Abstract
Cisplatin-induced acute kidney injury (AKI) is the main factor restraining the clinical application of cisplatin. The AKI is associated with high mortality and morbidity, but no effective pharmacological treatment is available at present. As increased levels of reactive oxygen species (ROS) may promote the progression of the injury, the elimination of ROS has been considered as an effective method to prevent the cisplatin-induced AKI. In addition, it has been revealed that an inducer of autophagy could protect kidney cells in the autophagy dependent manner. Induction of autophagy could also modulate the production of ROS in cases of renal injury. Therefore, kidney-targeted antioxidants and/or autophagy are urgently required for the better treatment of AKI. Accumulating evidence has indicated the important roles of gut microbiota in the pathogenesis of AKI. In addition, there is a scientific basis for considering future clinical applications of probiotics and/or prebiotics to treat cisplatin-induced AKI. Thus, gut microbiota might be a promising therapeutic target via the alteration of autophagy for the cancer therapy-induced nephrotoxicity.
1. Introduction
Cisplatin is one of the most widely used broad-spectrum anticancer agents, and is used for the treatment of various solid tumors such as ovarian cancer, prostate cancer, bladder cancer, head or neck cancer, and lung cancer [1,2]. The antitumor mechanisms of cisplatin are mainly DNA damage via the enhanced generation of reactive oxygen species (ROS) [3]. The excess ROS such as superoxide anion, hydrogen peroxide, and hydroxyl radical would cause oxidative damage to various important molecules including proteins, lipids, and/or DNAs, leading to the critical damage of cancer cells [4]. It had been suggested that hydrogen peroxide is involved in the cisplatin-induced necrosis, whereas hydroxyl radical is responsible for the cisplatin-induced apoptosis [5]. Accordingly, the protective effects of hydroxyl radical scavengers are associated with an inhibition of cytochrome c release and caspase activation [5]. In general, cancer cells have higher levels of ROS than normal cells as a result of hyper-metabolism [6]. In addition to their extreme cytotoxicity, cisplatin could also have a variety of non-specific adverse reactions in cancer patients [7]. Studies have suggested that the accumulation of intracellular ROS is a hallmark of cisplatin-induced acute kidney injury (AKI) [8]. Cisplatin may show high activity in the fast proliferating cells, thereby causing cellular damage. In particular, about 30% of cisplatin-administered patients suffer from renal dysfunction and/or injury [9]. Increased formation of ROS in renal proximal convoluted tubule cells may be associated with the cisplatin-induced AKI [10], which is a substantial complication of cisplatin chemotherapy related to the ROS-dependent death of renal cells [11]. The AKI may be associated with high morbidity and mortality. To date, there are few strategies for preventing cisplatin-induced AKI [12], and it is urgent to search for novel therapeutic procedures to protect the kidney against the nephrotoxicity of cisplatin. In recent years, remarkable advances have been made in effective protective regimens for the nephrotoxicity of cisplatin [13]. Basically, protection of kidney from cisplatin-induced AKI might be attainable with antioxidant-based therapeutic interventions that increase antioxidant levels and thus improve the damage from ROS. For example, it has been suggested that scavenging ROS might lessen cisplatin-induced mitochondrial dysfunction and thereby minimize the cisplatin-induced AKI [14,15]. The challenges of maintaining anti-cancer efficacy by regulating the ROS level in normal tissues and tumor tissues deserves to be thoroughly addressed [15]. Cisplatin may induce autophagy-associated apoptosis in part through the effect of ROS [16]. The autophagy has been demonstrated to alter the efficacy of conventional chemotherapy [17]. However, autophagy may not play a role in acquired resistance to cisplatin [16]. We believe it is important to comprehend the relationship between ROS and autophagy in cases of cisplatin-usage for prevention of cisplatin-induced AKI, and for the safe and effective cancer therapy [18].
2. ROS and Autophagy in Cisplatin-Induced Acute Kidney Injury
The excessive generation of ROS has been regarded as the critical role during the pathogenetic process induced by cisplatin, by which DNA damage and/or cell death could occur. Increased ROS production is also known to change the mitochondrial electron transport chain, and eventually lead to apoptosis [19]. In particular, the dysfunction of the mitochondrial respiratory chain results in the further excess production of ROS that contributes to severe kidney injury [20]. Consequently, the mitochondrial dysfunction induced by the treatment with cisplatin could be considered as due to increased levels of ROS resulting in various cellular apoptosis including kidney cells [2,21]. Increased levels of ROS can also contribute to the tubular cell apoptosis in kidney, thereby causing more severe kidney injury during AKI [22]. Further generation of ROS in the tubular cells mitochondria might thus possibly contribute to the exacerbation of cisplatin-induced AKI. Therefore, the elimination of ROS has long been considered as an effective procedure to prevent the cisplatin-induced AKI [23]. In addition to increased ROS levels, treatment with cisplatin also impairs the activity of antioxidants such as superoxide dismutases (SODs), catalase, and glutathione peroxidase, which could function to reduce ROS levels [24]. The SODs are ROS-eliminating super enzymes with several subcellular localizations [25]. Targeting the cisplatin-induced oxidative stress via manipulation of the cellular antioxidant system including the expression of SODs could be beneficial for protecting against cisplatin nephrotoxicity. In fact, some agents with antioxidant and/or anti-inflammation activities could alleviate the cisplatin-induced cell damage by reduced production of ROS [26]. These therapeutic approaches may enhance the tolerance to cisplatin and hence might enable greater dose intensity associated with better outcomes. It has been shown that sirt1 expression on proximal tubules in the kidney may save cisplatin-induced AKI by preserving the function of peroxisomes and the elimination of ROS, which could be a potential therapeutic target for the treatment of cisplatin-induced AKI [27] (Figure 1).
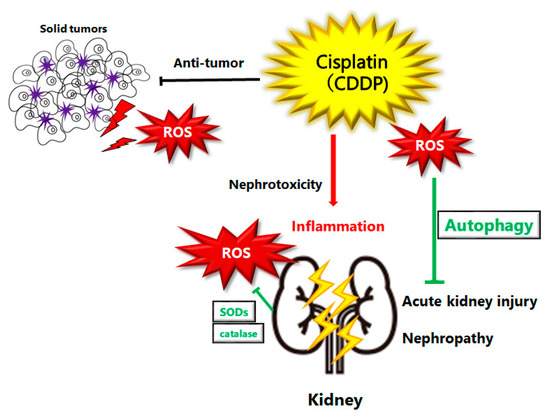
Figure 1.
Schematic illustration of pathogenesis of cisplatin induced acute kidney injury or nephropathy. Reactive oxygen species (ROS), inflammation, and autophagy are all involved in the pathogenesis of cisplatin induced acute kidney injury. ROS may damage DNA or organelles within a cell. The damage could be treated with autophagy to enhance the survival of kidney cells. If the damage is too severe to be repaired, cells might undergo cell-death leading to kidney injury or nephropathy. Note that several significant features have been omitted for clarity.
ROS-induced autophagy may also lead to a different outcome of cell fate that may result in cell survival or cell death, depending on the severity of ROS exposure [28]. ROS impact on autophagy is mediated by specific signaling pathways, which might reduce the oxidative damage by degrading and/or recycling intracellular oxidized macromolecules and dysfunctional organelles [29]. It is well known that autophagy could be modulated by oxidative stress [30], and that antioxidants may prevent the induction of ROS-induced autophagy [31]. In fact, correlative evidence on the roles of ROS in autophagy in renal diseases has been formerly reported [32]. Induction of autophagy could also modulate ROS production in renal injury. For example, cisplatin treatment in autophagy-deficient renal proximal tubular cells may increase ROS production, oxidative stress, and DNA damage [33], suggesting that autophagy provides protection by reducing ROS production and eliminating toxic oxidized protein aggregates and/or other macromolecules (Figure 1).
3. Autophagy as a Target for the Treatment of Cisplatin-Induced Acute Kidney Injury
Autophagy, a highly conserved multistep catabolic pathway in eukaryotic cells, degrades and/or recycles macromolecules and/or dysfunctional organelles, which also contributes to the maintenance of the homeostasis of the kidney. For example, an inducer of autophagy could protect cells in an autophagy dependent manner [34]. In addition, the activity of autophagy regulator beclin 1 brings kidney protection via the reduction of kidney damage, subsequently refining kidney recovery post-AKI [35]. The beneficial effect of autophagy has a potential clinical significance in minimizing or preventing cisplatin nephrotoxicity [36]. In fact, autophagy occurs in AKI, and this might be an imperative mechanism for protection of cell survival [37]. Autophagy can protect kidney proximal tubules against AKI, possibly by alleviating DNA damage and/or ROS production [33,38]. In general, adenosine-monophosphate activated-protein kinase (AMPK) and mammalian target of rapamycin (mTOR) are major positive and negative regulators of autophagy, respectively. Therefore, inhibition of AMPK could lead to autophagy in cisplatin-induced AKI, resulting in more cellular or tubular kidney damage [39]. It has been indicated that penicilliumin-B denotes a different AMPK activator that might provide considerable protection against the apoptosis of renal tubular cells through the activated AMPK-induced autophagy and/or mitochondrial regeneration [40]. Likewise, metformin might protect against the cisplatin-induced apoptosis of tubular cells and/or AKI through stimulating AMPK-activation and/or inducing autophagy [41]. In addition, it has been shown that the pre-activation of autophagy could improve the survival and differentiation of kidney cells by inhibiting the mTOR signaling pathway, which in turn could mitigate the cisplatin-induced AKI [42].
In this regard, various compounds have an impact on the autophagy within kidney diseases. For example, chlorogenic acids may decrease cisplatin-induced AKI through alterations of inflammation, oxidative stress, apoptosis and/or autophagy, with the improvement in kidney restoration [43]. Also, ginsenoside effectively protects against cisplatin-induced AKI by activating the autophagy-mediated pathway [44]. The ginsenoside mediated improvement has been found due to the regulation of AMPK and/or mTOR-mediated autophagy and the inhibition of apoptosis [45]. Autophagy-mediated inhibition of apoptosis might also play a crucial role in astragaloside-mediated protection against cisplatin-induced renotoxicity [46]. In addition, berberine could play a protecting role in cisplatin-induced AKI by up-regulating mitophagy that is a kind of autophagy [47]. Similarly, Pink1 or Parkin dependent mitophagy has also identified potential targets for the treatment of cisplatin-induced AKI [48]. Honokiol treatment may cause noticeable kidney protection and attenuation of the cisplatin-induced kidney changes via preventing mitochondrial dysfunction [49]. Morin hydrate, a natural flavonoid, could also improve autophagy and/or inflammatory responses and decrease the cellular death in kidney, suggesting morin hydrate as a potential therapeutic agent against cisplatin-induced nephrotoxicity [50]. Trehalose treatment similarly conserves mitochondrial function via the activation of autophagy, and then attenuates cisplatin-induced AKI [51]. Cordyceps cicadae, a traditional Chinese medicine, may have a potential kidney protective effectfor prevention of cisplatin-induced AKI through the inhibition of various oxidative stresses by activating AMPK [52]. It is reported that treatment with 3-dehydroxyceanothetric acid 2-methyl ester isolated from the root of Ziziphus jujuba has decreased the autophagic vesicles via the altered protein expressions of AMPK and/or mTOR dependent pathway against cisplatin-induced AKI [53]. Retinoic acids could also improve cisplatin-induced AKI through the activation of autophagy, and the retinoic acids might have some protective effects for cisplatin-based chemotherapy [54]. AMPK activation is probably essential for the protection of kidney via the lithium-induced tubular cell autophagy in cases of cisplatin-induced AKI [55]. In addition, IFN-γ could accelerate autophagic change in kidney and increase the viability of kidney tubular cells, thereby attenuating cisplatin-induced AKI [56]. Deficiency of neutral ceramidase, an enzyme responsible for converting ceramide into sphingosine, could protect against cisplatin-induced AKI by the mechanism of increased autophagy [57]. Amniotic fluid stem cells may lead to amelioration of cisplatin-induced AKI, which is mediated by inhibition of apoptosis and/or activation of autophagy [58]. However, persistent autophagy after AKI induces pro-fibrotic cytokines in renal tubular cells, promoting renal fibrosis and chronic kidney disease (CKD) [59]. As described in this overview, autophagy is deeply involved in the cisplatin-induced AKI, and autophagy could be a target for kidney protection (Figure 2).
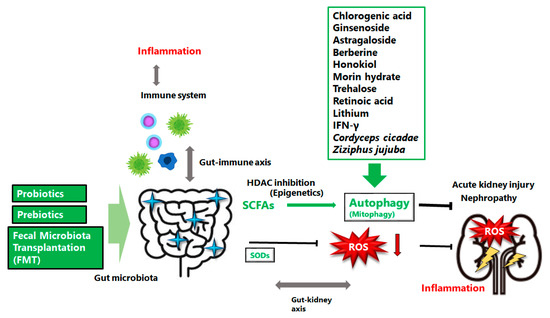
Figure 2.
The gut microbiota could support action, via the alteration of autophagy, against the cisplatin induced acute kidney injury or nephropathy. Several examples including natural compounds or medical agents which could affect the autophagy have also been shown. Prebiotics, probiotics, and/or fecal microbiota transplantation (FMT) might be potential therapy for the treatment of cisplatin induced acute kidney injury or nephropathy, which might lead to successful cancer therapy with low damage in normal tissues. The arrowhead indicates stimulation whereas the hammerhead shows inhibition. Note that several important activities such as cytokine-induction or anti-inflammatory reaction have been omitted for clarity. Abbreviations: FMT, fecal microbiota transplantation; SCFAs, short-chain fatty acids; ROS, reactive oxygen species; HDAC, histone deacetylase.
4. Favorable Roles of Gut-Kidney Axis for the Protection of Kidney
Autophagy is also critical for the homeostasis of intestinal bacteria and/or intestinal barrier function [60]. In addition, it has been indicated that exploring the mechanism of the interaction between autophagy and gut microbiota is beneficial for the study of autoimmune diseases [61]. Furthermore, gut microbiota dysbiosis has emerged as a significant factor leading to renal failure via the progression of renal dysfunction, and alteration of gut microbiota has been observed after treatment with cisplatin [62]. Dysbiosis of gut microbiota is also found in diabetic nephropathy, and oral butyrate supplementation might amend the kidney injury, possibly by boosting autophagy via modifying AMPK and/or mTOR signaling pathway [63]. Interestingly, it has been shown that total flavones of Abelmoschus manihot could improve renal injury and induce modifications in the gut microbiota, showing increased Erysipelotrichales, and decreased Lactobacillales and/or Bacteroidales [64]. The relationship between gut microbiota and kidney function is termed the gut-kidney axis [65], which is often implicated in the pathogenesis of IgA nephropathy and/or CKD [66]. It has been detected that several metabolites derived from the fermentation of gut microbiota may be associated with a systemic inflammatory response and/or kidney injury, via the gut-kidney axis [67]. In particular, short-chain fatty acids (SCFAs) are well-known fermentation products derived from dietary fiber sources, which are key substrates for regulating immune system and/or inflammatory responses [68]. SCFAs have anti-inflammatory and histone deacetylase (HDAC)-inhibiting properties, which could improve several organ functions after an injury most likely via the epigenetic modification [69]. Interestingly, it has been shown that induction of autophagy by SCFAs is associated with the activation of the AMPK catalytic subunit [70]. Likewise, inhibition of histone H3K27 acetylation could coordinate the protection of kidney in cases of cisplatin-induced AKI. [71]. Therefore, gut microbiota could mediate the nephron-protective effects potentially via increasing the production of SCFAs which are well-known inhibitors of HDAC [72]. Remarkably, it has been indicated that the HDAC-inhibitors may protect kidneys by activating autophagy in proximal tubular cells [73].
Accumulating evidence suggests that gut microbiota is involved in the pathogenesis of AKI [74]. In addition, recent research has demonstrated a substantial compositional and/or functional discrepancy in the gut microorganisms after cisplatin-treatment, contributing to further impairments of the gut structure and/or function [75]. Hence, the gut microbiota could be targeted to benefit the efficacy and reduce the toxicity of prevailing chemotherapy agents [76]. It is well known that the composition of gut microbiota could be changed promptly through diet alteration [77]. For example, pretreatment with probiotics is reported to slow the progression of AKI and CKD through increasing SCFAs production by the gut microbiota, suggesting a potential treatment to reduce kidney injury [78]. In addition, augmented levels of SCFAs could be reached through certain dietary changes [79]. Therefore, probiotics have been investigated as alternative therapeutic strategies supporting the concept that SCFAs-producing bacteria could become a tool for the prevention and/or treatment of inflammatory processes in kidney. These studies powerfully indicate that gut microbiota could be a latent ideal therapeutic target for cisplatin-induced AKI via the alteration of autophagy (Figure 2).
5. Future Perspectives
Cisplatin could cause dysbiosis in intestinal microflora, which might change the microbiota-derived metabolites and influence the gut-kidney axis, leading to nephrotoxicity. In clinical practice, Lactobacillus reuteri and Clostridium butyricum have been used for the treatment of dysbiosis and/or inflammation in gut or specific organ damage [80,81]. Modulation of intestinal microbiota by administering probiotics could be considered as a beneficial intervention to remove uremic toxins, promote SCFAs production, and amend renal function [82]. The role of probiotics and/or prebiotics as mediators of the gut-kidney axis for the kidney protection in cisplatin-induced AKI has been considered [81,83]. The probiotic kidney-protective effects of L. reuteri combined with C. butyricum might be mediated by the modulation of composition in gut microbiota, thereby inhibiting the production of uremic toxin and enhancing the production of SCFAs. These potential mechanisms might include indirect alterations in the structure of gut commensal bacteria such as SCFAs-producing bacteria and/or decreasing the pathogenic inflammatory bacteria. SCFAs could enhance the efficacy of cancer therapy, while protecting the normal mucosa cells from associated toxicity of the cancer therapy [84]. Too much toxicity in cancer therapy due to the adverse effects on normal cells may limit the efficacy of the treatment, through disturbing patients’ quality of life (QOL) [85]. To find an appropriate, not too much but not too little, level of effectiveness in cancer-therapy should be important and required for the good QOL of individuals. A focus of future studies should be the clarification of patients’ QOL with clinical applications of prebiotics and/or probiotics for the treatment against AKI. Since antibiotic resistant strains do not create an immediate danger to human health, detailed studies have shown that antibiotic resistant genes might be problematic [86]. In particular, the application of microrganisms such as L. reuterii and C. butyricum could transmit resistance characteristics to other microorganisms in the gut, which could cause serious infections in the host. Therefore, more studies are needed to identify promising strategies to evade antibiotic resistance-spread to pathogens through fermented food consumption.
Author Contributions
Conceptualization, S.Y. and S.M.; original draft preparation and editing, S.Y., K.T., H.S., Y.I., A.T. and S.M.; visualization, S.Y. and S.M.; supervision, S.M. Each author (S.Y., K.T., H.S., Y.I., A.T. and S.M.) has participated sufficiently in this work of drafting the article and/or revising the article for its substantive content. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AKI | acute kidney injury |
| AMP | Adenosine monophosphate |
| AMPK | AMP-activated protein kinase |
| CKD | chronic kidney disease |
| DNA | deoxyribonucleic acid |
| HDAC | histone deacetylase |
| mTOR | mammalian target of rapamycin |
| QOL | quality of life |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| SCFAs | short-chain fatty acids |
References
- Martinho, N.; Santos, T.C.B.; Florindo, H.F.; Silva, L.C. Cisplatin-Membrane Interactions and Their Influence on Platinum Complexes Activity and Toxicity. Front. Physiol. 2019, 9, 1898. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, S.; Disler, C.; Perego, P. The rediscovery of platinum-based cancer therapy. Nat. Rev. Cancer 2021, 21, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Fasnacht, M.; Polacek, N. Oxidative Stress in Bacteria and the Central Dogma of Molecular Biology. Front. Mol. Biosci. 2021, 8, 671037. [Google Scholar] [CrossRef]
- Baek, S.M.; Kwon, C.H.; Kim, J.H.; Woo, J.S.; Jung, J.S.; Kim, Y.K. Differential roles of hydrogen peroxide and hydroxyl radical in cisplatin-induced cell death in renal proximal tubular epithelial cells. J. Lab. Clin. Med. 2003, 142, 178–186. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.S.; Seo, Y.R. Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxid Med Cell Longev. 2019, 2019, 5381692. [Google Scholar] [CrossRef]
- Jordan, P.; Carmo-Fonseca, M. Molecular mechanisms involved in cisplatin cytotoxicity. Cell Mol. Life Sci. 2000, 57, 1229–1235. [Google Scholar] [CrossRef]
- Guan, J.; Tong, X.; Zhang, Y.; Xu, F.; Zhang, Y.; Liang, X.; Jin, J.; Jing, H.; Guo, L.; Ni, X.; et al. Nephrotoxicity induced by cisplatin is primarily due to the activation of the 5-hydroxytryptamine degradation system in proximal renal tubules. Chem. Biol. Interact. 2021, 349, 109662. [Google Scholar] [CrossRef]
- Tan, Z.; Guo, F.; Huang, Z.; Xia, Z.; Liu, J.; Tao, S.; Li, L.; Feng, Y.; Du, X.; Ma, L.; et al. P harmacological and genetic inhibition of fatty acid-binding protein 4 alleviated cisplatin-induced acute kidney injury. Cell Mol. Med. 2019, 23, 6260–6270. [Google Scholar] [CrossRef]
- Mapuskar, K.A.; Steinbach, E.J.; Zaher, A.; Riley, D.P.; Beardsley, R.A.; Keene, J.L.; Holmlund, J.T.; Anderson, C.M.; Zepeda-Orozco, D.; Buatti, J.M.; et al. Mitochondrial Superoxide Dismutase in Cisplatin-Induced Kidney Injury. Antioxidants (Basel) 2021, 10, 1329. [Google Scholar] [CrossRef]
- Soni, H.; Kaminski, D.; Gangaraju, R.; Adebiyi, A. Cisplatin-induced oxidative stress stimulates renal Fas ligand shedding. Ren. Fail. 2018, 40, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jiang, W.; Zhao, H.; He, C.; Tang, X.; Xu, S.; Xu, C.; Feng, R.; Li, J.; Ma, T.; et al. PSTPIP2 inhibits cisplatin-induced acute kidney injury by suppressing apoptosis of renal tubular epithelial cells. Cell Death Dis. 2020, 11, 1057. [Google Scholar] [CrossRef] [PubMed]
- Holditch, S.J.; Brown, C.N.; Lombardi, A.M.; Nguyen, K.N.; Edelstein, C.L. Recent Advances in Models, Mechanisms, Biomarkers, and Interventions in Cisplatin-Induced Acute Kidney Injury. Int. J. Mol. Sci. 2019, 20, 3011. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Kashihara, N.; Fujimoto, S.; Horike, H.; Tokura, T.; Namikoshi, T.; Sasaki, T.; Makino, H. A novel free radical scavenger, edarabone, protects against cisplatin-induced acute renal damage in vitro and in vivo. J. Pharmacol. Exp. Ther. 2003, 305, 1183–1190. [Google Scholar] [CrossRef]
- Xin, Q.; Ji, Q.; Zhang, Y.; Ma, W.; Tian, B.; Liu, Y.; Chen, Y.; Wang, F.; Zhang, R.; Wang, X.; et al. Aberrant ROS Served as an Acquired Vulnerability of Cisplatin-Resistant Lung Cancer. Oxid. Med. Cell Longev. 2022, 2022, 1112987. [Google Scholar] [CrossRef]
- Magnano, S.; Hannon Barroeta, P.; Duffy, R.; O’Sullivan, J.; Zisterer, D.M. Cisplatin induces autophagy-associated apoptosis in human oral squamous cell carcinoma (OSCC) mediated in part through reactive oxygen species. Toxicol. Appl. Pharmacol. 2021, 427, 115646. [Google Scholar] [CrossRef]
- Wang, K.; Liu, X.; Liu, Q.; Ho, I.H.; Wei, X.; Yin, T.; Zhan, Y.; Zhang, W.; Zhang, W.; Chen, B.; et al. Hederagenin potentiated cisplatin- and paclitaxel-mediated cytotoxicity by impairing autophagy in lung cancer cells. Cell Death Dis. 2020, 11, 611. [Google Scholar] [CrossRef]
- Ikeda, Y.; Nagase, N.; Tsuji, A.; Taniguchi, K.; Kitagishi, Y.; Matsuda, S. Comprehension of the Relationship between Autophagy and Reactive Oxygen Species for Superior Cancer Therapy with Histone Deacetylase Inhibitors. Oxygen 2021, 1, 22–31. [Google Scholar] [CrossRef]
- Choi, Y.M.; Kim, H.K.; Shim, W.; Anwar, M.A.; Kwon, J.W.; Kwon, H.K.; Kim, H.J.; Jeong, H.; Kim, H.M.; Hwang, D.; et al. Mechanism of Cisplatin-Induced Cytotoxicity Is Correlated to Impaired Metabolism Due to Mitochondrial ROS Generation. PLoS ONE 2015, 10, e0135083. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fu, Y.; Wang, P.; Liu, S.; Sha, Y.; Zhang, Y.; Zhang, A.; Jia, Z.; Ding, G.; Huang, S. Intervention of mitochondrial activity attenuates cisplatin-induced acute kidney injury. Int. Urol. Nephrol. 2019, 51, 1207–1218. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, T.; Wang, C.; Meng, Q.; Huo, X.; Wang, C.; Sun, P.; Sun, H.; Ma, X.; Wu, J.; et al. Catalpol-Induced AMPK Activation Alleviates Cisplatin-Induced Nephrotoxicity through the Mitochondrial-Dependent Pathway without Compromising Its Anticancer Properties. Oxid. Med. Cell Longev. 2021, 2021, 7467156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Shao, L.; Spitz, D.R. Reactive oxygen species in normal and tumor stem cells. Adv. Cancer Res. 2014, 122, 1–67. [Google Scholar] [PubMed]
- Li, R.; Hu, L.; Hu, C.; Wang, Q.; Lei, Y.; Zhao, B. Myricitrin protects against cisplatin-induced kidney injury by eliminating excessive reactive oxygen species. Int. Urol. Nephrol. 2020, 52, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Husain, K.; Morris, C.; Whitworth, C.; Trammell, G.L.; Rybak, L.P.; Somani, S.M. Protection by ebselen against cisplatin-induced nephrotoxicity: Antioxidant system. Mo.l Cell Biochem. 1998, 178, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Pechenino, A.S.; Brown, T.R. Superoxide dismutase in the prostate lobes of aging Brown Norway rats. Prostate 2006, 66, 522–535. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Xu, Y.; Yuan, Y.; Tian, L.; Wang, Q.; Xie, Y.; Shao, X.; Zhang, M.; Ni, Z.; Mou, S. Renoprotective mechanisms of Astragaloside IV in cisplatin-induced acute kidney injury. Free Radic. Res. 2017, 51, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Wakino, S.; Yoshioka, K.; Tatematsu, S.; Hara, Y.; Minakuchi, H.; Sueyasu, K.; Washida, N.; Tokuyama, H.; Tzukerman, M.; et al. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J. Biol. Chem. 2010, 285, 13045–13056. [Google Scholar] [CrossRef]
- Thévenod, F.; Lee, W.K. Cadmium and cellular signaling cascades: Interactions between cell death and survival pathways. Arch. Toxicol. 2013, 87, 1743–1786. [Google Scholar] [CrossRef]
- Kaushal, G.P.; Chandrashekar, K.; Juncos, L.A. Molecular Interactions Between Reactive Oxygen Species and Autophagy in Kidney Disease. Int. J. Mol. Sci. 2019, 20, 3791. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell. Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef]
- Levonen, A.L.; Hill, B.G.; Kansanen, E.; Zhang, J.; Darley-Usmar, V.M. Redox regulation of antioxidants, autophagy, and the response to stress: Implications for electrophile therapeutics. Free Radic. Biol. Med. 2014, 71, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Sureshbabu, A.; Ryter, S.W.; Choi, M.E. Oxidative stress and autophagy: Crucial modulators of kidney injury. Redox Biol. 2015, 4, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Kimura, T.; Takabatake, Y.; Namba, T.; Kaimori, J.; Kitamura, H.; Matsui, I.; Niimura, F.; Matsusaka, T.; Fujita, N.; et al. Autophagy guards against cisplatin-induced acute kidney injury. Am. J. Pathol. 2012, 180, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Lee, B.; Han, M.; Rhee, W.J.; Kwak, M.S.; Yoo, T.H.; Shin, J.S. Canagliflozin protects against cisplatin-induced acute kidney injury by AMPK-mediated autophagy in renal proximal tubular cells. Cell Death Discov. 2022, 8, 12. [Google Scholar] [CrossRef]
- Shi, M.; Maique, J.; Shepard, S.; Li, P.; Seli, O.; Moe, O.W.; Hu, M.C. In vivo evidence for therapeutic applications of beclin 1 to promote recovery and inhibit fibrosis after acute kidney injury. Kidney Int. 2022, 101, 63–78. [Google Scholar] [CrossRef]
- Kaushal, G.P.; Kaushal, V.; Herzog, C.; Yang, C. Autophagy delays apoptosis in renal tubular epithelial cells in cisplatin cytotoxicity. Autophagy 2008, 4, 710–712. [Google Scholar] [CrossRef]
- Periyasamy-Thandavan, S.; Jiang, M.; Wei, Q.; Smith, R.; Yin, X.M.; Dong, Z. Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int. 2008, 74, 631–640. [Google Scholar] [CrossRef]
- Kaushal, G.P.; Shah, S.V. Autophagy in acute kidney injury. Kidney Int. 2016, 89, 779–791. [Google Scholar] [CrossRef]
- Wei, L.; Chen, W.; Zou, Y.; Huang, H.; Pan, B.; Jin, S.; Huang, R.; Nie, S.; Kong, G. AMP-activated protein kinase regulates autophagic protection against cisplatin-induced tissue injury in the kidney. Genet. Mol. Res. 2015, 14, 12006–12015. [Google Scholar] [CrossRef]
- Shen, W.; Jia, N.; Miao, J.; Chen, S.; Zhou, S.; Meng, P.; Zhou, X.; Tang, L.; Zhou, L. Penicilliumin B Protects against Cisplatin-Induced Renal Tubular Cell Apoptosis through Activation of AMPK-Induced Autophagy and Mitochondrial Biogenesis. Kidney Dis. 2021, 7, 278–292. [Google Scholar] [CrossRef]
- Li, J.; Gui, Y.; Ren, J.; Liu, X.; Feng, Y.; Zeng, Z.; He, W.; Yang, J.; Dai, C. Metformin Protects Against Cisplatin-Induced Tubular Cell Apoptosis and Acute Kidney Injury via AMPKα-regulated Autophagy Induction. Sci. Rep. 2016, 6, 23975. [Google Scholar] [CrossRef] [PubMed]
- Awadalla, A.; Hussein, A.M.; El-Far, Y.M.; El-Senduny, F.F.; Barakat, N.; Hamam, E.T.; Abdeen, H.M.; El-Sherbiny, M.; Serria, M.S.; Sarhan, A.A.; et al. Rapamycin Improves Adipose-Derived Mesenchymal Stem Cells (ADMSCs) Renoprotective Effect against Cisplatin-Induced Acute Nephrotoxicity in Rats by Inhibiting the mTOR/AKT Signaling Pathway. Biomedicines 2022, 10, 1295. [Google Scholar] [CrossRef] [PubMed]
- Domitrović, R.; Cvijanović, O.; Šušnić, V.; Katalinić, N. Renoprotective mechanisms of chlorogenic acid in cisplatin-induced kidney injury. Toxicology 2014, 324, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Gao, H.; Wang, S.; Zhang, S.; Qu, X.; Zhang, Y.; Tao, L.; Sun, J.; Song, Y.; Fu, L. Ginsenoside Rg3 attenuates cisplatin-induced kidney injury through inhibition of apoptosis and autophagy-inhibited NLRP3. J. Biochem. Mol. Toxicol. 2021, 35, e22896. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.J.; Hou, J.G.; Ma, Z.N.; Wang, Z.; Ren, S.; Wang, Y.P.; Liu, W.C.; Chen, C.; Li, W. Ginsenoside Rb3 provides protective effects against cisplatin-induced nephrotoxicity via regulation of AMPK-/mTOR-mediated autophagy and inhibition of apoptosis in vitro and in vivo. Cell Prolif. 2019, 52, e12627. [Google Scholar] [CrossRef]
- Qu, X.; Gao, H.; Tao, L.; Zhang, Y.; Zhai, J.; Sun, J.; Song, Y.; Zhang, S. Astragaloside IV protects against cisplatin-induced liver and kidney injury via autophagy-mediated inhibition of NLRP3 in rats. J. Toxicol. Sci. 2019, 44, 167–175. [Google Scholar] [CrossRef]
- Qi, J.; Xue, Q.; Kuang, L.; Xie, L.; Luo, R.; Nie, X. Berberine alleviates cisplatin-induced acute kidney injury by regulating mitophagy via PINK 1/Parkin pathway. Transl. Androl. Urol. 2020, 9, 1712–1724. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, Z.; Xu, X.; An, X.; Duan, S.; Huang, Z.; Zhang, C.; Wu, L.; Zhang, B.; Zhang, A.; et al. Pink1/Parkin-mediated mitophagy play a protective role in cisplatin induced renal tubular epithelial cells injury. Exp. Cell Res. 2017, 350, 390–397. [Google Scholar] [CrossRef]
- Mao, R.W.; He, S.P.; Lan, J.G.; Zhu, W.Z. Honokiol ameliorates cisplatin-induced acute kidney injury via inhibition of mitochondrial fission. Br. J. Pharmacol. 2022, 179, 3886–3904. [Google Scholar] [CrossRef]
- Singh, M.P.; Chauhan, A.K.; Kang, S.C. Morin hydrate ameliorates cisplatin-induced ER stress, inflammation and autophagy in HEK-293 cells and mice kidney via PARP-1 regulation. Int. Immunopharmacol. 2018, 56, 156–167. [Google Scholar] [CrossRef]
- Zhu, L.; Yuan, Y.; Yuan, L.; Li, L.; Liu, F.; Liu, J.; Chen, Y.; Lu, Y.; Cheng, J. Activation of TFEB-mediated autophagy by trehalose attenuates mitochondrial dysfunction in cisplatin-induced acute kidney injury. Theranostics 2020, 10, 5829–5844. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.S.; Jiang, W.P.; Chen, C.C.; Lee, L.Y.; Li, P.Y.; Huang, W.C.; Liao, J.C.; Chen, H.Y.; Huang, S.S.; Huang, G.J. Cordyceps cicadae Mycelia Ameliorate Cisplatin-Induced Acute Kidney Injury by Suppressing the TLR4/NF-κB/MAPK and Activating the HO-1/Nrf2 and Sirt-1/AMPK Pathways in Mice. Oxid. Med. Cell Longev. 2020, 2020, 7912763. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kang, K.B.; Kim, H.W.; Park, J.S.; Hwang, G.S.; Kang, K.S.; Choi, S.; Yamabe, N.; Kim, K.H. Unique Triterpenoid of Jujube Root Protects Cisplatin-induced Damage in Kidney Epithelial LLC-PK1 Cells via Autophagy Regulation. Nutrients 2020, 12, 677. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zheng, C.; Wan, X.; Shi, M.; McMillan, K.; Maique, J.; Cao, C. Retinoic Acid Alleviates Cisplatin-Induced Acute Kidney Injury Through Activation of Autophagy. Front. Pharmacol. 2020, 11, 987. [Google Scholar] [CrossRef]
- Bao, H.; Zhang, Q.; Liu, X.; Song, Y.; Li, X.; Wang, Z.; Li, C.; Peng, A.; Gong, R. Lithium targeting of AMPK protects against cisplatin-induced acute kidney injury by enhancing autophagy in renal proximal tubular epithelial cells. FASEB J. 2019, 33, 14370–14381. [Google Scholar] [CrossRef]
- Kimura, A.; Ishida, Y.; Inagaki, M.; Nakamura, Y.; Sanke, T.; Mukaida, N.; Kondo, T. Interferon-γ is protective in cisplatin-induced renal injury by enhancing autophagic flux. Kidney Int. 2012, 82, 1093–1104. [Google Scholar] [CrossRef]
- Sears, S.M.; Dupre, T.V.; Shah, P.P.; Davis, D.L.; Doll, M.A.; Sharp, C.N.; Vega, A.A.; Megyesi, J.; Beverly, L.J.; Snider, A.J.; et al. Neutral ceramidase deficiency protects against cisplatin-induced acute kidney injury. J. Lipid. Res. 2022, 63, 100179. [Google Scholar] [CrossRef]
- Minocha, E.; Sinha, R.A.; Jain, M.; Chaturvedi, C.P.; Nityanand, S. Amniotic fluid stem cells ameliorate cisplatin-induced acute renal failure through induction of autophagy and inhibition of apoptosis. Stem Cell Res. Ther. 2019, 10, 370. [Google Scholar] [CrossRef]
- Fu, Y.; Xiang, Y.; Wu, W.; Cai, J.; Tang, C.; Dong, Z. Persistent Activation of Autophagy After Cisplatin Nephrotoxicity Promotes Renal Fibrosis and Chronic Kidney Disease. Front. Pharmacol. 2022, 13, 918732. [Google Scholar] [CrossRef]
- Pan, Q.; Guo, F.; Huang, Y.; Li, A.; Chen, S.; Chen, J.; Liu, H.F.; Pan, Q. Gut Microbiota Dysbiosis in Systemic Lupus Erythematosus: Novel Insights into Mechanisms and Promising Therapeutic Strategies. Front. Immunol. 2021, 12, 799788. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Eissa, N.T. Autophagy and autoimmunity crosstalks. Front. Immunol. 2013, 4, 88. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wilson, I.D.; Nicholson, J.K. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl. Res. 2017, 179, 204–222. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Ma, Y.; Cai, F.; Huang, X.; Xiao, L.; Zhong, C.; Ren, P.; Luo, Q.; Chen, J.; Han, F. Changes of gut microbiota in diabetic nephropathy and its effect on the progression of kidney injury. Endocrine 2022, 76, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Fang, Q.J.; Sun, W.; Liu, B.H.; Liu, Y.L.; Wu, W.; Yee, H.Y.; Yuan, C.C.; Wang, M.Z.; Wan, Z.Y.; et al. Total Flavones of Abelmoschus manihot Remodels Gut Microbiota and Inhibits Microinflammation in Chronic Renal Failure Progression by Targeting Autophagy-Mediated Macrophage Polarization. Front. Pharmacol. 2020, 11, 566611. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Shang, L.; Lu, Y.; Wang, Y. Gut Microbiome Characteristics in IgA Nephropathy: Qualitative and Quantitative Analysis from Observational Studies. Front. Cell Infect. Microbiol. 2022, 12, 904401. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hu, X.; Li, S.; Qiu, Y.; Cao, R.; Xu, C.; Lu, C.; Wang, Z.; Yang, J. Pharmacological targeting macrophage phenotype via gut-kidney axis ameliorates renal fibrosis in mice. Pharmacol. Res. 2022, 178, 106161. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, L.; Guo, H.; Xu, Y.; Xu, Y. The role of short-chain fatty acids in kidney injury induced by gut-derived inflammatory response. Metabolism 2017, 68, 20–30. [Google Scholar] [CrossRef]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1–24. [Google Scholar] [CrossRef]
- Andrade-Oliveira, V.; Amano, M.T.; Correa-Costa, M.; Castoldi, A.; Felizardo, R.J.; de Almeida, D.C.; Bassi, E.J.; Moraes-Vieira, P.M.; Hiyane, M.I.; Rodas, A.C.; et al. Gut Bacteria Products Prevent AKI Induced by Ischemia-Reperfusion. J. Am. Soc. Nephrol. 2015, 26, 1877–1888. [Google Scholar] [CrossRef]
- Iannucci, L.F.; Sun, J.; Singh, B.K.; Zhou, J.; Kaddai, V.A.; Lanni, A.; Yen, P.M.; Sinha, R.A. Short chain fatty acids induce UCP2-mediated autophagy in hepatic cells. Biochem. Biophys. Res. Commun. 2016, 480, 461–467. [Google Scholar] [CrossRef]
- Jiang, W.; Yuan, X.; Zhu, H.; He, C.; Ge, C.; Tang, Q.; Xu, C.; Hu, B.; Huang, C.; Ma, T. Inhibition of Histone H3K27 Acetylation Orchestrates Interleukin-9-Mediated and Plays an Anti-Inflammatory Role in Cisplatin-Induced Acute Kidney Injury. Front. Immunol. 2020, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.T.; Zhou, J.; Zhu, J.H.; Wu, C.Y.; Shen, H.; Zhang, W.; Zhou, S.S.; Xu, J.D.; Mao, Q.; Zhang, Y.Q.; et al. Gut Microbiota Mediates the Protective Effects of Traditional Chinese Medicine Formula Qiong-Yu-Gao against Cisplatin-Induced Acute Kidney Injury. Microbiol. Spectr. 2022, 10, e0075922. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Livingston, M.J.; Dong, G.; Tang, C.; Su, Y.; Wu, G.; Yin, X.M.; Dong, Z. Histone deacetylase inhibitors protect against cisplatin-induced acute kidney injury by activating autophagy in proximal tubular cells. Cell Death Dis. 2018, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Gharaie, S.; Noel, S.; Rabb, H. Gut Microbiome and AKI: Roles of the Immune System and Short-Chain Fatty Acids. Nephron 2020, 144, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Feng, Y.; Zeng, Y.; Zhang, H.; Pan, M.; He, F.; Wu, R.; Chen, J.; Lu, J.; Zhang, S.; et al. Gut microbiota accelerates cisplatin-induced acute liver injury associated with robust inflammation and oxidative stress in mice. J. Transl. Med. 2021, 19, 147. [Google Scholar] [CrossRef]
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Zhu, H.; Cao, C.; Wu, Z.; Zhang, H.; Sun, Z.; Wang, M.; Xu, H.; Zhao, Z.; Wang, Y.; Pei, G.; et al. The probiotic L. casei Zhang slows the progression of acute and chronic kidney disease. Cell Metab. 2021, 33, 1926–1942.e8. [Google Scholar] [CrossRef]
- Palacio, M.I.; Weisstaub, A.R.; Zuleta, Á.; Etcheverría, A.I.; Manrique, G.D. α-Galactosides present in lupin flour affect several metabolic parameters in Wistar rats. Food Funct. 2016, 7, 4967–4975. [Google Scholar] [CrossRef]
- Hayashi, A.; Sato, T.; Kamada, N.; Mikami, Y.; Matsuoka, K.; Hisamatsu, T.; Hibi, T.; Roers, A.; Yagita, H.; Ohteki, T.; et al. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe 2013, 13, 711–722. [Google Scholar] [CrossRef]
- Hsiao, Y.P.; Chen, H.L.; Tsai, J.N.; Lin, M.Y.; Liao, J.W.; Wei, M.S.; Ko, J.L.; Ou, C.C. Administration of Lactobacillus reuteri Combined with Clostridium butyricum Attenuates Cisplatin-Induced Renal Damage by Gut Microbiota Reconstitution, Increasing Butyric Acid Production, and Suppressing Renal Inflammation. Nutrients 2021, 13, 2792. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Yan, Y.; Chen, D.; Zhao, Y.; Dong, W.; Zeng, X.; Cao, Y. Ascorbic Acid Derivative 2-O-β-d-Glucopyranosyl-l-Ascorbic Acid from the Fruit of Lycium barbarum Modulates Microbiota in the Small Intestine and Colon and Exerts an Immunomodulatory Effect on Cyclophosphamide-Treated BALB/c Mice. J. Agric. Food Chem. 2020, 68, 11128–11143. [Google Scholar] [CrossRef] [PubMed]
- Denk, S.; Weckbach, S.; Eisele, P.; Braun, C.K.; Wiegner, R.; Ohmann, J.J.; Wrba, L.; Hoenes, F.M.; Kellermann, P.; Radermacher, P.; et al. Role of Hemorrhagic Shock in Experimental Polytrauma. Shock 2018, 49, 154–163. [Google Scholar] [CrossRef]
- Park, M.; Kwon, J.; Shin, H.J.; Moon, S.M.; Kim, S.B.; Shin, U.S.; Han, Y.H.; Kim, Y. Butyrate enhances the efficacy of radiotherapy via FOXO3A in colorectal cancer patient-derived organoids. Int. J. Oncol. 2020, 57, 1307–1318. [Google Scholar] [CrossRef]
- Niegisch, G.; Retz, M.; Siener, R.; Albers, P. Quality of life in patients with cisplatin- resistant urothelial cancer: Typical ailments and effect of paclitaxel-based salvage therapy. Urol. Oncol. 2016, 34, 256.e15-21. [Google Scholar] [CrossRef] [PubMed]
- Devirgiliis, C.; Zinno, P.; Perozzi, G. Update on antibiotic resistance in foodborne Lactobacillus and Lactococcus species. Front. Microbiol. 2013, 4, 301. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).