Abstract
Recent advances have been made in understanding molecular markers involved in cancer malignancy, resulting in better tumor staging and identifying new potential therapeutic targets. Ezrin (EZR), a member of the ezrin, radixin, moesin (ERM) protein family, is essential for linking the actin cytoskeleton to the cell membrane and participates in the signal transduction of key signaling pathways such as Rho GTPases and PI3K/AKT/mTOR. Clinical and preclinical studies in a wide variety of solid and hematological tumors indicate that (i) EZR is highly expressed and predicts an unfavorable clinical outcome, and (ii) EZR inhibition reduces proliferation, migration, and invasion in experimental models. The development of pharmacological inhibitors for EZR (or the signaling mediated by it) has opened a new round of investigation, but studies are still limited. The scope of the present review is to survey studies on the expression and clinical impact of EZR in cancer, as well as studies that perform interventions on the function of this gene/protein in cancer cells, providing proof-of-concept of its antineoplastic potential.
1. Introduction
Ezrin (EZR) is a protein member of the ezrin, radixin, moesin (ERM) family, and its function, when phosphorylated, is essential for linking the actin cytoskeleton to the cell membrane. The FERM domain is composed of three structural modules (F1, F2, and F3), which together form a compact clover-shaped structure and bind to integral membrane proteins, adhesion molecules, multidrug resistance proteins, scaffold proteins, Rho-related proteins, and tyrosine kinase proteins, and are involved in epithelial organization, villus morphogenesis, and PIP2 signaling. EZR plays an essential role as an activator of notable signal transduction pathways involved in cancer progression, such as PI3K/AKT/mTOR signaling, which provides a mechanism to anchor PI3K in the proximity of its substrate recruiting the p85 regulatory subunit. In contrast, the phosphorylation of EZR at Y353 is also essential to activate the PI3K signaling [1,2,3,4,5]. The activation of AKT protects cells from apoptosis by phosphorylating and inactivating BAD, a proapoptotic member of the BCL2 family, and increasing cell proliferation [6]. Furthermore, ERM phosphorylation, which appears to be regulated positively by Rho and (possibly) negatively by RAC, may activate downstream signaling of Rho proteins (including RAC) that are required for membrane ruffling and lamellipodium extension and CDC42 that induces the formation of filopodia; both GTPases are essential for cell migration and invasion [7,8] (Figure 1). Thus, EZR may serve as a proliferation- and metastasis-related oncogene by modulating multiple cellular processes [9]. EZR is widely expressed in normal tissues, but its expression is time-specific during human development. For example, EZR immunoreactivity is positive in early embryo stages but undetectable during later development and postnatal stages [10]. Recently, reports have shown that EZR is upregulated in several human tumors, as described in the following.
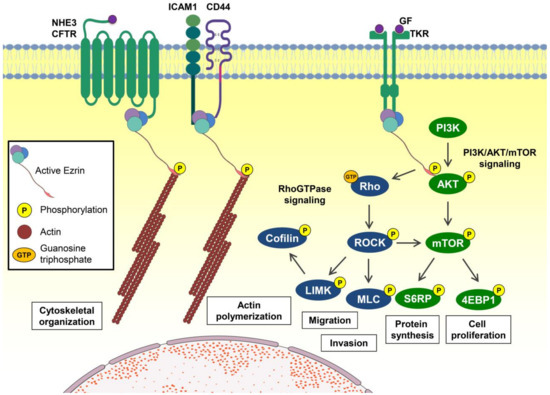
Figure 1.
Ezrin-mediated signaling pathways and cellular processes. When phosphorylated, ezrin (EZR) may bind membrane proteins such as G protein-coupled receptors (GPCRs) and other receptors allowing the binding of F-actin (actin filaments) with the membrane, promoting reorganization of the cytoskeleton. EZR also binds to several transmembrane receptors, such as growth factor or cytokine receptors, promoting the activation of multiple signaling pathways. Among the main signaling pathways triggered by EZR, we highlight the PI3K/AKT/mTOR pathway, which promotes protein synthesis and cell proliferation, and the Rho GTPase pathway, which promotes migration, invasion, and actin polymerization. Abbreviations: NHE3, Na+/H+ exchanger; CFTR, cystic fibrosis transmembrane conductance regulator; ICAM1, intercellular adhesion molecule 1; CD44, cell surface adhesion receptor; GF, growth factor; TKR, tyrosine kinase receptor; P, phosphorylation; GTP, guanosine triphosphate; PI3K, phosphatidylinositol 3-kinase; AKT, protein kinase B; mTOR, mammalian target of rapamycin; S6RP, ribosomal protein S6; 4EBP1, eukaryotic initiation factor 4E-binding protein 1; Rho, homologous Ras proteins; ROCK, Rho kinase; MLC, myosin light chain; LIMK, LIM kinase.
The EZR C-terminal domain, especially 34 amino acid residues, is highly conserved among ERM proteins and binds to the actin cytoskeleton. A coiled-coil structure connects the N-terminal FERM domain and the C-terminal actin-binding domain. The primary protein structure of EZR and its inactive and active conformations are illustrated in Figure 2.
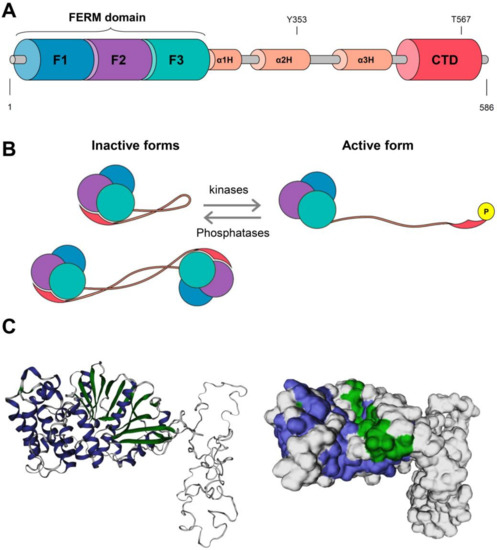
Figure 2.
Protein structure and activation model for ezrin (EZR). (A) The N-terminus consists of a domain called FERM (band 4.1 protein, ezrin, radixin, moesin) formed by three subunits F1, F2, and F3, after which there is a helical domain called α1H, α3H, and α3H followed by a C-terminal domain (CTD) subunit, where the ezrin phosphorylation sites (Y353 and T567) are found. (B) Representation of active and inactive forms of EZR. It can interact intermolecularly with itself when inactive, forming a monomer or even a dimer with another protein. When it experiences the action of kinases and is phosphorylated, ezrin is activated, “extends”, and passes to a new conformation. The action of phosphatases reverses this conformation. This illustration was adapted from Michie et al. [11]. The three subunits F1 (blue lobe), F2 (purple lobe), and F3 (green lobe) for the FERM domain, helical domain (orange line), and C-terminal domain (pink) are illustrated. (C) The 3D reconstitution of the EZR protein was constructed using the SWISS-MODEL platform (https://swissmodel.expasy.org/, accessed on 2 August 2021), and cartoon and surface versions of the protein are illustrated. Alpha-helix structures are shown in purple, beta-pleated sheet structures are in green, and other structures are in white.
2. EZR Activation
In the closed inactive state, the FERM domain is tightly associated with the ~80 residues of the C-terminal domain (CTD) from EZR, hiding the membrane association and F-actin-binding sites, and the change from closed to open ERMs requires phosphorylation of a specific threonine residue (T567 in ezrin, T564 in radixin, and T558 in moesin) [12]. Activation of EZR has been proposed to follow a two-step mechanism. The first activation occurs via phosphatidylinositol-4,5-bisphosphate (PIP2) binding at the membrane, which seems to facilitate the binding of EZR to membrane proteins. In other words, PIP2 may activate the dormant protein and expose the membrane binding site. Through this PIP2 binding, T567 in the CTD-ERM association domain becomes accessible for phosphorylation by ROCK, LOK, SLK, and some PKC isoforms [13,14,15,16].
3. Genomics of EZR
The entire EZR gene is approximately 53.6 Kb (start: 158765741 and end: 158819368bp; orientation: reverse strand). In the NCBI database (https://www.ncbi.nlm.nih.gov/gene, accessed on 2 August 2021), there are two transcript variants for EZR that encode for the same protein (586 aa). Transcript variant 1 (transcript length: 3069 bp) represents the longer transcript, while transcript variant 2 (transcript length: 3052 bp) differs from transcript variant 1 in the 5′ UTR region. In the Ensembl database (http://www.ensembl.org/, accessed on 2 August 2021), there is one additional transcript variant for EZR (ENST00000392177.8: 2933 bp), which also encodes for the same protein (586 aa).
Recurrent mutations in the EZR gene are rare, and most are variants of uncertain significance. Using The Cancer Genome Atlas (TCGA) cohorts (10,953 patients in 32 studies), a total of 192 patients (2%) presented EZR genetic alterations (mutations, amplifications, deep deletions, and multiple alterations), as reported on cBioPortal (http://www.cbioportal.org, accessed on 10 October 2022) (Figure 3A). A total of 110 somatic mutations were found: 84 missense substitutions, 16 truncating, one splice mutation, and nine gene fusions (Figure 3B). Among these mutations, two alterations stand out for having driver mutation potential: EZR::ROS1 and ARID1B::EZR gene fusions.
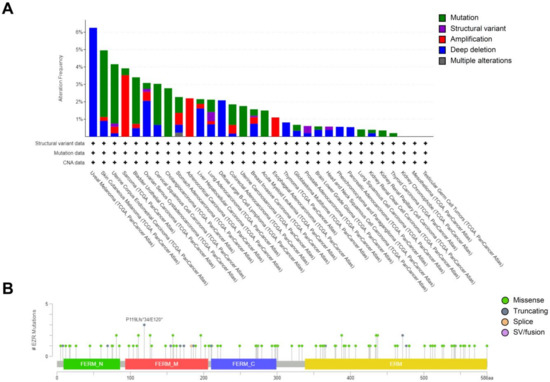
Figure 3.
EZR genomic alterations in cancer. (A) Distribution of EZR genetic alterations in The Cancer Genome Atlas (TCGA) cohorts (10953 patients in 32 studies). (B) A total of 110 somatic mutations were found: 84 missense substitutions, 16 truncating, one splice mutation, and nine gene fusions. The figures were obtained using the cBioPortal (http://www.cbioportal.org, accessed on 2 August 2021).
4. Expression and Functions for EZR in Human Cancers
4.1. Breast Cancer
EZR is upregulated in breast cancer tissues compared with normal breast tissues, especially in metastatic breast cancer, and also may be used as a biomarker in overall survival (OS) [17]. Indeed, it had been demonstrated that EZR was required for the invasion and metastasis of mammary carcinoma cells [18]. In metastatic breast cancer cells, MDA-MB-231, EZR silencing by shRNA reduced cell motility and invasion, c-Src signaling, and increased E-cadherin expression, a vital component of the adherent junctions [19]. EZR interacts with AKT in breast cancer cells, induces its downstream signaling pathway, and promotes tumor progression [9]. PI3K and proto-oncogene c-Src activities were modulated by EZR and are required for EZR-dependent cell invasion [18]. The co-expression of EZR with CD44, a glycoprotein involved in cell–cell interactions, adhesion, and migration, may also be a potential biomarker for the initiation, progression, and differentiation of breast cancer tumors [20].
4.2. Melanoma
Ilmonen et al. [21] showed that EZR expression was found in 76 out of 95 melanoma samples analyzed (80%), with 48 weakly positive and 28 strongly positive, as evidenced by an immunohistochemistry assay. Notably, EZR immunoreactivity of metastatic tumors was more substantial than in primary tumors, and none of the metastasis samples were negative for EZR expression (two samples were weakly positive, and ten were strongly positive). Additionally, EZR expression was associated with tumor proliferation index and tumor growth in primary melanoma [21]. Federici et al. [22] aimed to correlate EZR’s molecular interactions with major actors of the metastatic behavior of tumor cells (i.e., CD44) and showed that an EZR deletion mutant (146 N-terminal amino acids) abolished the in vivo metastatic dissemination of a melanoma model. In melanoma cells, EZR expression was also associated with phagocytic machinery and phagocytosis through actin cytoskeleton modulation [23]. Interestingly, miR-183 may serve as a tumor suppressor since it reduces migratory activity and invasiveness of A375 human melanoma cells. In this context, EZR was identified as a target for miR-183 [24].
4.3. Cervical Carcinoma
EZR protein was highly expressed in all cervical cancer cell lines and tissues compared with the normal cervical tissues and predicted poor prognosis in cervical cancer [25,26]. In HPV-associated squamous intraepithelial lesions, EZR expression was associated with the severity of the disease. Intense staining for EZR with diffuse intracellular distribution was also observed in in situ adenocarcinoma. In contrast, the distribution in normal endocervical tissue was strongly polarized to the apical side of columnar epithelial cells [27]. In a previous study, the authors observed slightly reduced EZR expression due to HPV 16 E5, an oncogene facilitating the early events in the neoplastic development, suggesting that increased expression of EZR would rather be a consequence of E6 and/or E7 oncogene expression [28]. In HeLa and SiHa cells, EZR silencing by siRNA reduced colony formation and cervical cancer cells’ ability to cross the chorioallantoic membrane surface and infiltrate the underlying stroma, consequently attenuating migration, invasion, mesenchymal marker expression, and PI3K/AKT activation [25].
4.4. Colorectal Cancer
Through tissue microarray and immunohistochemistry, Patara et al. [29] concluded that a higher cytoplasmatic EZR expression correlates with higher tumor aggressiveness and poor prognosis. Moreover, its expression is an important prognostic factor in colorectal cancer patients. One of the major causes of poor prognosis in colorectal cancer is lymph node metastasis initiated by epithelial–mesenchymal transition (EMT) due to the activation of several signaling pathways by EZR, which plays a significant role in protein signal transmission. Indeed, many growth factors and cytokines have been proven essential in stimulating EMT, including EGF. Li et al. [30] showed that the knockout of EZR inhibits EGF-induced lung metastasis of colorectal cancer xenografts. Abnormal activation of EZR and NFκB are related to colorectal cancer metastasis and poor prognosis. EZR expression was also associated with the degree of tumor differentiation, lymph node metastasis, and Dukes’ stage [29,31]. Gavert et al. [32] reported that the NFκB-mediated signaling participates in cellular changes induced by L1 (immunoglobulin-like cell-adhesion receptors) that lead to invasive phenotype in colorectal cancer. Moreover, the same authors found that NFκB- and EZR-mediated signaling are essential for the ability of L1 to induce metastasis in colorectal cancer cells. The decrease in NFκB transactivation, EZR levels, or an L1 mutant with an altered EZR-binding domain blocked the ability of L1 to induce liver metastasis. Accordingly, colorectal cancer patients with moderate to robust EZR expression (by immunohistochemistry) presented a reduced five-year overall survival rate compared to patients with a negative or low-intensity EZR expression [29]. Increased EZR phosphorylation at T567 was also reported in samples from colorectal cancer patients, which was associated with the IGF1R signaling pathway and expression of XIAP and BIRC5, both apoptosis inhibitors [33].
4.5. Endometrial Cancer
In endometrial cancer, EZR expression is higher in the high-metastasis Ishikawa subclone (named mEIIL) compared to its parental low-metastasis cell line. Treatment with EZR antisense phosphorothioate oligonucleotides reduced invasiveness, but not proliferation, in mEIIL cells [34]. High EZR protein expression was reported in uterine endometrioid adenocarcinomas. EZR protein levels were also significantly increased in endometrial hyperplasias that more frequently progressed to invasive cancer and metastatic lesions than in the matched primary lesions. In endometrioid carcinomas, EZR was at least focally expressed in 93% of cases, which was quite heterogeneous, with a wide range from only a few to nearly 100% positive tumor cells [35].
4.6. Gastric Cancer
EZR immunostaining was positive in 81.1% of intestinal-type and 40.9% of diffuse-type adenocarcinoma cases, suggesting that low EZR expression indicates the loss of adhesion in diffuse carcinomas. Furthermore, EZR overexpression was related to Helicobacter pylori infection [36]. In another study, EZR expression was detected, at low levels, in 11.2% of non-tumor mucosae and, at elevated levels, in 59.2% of gastric cancer samples. EZR overexpression was associated with age, tumor size, location, differentiation stage, depth of invasion, vessel invasion, lymph node and distant metastasis, and TNM stage, and it was an independent prognostic factor in patients with gastric carcinoma [37]. Jin et al. reported similar findings [38]. The high frequency of EZR expression suggests a central role in gastric cancer biology. Thus, EZR protein expression could be used as an early diagnostic marker of gastric cancer and its precancerous disease, and its overexpression may be a predictor of poor prognosis [38].
4.7. Head and Neck Squamous Cell Carcinoma
In head and neck squamous cell carcinoma patients, high cytoplasmic EZR expression was observed in 92% of the tumors and independently associated with a worse prognosis [39]. These data were validated in a large cohort in which the high EZR expression was associated with poor survival outcomes. Notably, tumors with high cytoplasmic EZR display a more invasive phenotype [40].
4.8. Hepatocellular Carcinoma
EZR expression was higher in tumor samples than hepatic tissues and associated with metastasis and differentiation degree [41,42]. High EZR levels have been significantly associated with advanced TNM stage, poor Edmondson’s histological grade, macroscopic portal vein invasion, tumor recurrence, and extrahepatic recurrence, and are independently associated with poor overall survival [43,44]. Increased levels of EZR were observed in hepatocarcinoma cell lines with higher metastatic potential, and EZR inhibition by siRNA reduced clonogenicity, cell proliferation, motility, and invasion [44,45]. EZR hyperphosphorylation has been identified as responsible for the invasion of hepatocellular cells, promoting intrahepatic metastasis in vivo and cell migration in vitro [46,47]. EZR overexpression promotes hepatocellular carcinoma cell proliferation, EMT, angiogenesis, and glycolysis reprogramming [46,47]. In hepatocellular carcinoma patients, EZR gene expression was significantly decreased after treatment with arsenic trioxide [48], suggesting a potential approach for pharmacological intervention on EZR expression.
4.9. Kidney Cancer
In renal cell cancer, the absence of EZR expression was an independent prognostic factor of disease-specific survival. EZR expression was also associated with incidental tumors, clinical stage, pT stage, synchronic metastasis, and ISUP histological grade [49,50].
4.10. Leukemia
In T-cell acute lymphoblastic leukemia cells, ERM phosphorylation has been found to promote invasion [51], and EZR deregulation has been related to the progression of mouse preleukemia erythroblasts toward malignancy [52]. In acute myeloid leukemia cells, functional studies have shown that EZR promotes survival and acts as an effector protein in cell signaling mediated by FLT3-ITD and mutated KIT receptors [52,53,54]. In a comprehensive analysis of cytoskeleton regulatory genes, Lipreri da Silva et al. [55] identified that high EZR expression is an independent marker of worse outcomes in acute myeloid leukemia patients, and EZR pharmacological inhibition reduced viability, proliferation, autonomous clonal growth, and cell cycle progression in leukemia cells. In chronic lymphocytic leukemia (CLL), EZR is highly expressed and associated with molecular signatures relevant to the disease’s development and maintenance, including TP53, PI3K/AKT/mTOR, NFκB, and MAPK pathways [56]. Pharmacological EZR inhibition with NSC305787 reduced viability, clonogenicity, and cell cycle progression and induced apoptosis in CLL primary and cell lines [56].
4.11. Lymphoma
In diffuse large B-cell lymphoma, EZR is essential to B-cell antigen receptor organization at the cell membrane and supports proliferation and survival signals. EZR inhibition by siRNA or pharmacological agents reduced cell viability in lymphoma cells [57]. Moreover, it has been shown that ERM proteins regulate B- and T-cell activation by controlling BCR and TCR dynamics, scaffolding protein assembly, and membrane-associated intracellular signaling [58].
4.12. Lung Cancer
EZR expression was associated with late-stage tumors, pleural invasion, and poor survival outcomes in non-small cell lung carcinomas [59]. EZR phosphorylation at T567 and Y353 was significantly upregulated in non-small cell lung carcinomas compared with normal tissues, which was also correlated with poor differentiation and late clinical stage. However, only EZR phosphorylation at T567 overexpression was associated with the presence of lymph node metastasis and overall survival [60]. EZR may also be a possible predictor to assess the prognosis in patients with non-small cell lung carcinoma since its gene expression was associated with lymphatic metastasis [61].
4.13. Malignant Pleural Mesothelioma
Pleural mesothelioma expresses activated ERM proteins, with EZR being the principal component of this family of proteins associated with proliferation and migration in this disease [62].
4.14. Oral Squamous Cell Carcinoma
EZR expression was significantly associated with N-classification, UICC stage, and lymphangitic carcinomatosis [63]. Another study, using tissue microarray, observed that EZR is overexpressed in primary tongue squamous cell carcinoma and may be implicated in these cells’ growth, migration, and invasiveness, possibly regulating the E-cadherin/β-catenin complex [64]. Evaluation of EZR expression in human tongue cancer tissues by immunohistochemical staining showed that this protein was highly expressed in invasive squamous cell carcinoma compared to in situ carcinoma [65]. Another study also suggested that EZR may be involved in the progression of tongue carcinoma in situ to squamous cell carcinoma [66]. Immunoexpression results confirmed a correlation between EZR expression in squamous cell carcinoma of the lip, suggesting cooperative participation of these proteins in cell movement and invasion [67]. In tongue squamous cell carcinoma clinical samples, EZR activation at Y353 is associated with metastases and poor patient prognosis [68].
4.15. Ovarian Cancer
In OVCA cells, estradiol increased EZR expression, which was associated with invasive behavior [69]. In ovarian carcinoma patients, EZR expression presents a high predictive value for tumor-related death, including late stage [70]. EZR regulates OVCA cell proliferation, migration, and invasion by modulating EMT and inducing the formation of actin stress fibers by regulating Rho GTPase activity [71]. In three-dimensional cultured ovarian carcinoma cell lines, reduced expression of EZR by shRNA impaired invasiveness and cell branching ability [72].
4.16. Pancreatic Cancer
Zhou et al. [73] showed that the levels of p-EZR T567/p-EZR Y353 protein expression in the cytoplasm of pancreatic cancer cells increased with the TNM stage of human pancreatic cancers. The survival time of the group positive for p-EZR T567/p-EZR Y353 protein expression was shorter than that of the negative group [73]. EZR levels were significantly upregulated in pancreatic cancer patients’ samples and correlated with poor prognosis [74]. Two active phosphorylated sites of EZR, Y353 and T567, were differentially expressed in pancreatic cancer tissues. High EZR phosphorylation at Y353, but not T567, was associated with malignant progression and clinical outcomes of pancreatic cancer patients [75]. In pancreatic cancer cells, EZR was involved in the cytoskeleton modulation, protrusions, and microvilli, which were related to migration and invasion [76]. Bioinformatic tools showed that EZR expression predicted worse survival outcomes in the pancreatic adenocarcinoma cohort, and gene signatures were associated with EZR expression, including apoptosis, PI3K/AKT/mTOR signaling, estrogen response early, NOTCH signaling, estrogen response late, and pancreatic beta cells [77]. Pharmacological EZR inhibitor, NSC305787, reduced viability, clonogenicity, migration, and induced apoptosis in pancreatic cancer cells [77].
4.17. Prostate Cancer
EZR expression was moderate or strong in samples from prostate cancer patients and negative or weakly expressed in benign cases [78]. EZR staining was more intense in high-grade prostatic intraepithelial neoplasia than in other prostate cancer cells [79]. EZR was found to be an essential intracellular mediator of cell invasion in hormone-sensitive and hormone-resistant prostate cancer cells. Androgen treatment induces EZR expression and phosphorylation at T567 and Y353 residues. Overexpression of mutated EZR variants (T567A and Y353F) or EZR silencing by siRNA inhibited androgen-induced invasion [80]. In metastatic prostate cancer cell lines (22RV1 and PC-3), EZR overexpression promoted migratory and invasive abilities. Accordingly, EZR expression was significantly increased in circulating tumor cells from metastatic prostate cancer patients [81].
After radical prostatectomy, circulating tumor cells from EZR-positive prostate cancer patients were susceptible to tumor metastases [81]. In nude mice bearing prostate cancer, treatment with baicalein decreased tumor volume and weight, which were much more pronounced in those with in vivo EZR knockdown [82]. Furthermore, baicalein treatment suppresses proliferative capacity in prostate cancer cell lines, interrupts the cell cycle progression, and stimulates apoptosis through EZR downregulation [82].
5. Concluding Remarks and Pharmacological Advances for EZR Inhibition
In most tumors studied to date, EZR is highly expressed and predicts an unfavorable clinical outcome. Studies with genetic intervention in experimental models highlight the importance of EZR in maintaining the malignant phenotype and directing this gene/protein (or the signaling mediated by it) as a potential target for antineoplastic therapy.
Two synthetic compounds that act as selective EZR inhibitors (NSC668394 and NSC305787) have been evaluated in preclinical studies (Figure 4). These compounds directly bind to EZR, inhibiting T567 phosphorylation with low micromolar affinity [83]. It has also been demonstrated that these EZR inhibitors have distinct target-binding profiles. For example, NSC305787 bound and dissociated more quickly from EZR than NSC668394 [83]. Another point worth mentioning is that NSC305787 and NSC668394 are well tolerated in murine models without any apparent acute toxicity [83]. Regarding pharmacokinetics, NSC305787 has the most prolonged plasma half-life (>6 h by intraperitoneal (i.p.) injection) compared to NSC668394 (<0.5 h i.p.) [84]. Although these compounds have potent antineoplastic activity in models of osteosarcomas, lung cancer, leukemia, and pancreatic cancer, their underlying molecular mechanisms and potential application in other cancer models remain poorly explored [55,77,83].
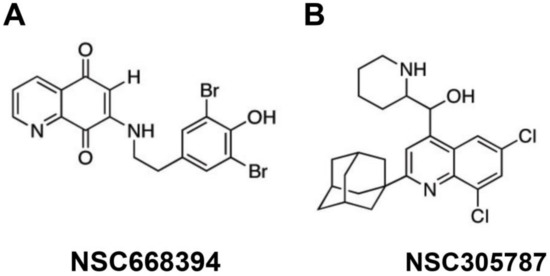
Figure 4.
Chemical structures of putative pharmacological EZR inhibitors NSC668394 (A) and NSC305787 (B).
A summary of EZR expression in cancer is presented in Table 1, and a summary of EZR functional assays in cancer cells is shown in Table 2.

Table 1.
Summary of EZR expression in cancer.

Table 2.
Summary of EZR functional assays in cancer cells.
Author Contributions
Conceptualization, J.C.L.d.S. and J.A.M.-N.; writing—original draft preparation, J.C.L.d.S. and J.A.M.-N.; writing—review and editing, H.P.V.; visualization, J.C.L.d.S. and H.P.V.; supervision, J.A.M.-N.; project administration, J.A.M.-N.; funding acquisition, J.A.M.-N. All authors have read and agreed to the published version of the manuscript.
Funding
J.C.L.d.S. and H.P.V. received fellowships from the São Paulo Research Foundation (FAPESP; grants #2020/12909-7 and #2021/01460-1). This study was supported by grants #2019/23864-7 and #2021/11606-3 from the FAPESP. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil (CAPES), Finance Code 001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Neisch, A.L.; Fehon, R.G. Ezrin, Radixin and Moesin: Key regulators of membrane-cortex interactions and signaling. Curr. Opin. Cell Biol. 2011, 23, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Asano, S. Pathophysiological Roles of Actin-Binding Scaffold Protein, Ezrin. Int. J. Mol. Sci. 2022, 23, 3246. [Google Scholar] [CrossRef] [PubMed]
- Clucas, J.; Valderrama, F. ERM proteins in cancer progression. J. Cell Sci. 2014, 127, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Bruce, B.; Hewitt, S.; Thomas, D.; Khanna, C.; Helman, L.J. Ezrin mediates growth and survival in Ewing’s sarcoma through the AKT/mTOR, but not the MAPK, signaling pathway. Clin. Exp. Metastasis 2006, 23, 227–236. [Google Scholar] [CrossRef]
- Gautreau, A.; Poullet, P.; Louvard, D.; Arpin, M. Ezrin, a plasma membrane-microfilament linker, signals cell survival through the phosphatidylinositol 3-kinase/Akt pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 7300–7305. [Google Scholar] [CrossRef]
- Del Peso, L.; Gonzalez-Garcia, M.; Page, C.; Herrera, R.; Nunez, G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 1997, 278, 687–689. [Google Scholar] [CrossRef]
- Ivetic, A.; Ridley, A.J. Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes. Immunology 2004, 112, 165–176. [Google Scholar] [CrossRef]
- Ridley, A.J. Rho family proteins: Coordinating cell responses. Trends Cell Biol. 2001, 11, 471–477. [Google Scholar] [CrossRef]
- Li, N.; Kong, J.; Lin, Z.; Yang, Y.; Jin, T.; Xu, M.; Sun, J.; Chen, L. Ezrin promotes breast cancer progression by modulating AKT signals. Br. J. Cancer 2019, 120, 703–713. [Google Scholar] [CrossRef]
- Xie, J.J.; Zhang, F.R.; Tao, L.H.; Lu, Z.; Xu, X.E.; Jian, S.; Xu, L.Y.; Li, E.M. Expression of ezrin in human embryonic, fetal, and normal adult tissues. J. Histochem. Cytochem. 2011, 59, 1001–1008. [Google Scholar] [CrossRef]
- Michie, K.A.; Bermeister, A.; Robertson, N.O.; Goodchild, S.C.; Curmi, P.M.G. Two Sides of the Coin: Ezrin/Radixin/Moesin and Merlin Control Membrane Structure and Contact Inhibition. Int. J. Mol. Sci. 2019, 20, 1996. [Google Scholar] [CrossRef] [PubMed]
- Pelaseyed, T.; Viswanatha, R.; Sauvanet, C.; Filter, J.J.; Goldberg, M.L.; Bretscher, A. Ezrin activation by LOK phosphorylation involves a PIP(2)-dependent wedge mechanism. Elife 2017, 6, e22759. [Google Scholar] [CrossRef] [PubMed]
- Shabardina, V.; Kramer, C.; Gerdes, B.; Braunger, J.; Cordes, A.; Schafer, J.; Mey, I.; Grill, D.; Gerke, V.; Steinem, C. Mode of Ezrin-Membrane Interaction as a Function of PIP2 Binding and Pseudophosphorylation. Biophys. J. 2016, 110, 2710–2719. [Google Scholar] [CrossRef] [PubMed]
- Herrig, A.; Janke, M.; Austermann, J.; Gerke, V.; Janshoff, A.; Steinem, C. Cooperative adsorption of ezrin on PIP2-containing membranes. Biochemistry 2006, 45, 13025–13034. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, A.; Reczek, D.; Berryman, M. Ezrin: A protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J. Cell Sci. 1997, 110 Pt 24, 3011–3018. [Google Scholar] [CrossRef] [PubMed]
- Viswanatha, R.; Ohouo, P.Y.; Smolka, M.B.; Bretscher, A. Local phosphocycling mediated by LOK/SLK restricts ezrin function to the apical aspect of epithelial cells. J. Cell Biol. 2012, 199, 969–984. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, S.; Xing, R.; Zhang, Q. High expression of EZR (ezrin) gene is correlated with the poor overall survival of breast cancer patients. Thorac. Cancer 2019, 10, 1953–1961. [Google Scholar] [CrossRef]
- Elliott, B.E.; Meens, J.A.; SenGupta, S.K.; Louvard, D.; Arpin, M. The membrane cytoskeletal crosslinker ezrin is required for metastasis of breast carcinoma cells. Breast. Cancer Res. 2005, 7, R365. [Google Scholar] [CrossRef]
- Li, Q.; Wu, M.; Wang, H.; Xu, G.; Zhu, T.; Zhang, Y.; Liu, P.; Song, A.; Gang, C.; Han, Z.; et al. Ezrin silencing by small hairpin RNA reverses metastatic behaviors of human breast cancer cells. Cancer Lett. 2008, 261, 55–63. [Google Scholar] [CrossRef]
- Ma, L.; Jiang, T. Clinical implications of Ezrin and CD44 coexpression in breast cancer. Oncol. Rep. 2013, 30, 1899–1905. [Google Scholar] [CrossRef]
- Ilmonen, S.; Vaheri, A.; Asko-Seljavaara, S.; Carpen, O. Ezrin in primary cutaneous melanoma. Mod. Pathol. 2005, 18, 503–510. [Google Scholar] [CrossRef]
- Federici, C.; Brambilla, D.; Lozupone, F.; Matarrese, P.; de Milito, A.; Lugini, L.; Iessi, E.; Cecchetti, S.; Marino, M.; Perdicchio, M.; et al. Pleiotropic function of ezrin in human metastatic melanomas. Int. J. Cancer 2009, 124, 2804–2812. [Google Scholar] [CrossRef] [PubMed]
- Lugini, L.; Lozupone, F.; Matarrese, P.; Funaro, C.; Luciani, F.; Malorni, W.; Rivoltini, L.; Castelli, C.; Tinari, A.; Piris, A.; et al. Potent phagocytic activity discriminates metastatic and primary human malignant melanomas: A key role of ezrin. Lab. Invest. 2003, 83, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, G. MicroRNA-183 inhibits A375 human melanoma cell migration and invasion by targeting Ezrin and MMP-9. Oncol. Lett. 2019, 17, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Di, C.; Piao, J.; Sun, J.; Han, L.; Chen, L.; Yan, G.; Lin, Z. Ezrin contributes to cervical cancer progression through induction of epithelial-mesenchymal transition. Oncotarget 2016, 7, 19631–19642. [Google Scholar] [CrossRef]
- Kong, J.; Li, Y.; Liu, S.; Jin, H.; Shang, Y.; Quan, C.; Lin, Z. High expression of ezrin predicts poor prognosis in uterine cervical cancer. BMC Cancer 2013, 13, 520. [Google Scholar] [CrossRef]
- Auvinen, E.; Carpen, O.; Korpela, T.; Ronty, M.; Vaheri, A.; Tarkkanen, J. Altered expression of ezrin, E-Cadherin and beta-Catenin in cervical neoplasia. Neoplasma 2013, 60, 56–61. [Google Scholar] [CrossRef]
- Greco, D.; Kivi, N.; Qian, K.; Leivonen, S.K.; Auvinen, P.; Auvinen, E. Human papillomavirus 16 E5 modulates the expression of host microRNAs. PLoS ONE 2011, 6, e21646. [Google Scholar] [CrossRef]
- Patara, M.; Santos, E.M.; Coudry Rde, A.; Soares, F.A.; Ferreira, F.O.; Rossi, B.M. Ezrin expression as a prognostic marker in colorectal adenocarcinoma. Pathol. Oncol. Res. 2011, 17, 827–833. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Chen, B.; Chen, S.; Jiang, Z.; Zhou, T.; Hou, Z.; Wang, Y. Ezrin/NF-kB activation regulates epithelial- mesenchymal transition induced by EGF and promotes metastasis of colorectal cancer. Biomed. Pharmacother. 2017, 92, 140–148. [Google Scholar] [CrossRef]
- Wang, H.J.; Zhu, J.S.; Zhang, Q.; Sun, Q.; Guo, H. High level of ezrin expression in colorectal cancer tissues is closely related to tumor malignancy. World J. Gastroenterol. 2009, 15, 2016–2019. [Google Scholar] [CrossRef] [PubMed]
- Gavert, N.; Ben-Shmuel, A.; Lemmon, V.; Brabletz, T.; Ben-Ze’ev, A. Nuclear factor-kappaB signaling and ezrin are essential for L1-mediated metastasis of colon cancer cells. J. Cell Sci. 2010, 123, 2135–2143. [Google Scholar] [CrossRef] [PubMed]
- Leiphrakpam, P.D.; Rajput, A.; Mathiesen, M.; Agarwal, E.; Lazenby, A.J.; Are, C.; Brattain, M.G.; Chowdhury, S. Ezrin expression and cell survival regulation in colorectal cancer. Cell Signal 2014, 26, 868–879. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, K.; Sakamoto, H.; Rutherford, T.; Chen, Z.; Satoh, K.; Naftolin, F. Ezrin, a membrane-cytoskeletal linking protein, is involved in the process of invasion of endometrial cancer cells. Cancer Lett. 1999, 147, 31–38. [Google Scholar] [CrossRef]
- Kobel, M.; Langhammer, T.; Huttelmaier, S.; Schmitt, W.D.; Kriese, K.; Dittmer, J.; Strauss, H.G.; Thomssen, C.; Hauptmann, S. Ezrin expression is related to poor prognosis in FIGO stage I endometrioid carcinomas. Mod. Pathol. 2006, 19, 581–587. [Google Scholar] [CrossRef]
- Bal, N.; Yildirim, S.; Nursal, T.Z.; Bolat, F.; Kayaselcuk, F. Association of ezrin expression in intestinal and diffuse gastric carcinoma with clinicopathological parameters and tumor type. World J. Gastroenterol. 2007, 13, 3726–3729. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.Y.; Zhao, Z.S.; Ma, J. Ezrin is associated with gastric cancer progression and prognosis. Pathol. Oncol. Res. 2011, 17, 909–915. [Google Scholar] [CrossRef]
- Jin, J.; Jin, T.; Quan, M.; Piao, Y.; Lin, Z. Ezrin overexpression predicts the poor prognosis of gastric adenocarcinoma. Diagn. Pathol. 2012, 7, 135. [Google Scholar] [CrossRef]
- Madan, R.; Brandwein-Gensler, M.; Schlecht, N.F.; Elias, K.; Gorbovitsky, E.; Belbin, T.J.; Mahmood, R.; Breining, D.; Qian, H.; Childs, G.; et al. Differential tissue and subcellular expression of ERM proteins in normal and malignant tissues: Cytoplasmic ezrin expression has prognostic signficance for head and neck squamous cell carcinoma. Head Neck 2006, 28, 1018–1027. [Google Scholar] [CrossRef]
- Schlecht, N.F.; Brandwein-Gensler, M.; Smith, R.V.; Kawachi, N.; Broughel, D.; Lin, J.; Keller, C.E.; Reynolds, P.A.; Gunn-Moore, F.J.; Harris, T.; et al. Cytoplasmic ezrin and moesin correlate with poor survival in head and neck squamous cell carcinoma. Head Neck Pathol. 2012, 6, 232–243. [Google Scholar] [CrossRef]
- Bakheet, A.M.H.; Mahmoud, S.A.; Huang, Y.; Zhang, J.; Wang, J.; Wei, Y.; Gamallat, Y.; Awadasseid, A.; Owusu, L.; Khidir, Y.; et al. Ezrin as a possible diagnostic and/or prognostic biomarker in mice lymphatic metastatic hepatocellular carcinoma in vivo. Biofactors 2017, 43, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Wang, S.; Ye, H.; Xu, S.; Ye, G. Ezrin expression in the primary hepatocellular carcinoma patients and associated with clinical, pathological characteristics. J. Cancer Res. Ther. 2016, 12, 291–294. [Google Scholar]
- Kang, Y.K.; Hong, S.W.; Lee, H.; Kim, W.H. Prognostic implications of ezrin expression in human hepatocellular carcinoma. Mol. Carcinog. 2010, 49, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, N.; Han, A.; Wang, Y.; Lin, Z.; Yang, Y. Ezrin promotes hepatocellular carcinoma progression by modulating glycolytic reprogramming. Cancer Sci. 2020, 111, 4061–4074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, M.Y.; Wu, W.Z.; Wang, Z.J.; Zhou, K.; Zha, X.L.; Liu, K.D. The membrane-cytoskeleton organizer ezrin is necessary for hepatocellular carcinoma cell growth and invasiveness. J. Cancer Res. Clin. Oncol. 2006, 132, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Song, X.; Li, Y.; Cao, Y.; Chu, F.; Durojaye, O.A.; Su, Z.; Shi, X.; Wang, J.; Cheng, J.; et al. Celastrol inhibits ezrin-mediated migration of hepatocellular carcinoma cells. Sci. Rep. 2020, 10, 11273. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, D.; Guo, Z.; Zhao, J.; Wu, B.; Deng, H.; Zhou, T.; Xiang, H.; Gao, F.; Yu, X.; et al. Rho kinase phosphorylation promotes ezrin-mediated metastasis in hepatocellular carcinoma. Cancer Res. 2011, 71, 1721–1729. [Google Scholar] [CrossRef]
- Lu, W.; Yang, C.; Du, P.; Zhang, J.L.; Zhang, J.C. Effects of arsenic trioxide on the expression of ezrin in hepatocellular carcinoma. Medicine 2017, 96, e7602. [Google Scholar] [CrossRef]
- Cetin, B.; Gonul, I.I.; Gumusay, O.; Afsar, B.; Bilgetekin, I.; Ozet, A.; Uner, A. Ezrin is a prognostic biomarker in patients with clear cell metastatic renal cell carcinoma receiving sunitinib. J. Cancer Res. Ther. 2021, 17, 408–413. [Google Scholar] [CrossRef]
- Ferrari, M.V.O.; da Costa, W.H.; Matushita, M.A.M.; Meduna, R.R.; Brazao, E.S., Jr.; Bezerra, S.M.; da Cunha, I.W.; Zequi, S.C. Immunohistochemical negative expression of ezrin predicts poor prognosis in clear cell renal cell carcinoma. Urol. Oncol. 2020, 38, 75.e1–75.e7. [Google Scholar] [CrossRef]
- Altaf, E.; Huang, X.; Xiong, J.; Yang, X.; Deng, X.; Xiong, M.; Zhou, L.; Pan, S.; Yuan, W.; Li, X.; et al. NHE1 has a notable role in metastasis and drug resistance of T-cell acute lymphoblastic leukemia. Oncol. Lett. 2017, 14, 4256–4262. [Google Scholar] [CrossRef] [PubMed]
- Habif, G.; Grasset, M.F.; Kieffer-Jaquinod, S.; Kuhn, L.; Mouchiroud, G.; Gobert-Gosse, S. Phosphoproteome analyses reveal specific implications of Hcls1, p21-activated kinase 1 and Ezrin in proliferation of a myeloid progenitor cell line downstream of wild-type and ITD mutant Fms-like tyrosine kinase 3 receptors. J. Proteomics 2013, 78, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, A.; Cotter, T.G. FLT3-driven redox-modulation of Ezrin regulates leukaemic cell migration. Free Radic Res. 2013, 47, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Monni, R.; Haddaoui, L.; Naba, A.; Gallais, I.; Arpin, M.; Mayeux, P.; Moreau-Gachelin, F. Ezrin is a target for oncogenic Kit mutants in murine erythroleukemia. Blood 2008, 111, 3163–3172. [Google Scholar] [CrossRef]
- Lipreri da Silva, J.C.; Coelho-Silva, J.L.; Lima, K.; Vicari, H.P.; Lazarini, M.; Costa-Lotufo, L.V.; Traina, F.; Machado-Neto, J.A. Comprehensive analysis of cytoskeleton regulatory genes identifies ezrin as a prognostic marker and molecular target in acute myeloid leukemia. Cell Oncol. 2021, 44, 1105–1117. [Google Scholar] [CrossRef]
- Lipreri da Silva, J.C.; Saldanha-Araujo, F.; de Melo, R.C.B.; Vicari, H.P.; Silva-Carvalho, A.E.; Rego, E.M.; Buccheri, V.; Machado-Neto, J.A. Ezrin is highly expressed and a druggable target in chronic lymphocytic leukemia. Life Sci. 2022, 311, 121146. [Google Scholar] [CrossRef]
- Pore, D.; Bodo, J.; Danda, A.; Yan, D.; Phillips, J.G.; Lindner, D.; Hill, B.T.; Smith, M.R.; Hsi, E.D.; Gupta, N. Identification of Ezrin-Radixin-Moesin proteins as novel regulators of pathogenic B-cell receptor signaling and tumor growth in diffuse large B-cell lymphoma. Leukemia 2015, 29, 1857–1867. [Google Scholar] [CrossRef]
- Pore, D.; Gupta, N. The ezrin-radixin-moesin family of proteins in the regulation of B-cell immune response. Crit. Rev. Immunol. 2015, 35, 15–31. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, E.H.; Oh, M.H. Clinicopathologic implication of ezrin expression in non-small cell lung cancer. Korean J. Pathol. 2012, 46, 470–477. [Google Scholar] [CrossRef]
- Jin, T.; Jin, J.; Li, X.; Zhang, S.; Choi, Y.H.; Piao, Y.; Shen, X.; Lin, Z. Prognostic implications of ezrin and phosphorylated ezrin expression in non-small cell lung cancer. BMC Cancer 2014, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Kolegova, E.S.; Kakurina, G.V.; Kostromitskiy, D.N.; Dobrodeev, A.Y.; Kondakova, I.V. Increases in mRNA and Protein Levels of the Genes for the Actin-Binding Proteins Profilin, Fascin, and Ezrin Promote Metastasis in Non-Small Cell Lung Cancer. Mol. Biol. 2020, 54, 285–292. [Google Scholar] [CrossRef]
- Pignochino, Y.; Dell’Aglio, C.; Inghilleri, S.; Zorzetto, M.; Basirico, M.; Capozzi, F.; Canta, M.; Piloni, D.; Cemmi, F.; Sangiolo, D.; et al. The combination of sorafenib and everolimus shows antitumor activity in preclinical models of malignant pleural mesothelioma. BMC Cancer 2015, 15, 374. [Google Scholar] [CrossRef] [PubMed]
- Safi, A.F.; Nickenig, H.J.; Rothamel, D.; Zirk, M.; Thiele, O.; Grandoch, A.; Scheer, M.; Zinser, M.; Zoller, J.; Drebber, U.; et al. Expression of ezrin in oral squamous cell carcinoma: Prognostic impact and clinicopathological correlations. J. Craniomaxillofac. Surg. 2015, 43, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Yamamoto, H.; Mukaisho, K.; Sato, S.; Higo, T.; Hattori, T.; Yamamoto, G.; Sugihara, H. Mechanisms underlying cancer progression caused by ezrin overexpression in tongue squamous cell carcinoma. PLoS ONE 2013, 8, e54881. [Google Scholar] [CrossRef] [PubMed]
- Noi, M.; Mukaisho, K.I.; Yoshida, S.; Murakami, S.; Koshinuma, S.; Adachi, T.; Machida, Y.; Yamori, M.; Nakayama, T.; Yamamoto, G.; et al. ERK phosphorylation functions in invadopodia formation in tongue cancer cells in a novel silicate fibre-based 3D cell culture system. Int. J. Oral. Sci. 2018, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Noi, M.; Mukaisho, K.I.; Murakami, S.; Koshinuma, S.; Machida, Y.; Yamori, M.; Nakayama, T.; Ogawa, T.; Nakata, Y.; Shimizu, T.; et al. Expressions of ezrin, ERK, STAT3, and AKT in tongue cancer and association with tumor characteristics and patient survival. Clin. Exp. Dent. Res. 2020, 6, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Assao, A.; Nonogaki, S.; Lauris, J.R.P.; Carvalho, A.L.; Pinto, C.A.L.; Soares, F.A.; Kowalski, L.P.; Oliveira, D.T. Podoplanin, ezrin, and Rho-A proteins may have joint participation in tumor invasion of lip cancer. Clin. Oral. Investig. 2017, 21, 1647–1657. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Z.; Sun, L.; Fan, S.; Huang, Z.; Zhang, D.; Yang, Z.; Li, J.; Chen, W. Akt/Ezrin Tyr353/NF-kappaB pathway regulates EGF-induced EMT and metastasis in tongue squamous cell carcinoma. Br. J. Cancer 2014, 110, 695–705. [Google Scholar] [CrossRef]
- Song, J.; Fadiel, A.; Edusa, V.; Chen, Z.; So, J.; Sakamoto, H.; Fishman, D.A.; Naftolin, F. Estradiol-induced ezrin overexpression in ovarian cancer: A new signaling domain for estrogen. Cancer Lett. 2005, 220, 57–65. [Google Scholar] [CrossRef]
- Kobel, M.; Gradhand, E.; Zeng, K.; Schmitt, W.D.; Kriese, K.; Lantzsch, T.; Wolters, M.; Dittmer, J.; Strauss, H.G.; Thomssen, C.; et al. Ezrin promotes ovarian carcinoma cell invasion and its retained expression predicts poor prognosis in ovarian carcinoma. Int. J. Gynecol. Pathol. 2006, 25, 121–130. [Google Scholar] [CrossRef]
- Li, M.J.; Xiong, D.; Huang, H.; Wen, Z.Y. Ezrin Promotes the Proliferation, Migration, and Invasion of Ovarian Cancer Cells. Biomed. Environ. Sci. 2021, 34, 139–151. [Google Scholar] [PubMed]
- Horwitz, V.; Davidson, B.; Stern, D.; Trope, C.G.; Tavor Re’em, T.; Reich, R. Ezrin Is Associated with Disease Progression in Ovarian Carcinoma. PLoS ONE 2016, 11, e0162502. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Feng, Y.; Tao, K.; Su, Z.; Yu, X.; Zheng, J.; Zhang, L.; Yang, D. The expression and phosphorylation of ezrin and merlin in human pancreatic cancer. Int. J. Oncol. 2014, 44, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Quan, C.; Sun, J.; Lin, Z.; Jin, T.; Dong, B.; Meng, Z.; Piao, J. Ezrin promotes pancreatic cancer cell proliferation and invasion through activating the Akt/mTOR pathway and inducing YAP translocation. Cancer Manag. Res. 2019, 11, 6553–6566. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, T.; Zhang, D.; Han, J. Expression of Ezrin and phosphorylated Ezrin (pEzrin) in pancreatic ductal adenocarcinoma. Cancer Invest. 2010, 28, 242–247. [Google Scholar] [CrossRef]
- Meng, Y.; Lu, Z.; Yu, S.; Zhang, Q.; Ma, Y.; Chen, J. Ezrin promotes invasion and metastasis of pancreatic cancer cells. J. Transl. Med. 2010, 8, 61. [Google Scholar] [CrossRef]
- Lipreri da Silva, J.C.; Carvalho, M.F.L.; de Miranda, L.B.L.; de Almeida, B.O.; Lima, K.; Machado-Neto, J.A. NSC305787, a pharmacological ezrin inhibitor, exhibits antineoplastic activity in pancreatic cancer cells. Invest. New Drugs 2022, 40, 728–737. [Google Scholar] [CrossRef]
- Valdman, A.; Fang, X.; Pang, S.T.; Nilsson, B.; Ekman, P.; Egevad, L. Ezrin expression in prostate cancer and benign prostatic tissue. Eur. Urol. 2005, 48, 852–857. [Google Scholar] [CrossRef]
- Pang, S.T.; Fang, X.; Valdman, A.; Norstedt, G.; Pousette, A.; Egevad, L.; Ekman, P. Expression of ezrin in prostatic intraepithelial neoplasia. Urology 2004, 63, 609–612. [Google Scholar] [CrossRef]
- Chuan, Y.C.; Pang, S.T.; Cedazo-Minguez, A.; Norstedt, G.; Pousette, A.; Flores-Morales, A. Androgen induction of prostate cancer cell invasion is mediated by ezrin. J. Biol. Chem. 2006, 281, 29938–29948. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Lu, Y.; Lai, C.; Qu, L.; Zhuo, Y. Ezrin expression in circulating tumor cells is a predictor of prostate cancer metastasis. Bioengineered 2022, 13, 4076–4084. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.C.; Chen, R.; Yang, T.N.; Zhang, F.; Zhao, D. Baicalein inhibits the proliferative activity of human prostate cancer cell line PC3 by downregulating Ezrin. J. Biol. Regul. Homeost. Agents 2020, 34, 885–892. [Google Scholar] [PubMed]
- Bulut, G.; Hong, S.H.; Chen, K.; Beauchamp, E.M.; Rahim, S.; Kosturko, G.W.; Glasgow, E.; Dakshanamurthy, S.; Lee, H.S.; Daar, I.; et al. Small molecule inhibitors of ezrin inhibit the invasive phenotype of osteosarcoma cells. Oncogene 2012, 31, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Celik, H.; Bulut, G.; Han, J.; Graham, G.T.; Minas, T.Z.; Conn, E.J.; Hong, S.H.; Pauly, G.T.; Hayran, M.; Li, X.; et al. Ezrin Inhibition Up-regulates Stress Response Gene Expression. J. Biol. Chem. 2016, 291, 13257–13270. [Google Scholar] [CrossRef]
- Ohtani, K.; Sakamoto, H.; Rutherford, T.; Chen, Z.; Kikuchi, A.; Yamamoto, T.; Satoh, K.; Naftolin, F. Ezrin, a membrane-cytoskeletal linking protein, is highly expressed in atypical endometrial hyperplasia and uterine endometrioid adenocarcinoma. Cancer Lett. 2002, 179, 79–86. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).