Plant Disease Symptomatology: Cucumber Green Mottle Mosaic Virus (CGMMV)-Infected Cucumber Plants Exposed to Fluctuating Extreme Temperatures †
Abstract
1. Introduction
2. Materials and Methods
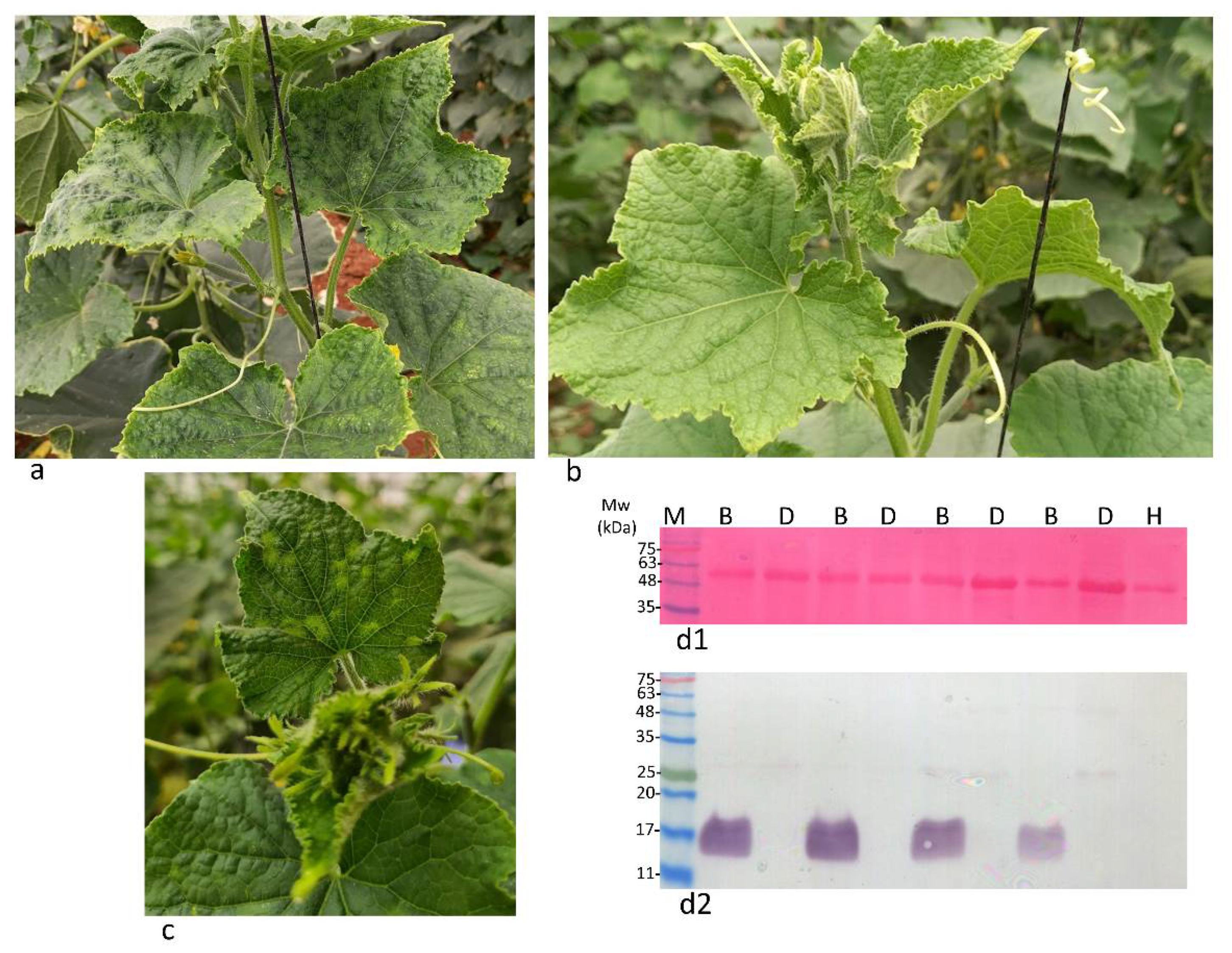
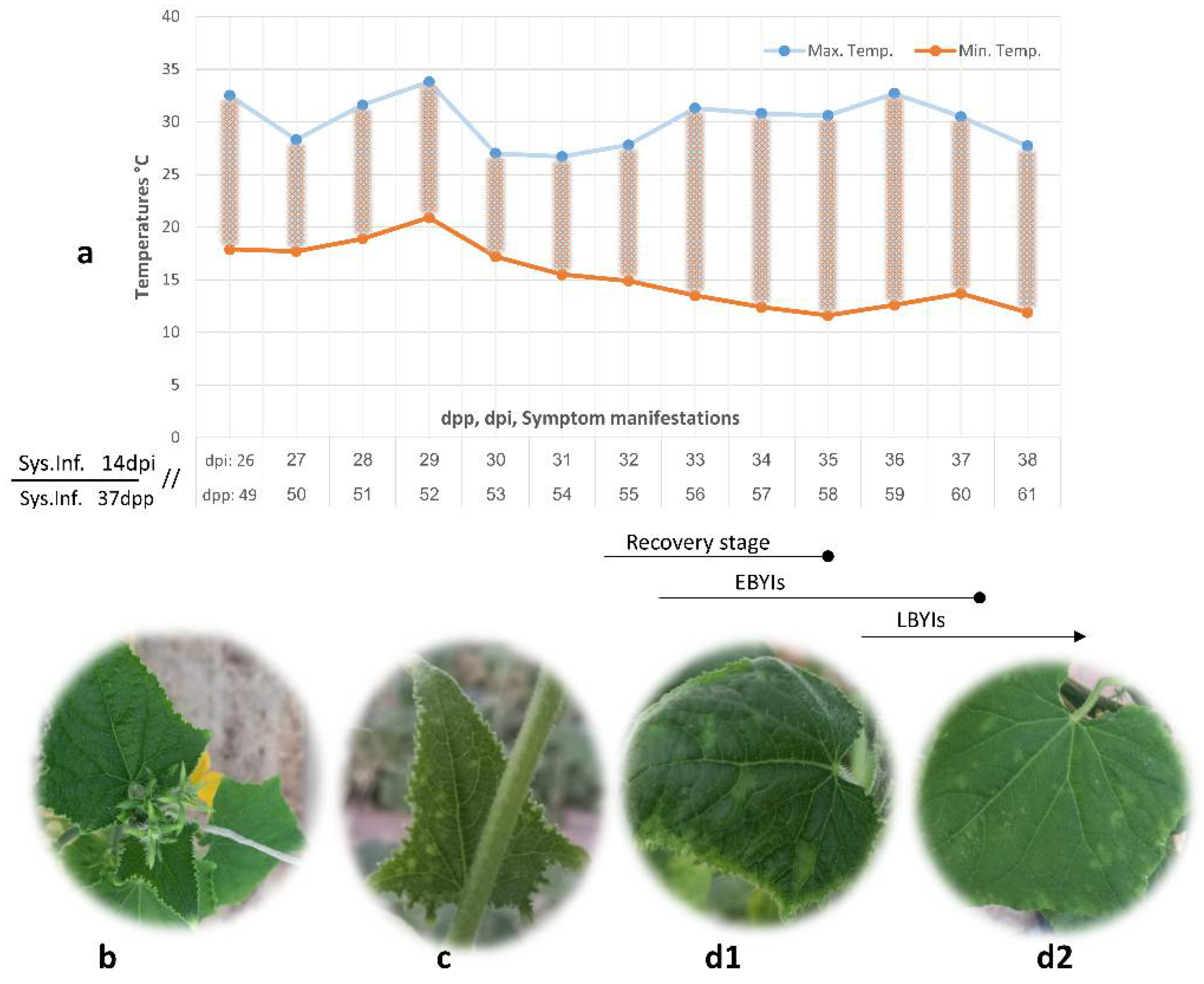
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ainsworth, G.C. Mosaic disease of cucumber. Ann. Appl. Biol. 1935, 22, 55–67. [Google Scholar] [CrossRef]
- Dombrovsky, A.; Tran-Nguyen, L.T.; Jones, R.A. Cucumber green mottle mosaic virus: Rapidly Increasing Global Distribution, Etiology, Epidemiology, and Management. Annu. Rev. Phytopathol. 2017, 55, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Dombrovsky, A.; Smith, E. Seed Transmission of Tobamoviruses: Aspects of Global Disease Distribution. In Advances in Seed Biology; InTech: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Ramakrishna, P.; Amritphale, D. The perisperm-endosperm envelope in Cucumis: Structure, proton diffusion and cell wall hydrolysing activity. Ann. Bot. 2005, 96, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Reingold, V.; Lachman, O.; Blaosov, E.; Dombrovsky, A. Seed disinfection treatments do not sufficiently eliminate the infectivity of Cucumber green mottle mosaic virus (CGMMV) on cucurbit seeds. Plant Pathol. 2015, 64, 245–255. [Google Scholar] [CrossRef]
- Shargil, D.; Zemach, H.; Belausov, E.; Lachman, O.; Luria, N.; Molad, O.; Smith, E.; Kamenetsky, R.; Dombrovsky, A. Insights into the maternal pathway for Cucumber green mottle mosaic virus infection of cucurbit seeds. Protoplasma 2019, 256, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Darzi, E.; Lachman, O.; Smith, E.; Koren, A.; Klein, E.; Pass, N.; Frenkel, O.; Dombrovsky, A. Paths of cucumber green mottle mosaic virus disease spread and disinfectant-based management. Ann. Appl. Biol. 2020, 177, 374–384. [Google Scholar] [CrossRef]
- Chanda, B.; Shamimuzzaman, M.; Gilliard, A.; Ling, K.-S. Effectiveness of disinfectants against the spread of tobamoviruses: Tomato brown rugose fruit virus and Cucumber green mottle mosaic virus. Virol. J. 2021, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Ohara, T.; Sakata, Y. Inheritance of Resistance to Cucumber green mottle mosaic virus in Cucumis melo L. ‘Chang Bougi’. J. Jpn. Soc. Hortic. Sci. 2007, 76, 316–318. [Google Scholar] [CrossRef]
- Smith, E.; Luria, N.; Reingold, V.; Frenkel, O.; Koren, A.; Klein, E.; Bekelman, H.; Lachman, O.; Dombrovsky, A. Aspects in tobamovirus management in modern agriculture: Cucumber green mottle mosaic virus. In Proceedings of the XXX International Horticultural Congress IHC2018: International Symposium on Tropical and Subtropical Vegetable Production: 1257, Istanbul, Turkey, 12–16 August 2018; pp. 1–8. [Google Scholar]
- Smith, E.; Dombrovsky, A. Aspects in Tobamovirus management in intensive agriculture. In Plant Diseases-Current Threats and Management Trends; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Crespo, O.; Janssen, D.; Robles, C.; Ruiz, L. Resistance to Cucumber green mottle mosaic virus in Cucumis sativus. Euphytica 2018, 214, 1–11. [Google Scholar] [CrossRef]
- Ellouze, W.; Mishra, V.; Howard, R.J.; Ling, K.-S.; Zhang, W. Preliminary Study on the Control of Cucumber Green Mottle Mosaic Virus in Commercial Greenhouses Using Agricultural Disinfectants and Resistant Cucumber Varieties. Agronomy 2020, 10, 1879. [Google Scholar] [CrossRef]
- Liu, H.; Luo, L.; Liang, C.; Jiang, N.; Liu, P.; Li, J. High-throughput sequencing identifies novel and conserved cucumber (Cucumis sativus L.) microRNAs in response to cucumber green mottle mosaic virus infection. PLoS ONE 2015, 10, e0129002. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-W.; Liang, C.-Q.; Liu, P.-F.; Luo, L.-X.; Li, J.-Q. Quantitative proteomics identifies 38 proteins that are differentially expressed in cucumber in response to cucumber green mottle mosaic virus infection. Virol. J. 2015, 12, 1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, X.; An, M.; Xia, Z.; Bai, X.; Wu, Y. Transcriptome analysis of watermelon (Citrullus lanatus) fruits in response to Cucumber green mottle mosaic virus (CGMMV) infection. Sci. Rep. 2017, 7, 16747. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Liu, H.; Hao, J.; Li, J.; Luo, L. Expression profiling and regulatory network of cucumber microRNAs and their putative target genes in response to cucumber green mottle mosaic virus infection. Arch. Virol. 2019, 164, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Molad, O.; Smith, E.; Luria, N.; Sela, N.; Lachman, O.; Bakelman, E.; Leibman, D.; Dombrovsky, A. New early phenotypic markers for cucumber green mottle mosaic virus disease in cucumbers exposed to fluctuating extreme temperatures. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Ghoshal, B.; Sanfaçon, H. Temperature-dependent symptom recovery in Nicotiana benthamiana plants infected with tomato ringspot virus is associated with reduced translation of viral RNA2 and requires ARGONAUTE 1. Virology 2014, 456, 188–197. [Google Scholar] [CrossRef]
- Zhao, F.; Li, Y.; Chen, L.; Zhu, L.; Ren, H.; Lin, H.; Xi, D. Temperature dependent defence of Nicotiana tabacum against Cucumber mosaic virus and recovery occurs with the formation of dark green islands. J. Plant Biol. 2016, 59, 293–301. [Google Scholar] [CrossRef]
- Samuel, G. Some experiments on inoculating methods with plant viruses, and on local lesions. Ann. Appl. Biol. 1931, 18, 494–507. [Google Scholar] [CrossRef]
- Makowski, K.; Wild, M.; Ohmura, A. Diurnal temperature range over Europe between 1950 and 2005. Atmos. Chem. Phys. 2008, 8, 6483–6498. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molad, O.; Smith, E.; Luria, N.; Sela, N.; Lachman, O.; Bakelman, E.; Leibman, D.; Dombrovsky, A. Plant Disease Symptomatology: Cucumber Green Mottle Mosaic Virus (CGMMV)-Infected Cucumber Plants Exposed to Fluctuating Extreme Temperatures. Biol. Life Sci. Forum 2022, 11, 58. https://doi.org/10.3390/IECPS2021-11991
Molad O, Smith E, Luria N, Sela N, Lachman O, Bakelman E, Leibman D, Dombrovsky A. Plant Disease Symptomatology: Cucumber Green Mottle Mosaic Virus (CGMMV)-Infected Cucumber Plants Exposed to Fluctuating Extreme Temperatures. Biology and Life Sciences Forum. 2022; 11(1):58. https://doi.org/10.3390/IECPS2021-11991
Chicago/Turabian StyleMolad, Ori, Elisheva Smith, Neta Luria, Noa Sela, Oded Lachman, Elena Bakelman, Diana Leibman, and Aviv Dombrovsky. 2022. "Plant Disease Symptomatology: Cucumber Green Mottle Mosaic Virus (CGMMV)-Infected Cucumber Plants Exposed to Fluctuating Extreme Temperatures" Biology and Life Sciences Forum 11, no. 1: 58. https://doi.org/10.3390/IECPS2021-11991
APA StyleMolad, O., Smith, E., Luria, N., Sela, N., Lachman, O., Bakelman, E., Leibman, D., & Dombrovsky, A. (2022). Plant Disease Symptomatology: Cucumber Green Mottle Mosaic Virus (CGMMV)-Infected Cucumber Plants Exposed to Fluctuating Extreme Temperatures. Biology and Life Sciences Forum, 11(1), 58. https://doi.org/10.3390/IECPS2021-11991






