Abstract
Micronutrients play a critical role in plant growth and development, and their deficiency can have adverse effects on plant performance. These elements can also influence plant physiological processes as they are incorporated into the molecular structure of enzymes as cofactors. In this study, the impact of a micronutrient solution containing manganese (125 ppm), iron (200 ppm), zinc (60 ppm), and copper (20 ppm) was investigated on the growth parameters, yield, and antioxidant enzyme activity of tomato (Solanum lycopersicum) plants. Greenhouse tomatoes (cultivar Jet Star F1) were irrigated with the above-mentioned concentrations of elements in a completely randomized design, with five independent biological replicates. The micronutrient treatment increased the specific activities of superoxide dismutase, ascorbate peroxidase, glutathione reductase, guaiacol peroxidase, catalase, and phenylalanine ammonia-lyase, as well as the phenol and salicylic acid contents in tomato leaves. However, the malondialdehyde level and electrolyte leakage index were unaffected. Analysis of the plant growth parameters revealed that the micronutrients increased the stem diameter, root length, number of leaves, stem height, and fruit’s fresh weight in the treated plants. Overall, our results indicated that micronutrients positively affected the growth and development of tomato plants without adverse effects on the health indices. Moreover, the application of micronutrients can magnify the antioxidant capacity of tomato plants through increasing enzyme activity, as well as the phenol and salicylic acid levels. These changes would benefit those plants under abiotic/biotic stress conditions, where elevated levels of antioxidant activities are crucial.
1. Introduction
Micronutrients have a critical role in plant growth and development and serve numerous functions in plants, such as being cofactors of antioxidant enzymes [1] and structural components in osmolites under stress conditions [2]. In addition, it is well-established that the loss of micronutrients can lead to a decrease in plant performance and yield and may have adverse effects on sustainable agriculture [3]. Microelements consisting of manganese, iron, zinc, and copper are required in small amounts and are essential for agricultural plants production [4]. Tomato (Solanum lycopersicum) is the most cost-effective vegetable for growers where micronutrient fertilizers are used to improve the yield [5].
Due to the importance of tomato growing around the world, this paper describes the effects induced by micronutrient application on the antioxidant capacity and performance of tomato plants. The output of this study will help farmers in obtaining a maximum yield through nutritional programs in tomato greenhouses, especially under stressful growing conditions.
2. Materials and Methods
Greenhouse tomato seeds (cultivar Jet Star F1) were planted and grown in plastic pots of sterilized soil, composed of 1:1:2 cocopeat: peat moss: perlite. Plant growth was conducted in a greenhouse under optimal conditions. Then, a micronutrient solution containing manganese (125 ppm), iron (200 ppm), zinc (60 ppm), and copper (20 ppm) was irrigated at different doses in the different growth stages of tomato seedlings (Table A1). Simultaneously, the control plants were irrigated with distilled water. The physiological and morphological parameters of the treated and control plants were investigated at the harvesting stage.
Biochemical analysis of harvested leaves was performed, after preparation in a suitable buffer. For superoxide dismutase (SOD), ascorbate peroxidase (APX), and glutathione reductase (GR) activities, the method of Homayoonzadeh et al. [6] was adopted. After homogenizing 1 g of fresh weight in 1 mL phosphate buffer (50 mM, pH 7) and centrifugation at 16,000× g for 15 min at 4 °C, the supernatant was used as the enzyme source. SOD activity was assayed after mixing the enzyme source with EDTA, methionine, NBT, and riboflavin, and was spectrophotometrically measured at 560 nm. APX activity was assessed by mixing the enzyme source with H2O2 as substrate and ascorbic acid as a reductant, then absorbance was measured at 290 nm. The GR activity was spectrophotometrically evaluated at 412 nm, using a reaction mixture of NADPH, DTNB, and GSSG.
The assessment of guaiacol peroxidase (GPX), catalase (CAT), and phenylalanine ammonia-lyase (PAL)-specific activities was performed based on the method of Homayoonzadeh et al. [7]. For this assessment, after homogenization of 1 g of fresh leaf tissue in Tris-HCl buffer (50 mM, pH 7.5) and centrifugation at 15,000× g for 10 min at 4 °C, the enzyme source was obtained by using the supernatant. In the GPX activity assay, the absorbance of the reaction mixture, consisting of the enzyme source with H2O2 as a substrate, and guaiacol as an electron donor, was measured at 470 nm by spectrophotometer. The activity of CAT was recorded at 240 nm after mixing the enzyme source with H2O2 as a substrate. PAL activity was estimated using phenylalanine as substrate and cinnamic acid production at 290 nm.
The contents of phenols and salicylic acid were measured using the method reported by Homayoonzadeh et al. [8]. The phenol content was quantified spectrophotometrically at 760 nm using Folin–Ciocalteu as a reagent and gallic acid solution as a standard. The salicylic acid was extracted by homogenization in methanol and was then analyzed with an HPLC apparatus equipped with a UV/VIS detector at 235 nm and a GLC-ODS C18 column (150 mm × 6 mm internal diameter). The mobile phase consisted of methanol/water (70/30) at 1 mL min−1. The concentration of malondialdehyde, as well as the electrolyte leakage index, was estimated according to the method described by Homayoonzadeh et al. [9]. Thiobarbituric acid was utilized for the malondialdehyde test, then absorbance was recorded at 600 nm. The assessment of ELI was performed using a platinum electrode, and the percentages of initial to final conductivity were recorded.
The morphological parameters related to plant growth and yield, comprising stem diameter, root length, number of leaves, stem height, and fruit’s fresh weight, were also evaluated at the harvesting stage in both treated and control tomato plants.
Experiments were consigned to a completely randomized design, with five independent biological replicates. After the data passed the Shapiro–Wilk test for normality and Levene’s test for the equality of variances, an unpaired Student’s t-test was used for comparisons between the treatments. All analyses were carried out using GraphPad Prism, version 8.2.0.
3. Results
The results showed that the antioxidant capacity of tomato plants was amplified in response to the micronutrient solution without adverse effects on the plant’s health indices. The specific activities of superoxide dismutase (p = 0.0036, t = 2.164, 1.33-fold), ascorbate peroxidase (p = 0.0190, t = 3.256, 1.25-fold), glutathione reductase (p = 0.0091, t = 4.369, 1.99-fold), guaiacol peroxidase (p = 0.0028, t = 2.279, 1.35-fold), catalase (p = 0.0401, t = 3.387, 1.14-fold), and phenylalanine ammonia-lyase (p = 0.0299, t = 4.489, 1.86-fold) were significantly higher in the treated plants, compared with the control ones (Figure 1A–F). Moreover, the analysis of phenol (p = 0.0213, t = 2.348) and salicylic acid (p = 0.0225, t = 3.856) contents revealed their significant increase in treated plants compared to the controls by 1.22-fold and 1.41-fold, respectively (Figure 2A,B). In contrast, there were no significant changes in malondialdehyde content (p = 0.4420, t = 0.412) or electrolyte leakage index (p = 0.5200, t = 0.325) in response to the micronutrient treatment (Figure 2C,D).
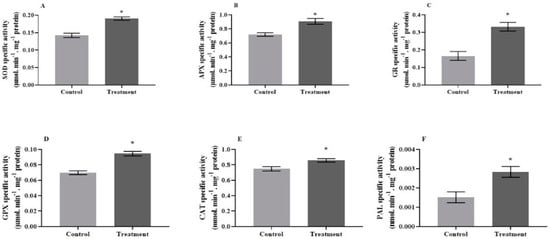
Figure 1.
Mean (±SE) specific activities of (A) superoxide dismutase, (B) ascorbate peroxidase, (C) glutathione reductase, (D) guaiacol peroxidase, (E) catalase, and (F) phenylalanine ammonia-lyase in tomato leaves, when plants were treated with micronutrient solution (Treatment) or without (Control). The error bar shows standard errors. Asterisks are used to show statistically significant differences between treated and control plants.
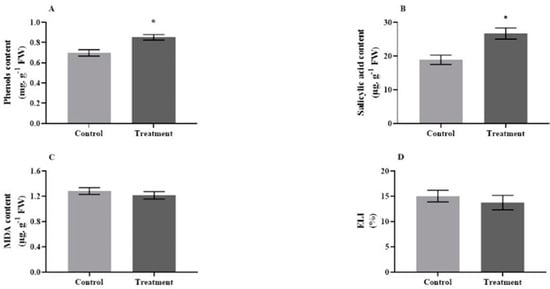
Figure 2.
Mean (±SE) contents of (A) phenols, (B) salicylic acid, (C) malondialdehyde, and (D) electrolyte leakage index in tomato leaves, when plants were treated with micronutrient solution (Treatment) or without (Control). The error bar shows standard errors. Asterisks are used to show statistically significant differences between treated and control plants.
Further analysis of plant growth and yield clearly showed that the performance of tomato plants treated with micronutrient solution was improved. Morphological parameters, including stem diameter (1.32-fold), root length (1.39-fold), number of leaves per plant (1.36-fold), stem height (1.14-fold), and fruit’s fresh weight (1.17-fold), were significantly higher in the treated tomato plants compared with the controls (Table 1).

Table 1.
T Mean (±SE) tomato plant growth and yield, following treatment with micronutrient solution (Treatment) or without (Control). Asterisks are used to show statistically significant differences between treated and control plants.
4. Discussion
This paper proposes a framework of micronutrient application in tomato crops in greenhouses that can have positive effects on the plants’ antioxidant system, as well as on their performance. Some microelements are important cofactors of antioxidative enzymes involved in plant defense. Manganese is a cofactor in the activation of SOD, CAT, and PAL [10]. Iron plays an activator role for APX, GPX, and CAT [11]. Zinc is a cofactor of transcriptional factors commonly involved in the expression of genes encoding antioxidative defense enzymes, such as SOD, APX, and GR, which results in higher enzyme activity [12]. Copper is a cofactor of SOD, APX, and GST, which increases the catalysis of reactions [13]. According to results that demonstrate increases in antioxidant activities, it is plausible that treatment with micronutrients has positive and profound effects on the tomato plant’s defense systems, which may protect it against both biotic and abiotic stresses.
Phenolics, as reactive oxygen species quenchers, are produced by PAL activity because PAL is the key enzyme in the plant’s secondary metabolism, which catalyzes the first step in the phenylpropanoid pathway, leading to the synthesis of phenolic compounds [14]. Salicylic acid is a small phenolic compound that makes a substantial contribution to multiple physiological processes and the activation of the plant’s defense system against biotic and abiotic stresses, which, in turn, could result in systemic resistance [15]. By contrast, the malondialdehyde level and electrolyte leakage index, which did not significantly change in tomato plants in response to micronutrient treatment, may be related to the inhibition of lipid peroxidation and cell injury by elevated levels of phenols [14] and salicylic acid [15], since they act as non-enzymatic antioxidants and cause a decrease in membrane permeability and an increase in cell viability.
Micronutrients, such as manganese, iron, zinc, and copper, have crucial roles in plant performance [16] and plants use these essential micronutrients to grow and complete their life cycle [17]. It is widely recognized that micronutrients promote plant growth and development by the biosynthesis of free amino acids, carbohydrates, and protein, as well as plant yield through improving photosynthetic pigment function [18]. Thus, it can be concluded that the micronutrient regime utilized in this study has substantial benefits for tomato plant farming by amplifying the antioxidant capacity and improving growth and yield.
Author Contributions
Conceptualization, K.T. and H.A.; methodology, M.H. and E.T.; validation, H.A. and J.N.; formal analysis, M.H. and H.A.; investigation, M.H. and E.T.; writing original draft preparation, M.H. and E.T.; review and editing, K.T.; visualization, J.N.; supervision, K.T.; project administration, H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available in a publicly accessible repository.
Acknowledgments
This research was supported by the Research and Technology Deputy of the University of Tehran.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Nutrition regime at used doses on different phenological stages of tomato plants.
Table A1.
Nutrition regime at used doses on different phenological stages of tomato plants.
| Growth Stage | Days from Planting | Stage Duration (days) | Crop Age (days) | Dose (%) | Watering Volume (mL plant−1) | Watering Duration |
|---|---|---|---|---|---|---|
| Vegetative | 1–14 | 14 | 14 | 0.5 | 300 mL | Every 7 Days |
| Budding | 15–28 | 14 | 28 | 1.0 | 300 mL | Every 7 Days |
| Flowering | 29–35 | 7 | 35 | 1.5 | 300 mL | Every 7 Days |
References
- Mondal, S.; Bose, B. Impact of micronutrient seed priming on germination, growth, development, nutritional status and yield aspects of plants. J. Plant Nutr. 2019, 42, 2577–2599. [Google Scholar] [CrossRef]
- Welch, R.M.; Shuman, L. Micronutrient nutrition of plants. Crit. Rev. Plant Sci. 1995, 14, 49–82. [Google Scholar] [CrossRef]
- Khoshgoftarmanesh, A.H.; Schulin, R.; Chaney, R.L.; Daneshbakhsh, B.; Afyuni, M. Micronutrient-efficient genotypes for crop yield and nutritional quality in sustainable agriculture. Agron. Sustain. Dev. 2010, 30, 83–107. [Google Scholar] [CrossRef] [Green Version]
- Graham, R.D. Micronutrient deficiencies in crops and their global significance. In Micronutrient Deficiencies in Global Crop Production; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands; Berlin, Germany, 2008; pp. 41–61. [Google Scholar] [CrossRef]
- Mihalache, G.; Peres, C.I.; Bodale, I.; Achitei, V.; Gheorghitoaie, M.V.; Teliban, G.C.; Cojocaru, A.; Butnariu, M.; Muraru, V.; Stoleru, V. Tomato crop performances under chemical nutrients monitored by electric signal. Agronomy 2020, 10, 1915. [Google Scholar] [CrossRef]
- Homayoonzadeh, M.; Hosseininaveh, V.; Reyhaniaghighi, S.; Talebi, K.; Roessner, U.; Maali-Amiri, R. Evaluation of physiological and biochemical responses of pistachio plants (Pistacia vera L.) exposed to pesticides. Ecotoxicology 2021, 30, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Homayoonzadeh, M.; Moeini, P.; Talebi, K.; Allahyari, H.; Torabi, E.; Michaud, J.P. Physiological responses of plants and mites to salicylic acid improve the efficacy of spirodiclofen for controlling Tetranychus urticae (Acari: Tetranychidae) on greenhouse tomatoes. Exp. Appl. Acarol. 2020, 82, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Homayoonzadeh, M.; Esmaeily, M.; Talebi, K.; Allahyari, H.; Nozari, J.; Michaud, J.P. Micronutrient fertilization of greenhouse cucumbers mitigates pirimicarb resistance in Aphis gossypii (Hemiptera: Aphididae). J. Econ. Entomol. 2020, 113, 2864–2872. [Google Scholar] [CrossRef] [PubMed]
- Homayoonzadeh, M.; Moeini, P.; Talebi, K.; Roessner, U.; Hosseininaveh, V. Antioxidant system status of cucumber plants under pesticides treatment. Acta Physiol. Plant 2020, 42, 161–172. [Google Scholar] [CrossRef]
- Millaleo, R.; Reyes-Díaz, M.; Ivanov, A.G.; Mora, M.L.; Alberdi, M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 470–481. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, D.K.; Singh, S.; Gaur, S.; Singh, S.; Yadav, V.; Liu, S.; Singh, V.P.; Sharma, S.; Srivastava, P.; Prasad, S.M.; et al. Acquisition and homeostasis of iron in higher plants and their probable role in abiotic stress tolerance. Front. Environ. Sci. 2018, 5, 86. [Google Scholar] [CrossRef] [Green Version]
- Hansch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Burkhead, J.L.; Gogolin Reynolds, K.A.; Abdel-Ghany, S.E.; Cohu, C.M.; Pilon, M. Copper homeostasis. New Phytol. 2009, 182, 799–816. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Parihar, P.; Singh, R.; Prasad, S.M. An assessment to show toxic nature of beneficial trace metals: Too much of good thing can be bad. Int. J. Curr. Multidiscip. Stud. 2016, 2, 141–144. [Google Scholar]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef] [Green Version]
- Arif, N.; Yadav, V.; Singh, S.; Singh, S.; Ahmad, P.; Mishra, R.K.; Sharma, S.; Tripathi, D.K.; Dubey, N.K.; Chauhan, D.K. Influence of high and low levels of plant-beneficial heavy metal ions on plant growth and development. Front. Environ. Sci. 2016, 4, 69. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).