Mitochondria-Stimulating and Antioxidant Effects of Slovak Propolis Varieties on Bovine Spermatozoa
Abstract
1. Introduction
2. Materials and Methods
2.1. Propolis Collection and Extract Preparation
2.2. Characterization of the Extract
2.3. Sperm Cultures and Analysis
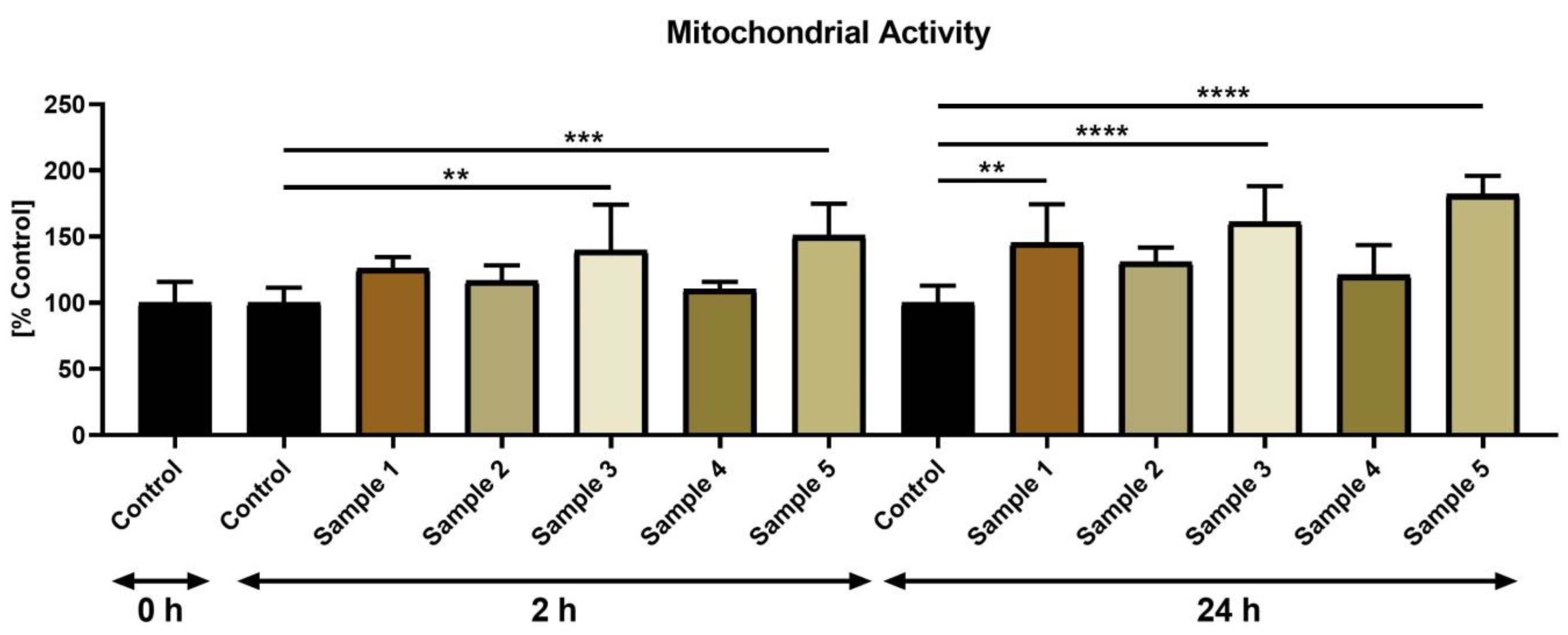
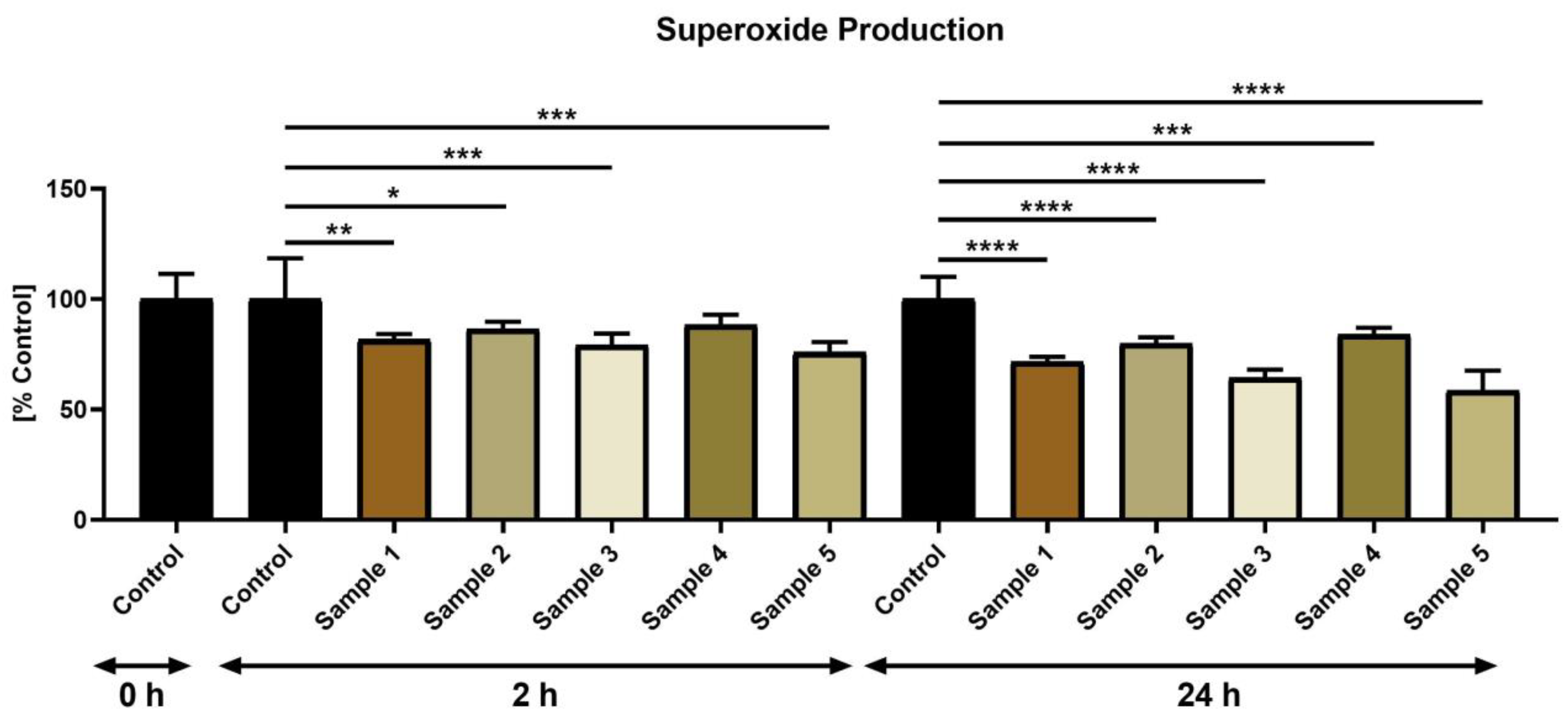
3. Results
3.1. Chemical Properties of the Propolis Extract
3.2. Sperm Characteristics
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barszcz, K.; Wiesetek, D.; Wasowicz, M.; Kupczynska, M. Bull semen collection and analysis for artificial insemination. J. Agric. Sci. 2007, 4, 1–10. [Google Scholar] [CrossRef]
- Khan, I.M.; Cao, Z.; Liu, H.; Khan, A.; Rahman, S.U.; Khan, M.Z.; Sathanawongs, A.; Zhang, Y. Impact of Cryopreservation on Spermatozoa Freeze-Thawed Traits and Relevance OMICS to Assess Sperm Cryo-Tolerance in Farm Animals. Front. Vet. Sci. 2021, 8, 609180. [Google Scholar] [CrossRef]
- Bucak, M.N.; Ataman, M.B.; Başpınar, N.; Uysal, O.; Taşpınar, M.; Bilgili, A.; Öztürk, C.; Güngör, Ş.; İnanç, M.E.; Akal, E. Lycopene and resveratrol improve post-thaw bull sperm parameters: Sperm motility, mitochondrial activity and DNA integrity. Andrologia 2015, 47, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Riesco, M.F.; Alvarez, M.; Anel-Lopez, L.; Neila-Montero, M.; Palacin-Martinez, C.; Montes-Garrido, R.; Boixo, J.C.; de Paz, P.; Anel, L. Multiparametric Study of Antioxidant Effect on Ram Sperm Cryopreservation—From Field Trials to Research Bench. Animals 2021, 11, 283. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M. A review study on the effect of various antioxidant supplements on maintaining and improving the performance of sperm parameters. Iran. J. Biol. 2021, 5, 69–77. [Google Scholar]
- Martins, R.V.L.; Silva, A.M.S.; Duarte, A.P.; Socorro, S.; Correia, S.; Maia, C.J. Natural Products as Protective Agents for Male Fertility. BioChem 2021, 1, 122–147. [Google Scholar] [CrossRef]
- Braakhuis, A. Evidence on the Health Benefits of Supplemental Propolis. Nutrients 2019, 11, 2705. [Google Scholar] [CrossRef]
- Capucho, C.; Sette, R.; de Souza Predes, F.; de Castro Monteiro, J.; Pigoso, A.A.; Barbieri, R.; Dolder, M.A.; Severi-Aguiar, G.D. Green Brazilian propolis effects on sperm count and epididymis morphology and oxidative stress. Food Chem. Toxicol. 2012, 50, 3956–3962. [Google Scholar] [CrossRef]
- Handayani, N.; Gofur, A. Does Propolis Extract Alleviate Male Reproductive Performance Through Gonadotropic Hormone Levels and Sperm Quality? IOP Conf. Ser. Earth Environ. Sci. 2019, 276, 012056. [Google Scholar] [CrossRef]
- Seven, I.; Tatli Seven, P.; Gul Baykalir, B.; Parlak Ak, T.; Ozer Kaya, S.; Yaman, M. Bee glue (propolis) improves reproductive organs, sperm quality and histological changes and antioxidant parameters of testis tissues in rats exposed to excess copper. Andrologia 2020, 52, e13540. [Google Scholar] [CrossRef]
- Cedikova, M.; Miklikova, M.; Stachova, L.; Grundmanova, M.; Tuma, Z.; Vetvicka, V.; Zech, N.; Kralickova, M.; Kuncova, J. Effects of the Czech propolis on sperm mitochondrial function. Evid. Based Complement. Altern. Med. 2014, 2014, 248768. [Google Scholar] [CrossRef]
- Al-Nawab, N.; Al-Dujaily, S.S.; Al-Obaidi, Z.F.H. Effects of Propolis Extract Medium on In Vitro Sperms Activation of Infertile Asthenozoospermic Men. Ann. Rom. Soc. Cell Biol. 2021, 25, 5050–5059. [Google Scholar]
- Barbarić, M.; Mišković, K.; Bojić, M.; Lončar, M.B.; Smolčić-Bubalo, A.; Debeljak, Z.; Medić-Šarić, M. Chemical composition of the ethanolic propolis extracts and its effect on HeLa cells. J. Ethnopharmacol. 2011, 135, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Blainski, A.; Lopes, G.C.; de Mello, J.C. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; Michalko, J.; Árvay, J.; Vukovic, N.L.; Ivanišová, E.; Ďuračka, M.; Matušíková, I.; Kačániová, M. Characterization of the Omija (Schisandra chinensis) Extract and Its Effects on the Bovine Sperm Vitality and Oxidative Profile during In Vitro Storage. Evid. Based Complement. Altern. Med. 2020, 2020, 7123780. [Google Scholar] [CrossRef]
- Willett, W.C. Balancing life-style and genomics research for disease prevention. Science 2002, 296, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Sánchés-Moreno, C.; Larrauri, A.; Saura-Calixto, F. A procedure to measure the antioxidant efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Benko, F.; Mohammadi-Sangcheshmeh, A.; Ďuračka, M.; Lukáč, N.; Tvrdá, E. In vitro versus cryo-induced capacitation of bovine spermatozoa, part 1: Structural, functional, and oxidative similarities and differences. PLoS ONE 2022, 17, e0276683. [Google Scholar] [CrossRef]
- Galimov, S.N.; Gromenko, J.Y.; Bulygin, K.V.; Galimov, K.S.; Galimova, E.F.; Sinelnikov, M.Y. (2021). The level of secondary messengers and the redox state of NAD+/NADH are associated with sperm quality in infertility. J. Reprod. Immunol. 2021, 148, 103383. [Google Scholar] [CrossRef]
- Ahmadi, S.; Bashiri, R.; Ghadiri-Anari, A.; Nadjarzadeh, A. Antioxidant supplements and semen parameters: An evidence based review. Int. J. Reprod. Biomed. 2016, 14, 729–736. [Google Scholar] [CrossRef]
- Tvrdá, E.; Benko, F.; Slanina, T.; du Plessis, S.S. The Role of Selected Natural Biomolecules in Sperm Production and Functionality. Molecules 2021, 26, 5196. [Google Scholar] [CrossRef] [PubMed]
- Seven, P.T.; Seven, I.; Baykalir, B.G.; Mutlu, S.I.; Salem, A.Z.M. Nanotechnology and nano-propolis in animal production and health: An overview. Ital. J. Anim. Sci. 2018, 17, 921–930. [Google Scholar] [CrossRef]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid. Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.E.E.; Alqurashi, R.M. Anti-fungal and antioxidant properties of propolis (bee glue) extracts. Int. J. Food Microbiol. 2022, 361, 109463. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Keskin, Ş.; Bobis, O.; Felitti, R.; Górecki, M.; Otręba, M.; Stojko, J.; Olczyk, P.; Kolayli, S.; Rzepecka-Stojko, A. Comparison of the Antioxidant Activity of Propolis Samples from Different Geographical Regions. Plants 2022, 11, 1203. [Google Scholar] [CrossRef]
- Romero, M.; Freire, J.; Pastene, E.; García, A.; Aranda, M.; Gonzáles, C. Propolis polyphenolic compounds affect the viability and structure of Helicobacter pylori in vitro. Rev. Bras. Farmacogn. 2019, 29, 325–332. [Google Scholar] [CrossRef]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Betances-Salcedo, E.; Revilla, I.; Vivar-Quintana, A.M.; González-Martín, M.I. Flavonoid and Antioxidant Capacity of Propolis Prediction Using Near Infrared Spectroscopy. Sensors 2017, 17, 1647. [Google Scholar] [CrossRef]
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical Composition and Antioxidant Activity of Propolis Prepared in Different Forms and in Different Solvents Useful for Finished Products. Foods 2018, 7, 41. [Google Scholar] [CrossRef]
- Volpi, N.; Bergonzini, G. Analysis of flavonoids from propolis by on-line HPLC-electrospray mass spectrometry. J. Pharm. Biomed. Anal. 2006, 42, 354–361. [Google Scholar] [CrossRef]
- Rosli, N.L.; Roslan, H.R.; Omar, E.A.; Mokhtar, N.; Abdul Hapit, N.H.; Asem, N. Phytochemical Analysis and Antioxidant Activities of Trigona Apicalis Propolis Extract. IOP Conf. Proc. 2016, 1791, 010001. [Google Scholar]
- Shehata, M.G.; Ahmad, F.T.; Badr, A.N.; Masry, S.H.; El-Sohaimy, S.A. Chemical analysis, antioxidant, cytotoxic and antimicrobial properties of propolis from different geographic regions. Ann. Agric. Sci. 2020, 65, 209–217. [Google Scholar] [CrossRef]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2012, 35, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, E. Mitochondrial free radical production and cell signaling. Mol. Asp. Med. 2004, 25, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, J.; Samadder, A.; Boujedaini, N.; Khuda-Bukhsh, A.R. Apigenin-induced apoptosis in A375 and A549 cells through selective action and dysfunction of mitochondria. Exp. Biol. Med. 2012, 237, 1433–1448. [Google Scholar] [CrossRef]
- Cimen, H.; Han, M.J.; Yang, Y.; Tong, Q.; Koc, H.; Koc, E.C. Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry 2010, 49, 304–311. [Google Scholar] [CrossRef]
- Silva Santos, L.F.; Stolfo, A.; Calloni, C.; Salvador, M. Catechin and epicatechin reduce mitochondrial dysfunction and oxidative stress induced by amiodarone in human lung fibroblasts. J. Arrhythm. 2017, 33, 220–225. [Google Scholar] [CrossRef]
- Parihar, P.; Jat, D.; Ghafourifar, P.; Parihar, M.S. Efficiency of mitochondrially targeted gallic acid in reducing brain mitochondrial oxidative damage. Cell. Mol. Biol. 2014, 60, 35–41. [Google Scholar]
- Semaming, Y.; Sripetchwandee, J.; Sa-Nguanmoo, P.; Pintana, H.; Pannangpetch, P.; Chattipakorn, N.; Chattipakorn, S.C. Protocatechuic acid protects brain mitochondrial function in streptozotocin-induced diabetic rats. Appl. Physiol. Nutr. Metab. 2015, 40, 1078–1081. [Google Scholar] [CrossRef]




| Sample Number | Location | GPS Coordinates |
|---|---|---|
| 1 | Podhorany, Nitra region | 48°23′09.4″ N, 18°07′00.2″ E |
| 2 | Jablonov nad Turňou, Rožňava region | 48°34′35.6″ N, 20°39′30.9″ E |
| 3 | Hrabské, Bardejov region | 49°20′28.1″ N, 21°04′53.4″ E |
| 4 | Jakubov, Malacky region | 48°24′14.2″ N, 16°56′43.2″ E |
| 5 | Liptovský Hrádok, Liptov region | 49°02′04.4″ N, 19°43′45.3″ E |
| Sample Number | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Total phenols [mg GAE/g d. e.] | 72.65 ± 6.18 | 71.21 ± 5.05 | 82.58 ± 7.58 | 61.76 ± 7.04 | 89.55 ± 9.47 |
| Total flavonoids [mg QUE/g d. e.] | 25.59 ± 4.26 | 23.51 ± 2.36 | 30.68 ± 3.16 | 20.41 ± 3.09 | 38.73 ± 5.05 |
| DPPH assay [mM TEAC/g d. e.] | 5.45 ± 0.47 | 4.77 ± 0.54 | 5.98 ± 0.61 | 4.47 ± 0.43 | 6.21 ± 0.60 |
| Compound [mg/L] | Rt [min] | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| Caffeic acid | 8.55 | 6.55 ± 0.78 | 2.38 ± 0.19 | 7.61 ± 0.84 | 3.37 ± 0.51 | 6.36 ± 0.59 |
| Syringic acid | 10.05 | N.D. | 3.12 ± 0.35 | N.D. | N.D. | N.D. |
| p-Coumaric acid | 12.80 | 4.94 ± 0.52 | 26.57 ± 2.03 | 12.70 ± 1.52 | 6.54 ± 0.51 | 4.85 ± 0.39 |
| trans-Ferulic acid | 15.02 | 2.62 ± 0.31 | 20.78 ± 3.02 | 6.08 ± 0.67 | 6.25 ± 0.79 | 2.82 ± 0.33 |
| Rutin | 22.05 | 4.81 ± 0.52 | N.D. | 7.01 ± 0.58 | 2.81 ± 0.37 | N.D. |
| Cinnamic acid | 25.17 | 0.65 ± 0.07 | 0.51 ± 0.06 | 0.79 ± 0.06 | 0.44 ± 0.05 | 0.94 ± 0.08 |
| Quercetin | 29.42 | 3.51 ± 0.25 | 2.44 ± 0.36 | 4.16 ± 0.55 | 2.29 ± 0.30 | 3.91 ± 0.44 |
| Kaempferol | 31.92 | 2.91 ± 0.31 | 2.76 ± 0.27 | 3.09 ± 0.29 | 1.94 ± 0.15 | 3.36 ± 0.32 |
| Apigenin | 33.02 | 5.18 ± 0.46 | 1.03 ± 0.10 | 6.11 ± 0.56 | 2.52 ± 0.21 | 6.60 ± 0.58 |
| Chrysin | 37.16 | 12.97 ± 1.11 | 1.93 ± 0.12 | 10.33 ± 1.05 | 6.83 ± 0.65 | 11.97 ± 1.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tvrdá, E.; Árvay, J.; Ďuračka, M.; Kačániová, M. Mitochondria-Stimulating and Antioxidant Effects of Slovak Propolis Varieties on Bovine Spermatozoa. Oxygen 2023, 3, 179-189. https://doi.org/10.3390/oxygen3020013
Tvrdá E, Árvay J, Ďuračka M, Kačániová M. Mitochondria-Stimulating and Antioxidant Effects of Slovak Propolis Varieties on Bovine Spermatozoa. Oxygen. 2023; 3(2):179-189. https://doi.org/10.3390/oxygen3020013
Chicago/Turabian StyleTvrdá, Eva, Július Árvay, Michal Ďuračka, and Miroslava Kačániová. 2023. "Mitochondria-Stimulating and Antioxidant Effects of Slovak Propolis Varieties on Bovine Spermatozoa" Oxygen 3, no. 2: 179-189. https://doi.org/10.3390/oxygen3020013
APA StyleTvrdá, E., Árvay, J., Ďuračka, M., & Kačániová, M. (2023). Mitochondria-Stimulating and Antioxidant Effects of Slovak Propolis Varieties on Bovine Spermatozoa. Oxygen, 3(2), 179-189. https://doi.org/10.3390/oxygen3020013








