1. Introduction
Water quality problems associated with fish biomass and species composition have been known for several decades [
1,
2,
3,
4]. Key to understanding these relationships is that total fish biomass is generally more important than species composition in affecting water quality in lakes and reservoirs [
5,
6]. Nevertheless, species composition can be important because omnivorous and planktivorous species have greater opportunity to achieve high biomass relative to piscivores. Efforts to control cyprinids and centrarchids using stocking of predacious fish have had some success, assuming that a favorable predator:prey ratio can be maintained [
7]. Other techniques to manage populations of unwanted fish taxa include the application of piscicides, such as was done upstream in Diamond Lake [
8], however many lakes, including Lemolo Lake, are not suitable candidates for the use of piscicides [
9]. Other investigators have employed various types of netting to reduce the biomass of unwanted fish taxa such as ictalurids [
10] and gizzard shad [
11]. After a review of available techniques, it was decided by the Technical Working Group of the Resource Advisory Council, an entity composed of local resource agency staff, that mechanical removal of the unwanted fish taxa in Lemolo Lake would best address the large population of unwanted cyprinids, at least on an experimental basis.
As with many lake management projects, there is often more than one variable changing at a time. In the case of this hydropower impoundment, a license renewal had been granted by the Federal Energy Power Commission (FERC) that required two changes to the operation of the impoundment. One change was an increase in the discharge to the original river channel (referred to as the bypass reach) and the second was a reduction in the magnitude of the winter drawdown. Both changes were implemented in 2006, the same time when the lake began to experience a dramatic increase in cyanobacteria. Consequently, it was unknown what factor or factors were contributing to the changes in the lake. Additionally, there were concerns that changes in climate also could have triggered a response in phytoplankton.
Here we report on an experimental netting program to reduce the biomass of a cyprinid (tui chub;
Gila bicolor) in an attempt to reduce the intensity and duration of the
Anabaena (=
Dolichospermum) blooms [
12,
13]. We also report on an unanticipated response from the fisheries and the need to adapt to changing conditions that were not recognized at the outset of the study. The water quality observations were used to calibrate a hydrodynamic model and a Bayesian model, to evaluate hypotheses for the sudden onset of the cyanobacterial blooms and to evaluate alternative methods to maintain both a viable sport fishery and achieving water quality goals. The results were used to inform management decisions regarding the role of fisheries in the lake.
3. Materials and Methods
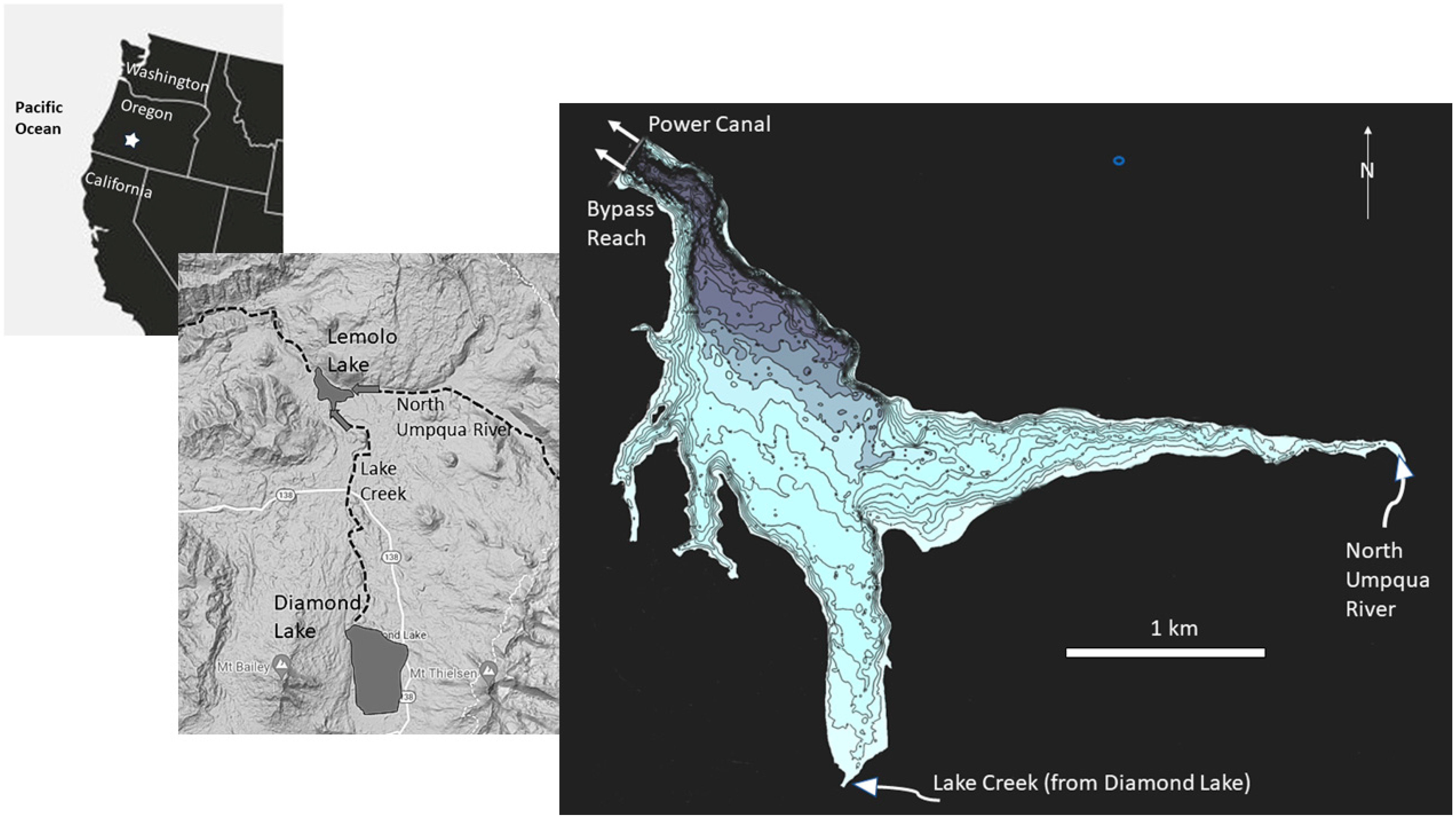
A monitoring program was designed to collect information regarding the limnology of Lemolo Lake, primarily from June into October when snow would not block access to the lake. Elements of the monitoring program consisted of documenting weather conditions, measuring water temperature in the lake and its tributaries and outlets, measuring nutrient chemistry, and sampling phytoplankton and zooplankton. A weather station was installed on the south intake tower at Lemolo Lake and was operated through the active sampling periods. The weather station was an Onset Computer weather station (Model H21-SYS-A) equipped with sensors to measure air temperature, relative humidity, solar radiation, wind speed, and wind direction. The station was installed at a height of 2 m above the base of the tower and was programmed to record observations every 15 min. Long-term meteorological data were available from the Diamond Lake SNOTEL site (#442) maintained by the US Department of Agriculture. The site is located at latitude 43°ll′ N and longitude 122°8′ W at elevation 1609 m.
A buoy was installed in the forebay of Lemolo Lake (site = LEMLL) at the beginning of each sampling season approximately 200 m east of the dam face at a depth of about 28 m. Onset Computer thermistors (Model HOBO Water Temp Pro v2) were attached to the buoy line at depths of 0.4, 1.4, 4.4, 7.9, 11.9, 19.9, and 27.7 m below the lake surface. Thermistors were also placed near the mouth of Lake Creek above the culvert at FS Road 2614 (LCM), at the inlet from the North Umpqua River above the bridge crossing at FS Road 2614 (NUR), at the Bypass Reach 50 m below Lemolo Dam (LEM1T), and in the Power Canal (LEM1C1) from Lemolo Lake. A conductivity/temperature logger (Onset Computer model U24-001) was installed at LEM1C1 during 2012 and 2013 to provide additional information regarding the source of water drawn from the lake. One conductivity/temperature logger was installed at LCM and another at NUR at the sites used for the temperature measurements. Thermistors and other loggers were programmed to record at 15-minute intervals.
Samples were pumped into an 8 L Wildco HPDE sample splitter (Model 1831-C80, Yulee, FL, USA) using a RolaTech peristaltic pump through plasticizer-free Tygon
® tubing that had been purged with multiple rinses of the sample water prior to collecting the sample. Water was drawn from the sample splitter into HPDE Nalgene
® bottles that had been rinsed three times with the sample water prior to collecting the sample, unless the sample bottle contained a preservative for specific aliquots. Samples requiring filtration for analysis of ortho-phosphorus were pumped through a GeoTech 0.45-micron capsule filter (medium capacity) that had been first rinsed with two volumes of the sample water before retaining the sample. Analyses of TP and TN were performed on unfiltered samples. Samples were placed immediately into a cooler for storage prior to shipping samples or freezing them before shipping them. Samples for analysis of nutrient chemistry were shipped to the Cooperative Chemical Analytical Laboratory (CCAL) on the campus of Oregon State University, Corvallis, for analysis. Their methods are available at
http://ccal.oregonstate.edu/methodology.htm (accessed on 8 August 2023). CCAL changed methods for analysis of total and dissolved phosphorus on 16 June 2010. The change in methodology resulted in a slight negative bias in the new method relative to the previous method. This bias was reported to be greater with waters with silica concentrations greater than 4 mg/L (as Si), which includes Lemolo Lake. However, an analysis of phosphorus before and after the change in methods showed no statistically significant impact on the results. Samples were analyzed for nitrate and ammonia during the early part of the study, but most of the sites exhibited concentrations below detection limits with the major exception being the inlet from Lake Creek.
Samples for analysis of phytoplankton were preserved with Lugol’s solution and were shipped to Aquatic Analysts (Friday Harbor, WA, USA) for analysis of taxonomic composition. Aquatic Analysts counted 100 algal units and reported the biovolumes from a table of estimated algal volumes by taxa. Samples collected for analysis of chlorophyll a were placed into a 1 L Nalgene bottle with no preservative and shipped overnight, with ice in a cooler, to IEH-Aquatic Inc. in Seattle, WA, USA. Ten replicate collections of water were made at the mouth of Lake Creek in 2013 by collecting a raw water sample, transferring it to a graduated cylinder and counting the number of Gloeotrichia colonies, and extrapolating to daily inputs based on measured discharge.
Zooplankton samples were collected by lowering a net to a depth of 20 m and retrieving the net at a rate of about 1 m per second. Zooplankton samples from 2008–2011 were collected using a net with 64-micron mesh and a 20 cm opening with a 30 cm reduction collar. In subsequent years, a similar net was used, but the mesh size was increased to 125-micron because only large cladocerans (Daphnia pulicaria + D. rosea) were being counted to reduce monitoring costs. The zooplankton samples were preserved in ethanol and shipped to ZP Taxonomic Services, Olympia, WA, USA for analysis of large cladocerans.
Measurements of water column profile conditions in Lemolo Lake were conducted using an In-Situ Troll 9000 multi-parameter sonde equipped with sensors for measuring temperature, dissolved oxygen (RDO sensor), conductivity, barometric pressure, and pH. The dissolved oxygen sensor was calibrated at the lake prior to deployment using an air-saturated chamber and setting the instrument to the local elevation. The pH was calibrated on site using buffers of 7.00 and 10.01 in that order. Field conductivity measurements collected with the In-Situ Troll 9000 were normalized to consistent measurements by regressing the field data with laboratory measurements of specific conductance.
Lemolo Lake Resort, the Oregon Department of Fish and Wildlife, and PacifiCorp Energy cooperated on fisheries studies throughout the project. Tui chub removal was conducted by staff from Lemolo Lake Resort deploying three Oneida trap nets. The nets had a box opening of 1.8 × 2.1 × 1.8 m, wings of 12.8 m, and a leader panel that was 30.5 m long and 1.8 m high with a mesh size of 0.95 cm. The nets were deployed beginning in June and proceeding through August, with nets generally checked daily. The nets were initially deployed at various locations around the lake to assess which areas of the lake yielded the greatest harvest rate per deployment. It was determined that the most favorable catch rate was achieved in the arm of the lake receiving inflow from Lake Creek. Hatchery rainbow trout were stocked in the reservoir by ODFW during the study. Brown trout and kokanee redds were counted in the North Umpqua River above Lemolo Lake in the fall by ODFW staff.
An updated bathymetric map was commissioned by Lemolo Lake Resort and the hydroacoustic data were collected in the summer of 2012 and 2013 when the lake was near full pool. The data were collected using a Lowrance HDS7 Gen 2 hydroacoustic system equipped with 200 kHz and 455 kHz transducers and a GPS receiver placed over the transducers. The data were collected and processed using the ciBioBase® technology.
Water quality modeling was conducted using the 2-dimensional hydrodynamic model CE-QUAL-W2 [
17]. The model (version 3.7) was calibrated to current conditions and was used to test hypotheses for the onset of the cyanobacteria blooms and to evaluate scenarios for improving water quality in the impoundment. A simple Bayesian model (Norsys Netica, version 6.05) was used to assess the likelihood of lake response to manipulations of the fisheries that were deemed too complex to incorporate into the hydrodynamic model at this time. The probabilities of events were populated using data collected during the study and expert judgment.
4. Results
Physical Limnology: The water budget for the impoundment indicates that about 85 percent of the inflow is derived from the North Umpqua River, with most of the balance derived from Lake Creek. Lake Creek is the natural outlet from Diamond Lake located 14 km upstream. The flow in the North Umpqua River as it enters Lemolo Lake is derived largely from spring discharge and enters the lake at a temperature typically from 6–8 °C. In contrast, Lake Creek is comprised primarily of surface discharge from Diamond Lake, and in the summer Lake Creek flows enter Lemolo Lake at temperatures typically from about 12–20 °C. The water temperatures in Lake Creek vary diurnally: when the waters are warmer (18–20 °C) in the late afternoon, they enter the epilimnion with relatively little mixing. As the tributary waters cool later in the night (12–14 °C), the inflows mix into the metalimnion as well. The consistently cold waters from the North Umpqua River enter the hypolimnion with some mixing of the surface waters. Water exits the impoundment normally through two paths: (1) an outlet at the base of the dam (invert opening at 30 m) to the natural river channel, and (2) a mid-depth outlet (invert at 15 m) in the impoundment to the power canal, which leads to a turbine further downstream. About 75 percent of the water from the reservoir is delivered to the power canal and the remainder is passed to the natural river channel. The flows to the power canal are typically greatest from 09:00 to 19:00 during the study, although these times change depending on market demands for electrical power. Annual precipitation for the watershed, based on the SNOTEL site, averaged 122 cm during the study, ranging from a low of 72 cm in 2010 and a high of 165 cm in 2011.
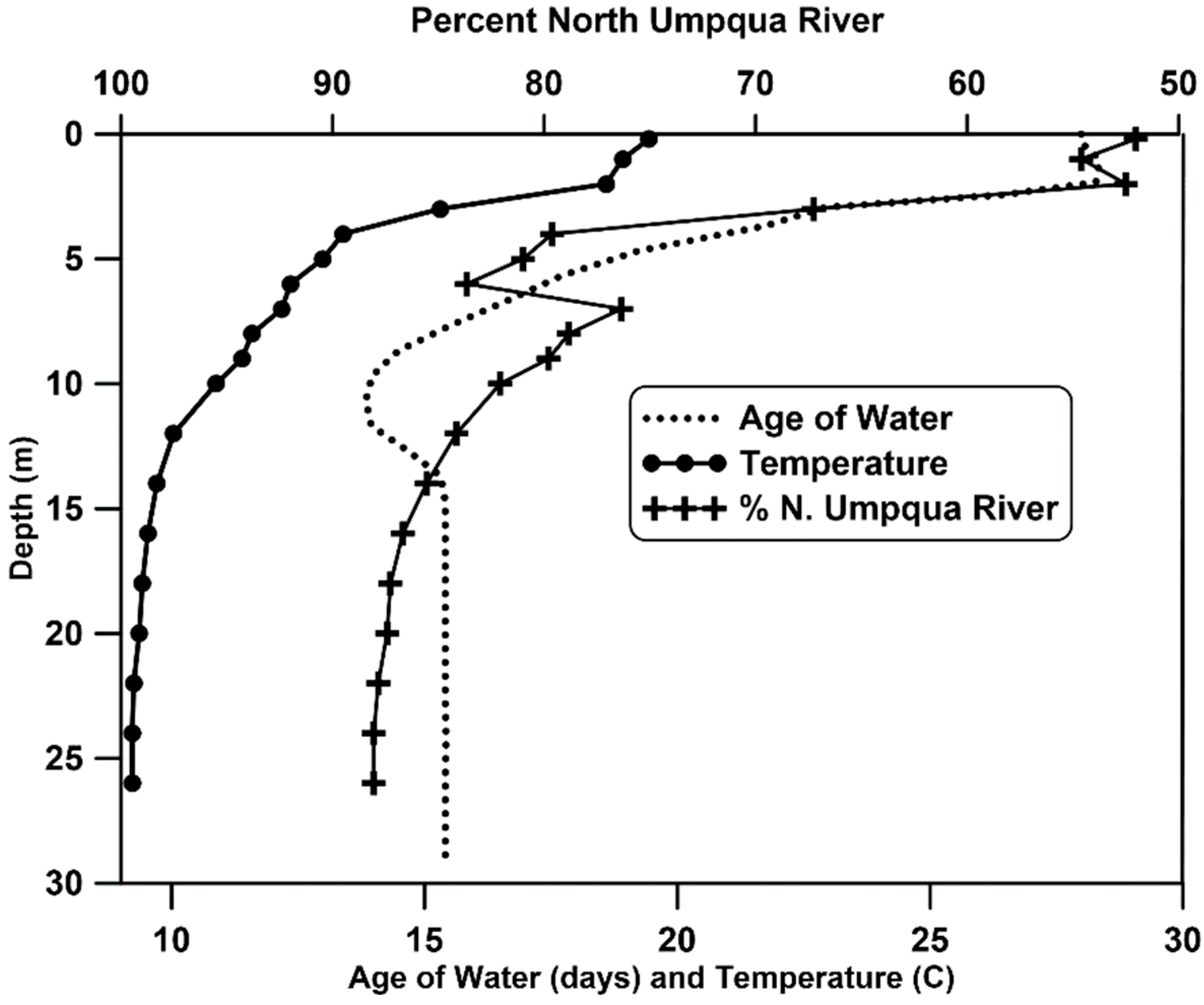
Lemolo Lake develops strong thermal stratification in the summer with a metalimnion beginning at about 4 m (
Figure 2). Temperature and conductivity profiles in the impoundment compared with data from the two outlets indicate that water is being withdrawn from higher in the water column than the depth of the withdrawal openings. Matching water temperature and conductivity shows that the water leaving the power canal during stratification and high flows to the power canal is derived from water at depths of about 7 to 10 m, whereas water passing to the river channel is derived from a depth of about 16 to 21 m. The depth of withdrawal corresponds to periods when discharge to the power canal is high; at lower rates of flow depth of the flow to the intake approaches the depth of the intake. The influence of the inflow from the North Umpqua River is evident in the examining age of water from the CE-QUAL-W2 model output illustrating the youthful age of water in the hypolimnion even into December (
Figure 2). This allows warmer water from the Lake Creek inflow to dominate much of the epilimnion, despite the lower volume of discharge from this source.
The depth to the metalimnion varies because of a pronounced seiche that passes through and under the metalimnion as illustrated by the changing temperatures in this region (
Figure 3). The seiche is driven by prevailing winds from the WNW that drive warmer surface waters to the east extent of the impoundment. The seiche provides opportunities for additional mixing both between the epilimnion and deeper waters and the inlet from the North Umpqua River and the east arm of the impoundment.
Chemical Limnology: The summer thermal stratification reflects the greatly different temperatures of the two inlets, solar radiation, and wind, combined with the depth and rate of the hydropower withdrawal. These factors also affect chemical stratification. Lemolo Lake is a distinct “two-story” system in which the surface waters are more heavily enriched in nitrogen and the bottom waters are dominated by phosphorus. This is a consequence of the cold, P-rich waters from the North Umpqua River comprising the majority of the hypolimnion and the warmer, N-rich waters from Lake Creek being retained largely in the epilimnion (
Table 2). The nutrient ratios in the metalimnion are intermediate between the more extreme endpoints observed in the epilimnion and the hypolimnion. The relatively high concentrations of phosphorus in the hypolimnion are the result of high concentrations of phosphorus entering from the North Umpqua River and are not associated with anoxia in this well-oxygenated impoundment. Because there is little dissolution of minerals from the sediments, conductivity provides a reliable indicator of the mixing of the two tributaries in the impoundment.
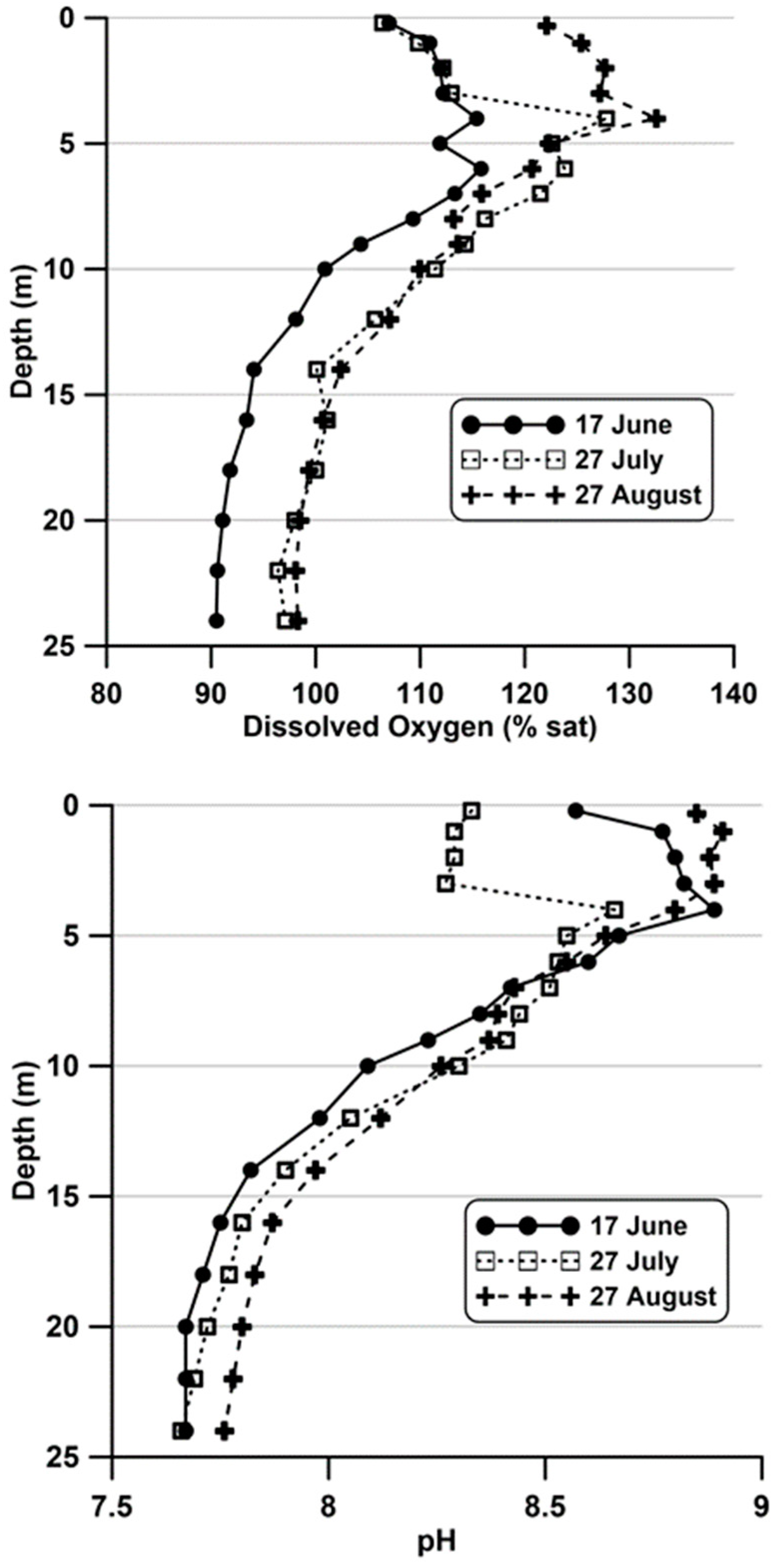
The relatively shallow depth to the metalimnion (<5 m) and favorable nutrient supply allow for high rates of photosynthesis resulting in metalimnetic maxima for pH and dissolved oxygen (
Figure 4). The metalimnion has moderate concentrations of nitrogen and phosphorus, thus facilitating phytoplankton growth.
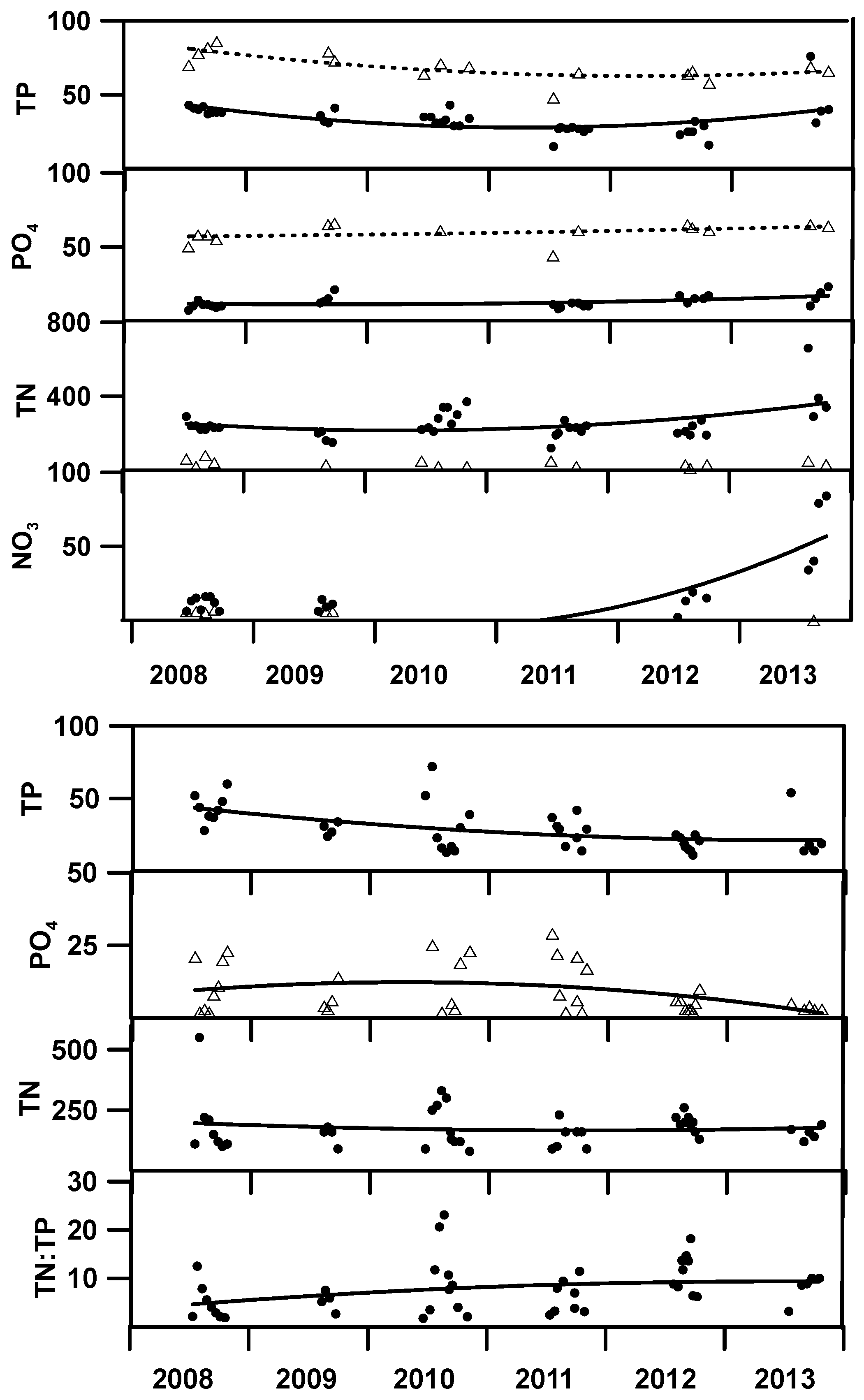
Although annual variability in physical limnological features remained stable throughout the study period, chemical attributes exhibited significant changes (
Figure 5). Most notable among these were the declines in total phosphorus and ortho-phosphorus. With total nitrogen remaining relatively unchanged and inorganic nitrogen remaining near detection limits, the ratio of TN:TP declined to values that suggest the epilimnetic nutrient chemistry transitioned from one of N-limitation to P-limitation. The presence of favorable N:P ratios and moderate transparency allowed for metalimnetic maxima in pH and dissolved oxygen under summer conditions (
Figure 6).
Both tributaries showed significant change in chemistry between 2008 and 2013 which complicates the assessment regarding the role of fisheries and reservoir operations in altering lake chemistry (
Table 3). Nevertheless, Lemolo Lake experienced a decline in total phosphorus of 35 µg/L, far greater than the decline of 12 µg/L measured in the North Umpqua River. Additionally, the inflow from Lake Creek, which has a much larger effect on water quality in the epilimnion, showed no change in TP. The mass ratio of TN:TP in Lemolo Lake increased substantially, caused by a large decrease in TP and a smaller increase in TN. Concentrations of nitrate were typically less than the detection limit for samples collected from Lemolo Lake and the North Umpqua River. However, nitrate exhibited a large increase in Lake Creek which we attribute to mineralization of organic nitrogen during transport from Diamond Lake to Lemolo Lake.
Biological Limnology: Concern regarding Lemolo Lake started in 2006 because of the intense blooms of
Anabaena. These blooms continued in 2007 leading to the initiation of the project in 2008. The intensity and duration of the
Anabaena blooms declined during the study, but the density of
Gloeotrichia echinulata increased markedly towards the end of the study (
Figure 6). Sampling of the outlet from Diamond Lake and the inlet to Lemolo Lake showed that about 45 percent of the
Gloeotrichia colonies leaving Diamond Lake (120 colonies/L) were still intact and viable on entering Lemolo Lake (54 colonies/L). Large cladocerans consisted primarily of
Daphnia pulicaria, with small numbers of
D. rosea. Zooplankton were not sampled intensively enough to provide for full characterization of the group. However, populations of
Daphnia were relatively modest with peak densities of less than 10,000 individuals per cubic meter typically observed during the summer. By comparison, Diamond Lake, whose outlet flows into Lemolo Lake, experiences peak densities of 50,000 to 70,000 individuals per cubic meter. Benthic macroinvertebrate communities were not sampled in Lemolo Lake, in part, because of available resources, but also because the benthic macroinvertebrate community is perceived to be less important in this impoundment compared to natural lakes in the area. The lake undergoes an annual winter drawdown of over 7 m, freezing the exposed sediments and eliminating the development of macrophyte beds.
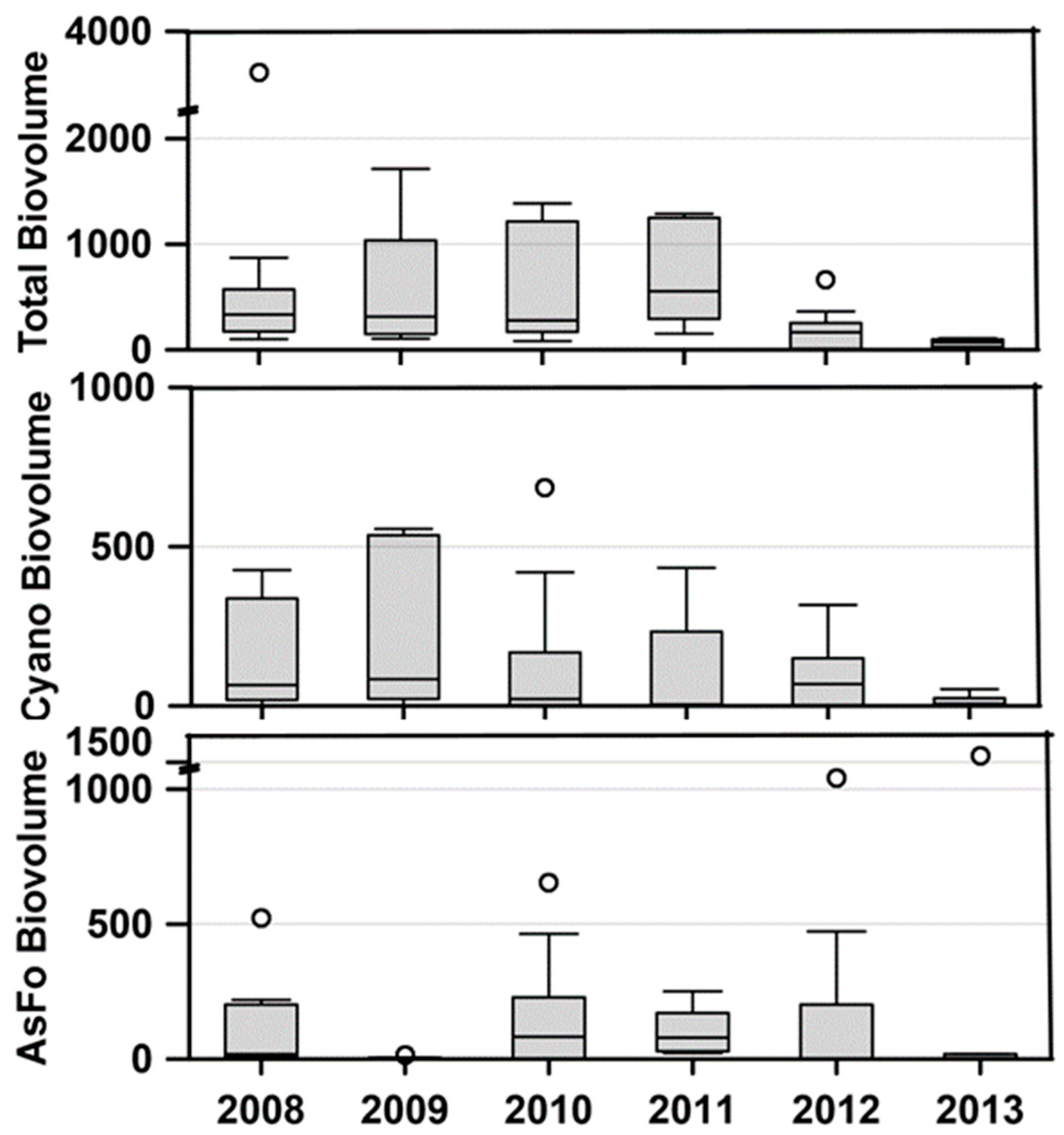
The primary reason for initiating the study of Lemolo Lake was the repeated intense blooms of cyanobacteria that resulted in closure to recreational use. These blooms began in 2006 during the drawdown of Diamond Lake prior to its treatment with rotenone to remove the tui chub [
8] Total phytoplankton biovolume remained high in Lemolo Lake through 2011; however, the biovolume of cyanobacteria exhibited a notable decline starting in 2010 (
Figure 7). The primary cyanobacteria taxa present during these blooms were
Anabaena and
Gloeotrichia. A portion of the
Gloeotrichia colonies were derived from Diamond Lake which flows into Lemolo Lake via Lake Creek. Measurements on 27 July 2013 showed that 1.6 × 10
10 Gloeotrichia colonies entered Lemolo Lake from Lake Creek during that 24-h period. The density of
Gloeotrichia colonies ranged from 60–560/L in the surface waters of Lemolo Lake. This event corresponded with a bloom of
Gloeotrichia in Diamond Lake which resulted in the release of 120 colonies/L being discharged to Lake Creek [
18]. Diatoms were also an important component of the phytoplankton community in Lemolo Lake. One taxon,
Asterionella formosa (AsFo), represented the largest diatom component of the phytoplankton biovolume.
Lemolo Lake is located above Tokatee Falls (34 m), a natural barrier to fish migration. The impoundment first filled in 1954 when Diamond Lake was being drawn down in preparation for a rotenone treatment to eradicate tui chub [
19]. During the drawdown, tui chub are inferred to have entered Lemolo Lake based on netting in 2007 which showed high catch per unit effort and the maturity and uniform age of the catch. Lemolo Lake has been stocked at various times with rainbow trout (
Oncorhynchus mykiss), brown trout (
Salmo trutta), and kokanee (
O. nerka). Rainbow trout have been unable to reproduce in the system, but brown trout and kokanee spawn in Spring River, a tributary to the North Umpqua River. Brown trout and kokanee are no longer stocked in the lake, but rainbow trout have been stocked regularly in recent years (
Table 4). The abundance of tui chub in Lemolo Lake was believed to be low prior to 2005, but apparently increased dramatically during the drawdown of Diamond Lake prior to a second rotenone treatment conducted in September 2006 [
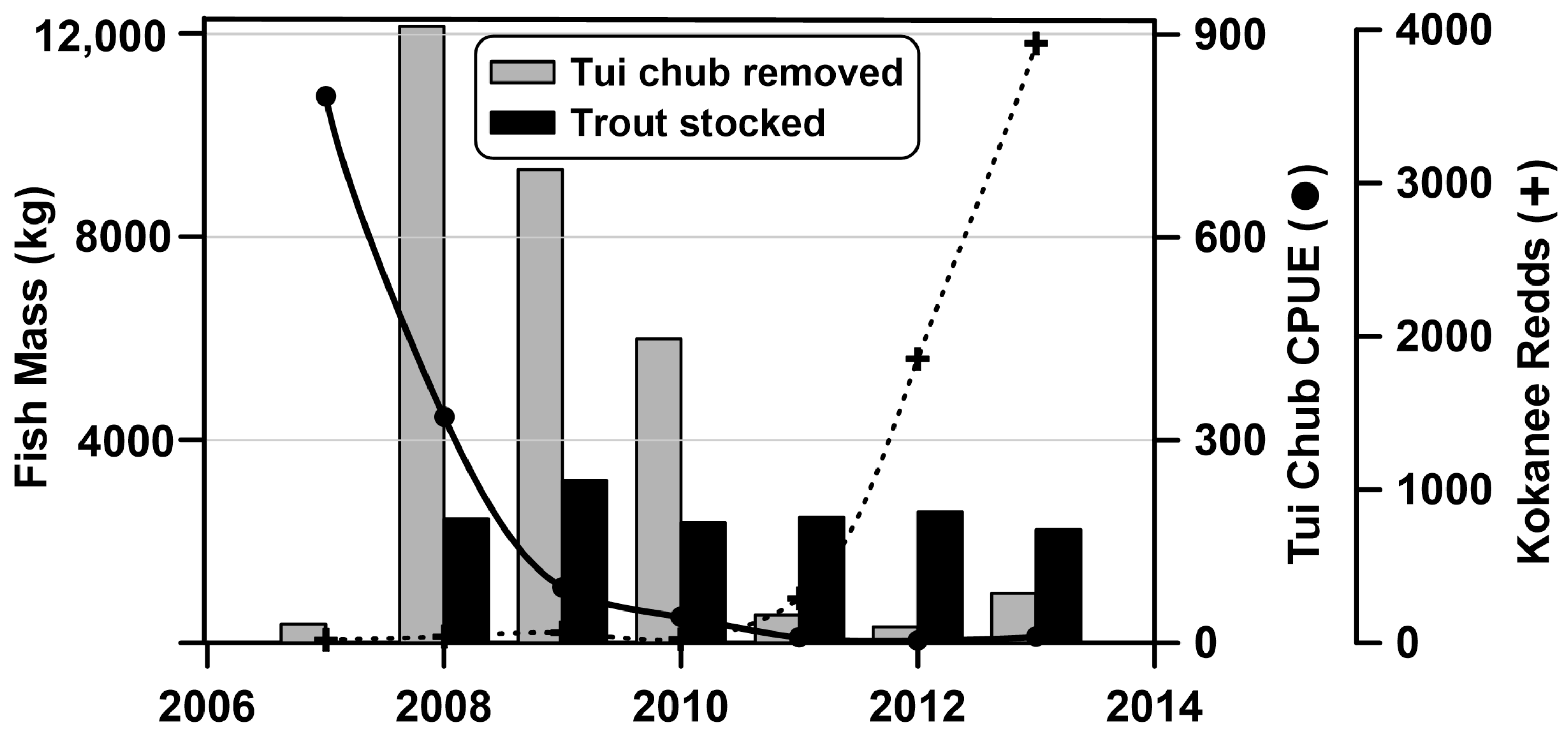
15]. A test trap net deployment by ODFW in 2007 showed a high abundance of tui chub in Lemolo Lake and it was decided to pursue a more concerted effort to remove the chub with trap netting. Chub harvesting was successful in the early years and declined as increasing effort yielded fewer fish (
Figure 8). As the chub netting effort proceeded, the kokanee population increased based on redd counts in the preferred spawning areas. The stocking rate of rainbow trout remained relatively stable throughout the study with an average rate of 14 kg/ha/year.
Entrainment of fish in the power canal was not formally investigated during this study, although an entrainment study was conducted prior to the recent relicensing [
16]. However, power shutdowns associated with forest fires and maintenance operations offered insight into the effects of hydropower operations on the current fishery. Two power canal shutdowns in 2013 resulted in fish being stranded in the power canal, which required PacifiCorp personnel to salvage trapped fish and return them to the lake. The results showed that 75 percent of the fish salvaged were tui chub and kokanee (
Table 5). These results likely underrepresent the proportion of chub and kokanee entrained because these taxa are more likely to pass quickly through the power canal, whereas the larger brown trout and rainbow trout are able to hold in the strong flows of the canal for a longer period. The high proportion of kokanee and tui chub entrained in 2013 is consistent with previous observations that these lacustrine taxa are preferentially entrained [
16].
The trap netting effort was highly successful in removing tui chub from Lemolo Lake, but these reductions in fish biomass were partially offset by the continued stocking of rainbow trout and the increase in the kokanee population (
Figure 7). The mass of trout stocking exceeded the mass of tui chub removed from 2011–2013. The mass of kokanee in 2013 likely exceeded the mass of the trout stocking by a factor of two. Kokanee naturally exhibit large fluctuations and it is unclear if their abundance in 2012–2013 was a result of reduced entrainment associated with a decreased reservoir drawdown or whether reduced competition from tui chub exerted a role in their increased abundance.
CE-QUAL-W2 Model: The “W2” model was calibrated to observed conditions and the model was able to reproduce temperature profiles throughout the study period (
Figure 7). Absolute mean errors (AME) were greatest at the beginning of each field season, likely because the weather station also began operation at the beginning of sampling. Without the preceding weather data, the model was unable to more accurately reproduce the early sampling data. However, once the season advanced, AME declined to acceptable levels. The ability of the model to reproduce the temperature regime in Lemolo Lake is impressive given the two outlets from the impoundment and the presence of a pronounced internal seiche. As expected, our ability to simulate water chemistry such as pH and dissolved oxygen declined compared to results observed for water temperature.
The W2 model was used to simulate a variety of scenarios to help resolve potentially confounding factors contributing to the onset of cyanobacteria blooms. These simulations included assessments of the effects of changing climate (increased precipitation, increased air temperature), changes in reservoir operations (increased flow to the bypass reach, decreased summer lake stage), and external manipulations. Of these, the only scenario that showed a substantial effect on water quality in the reservoir was increased precipitation. Although increased precipitation will reduce hydraulic residence time, the majority of flows enter the lake in the hypolimnion and pass through the impoundment with relatively small interaction with the epilimnetic waters. Consequently, the benefits of a reduced hydraulic residence time appear to be outweighed by the increased flux of nutrients into the lake. A simulation was run in which nitrate was added to the inlet from Lake Creek. The model results indicated that this manipulation would likely suppress the appearance of Anabaena blooms, although it would have little effect on the influx of Gloeotrichia exported from Diamond Lake.
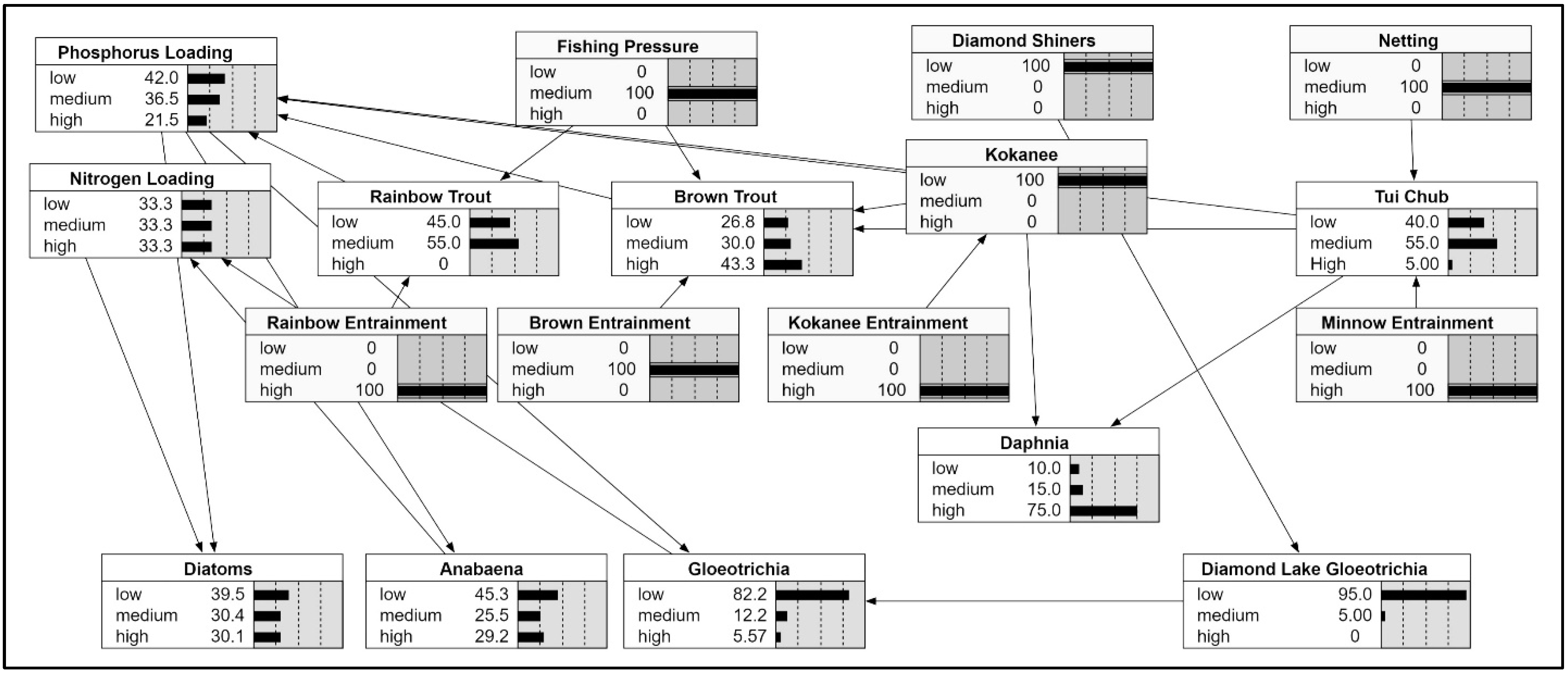
Bayesian Model: Although it is possible to add a fish subroutine to the hydrodynamic model, we resisted this approach because of the number of fish taxa present in the lake and uncertainties in the abundance of some of these taxa. We elected to develop a simple Bayesian model that showed some of the major interactions of the fish with one another and with reservoir operations. The resulting model depicts likely interactions, allowing us to explore possible solutions involving fishery and reservoir management (
Figure 8). The model was run using various assumptions regarding external inputs such as
Gloeotrichia from Diamond Lake, the magnitude of winter drawdown, fishing pressure, and stocking regimes. The model results that suggested the most favorable lake response (as reflected in decreased
Anabaena blooms) included continued trap netting of tui chub and a return to a deep drawdown used prior to relicensing.
5. Discussion
Lemolo Lake is typical of many lakes throughout North America in which multiple fish taxa have been introduced either by fish management agencies or through citizen actions [
20]. The complex fisheries that result add to the challenges in managing these resources for both water quality concerns and desired sport fish experiences. Here we attempted to resolve the primary causes of cyanobacterial blooms that coincided with the inadvertent introduction of a cyprinid previously associated with the onset of
Anabaena blooms in an upstream lake [
15]. However, the large influx of tui chub occurred in 2006 concomitantly with two changes in reservoir operations and a change to a period of above-normal annual precipitation. The hydrodynamic modeling indicated that the change in reservoir operations had little impact on the epilimnetic hydraulic residence time or other relevant physical properties that might have altered the phytoplankton dynamics. The increased precipitation increased the inflow to the impoundment, although the flux of phosphorus to the lake remained relatively stable during the study period. The hydrodynamic modeling indicated that one could expect a positive impact on increased phytoplankton production from increased hydraulic inputs, however only if phosphorus concentrations in the inflows remained stable.
Harvesting of tui chub resulted in the removal of nearly 30,000 kg of fish (163 kg/ha), whereas stocking introduced over 15,000 kg of rainbow trout, thus offsetting some of the reductions in fish biomass achieved with the trap netting. Rainbow trout were the most common fish caught by anglers in Lemolo Lake with nearly 2000 caught in 2013 and over 900 retained by anglers. Additionally, some of the rainbow trout were entrained in the power intake canal and some were consumed by the larger brown trout, presumably reducing the overall impact of the fish stocking on fish biomass. Initial gains in growth and mass of brown trout were minor and were overwhelmed by the rapid expansion of the kokanee population following the removal of much of the tui chub. By 2013, nearly 4000 kokanee redds were counted in the spawning areas. Assuming that there are two adults per redd with an average length of 400 mm and a weight of 0.35 kg/adult kokanee yields 2800 kg of adult kokanee present. The younger year class could easily exceed the biomass estimate for the spawning adults. Because kokanee are obligate planktivores, they have the potential to encourage the growth of phytoplankton through both the consumption of large cladocerans and the excretion of soluble nutrients.
The biovolume of cyanobacteria in Lemolo Lake exhibited a significant decline starting in 2010, two years after the initiation of tui chub netting which by then had achieved the largest reduction in tui chub biomass based on netting success. Additional reductions in total algal biovolume and cyanobacterial biovolume were observed in 2012 and 2013. These later reductions in algal biovolume coincided with dramatic increases in nitrate concentrations in Lake Creek from near-detection values to over 80 µg/L in 2013. A W2 model simulation based on adding nitrate to the Lake Creek inlet forecast that this would suppress the abundance of cyanobacteria. Although the reduction in cyanobacteria biomass from 2010–2011 was not accompanied by an increase in nitrate from Lake Creek, the additional reduction in cyanobacteria, particularly in 2013, may have been driven by the external change in nitrate loading from Diamond Lake rather than by internal changes to fish abundance in Lemolo Lake.
The question of how best to deal with cyanobacteria-induced growth from high fish biomass in Lemolo Lake was examined through a simple Bayesian model. The model results indicated that there were several actions that could be pursued to achieve lower fish biomass and improved water quality. These include (1) reducing the stocking rate of rainbow trout, (2) continued trap netting of tui chub, and (3) returning to a deep winter drawdown to preferentially increase the entrainment of tui chub and kokanee. The rainbow stocking rate employed during this study resulted in an average stocking rate of 75 fish per ha, which by local standards is not particularly high, but when combined with the other taxa present appears to be excessive. Trap netting of tui chub is completely selective since salmonids are returned to the lake unharmed. A modest trap netting program will ensure that the tui chub remain suppressed and avoids a return to the high densities observed at the outset of the study. Trap netting had no impact on the exclusively pelagic kokanee and sport angling had had little effect on the growing population of kokanee. However, a return to periodic deep winter drawdown of 15 m would preferentially increase entrainment of kokanee and tui chub [
16]. The incidental loss of brown trout and rainbow trout associated with the deeper drawdown likely would be offset by reduced competition for food resources from the tui chub and kokanee. All of the above fish-related management changes are expected to decrease the incidence of blooms of
Anabaena; however, they will have little impact on the transport of
Gloeotrichia into Lemolo Lake from Diamond Lake. Those colonies may remain a nuisance until they are controlled at the source.
The approach used here of monitoring, combined with experimental trap netting and modeling, helped to identify the likely cause(s) of the cyanobacteria blooms in Lemolo Lake. The success of trap netting tui chub greatly reduced this source of undesirable fish biomass, but the removal of the cyprinid opened a niche for kokanee to exploit. Two activities that were believed to enhance the sport fisheries by resource management agencies, rainbow trout stocking and reduced winter drawdown of the impoundment, were found to be counterproductive in maintaining low densities of cyanobacteria. The rainbow trout stocking added to the angler success, but also likely exacerbated the cyanobacteria problems by increasing fish biomass. The 15 m reservoir drawdown was discontinued with the new licensing for the express purpose of reducing entrainment of fish and hopefully leading to a more productive reservoir fishery. However, the periodic drawdowns were preferentially removing tui chub and kokanee, both of which likely contributed to biomass-induced water quality problems. A return to the use of a deeper drawdown will restore this previously under-appreciated mechanism for reducing the biomass of fish taxa that were likely to preferentially enhance cyanobacteria in the impoundment.
The study illustrated that mechanical removal of tui chub can successfully reduce a nuisance population of minnows and contribute to an improvement in water quality. However, the study also indicated that the decision to reduce the magnitude of the winter drawdown for the purpose of improving the trout fishery had the unanticipated consequence of selectively reducing entrainment of planktivorous fish such as the tui chub and kokanee. Additionally, the decision by ODFW to continue stocking rainbow trout at moderately high rates contributed to total fish biomass during a study designed to decrease fish biomass. It is ironic that the cyanobacteria blooms observed in Lemolo Lake starting in 2006 appeared to be the result of an influx of a massive population of tui chub from the drawdown of Diamond Lake prior to rotenone treatment. The initial plan for the treatment of Diamond Lake called for netting the outlet to Lake Creek to collect the tui chub during the drawdown, but the plan was abandoned when it was judged by ODFW to be too difficult to manage the logistics associated with the activity. Thus, one lake restoration effort compromised the water quality of a downstream system. The mechanical harvesting was highly successful in reducing the biomass of the cyprinids and appeared to have a positive effect in reducing the biovolume of cyanobacteria during the first three years of operation. The biomass of cyanobacteria continued to decline in the final two years of netting, although the harvest rate of cyprinids had declined greatly by this point. Other factors such as increased loading of nitrate to the lake, complex trophic dynamics associated with increased kokanee production, and variation in watershed runoff may have contributed to further changes to the lake.