebrun, Giovanni Renzone, Sylvain Bourgerie, Gabriella Stefania Scippa, Domenico Morabito, Andrea Scaloni, and Dalila Trupiano. 2022. "Bioinformatics-Assisted Proteomics of Metal(Loid) Tolerance in Arabidopsis" Biology and Life Sciences Forum 11, no. 1: 65. https://doi.org/10.3390/IECPS2021-12050
APA StyleSimiele, M., Sferra, G., Lebrun, M., Renzone, G., Bourgerie, S., Scippa, G. S., Morabito, D., Scaloni, A., & Trupiano, D. (2022). Bioinformatics-Assisted Proteomics of Metal(Loid) Tolerance in Arabidopsis. Biology and Life Sciences Forum, 11(1), 65. https://doi.org/10.3390/IECPS2021-12050










