Three-Dimensional Cell Culture Methods in Infectious Diseases and Vaccine Research
Abstract
1. Introduction
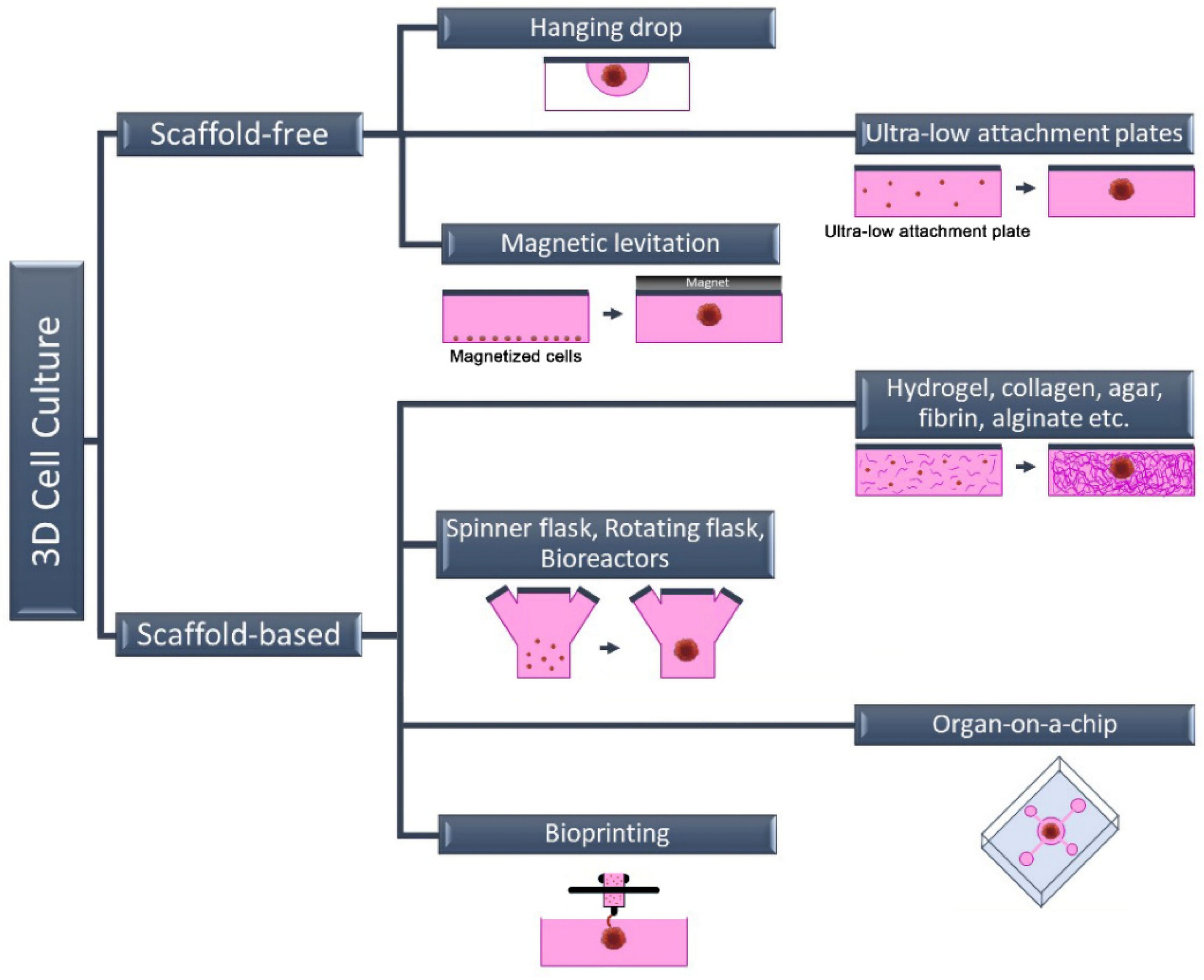
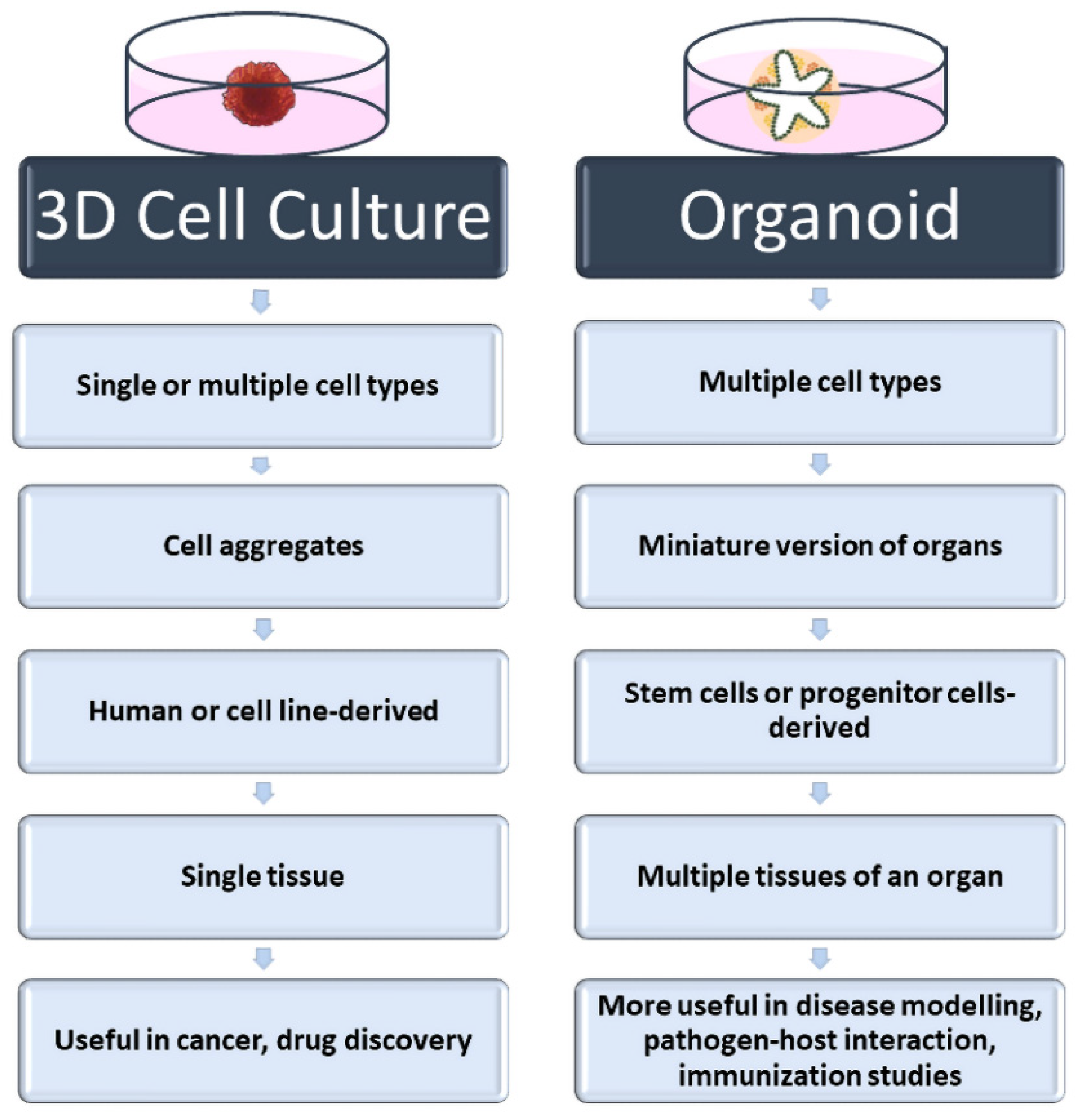
2. Three-Dimensional Cell Culture Methods
2.1. Scaffold-Free 3D Cell Culture Method
2.2. Scaffold-Based 3D Cell Culture Method
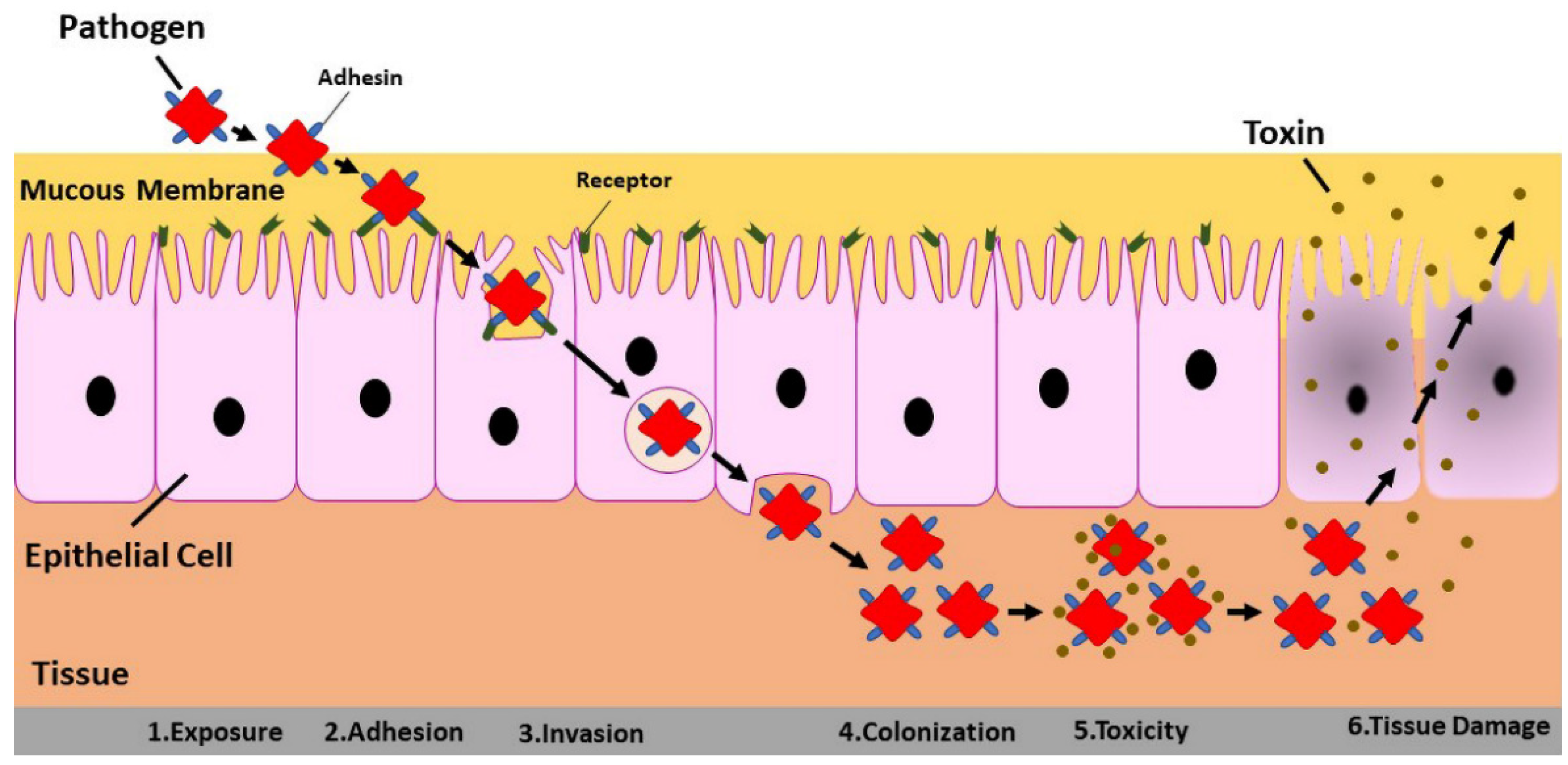
3. Three-Dimensional Cell Culture in Virus–Host Interaction
4. Three-Dimensional Cell Culture in Vaccine Development Studies: Future Direction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dolskiy, A.A.; Grishchenko, I.V.; Yudkin, D.V. Cell Cultures for Virology: Usability, Advantages, and Prospects. Int. J. Mol. Sci. 2020, 21, 7978. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Wang, J.; Ding, L.; Hu, Y.; Li, W.; Yuan, Z.; Guo, Q.; Zhu, C.; Yu, L.; Wang, H.; et al. Tissue Engineering and Regenerative Medicine: Achievements, Future, and Sustainability in Asia. Front. Bioeng. Biotechnol. 2020, 8, 83. [Google Scholar] [CrossRef]
- Hudu, S.A.; Alshrari, A.S.; Syahida, A.; Sekawi, Z. Cell Culture, Technology: Enhancing the Culture of Diagnosing Human Diseases. J. Clin. Diagn. Res. 2016, 10, DE01–DE05. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Verma, M.; Singh, A. Animal tissue culture principles and applications. In Animal Biotechnology; Academic Press: Cambridge, MS, USA, 2020; pp. 269–293. [Google Scholar]
- O’Flaherty, R.; Bergin, A.; Flampouri, E.; Mota, L.M.; Obaidi, I.; Quigley, A.; Xie, Y.; Butler, M. Mammalian cell culture for production of recombinant proteins: A review of the critical steps in their biomanufacturing. Biotechnol. Adv. 2020, 43, 107552. [Google Scholar] [CrossRef]
- Ju, X.; Zhu, Y.; Wang, Y.; Li, J.; Zhang, J.; Gong, M.; Ren, W.; Li, S.; Zhong, J.; Zhang, L.; et al. A novel cell culture system modeling the SARS-CoV-2 life cycle. PLoS Pathog. 2021, 17, e1009439. [Google Scholar]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Graham, B.S. Advances in antiviral vaccine development. Immunol. Rev. 2013, 255, 230–242. [Google Scholar] [CrossRef]
- Josefsberg, J.O.; Buckland, B. Vaccine process technology. Biotechnol. Bioeng. 2012, 109, 1443–1460. [Google Scholar]
- Cacciamali, A.; Villa, R.; Dotti, S. 3D Cell Cultures: Evolution of an Ancient Tool for New Applications. Front. Physiol. 2022, 13, 836480. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Barrila, J.; Crabbé, A.; Yang, J.; Franco, K.; Nydam, S.D.; Forsyth, R.J.; Davis, R.R.; Gangaraju, S.; Ott, C.M.; Coyne, C.B.; et al. Modeling Host-Pathogen Interactions in the Context of the Microenvironment: Three-Dimensional Cell Culture Comes of Age. Infect. Immun. 2018, 86, e00282-18. [Google Scholar] [CrossRef] [PubMed]
- Häfner, S.J. Level up for culture models - How 3D cell culture models benefit SARS-CoV-2 research. Biomed. J. 2021, 44, 1–6. [Google Scholar] [CrossRef]
- Harb, A.; Fakhreddine, M.; Zaraket, H.; Saleh, F.A. Three-Dimensional Cell Culture Models to Study Respiratory Virus Infections Including COVID-19. Biomimetics 2021, 7, 3. [Google Scholar] [CrossRef]
- Hsiao, A.Y.; Tung, Y.C.; Qu, X.; Patel, L.R.; Pienta, K.J.; Takayama, S. 384 hanging drop arrays give excellent Z-factors and allow versatile formation of co-culture spheroids. Biotechnol. Bioeng. 2012, 109, 1293–1304. [Google Scholar] [CrossRef]
- Alghuwainem, A.; Alshareeda, A.T.; Alsowayan, B. Scaffold-Free 3-D Cell Sheet Technique Bridges the Gap between 2-D Cell Culture and Animal Models. Int. J. Mol. Sci. 2019, 20, 4926. [Google Scholar] [CrossRef]
- Foty, R. A simple hanging drop cell culture protocol for generation of 3D spheroids. J. Vis. Exp. 2011, 51, e2720. [Google Scholar] [CrossRef]
- Shri, M.; Agrawal, H.; Rani, P.; Singh, D.; Onteru, S.K. Hanging Drop, A Best Three-Dimensional (3D) Culture Method for Primary Buffalo and Sheep Hepatocytes. Sci. Rep. 2017, 7, 1203. [Google Scholar] [CrossRef]
- Hu, M.; Ling, Z.; Ren, X. Extracellular matrix dynamics: Tracking in biological systems and their implications. J. Biol. Eng. 2022, 16, 13. [Google Scholar] [CrossRef]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Takebe, T. Generating Mini-Organs in Culture. Curr. Pathobiol. Rep. 2016, 4, 59–68. [Google Scholar] [CrossRef]
- Dutta, D.; Clevers, H. Organoid culture systems to study host-pathogen interactions. Curr. Opin. Immunol. 2017, 48, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Gunti, S.; Hoke, A.T.K.; Vu, K.P.; London, N.R., Jr. Organoid and Spheroid Tumor Models: Techniques and Applications. Cancers 2021, 13, 874. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, M.; Cortesi, M.; Zamagni, A.; Arienti, C.; Pignatta, S.; Tesei, A. Modeling neoplastic disease with spheroids and organoids. J. Hematol. Oncol. 2020, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Altmann, B.; Grün, C.; Nies, C.; Gottwald, E. Advanced 3D Cell Culture Techniques in Micro-Bioreactors, Part II: Systems and Applications. Processes 2021, 9, 21. [Google Scholar] [CrossRef]
- Sangeeta, B.; Ankita Jaywant, D.; Shafina, S.; Jyotirmoi, A.; Soumya, B. Two-Dimensional and Three-Dimensional Cell Culture and Their Applications, in Cell Culture; Zhan, X., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Dey, M.; Ozbolat, I.T. 3D bioprinting of cells, tissues and organs. Sci. Rep. 2020, 10, 14023. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Datta, P.; Oh, J.; Williams, A.; Ozbolat, V.; Unutmaz, D.; T Ozbolat, I. 3D Bioprinting for fabrication of tissue models of COVID-19 infection. Essays Biochem. 2021, 65, 503–518. [Google Scholar]
- Koban, R.; Lam, T.; Schwarz, F.; Kloke, L.; Bürge, S.; Ellerbrok, H.; Neumann, M. Simplified Bioprinting-Based 3D Cell Culture Infection Models for Virus Detection. Viruses 2020, 12, 1298. [Google Scholar] [CrossRef]
- Grün, C.; Altmann, B.; Gottwald, E. Advanced 3D Cell Culture Techniques in Micro-Bioreactors, Part I: A Systematic Analysis of the Literature Published between 2000 and 2020. Processes 2020, 8, 1656. [Google Scholar] [CrossRef]
- Kizilova, N.; Rokicki, J. 3D Bioreactors for Cell Culture: Fluid Dynamics Aspects. In Biomechanics in Medicine, Sport and Biology; Springer International Publishing: Cham, Switzerland, 2022. [Google Scholar]
- Yi, T.; Huang, S.; Liu, G.; Li, T.; Kang, Y.; Luo, Y.; Wu, J. Bioreactor Synergy with 3D Scaffolds: New Era for Stem Cells Culture. ACS Appl. Bio. Mater. 2018, 1, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Luo, Z.; Lee, J.; Kim, H.-J.; Lee, K.; Tebon, P.; Feng, Y.; Dokmeci, M.R.; Sengupta, S.; Khademhosseini, A. Organ-on-a-Chip for Cancer and Immune Organs Modeling. Adv. Healthc. Mater. 2019, 8, 1801363. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Abouleila, Y.; Si, L.; Ortega-Prieto, A.M.; Mummery, C.L.; Ingber, D.E.; Mashaghi, A. Human Organs-on-Chips for Virology. Trends Microbiol. 2020, 28, 934–946. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, P.; Qin, J. Human Organoids and Organs-on-Chips for Addressing COVID-19 Challenges. Adv. Sci. 2022, 9, 2105187. [Google Scholar] [CrossRef]
- Li, G.; He, X.; Zhang, L.; Ran, Q.; Wang, J.; Xiong, A.; Wu, D.; Chen, F.; Sun, J.; Chang, C. Assessing ACE2 expression patterns in lung tissues in the pathogenesis of COVID-19. J. Autoimmun. 2020, 112, 102463. [Google Scholar]
- Galán, J.E. The cell biology of microbial infections: Coming of age. J. Cell Biol. 2002, 158, 387–388. [Google Scholar] [CrossRef]
- Geiser, J.; Boivin, G.; Huang, S.; Constant, S.; Kaiser, L.; Tapparel, C.; Essaidi-Laziosi, M. RSV and HMPV Infections in 3D Tissue Cultures: Mechanisms Involved in Virus–host and Virus-Virus Interactions. Viruses 2021, 13, 139. [Google Scholar] [CrossRef]
- Heo, I.; Dutta, D.; Schaefer, D.A.; Iakobachvili, N.; Artegiani, B.; Sachs, N.; Boonekamp, K.E.; Bowden, G.; Hendrickx, A.P.A.; Willems, R.J.L.; et al. Modelling Cryptosporidium infection in human small intestinal and lung organoids. Nat. Microbiol. 2018, 3, 814–823. [Google Scholar] [CrossRef]
- Berg, J.; Hiller, T.; Kissner, M.S.; Qazi, T.H.; Duda, G.N.; Hocke, A.C.; Hippenstiel, S.; Elomaa, L.; Weinhart, M.; Fahrenson, C.; et al. Optimization of cell-laden bioinks for 3D bioprinting and efficient infection with influenza A virus. Sci. Rep. 2018, 8, 13877. [Google Scholar] [CrossRef]
- Berg, J.; Weber, Z.; Fechler-Bitteti, M.; Hocke, A.C.; Hippenstiel, S.; Elomaa, L.; Weinhart, M.; Kurreck, J. Bioprinted Multi-Cell Type Lung Model for the Study of Viral Inhibitors. Viruses 2021, 13, 1590. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.Z.; Zheng, Y.W.; Miyakawa, K.; Murata, S.; Zhang, R.R.; Sekine, K.; Ueno, Y.; Takebe, T.; Wakita, T.; Ryo, A.; et al. Recapitulation of hepatitis B virus–host interactions in liver organoids from human induced pluripotent stem cells. EBioMedicine 2018, 35, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Long, R.K.M.; Moriarty, K.P.; Cardoen, B.; Gao, G.; Vogl, A.W.; Jean, F.; Hamarneh, G.; Nabi, I.R. Super resolution microscopy and deep learning identify Zika virus reorganization of the endoplasmic reticulum. Sci. Rep. 2020, 10, 20937. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, A.Z.; Møller, R.; Makovoz, B.; Uhl, S.A.; tenOever, B.R.; Blenkinsop, T.A. SARS-CoV-2 infects human adult donor eyes and hESC-derived ocular epithelium. Cell Stem Cell 2021, 28, 1205–1220. [Google Scholar] [CrossRef]
- Jacob, F.; Pather, S.R.; Huang, W.K.; Zhang, F.; Wong, S.Z.H.; Zhou, H.; Cubitt, B.; Fan, W.; Chen, C.Z.; Xu, M.; et al. Human Pluripotent Stem Cell-Derived Neural Cells and Brain Organoids Reveal SARS-CoV-2 Neurotropism Predominates in Choroid Plexus Epithelium. Cell Stem Cell 2020, 27, 937–950. [Google Scholar] [CrossRef]
- Ramani, A.; Müller, L.; Ostermann, P.N.; Gabriel, E.; Abida-Islam, P.; Müller-Schiffmann, A.; Mariappan, A.; Goureau, O.; Gruell, H.; Walker, A.; et al. SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J. 2020, 39, e106230. [Google Scholar] [CrossRef]
- Zhang, B.Z.; Chu, H.; Han, S.; Shuai, H.; Deng, J.; Hu, Y.F.; Gong, H.R.; Lee, A.C.; Zou, Z.; Yau, T.; et al. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res. 2020, 30, 928–931. [Google Scholar] [CrossRef]
- Heuberger, J.; Trimpert, J.; Vladimirova, D.; Goosmann, C.; Lin, M.; Schmuck, R.; Mollenkopf, H.J.; Brinkmann, V.; Tacke, F.; Osterrieder, N.; et al. Epithelial response to IFN-γ promotes SARS-CoV-2 infection. EMBO Mol. Med. 2021, 13, e13191. [Google Scholar] [CrossRef]
- Jang, K.K.; Kaczmarek, M.E.; Dallari, S.; Chen, Y.H.; Tada, T.; Axelrad, J.; Landau, N.R.; Stapleford, K.A.; Cadwell, K. Variable susceptibility of intestinal organoid-derived monolayers to SARS-CoV-2 infection. PLoS Biol. 2022, 20, e3001592. [Google Scholar] [CrossRef]
- Zhou, J.; Li, C.; Liu, X.; Chiu, M.C.; Zhao, X.; Wang, D.; Wei, Y.; Lee, A.; Zhang, A.J.; Chu, H.; et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat. Med. 2020, 26, 1077–1083. [Google Scholar] [CrossRef]
- Lamers, M.M.; van der Vaart, J.; Knoops, K.; Riesebosch, S.; Breugem, T.I.; Mykytyn, A.Z.; Beumer, J.; Schipper, D.; Bezstarosti, K.; Koopman, C.D.; et al. An organoid-derived bronchioalveolar model for SARS-CoV-2 infection of human alveolar type II-like cells. EMBO J. 2021, 40, e105912. [Google Scholar] [CrossRef] [PubMed]
- Milewska, A.; Kula-Pacurar, A.; Wadas, J.; Suder, A.; Szczepanski, A.; Dabrowska, A.; Owczarek, K.; Marcello, A.; Ochman, M.; Stacel, T.; et al. Replication of Severe Acute Respiratory Syndrome Coronavirus 2 in Human Respiratory Epithelium. J. Virol. 2020, 94, e00957-20. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.M.; Grimley, S.L.; McAuley, J.L.; Hachani, A.; Earnest, L.; Wong, S.L.; Caly, L.; Druce, J.; Purcell, D.F.J.; Jackson, D.C.; et al. Air-Liquid-Interface Differentiated Human Nose Epithelium: A Robust Primary Tissue Culture Model of SARS-CoV-2 Infection. Int. J. Mol. Sci. 2022, 23, 835. [Google Scholar] [CrossRef]
- Han, Y.; Duan, X.; Yang, L.; Nilsson-Payant, B.E.; Wang, P.; Duan, F.; Tang, X.; Yaron, T.M.; Zhang, T.; Uhl, S.; et al. Identification of SARS-CoV-2 inhibitors using lung and colonic organoids. Nature 2021, 589, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Garcez, P.P.; Loiola, E.C.; Madeiro da Costa, R.; Higa, L.M.; Trindade, P.; Delvecchio, R.; Nascimento, J.M.; Brindeiro, R.; Tanuri, A.; Rehen, S.K. Zika virus impairs growth in human neurospheres and brain organoids. Science 2016, 352, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Weiss, A.A. Probiotic Properties of Escherichia coli Nissle in Human Intestinal Organoids. mBio 2020, 11, e01470-20. [Google Scholar] [CrossRef] [PubMed]
- Koster, S.; Gurumurthy, R.K.; Kumar, N.; Prakash, P.G.; Dhanraj, J.; Bayer, S.; Berger, H.; Kurian, S.M.; Drabkina, M.; Mollenkopf, H.J.; et al. Modelling Chlamydia and HPV co-infection in patient-derived ectocervix organoids reveals distinct cellular reprogramming. Nat. Commun. 2022, 13, 1030. [Google Scholar] [CrossRef]
- Riedel, S. Edward Jenner and the History of Smallpox and Vaccination. In Baylor University Medical Center Proceedings; Taylor Francis Group: Abingdon, UK, 2005; Volume 18, pp. 21–25. [Google Scholar]
- Strassburg, M.A. The global eradication of smallpox. Am. J. Infect. Control. 1982, 10, 53–59. [Google Scholar] [CrossRef]
- Lawko, N.; Plaskasovitis, C.; Stokes, C.; Abelseth, L.; Fraser, I.; Sharma, R.; Kirsch, R.; Hasan, M.; Abelseth, E.; Willerth, S.M. 3D Tissue Models as an Effective Tool for Studying Viruses and Vaccine Development. Front. Mater. 2021, 8, 80. [Google Scholar] [CrossRef]
- Excler, J.-L.; Saville, M.; Berkley, S.; Kim, J.H. Vaccine development for emerging infectious diseases. Nat. Med. 2021, 27, 591–600. [Google Scholar] [CrossRef]
- Han, Y.; Yang, L.; Lacko, L.A.; Chen, S. Human organoid models to study SARS-CoV-2 infection. Nat. Methods 2022, 19, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.J.; Seo, S.H. Organoid as a culture system for viral vaccine strains. Clin. Exp. Vaccin. Res. 2018, 7, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, L.; Chen, T. The Effects of Secretory IgA in the Mucosal Immune System. BioMed Res. Int. 2020, 2020, 2032057. [Google Scholar] [CrossRef]
- Kessie, D.K.; Rudel, T. Advanced human mucosal tissue models are needed to improve preclinical testing of vaccines. PLoS Biol. 2021, 19, e3001462. [Google Scholar] [CrossRef]
- Wagar, L.E.; Salahudeen, A.; Constantz, C.M.; Wendel, B.S.; Lyons, M.M.; Mallajosyula, V.; Jatt, L.P.; Adamska, J.Z.; Blum, L.K.; Gupta, N.; et al. Modeling human adaptive immune responses with tonsil organoids. Nat. Med. 2021, 27, 125–135. [Google Scholar] [CrossRef]
| Pathogen/Targeted Organ | Cell line(s)/3D Method | Results | Ref |
|---|---|---|---|
| Respiratory Syncytial virus and human Metapneumovirus/ Lung | Ciliated cells, Goblet cells, Basal Cells / Commercially available in vitro reconstituted 3D airway epithelium. | HMPV was less pathogenic and more sensitive to IFNs than RSV. RSV-infected epithelia were less receptive to HMPV in dual infections. Including the innate immunological response of the epithelium in both single- and dual-infection viral-host interactions. | [40] |
| Cryptosporidium parvum/ small intestinal and lung | Small intestinal: duodenal biopsy samples were embedded in Matrigel. Lung: bronchial airway tissue resection from cancer patients embedded in BME type 2. | Organoids used to demonstrate the complex life cycle of a parasite. The oocysts produced in organoids are infectious and comparable to those seen in infected host animals. | [41] |
| Influenza A virus / lung | Human epithelial lung carcinoma cells containing bioink / 3D Bioprinting. | A549 cells are effectively infected by the virus in both 2D and 3D culture, but the clustered. infection pattern of 3D culture is more similar to the normal biological state seen in human lung cells. Antiviral IL-29 was released in 3D printed cells. | [42] |
| Influenza A virus/Lung | Monocytic THP-1 and A549 alveolar epithelial cells / Bioprinted human lung model. | Immune response generated through proinflammatory cytokines in organoids treated with bacterial toxins. | [43] |
| Hepatitis B virus / liver | Human induced pluripotent stem cell, HUVEC and BM-MSCs / 3D microwell plate. | Compared to conventionally grown cells, organoids demonstrated increased sensitivity toward HBV infection. Organoids with viral infections may acquire hepatic dysfunction. | [44] |
| Zika virus / brain | hPSC-derived cerebral organoids / Cerebral Organoid Kit. | The endoplasmic reticulum is specially reorganized by pathogen. | [45] |
| SARS-CoV-2/ eye | Whole-eye organoid model from human embryonic stem cells onto Matrigel-coated dishes. | Eye organoids and cadaver samples both showed comparable viral replication. | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varan, G.; Unal, S. Three-Dimensional Cell Culture Methods in Infectious Diseases and Vaccine Research. Future Pharmacol. 2023, 3, 48-60. https://doi.org/10.3390/futurepharmacol3010004
Varan G, Unal S. Three-Dimensional Cell Culture Methods in Infectious Diseases and Vaccine Research. Future Pharmacology. 2023; 3(1):48-60. https://doi.org/10.3390/futurepharmacol3010004
Chicago/Turabian StyleVaran, Gamze, and Serhat Unal. 2023. "Three-Dimensional Cell Culture Methods in Infectious Diseases and Vaccine Research" Future Pharmacology 3, no. 1: 48-60. https://doi.org/10.3390/futurepharmacol3010004
APA StyleVaran, G., & Unal, S. (2023). Three-Dimensional Cell Culture Methods in Infectious Diseases and Vaccine Research. Future Pharmacology, 3(1), 48-60. https://doi.org/10.3390/futurepharmacol3010004










