Abstract
Large areas of the Pacific coast of the Americas remain unstudied regarding their intertidal ecosystems. Given the increasing disturbance related to human impacts on intertidal ecosystems, it is essential to gather census data on the biological composition of poorly studied regions so that a framework for future monitoring and management can be developed. Here, we synthesize the available research on intertidal communities along the Pacific rim to support the goal to fill bioregional gaps in knowledge in three less-studied areas in Southeast Alaska and Peru. A census of taxonomic and functional group hierarchies in these communities should include the use of various measures of alpha and beta diversity to provide a metric of temporal and spatial comparisons. A narrower-scale approach focusing on foundation species that harbor algal and invertebrate communities and serve as buffers against environmental stresses should also be conducted. Conducting a comprehensive census in poorly studied or unstudied areas will contribute to a better understanding of the response to disturbances caused by oil spills, El Niño and marine heatwaves and provide a latitudinal continuum of scientific knowledge about the biodiversity and ecosystem functioning in rocky intertidal systems on a trans-regional scale.
1. Introduction
The rocky intertidal zone is a unique and biologically challenging ecosystem that exists at an interface between terrestrial and marine ecosystems, with unique species of higher plants, algae, and animals that have developed the physiological adaptations to survive a spectrum of physical and biological thresholds such as solar radiation, air and seawater temperatures, ocean waves, desiccation, and predation during periods of low tide [1,2,3,4]. It is a highly productive system with intrinsic ecological and economic value that plays an integral role as a food source for humans as well as in marine food webs and provides habitat and recruitment for a diverse array of marine life including commercially important fish and shellfish [5,6,7]. Global patterns of intertidal zonation are characterized by those very adaptations, as well as competition for space and varying levels of environmental disturbance [1,8,9], with regional-scale differences that include variations in species niches as a result of environmental factors and the incidence and abundance of certain taxa with varying degrees of range overlap among conspecifics [10,11,12,13,14]. The theory of a latitudinal diversity gradient has been widely analyzed for terrestrial biomes, with a general agreement that a broad-scale inverse pattern of richness decreasing with latitude exists [15,16]. Unlike in terrestrial systems, examination of latitudinal diversity in marine benthic and pelagic systems has revealed a more complex, bi-modal pattern of diversity, with higher richness and biomass found primarily at temperate latitudes [8,17,18]. Fish and invertebrate populations in both deep-sea and shallow areas of the eastern and western Atlantic Ocean demonstrate an asymmetric diversity gradient, with richness driven by hydrogeographic features that facilitate gradients of productivity [15,17]. In intertidal regimes, beta diversity (species turnover) defines latitudinal gradients along the western and eastern seaboards of South America [11,19].
Intertidal systems are found on most shorelines of the world with biogeographical features that vary from region to region [20]. The sheer magnitude of the area covered by intertidal ecosystems belies that only a fraction of these systems has been investigated in detail, which underscores the significant and vast regional gaps in our understanding of the function and structure of intertidal communities on a continuum across and among continents [8,10,20,21,22]. These gaps in our knowledge contribute to our lack of understanding regarding effects on the functional structure, abundance, and richness of intertidal ecosystems following catastrophic events from climate change, pollution, and the introduction of invasive species [23]. Over the past several decades, research on intertidal communities has mainly focused on identifying local-scale patterns of species diversity and abundance coinciding with latitudinal-scale trophic-level patterns following climatic variations to better understand regional-scale resiliency in these systems [24,25,26,27,28,29]. Insights into regional-scale comparisons in Pacific systems include a correlation between biomass and latitude, spatial similarity and functional group structure coinciding with oceanographic features, shifts in species abundance following the El Niño phenomenon, and new reports of foundation species [8,10,30,31,32]. Regional-scale efforts to describe and compare diversity, richness, and biological compositions of intertidal ecosystems have been underway in many parts of the eastern and western Pacific (Figure 1); therefore, research attention to poorly studied or unstudied areas will serve to provide more information about temporal shifts in abundances and ranges of species and functional group structure from naturally occurring oscillations in the environment to acute disturbances.
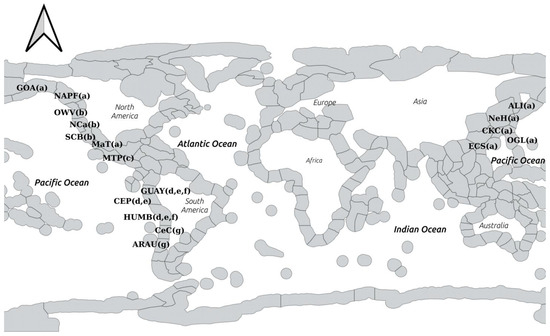
Figure 1.
Open-access map courtesy of Marine Ecoregions of the World available on the website (www.databasin.org) showing the ecoregions in the eastern Pacific. Ecoregions are given as abbreviations, from left to right: GOA = Gulf of Alaska; NAPF = North American Pacific Fjordland; OWV = Oregon Washington Vancouver; Nca = Northern California; SCB = Southern California Bight; MaT = Magdalena Transition; MTP = Mexican Tropical Pacific; GUAY = Guayaquil; CeP = Central Peru; HUMB = Humboldtian; CeC = Central Chile; ARAU = Araucanian; ECS = East China Sea; OgI = Ogasawara Islands; CKC = Central Kuroshio Islands; NeH = Northeastern Honshu; ALI = Aleutian Islands. Complementary research effort among ecoregions cited in this paper is indicated by matching small case letters in parentheses. Shapefile for QGIS projection courtesy of the author/The Nature Conservancy. More details on Copyright and Licensing are available at the following link: https://geospatial.tnc.org/datasets/903c3ae05b264c00a3b5e58a4561b7e6/about.
Although there is a considerable amount of information available regarding the vertical and latitudinal ranges of intertidal species, an analysis of diversity, abundance, and community structure in a few regions of the eastern Pacific is lacking. Furthermore, the reported range of any given species may be unreliable due to sampling bias, i.e., missed species as a result of regional gaps in census, or, conversely, the assumption of a species’ distribution absent a complete regional census [23]. Filling regional gaps in our knowledge and understanding of the countless unstudied intertidal areas of the world requires a universal agreement that research effort be focused on these areas regardless of perceptions of economic and development potential of the landscape. Employing a variety of diversity measures provides a means to untangle the underlying characteristics of each community, e.g., richness, evenness, and the limits of species ranges in poorly studied regions, which is particularly useful for measuring impacts from species invasions or where local extirpations of a population have occurred [11,14]. Additionally, the census methods used to collect data for diversity analysis are also suitable for identifying invasive species, which would otherwise go unnoticed in the absence of census effort. A good example is the spread of the invasive European Green Crab Carcinus maenas, with populations confirmed to be established in Washington state, British Columbia, and Southeast Alaska. Where detection and monitoring effort is consistent, management strategies have commenced, while alternately, a lack of monitoring and management in British Columbia has led to much a poorer understanding of the magnitude of the invasion [33]. The same case can be made for determining the magnitude of marine disease outbreaks. For example, Cornell University in the United States led a citizen-science-based effort that identified the onset of the sea star wasting event along the coasts of California, Washington, Oregon, and Alaska following the marine heatwave of 2014 [34].
One of the most compelling examples of the difficulty in assessing damage to ecosystems without the benefit of a baseline census of biota is the Exxon Valdez Oil Spill in Prince William Sound (PWS) Alaska, which covered over 2000 km of coastline in the spring of 1989. PWS was a woefully understudied region with significant deficiencies in the baseline knowledge of the biological composition of the communities, and in the years following the spill the scientific consensus that significant and long-lasting declines in diversity and abundance of foundation species such as canopy-forming macroalgae were underway was based on post-spill analysis only [21,35,36,37]. The oil spill in PWS not only revealed that deficiencies in baseline knowledge hampered efforts in terms of assessing damage to the ecosystem, but served as a catalyst for a broader regional effort to inventory intertidal taxa and measure the abundance of algal standing stock in this previously understudied region; in other words, to obtain a baseline census that could be integrated into a management plan before a significant level of impact occurs [5,38,39,40]. One such effort has been coordinated by the Bureau of Ocean Energy Management (BOEM), which developed the Multi-Agency Rocky Intertidal Network (MARINe) working group. MARINe is a consortium of citizen scientists and researchers with the objective of censusing rocky intertidal systems over a continuum of sites from Baja California to Washington, with a focus on the efficacy of marine protected areas including the Channel Islands, the Greater Farallones, and the Olympic Coast National Marine Sanctuaries [41,42]. MARINe employs a set of well-defined protocols using multi-scale survey techniques for collecting temporal incidence and abundance data, thus providing a baseline for forecasting changes in the biological composition of the communities as a result of catastrophic human-caused impacts.
There exists a scientific consensus that marine ecosystems along the Pacific coast are vulnerable to oil spills thanks to an ever-increasing demand for fossil fuels, and these systems are under threat from increasing sea surface temperatures as a result of climate change and increasingly stronger El Niño events. What is well understood about intertidal communities in meticulously studied areas of the Pacific should serve to compel an eagerness within the scientific community to fill the remaining gaps in our knowledge of bio-regional compositions in poorly studied or unstudied areas, particularly since they face increasing threats from climate change. A baseline understanding of biological communities from every region possible is essential for assessing damage and mitigating loss from catastrophic impacts, as mentioned previously. In this review, we will discuss the usefulness of census surveys as a means to provide regional baseline datasets of intertidal biota and to produce diversity indices that provide a coherent picture of biological community compositions in poorly studied or unstudied highly productive areas of the eastern Pacific. Regional specific baseline data can provide a “before” picture of the community, which is a necessary tool necessary for coastal managers and stakeholders to be able to forecast effects and mitigate responses to extreme pollution and climatic events [5,36,40,43]. We acknowledge the historical importance of the intertidal zone and the role it has played in human culture over time, and we present a summary of the characteristics of a biological census along with a background of the human relationship with the intertidal zone as well as existing research that provides trans-regional descriptions and comparisons of intertidal communities across the Pacific. We propose that a trans-regional biological census and comparison of community structures be undertaken in three poorly studied eastern Pacific ecoregions, with the objective of describing patterns of zonation, abundance, richness, and diversity. Additionally, we support an effort to unite stakeholders in a healthy coastal environment (tribal groups, university students, municipalities, fishermen’s co-ops, etc.) into localized working groups that can provide long-term monitoring, thus providing the valuable tools necessary for responding to threats posed by climate change and oil pollution.
2. Background
2.1. Measures of Diversity and the Disturbance Hypothesis
Biologists seek to measure the composition of communities in a way that describes the properties of any given assemblage, e.g., the number of species present (richness) and the ratios of abundances across species (evenness), and it is ideal to use an array of measures to effectively capture and compare community properties, including rare and unique species [44,45,46]. Species richness (S) is widely used because it is a simple calculation of the number of species in a sampled area, but the accuracy and precision of the resulting index values vary with sampling effort. Estimators such as the Chao Index are designed to give equal weight to the “unequal catchability” of incidence or abundance of rare species [47]. Increasingly, the functional group organization of assemblages categorized by structural, taxonomic, and trophic regimes has been used for describing community diversity and community composition across large regional and temporal scales [48,49,50,51,52]. Analysis of functional and taxonomic richness for abundance data using accumulation curves similar to methods used for species–area curves has been proposed as an effective method for describing gamma diversity among regions, particularly when there exists variance in sampling effort [53]. In the context of diversity, it is worth mentioning Rapoport’s rule, which suggests that a latitudinal gradient of terrestrial diversity exists that is driven by species dispersal and range, with higher diversity typical in lower, tropical latitudes, but this is not necessarily true for marine biological systems due to variations in nutrient delivery from oceanographic processes [19,54,55]. Accumulation curves for marine systems have revealed that levels of diversity are not necessarily monotonic with latitude and in fact can be variable across large regional scales because dispersal and range are driven by ocean processes and nutrient availability [3,11,14,56].
The Intermediate Disturbance Hypothesis (IDH) introduced in the early 1970s has been a subject of broad debate among marine ecosystem researchers because of the inherent extremes in environmental variability [9]. Nevertheless, it suggests that the scale of diversity in an ecosystem is a function of the magnitude of disturbance, with competitive exclusion, rapid colonization, and local extinctions being among the mechanisms that drive changes in diversity [57,58]. Among the objectives for measuring species diversity in community ecology are classifying systems on bioregional scales, determining changes to the community over time, drawing comparisons among communities, and measuring the response of a community to disturbance, which is defined as a variation or disruption in the ecosystem that results in negative or positive effects on species diversity (some examples of disturbance include species invasions, predation, pollution, human-induced conversion of the natural landscape, and sudden changes in environmental variables). The most commonly used measures of diversity are the Shannon–Wiener and the Simpson indices (16, 17, 53). Both are compound indices integrating both richness and evenness, while the Simpson index also measures the probability that two species randomly selected from a sample will differ [59,60]. Fisher’s alpha is a diversity index useful for incompletely sampled communities; it is more sensitive to rare species than are the Shannon and Simpson indices, with a formula designed to predict abundances to the ith species and to rank those species on a logarithmic scale [61]. The drawback to using the Shannon–Wiener and Simpson measures is based in their formulae as dominance indicators; in other words, changes in diversity indices are typically driven by changes in abundance of the dominant species measured, while the index values calculated from Fisher’s alpha measures may be zero, or difficult to compare among communities if sample sizes vary. A means to classify three of the above-mentioned commonly used diversity measures in terms of sensitivity to rare species has been proposed by Hill (1973) [62]. Hill numbers are a continuum of diversity indices that include the following indices, which are ranked from greatest to least sensitivity to rare species: species richness, exponential Shannon diversity, and Simpson’s inverse. The resulting values, when plotted to a graph, show a steeper curve as an indicator of higher variation in abundance between measures. In a similar fashion, rarefaction curves are used to draw comparisons among species richness levels from sampled communities [63].
Intermediate to high levels of disturbance, particularly from human-caused disruptions, can cause changes to the abundance and structure of functional groups [49,50,64]. Fortunately, in the mid-20th century the marine ecologist E.Y. Dawson recognized the importance of conducting a census of intertidal algal communities under threat of catastrophic disturbance, which influenced the “before-and-after-control” approach to research design on impact assessment [65]. It was Dawson’s pre-spill surveys following the catastrophic 1969 Santa Barbara oil spill in California that provided a reference standard in the days immediately following the spill and were instrumental in concluding that widespread destruction of marine biota and disappearance of kelp beds was underway [66]. Variations in seaweed abundance with localized declines of the brown alga Fucus gardneri (a foundation species) have been revealed in the decades following the Exxon Valdez oil spill; these two events underscore the significance of baseline studies as reference standards necessary for predicting temporal responses to disturbance [36,43]. Algal cover provided by the leathery brown macrophyte Fucus distichus has been identified as a primary substrate for North Pacific herring roe (Clupea pallasii), a forage fish with a seasonal spawning cycle [67] that is the focus of a large-scale commercial roe fishing industry in British Columbia and Alaska that has been linked to the collapse of herring populations throughout much of the Pacific [68]. Disturbance from overfishing has been found to cause shifts in the functional hierarchy of algal groups from higher to lower complexity [40,50], which poses significant implications for populations of fish that spawn close to shore.
As mentioned previously, the MARINe working group began as a citizen science response to catastrophic oil spills, but only recently has a census of intertidal communities commenced in Southeast Alaska, which is at the forefront of marine heatwaves originating in the Gulf of Alaska; nevertheless, their census of sea stars was instrumental in assessing damage following the sea star wasting event that commenced during the marine heatwave in the summer of 2014 when a decline in abundances of a multitude of asteroid species, including keystone asteroids, occurred [34,69]. This resulted in a trophic cascade and an increase in the abundance of primary consumers and benthic grazers and a significant decrease in kelp forests throughout Southeast Alaska. Threats from disturbance to the intertidal zone in Peru include the unregulated harvesting of invertebrates and seaweeds for the restaurant industry and phycocolloid (agar and carrageenans) extraction, respectively, most notably the gastropod Concholepus concholepus and the sea star Heliaster helianthus. Disturbance caused by the removal of a species has the potential to result in a cascade of trophic effects and varies depending upon the source of disturbance and accompanying environmental and biological patterns [70,71]. Another significant threat includes the shore-based transfer of fossil fuels, with five major ports with fuel loading docks in close proximity to rocky intertidal systems. The most recent spill occurred following a seismic event that resulted in the hull rupture of an Italian tanker, the Mare Doricum, while in port in Callao in January 2022. Similar to the Santa Barbara and Exxon Valdez spills, the spread of the Mare Doricum slick was driven by ocean currents and covered nearly two million square meters of intertidal habitat, although the true area is still unknown. The spill affected islets and coastlines within the Reserva Nacional Sistema des Islas Islotas y Puntas Guaneras (RNSIIPG), a UNESCO Heritage site created in 2009 consisting of 33 sites along the coast of Peru. Cleanup efforts for the Mare Doricum spill mirrored efforts for the Exxon Valdez spill, with catastrophic management of ecosystems and biota but no real assessment of disturbance to intertidal algal standing stock [72].
2.2. The Human Relationship with the Intertidal Zone
The cultural and historical importance of the intertidal zone as a source of food for indigenous cultures is often under-represented in scientific literature, and although a thorough discussion of current research in the archaeological connection to the intertidal zone is beyond the scope of this review, we believe the subject is deserving of attention in our synthesis because of the health implications that this system poses in coastal cultures today, as well as to acknowledge our relationship with the past. Coastal-dwelling civilizations of the Pacific have long had a strong relationship with the intertidal zone and managed it for sustenance, commerce (e.g., the shells of invertebrates were a form of currency), and as a spawning resource for commercially valuable fish and invertebrates. The Macah of northwestern Washington, the Bella Coola of British Columbia, and the Chumash of present-day Los Angeles and surrounding areas in southern California are examples of indigenous societies that engaged in the exchange in commerce and culture. The Tlingit people of Southeast Alaska and northern British Columbia have a distinct language with roots in the Copper River Delta of Southcentral Alaska as well as a distinct form of art that depicts marine life as the identity of the Tlingit clan houses throughout Southeast Alaska and British Columbia [73]. Their relationship with the intertidal zone is embodied in the saying of the Gaawt’ak.aan Clan of Glacier Bay: “When the tide is out, the table is set”. Today, Tlingit cultural immersion learning in after-school programs re-introduce children to traditional and cultural harvesting activities and original Tlingit names for intertidal animals that are an important and historical source of food such as Nées (sea urchin), Yein (sea cucumber, Stichopus californicus), Shaaw (gumboot chiton, Katharina tunicata), Yaak (mussels), and Gáal (clams) [74]. Stories passed down from generation to generation (the “word-of-mouth” cultural tradition) tell of Tlingit paddlers embarking in their canoes on trans-equatorial explorations in the South Pacific to engage in commerce with other indigenous nations. Traditional methods of harvesting herring roe from intertidal seaweeds, kelps, and seagrasses are passed down from generation to generation and are still in practice today.
Intertidal organisms have been linked to Peruvian culture since ancient times (13,000 BCE) for subsistence (small clams and mussels—e.g., Donax spp., Perumytilus purpuratus), and later for rituals (Spondylus calcifer) [75,76]. Recently unearthed artifacts have revealed the Mochica, an ancient coastal society with a complex social structure and a lifestyle that included subsistence harvesting from the ocean. Elaborate pottery detailed with fine line drawings depict beach seining for fish and portray traditional coming-of-age rituals between young Mochican warriors and juvenile South American sea lions; shell middens and the remnants of hand-crafted fishing rafts reveal a rich and integral relationship with their coastal environment that paralleled the Tlingits’ relationship with the sea [77]. Reportedly, intertidal biota might have provided subsistence for coastal populations even through challenging climate conditions from El Niño Southern Oscillation (ENSO) [78]. Currently, intertidal communities are affected not only by ENSO cycles but also by pollution and overfishing for bait and human consumption [79,80]. Assessing the state of intertidal communities is challenged by the lack of long-term monitoring and the lack of knowledge of non-commercial species [10,81].
2.3. Research Conducted on Intertidal Ecosystems in the Pacific
The early 20th-century marine ecologist Ed Ricketts pioneered the concept of ecology in marine research as he sought to describe the continuum of trophic-level relationships among various intertidal organisms from Baja California to Southeast Alaska during his first surveys of intertidal ecosystems in 1932, and these relationships were first addressed in an academic context in his book Between Pacific Tides, published in 1939, and Sea of Cortez, published in 1941 [82]. His exposure to the subsistence traditions of the coastal Haida and Tlingit tribes of Southeast Alaska and the significance of the intertidal zone as a resource for hunter-gatherer cultures, as well as a productive system in coastal marine food webs, resulted in his writings for audiences from an industrialized western cultural perspective [82]. Today, much of the intertidal research in the eastern and western Pacific has occurred along semi-continuous coastlines or a patchwork of latitudes with a focus on characterizing local-scale species diversity and abundance, spatial turnover, hierarchical structure, range shifts, and the relationship between biological and oceanographic processes [1,8,11,13,19,83,84]. In the western Pacific, Okuda et al. [13] have discerned regional-scale gradients in richness and beta (turnover) diversity, and more recently, small-scale accumulative carryover effects have been identified among functional and trophic groups during and after chronic marine heatwaves in Hokkaido [85]. In the central north Pacific, Konar et al. [86] have identified depth-stratified differences in taxonomic hierarchies, invertebrate abundance and macro-algal biomass in intertidal and subtidal communities in Kodiak, Kachemak Bay, and Prince William Sound. Blanchette et al. [30] have discovered patterns of diversity on a broad regional scale from southeast Alaska to southern California, with seven distinct biogeographical groups defined based on patterns in similarity. In the Southeast Pacific, Ibanez et al. [11] and Valqui et al. [10] have accomplished similar work by identifying localized patterns, shifts in biological compositions, and a shifting zone of transition following disturbance from El Niño in Peru between 4° S and 14° S. Each work concludes with a statement of need to carry forward census efforts on large- and small-region scales in unstudied or poorly studied areas in order to collect the necessary biogeographic data on contiguous and temporal scales [8,10,11,26,85].
Mussels (family Mytilidae) are a foundation species found throughout the intertidal zone and have increasingly become the subject of research due to their abundance world-wide, which positions them as a sentinel species for measuring the physiological responses and rates of mortality from environmental stress and human disturbance [1,87,88,89,90,91]. Mussels are also recognized as a bioengineer species with the ability to mitigate ambient temperatures and desiccation within the assemblage and within certain thresholds, moderate the density of their own matrices, form dense assemblages of mixed age- and size-class matrices, enhance diversity in the associated communities, and provide increased areas of substrate as habitats for larval fish and for the settlement of algae and invertebrates that are challenged by inter-species competition for space [89,92,93,94,95,96] as well as a food resource for seabirds and megafauna. The Mytilidae account for at least 9000 known species on nearly every continent [30] and were important in indigenous culture as food and trade items since before recorded history. Today, mussels have economic value as a product of commercial-scale aquaculture [97]. The term “mussel matrix” is a term used to describe the layers and depth of mussel assemblages, and the magnitude of layers in a mussel matrix has been correlated with mussel spat recruitment and shell size with varying wave factors as well as their function as islands of habitat for intertidal species [93,98,99]. Matrix depth, sedimentation, and algal canopy are factors that play a significant role in species richness. In the eastern Pacific, positive correlations have been identified between multi-layer mussel matrices and biodiversity, while an inverse relationship exists between multi-layer matrices and species evenness [92,99]. Furthermore, the relationship between mussel matrices and algae is a commensal one; the matrices provide space for the settlement of algal sporelings, and the algal sporophytes form canopies that retain moisture and thereby minimize desiccation, thus providing a benefit to intertidal biota living near the threshold of their thermal tolerance limits [92,94,100].
The mussel assemblages in Southeast Alaska are comprised primarily of the native blue mussel Mytilus trossulus and a suggested hybrid of M. trossulus and the non-native species M. edulis and M. galloprovincialis [101,102,103,104], although patterns of hybrid species versus stands of pure M. trossulus have not been explored. In Peru, the most common species north of 5°S are in the genus Brachidontes, while south of 5°S the dominant species are Perumytilus purpuratus and Semimytilus algosus. The role of temperature in the structuring of communities associated with intertidal mussel assemblages in the eastern Pacific and the physiological response to heat stress has been researched exclusively on small regional scales. The use of in situ temperature loggers to collect thermal data has been used in specific locations in the northeastern Pacific; however, a broader regional effort is unlikely due to the high cost of the study design. Mussel assemblages exhibit mosaics of heat stress and are vulnerable to localized extinction events based on a host of environmental variables following extreme heat events [89,105]. Mussel research in western and south-central Alaska has focused on commercial aquaculture, paralytic shellfish poisoning, the introduction of non-native mussel species and the assignment of mussels as a Mytilus complex following regional-scale hybridization of non-native with native species and commercial aquaculture [106]. A lack of research focus on the biological and structural complexity of wild mussel assemblages has contributed to a lack of understanding on the diversity of invertebrate and algal communities associated with mussels as well as the response of these communities to environmental stress and disturbance, and the role they play in temperate nearshore food webs has been largely unexplored. The effect of environmental factors (nutrient inputs, sediment loading, area of the assemblage, environmental heterogeneity, emersion times, etc., on the structural complexity and species richness in mussel assemblages have all been examined on the coasts of Japan, Washington, Chile, and central Peru with variable effect size based on the explanatory factors used in the analysis [90,92,96,98,99,100,101,107,108]. Only recently have efforts been made on the outer coast of Southeast Alaska and the northern subtropical and warm temperate coasts of Peru to study the biological and environmental variables that drive mussel assemblage complexity, with the discovery of a physical biomarker as a predictor of diversity in algal and invertebrate communities living within mussel assemblages [99].
3. The Focal Regions
3.1. The Need for Intertidal Census
Local-scale patterns of diversity, abundance, and turnover of taxa are typically driven by local-scale environmental processes [11,109]. The coastal areas of Southeast Alaska and Peru are proximal to the processes (downwelling and upwelling) that provide for highly productive marine ecosystems and are considered ground zero in terms of these respective processes. Larger-scale processes include the North Pacific Decadal Oscillation (NPO), a fluctuating atmospheric pressure regime that directly influences sea surface temperatures in the Gulf of Alaska, and the El Niño Southern Oscillation (ENSO) that also directly influences sea surface temperatures in the equatorial South Pacific. The cascade effects from NPO and ENSO on the hierarchical turnover of biologically important resources in the northeast and southeast Pacific [110,111,112,113] have been recognized, and while the effect of ENSO on higher-trophic-level organisms such as seabirds and marine mammals has been studied, along with more limited-in-scope catch-per-unit abundance studies on commercially important bivalves and other benthic invertebrates, there has been little effort that measures effects on the health of the intertidal ecosystem [114]. The effects of ENSO and marine heatwaves on intertidal kelps have been determined to be detrimental in terms of abundance and cover [115]; however, the response of intertidal communities to NPO has largely been ignored.
Marine heatwaves are independent of the processes that drive NPOs and ENSOs. NPOs are Kelvin waves that deliver abnormally high sea-surface temperatures to coastal areas, while the driver of marine heatwaves has only recently been suggested [116]. These events are responsible for acute, local-scale cascading events in marine ecosystems; the abundances of intertidal mussels are influenced by environmental extremes with varying effects that impact the entire ecosystem [117]. The marine heatwave in the spring of 2014 served as a bellwether in the eastern North Pacific and persisted throughout much of the boreal summer [118], with impacts on biological systems including reductions in phytoplankton biomass, seabird die-offs, commercial fish declines, and temporal range expansion of subtropical pelagic species to the Gulf of Alaska [110,119]. In the South Pacific, the “Coastal El Niño” marine heatwave manifested in the austral summer of 2017, resulting in range shifts and changes in abundance of intertidal taxa on local scales [10] as well as shifts in the functional group structure and species composition of intertidal mussel communities along the coast of Peru [120]. As marine heatwaves become more frequent, the question becomes one of predicting cumulative carryover effects identified by Ishida et al. [85] and changes in algal biomass and patterns of growth, particularly in the intertidal communities of Southeast Alaska as the climate continues to warm [121] (Figure 2).
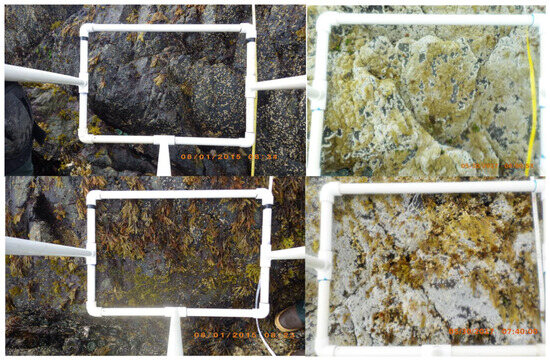
Figure 2.
Two intertidal strata photographed in Southeast Alaska following the marine heatwave of 2014–2016 demonstrate significant changes to the abundance and hierarchy of intertidal assemblages. The photos on the left were taken in 2015 and show assemblages of the brown seaweed Fucus gardneri, the red seaweed Mastocarpus jardinii and the acorn barnacle Balanus glandula. The photos on the right were taken at the exact same locations in 2017; note that F. gardneri cover is reduced and the substrate is dominated by the non-native barnacle Amphibalanus sp.
The relevancy of predicting carryover effects in intertidal systems in poorly studied or unstudied regions is attributed to the potential for these systems to become significantly altered by localized and regional shifts in trophic and functional groups, as well as alterations in the services provided by decreased algal biomass. For example, marine algae perform an important trophic-level role by contributing oxygen through photosynthesis, providing substrates for recruitment and shelter for marine invertebrates and larval fish [25,83]; thus, a decrease or vertical shift in the algal biomass as a result of climate change could lead to a significant negative impact on food webs and food resources. The regional-scale gaps that exist in intertidal research contribute to contemporaneous gaps in information on the biogeographic structure of rocky intertidal communities in poorly studied and unstudied regions. Until these gaps in knowledge are filled, voids will continue to exist in our broad regional understanding of how communities will adapt and re-structure following extreme disturbance events.
3.2. Bioregional Classifications
Southeast Alaska and Peru are part of a geographically contiguous coastline on the eastern Pacific separated by tens of degrees of latitude and thousands of kilometers. The terrestrial climate of Southeast Alaska is defined as a temperate boreal rainforest, while the climate of coastal Peru is defined as an arid to semi-arid coastal desert; nevertheless, similarities exist in the marine realm, namely the taxa and functional group structure of rocky intertidal communities that were important food items in indigenous cultures. For example, the upper rocky intertidal zones of both regions consist of stratified bio-bands of barnacle, mussel, and gastropod (Littorinidae) communities with transitional overlap occurring between at least two different species from the same genus. In both regions, species of the green algal (Ulva spp.) make up a considerable portion of the bands in the upper to mid zones, and the red alga (Pyropia lanceolata, Setchell and Hus 1900) occurs in stands associated with foliose green algae (Ulva linza, Linnaeus 1753). The composition of algal bio-bands in the intertidal zone in Southeast Alaska is tightly linked with a seasonal spawning event involving Pacific herring (Clupea pallasii, Valenciennes 1847); the herring roe on kelp is harvested for human consumption [67], and the harvesting of intertidal kelps, bivalves, univalves, echinoderms and crustaceans is still in practice in coastal towns in Peru.
A framework of bioregional classifications is helpful for making coherent comparison of communities across broad regional scales such as we propose, and several biogeographical provinces have been proposed for the eastern North Pacific with seven delineations spanning from 25° N to 60° N based on similarities and patterns of abundance and distribution of intertidal species [30]. The Census of Marine Life Mapping and Visualization program (also known as Marine Ecoregions of the World, or MEOW) is a global database that organizes all the world’s marine habitats and species into broad-scale (Realms), medium-scale (Provinces), and local-scale (Ecoregions) classifications (Table 1). Ecoregions are defined by a relative and distinct homogeneity of species composition that varies from one region to the next, and the geographic drivers of ecoregional species composition may be diverse from among locations within each ecoregion and include upwelling, freshwater influences, ice, currents, and nutrient inputs [20]. The Gulf of Alaska is a cold temperate realm in the North Pacific Ocean with a productive coastal downwelling system influenced by the Kuroshio and sub-Arctic Oyashio currents [122,123]. Southeast Alaska harbors rich yet poorly studied intertidal communities and makes up much of the North American Pacific Fjordland (NAPF) ecoregion stretching 480 km from 54° N to 59° N. The fjords and islands that make up the archipelago were carved by the Cordilleran ice sheet during the Late Wisconsin glaciation [124], with a coastline that is still undergoing isostatic rebound on a scale of centimeters per year since the end of the Little Ice Age approximately 200 years ago [125,126]. Shoreline geographies within the archipelago include tidewater glaciers, elongate bays, mud flats, and shorelines with rock vertices, benches, and terraces (Figure 3). Tides are mixed diurnal, with periods of extreme low tides occurring during daylight hours in the summer. Outer coastal areas of the archipelago are exposed to strong wave action and swell from the Gulf of Alaska, while the sounds, bays, and inside passages are relatively protected from waves.

Table 1.
Definitions of the three spatial marine categories for the ecoregions from Spalding et al. [20] that are the focus of this study starting with the largest regional scale (left column) to the smallest regional scale (second from left column). Decimal coordinates are in shown in third column from left.

Figure 3.
Examples of some of the shoreline geographic features of the intertidal zone of Southeast Alaska in the North American Pacific Fjordland ecoregion. Clockwise from top left: A glacially carved fjord leading to a tidewater glacier in Glacier Bay National Park; a view from the mouth of Dundas Bay, an elongate bay approximately 22 km in length; a typical shallow tidal mudflat in Dundas Bay; vertical rocky intertidal habitat in Endicott Arm; an intertidal terrace in the Kashevarof Islands showing “biobands”, the stratification of algae prominent for each zone; a rocky intertidal bench with a haul-out of Steller Sea Lions in Glacier Bay National Park.
Located approximately 10,000–13,000 km from Southeast Alaska are the Guayaquil (GUAY) and Humboldtian ecoregions of the eastern South Pacific, which include the coasts of Peru and Chile. The Peruvian coastline stretches over 5000 km from 3° S to 18° S with a coastal geography ranging from desert and relatively small sand dunes in the subtropical GUAY ecoregion to a combination of desert and foothills in the central region, with larger foothills, plains, wetlands and river valleys in the temperate Humboldtian ecoregion [127,128]. The intertidal zone is characterized by sandstone benches in the GUAY ecoregion, while farther south of 6° the coastline is primarily formed by uplift from tectonic activity, and the intertidal platforms are primarily composed of granite benches, terraces, vertices, and boulders in the Humboldtian ecoregion (Figure 4). Areas of near-shore upwelling along the southeastern boundary of the South Pacific are a result of the Humboldt Current System, while offshore upwelling between 3° S and 6° S is influenced by the Humboldt Current, the Cromwell Current, the Peru Counter Current, and the Southern Trade Winds [129,130,131]. Upwelling is depressed during El Niño Southern Oscillation (ENSO) events every 3–7 years, with the entire coast from the GUAY ecoregion to the Humboldtian ecoregion exposed to sea surface temperature anomalies.

Figure 4.
Examples of shoreline geographic features in the ecoregions in Peru that are the focus of research in this review. Clockwise from top left: a sandstone bench in the GUAY ecoregion; a granite bench in the HWS ecoregion; emerging granite terraces near a recreational beach in the south of Lima City (HWS); vertical walls in the HWS ecoregion; granite boulders and slabs in the intertidal zone of the HNS ecoregion, likely the result of the toppling of adjacent cliffs during tectonic activity.
The NAPF, GUAY, CeP, and Humboldtian ecoregions are located along a contiguous coastline that shares productive commercial fishing and aquaculture industries, with a rich history of commerce and trade among the major port cities established within those regions. Disturbance in marine systems stems primarily from the drilling and transport of fossil fuels, large-scale commercial fishing, and changes in oceanographic patterns as a result of global warming. In Southeast Alaska, harvesting in the intertidal zone is closely regulated, while in Peru regulations are weak or non-existent with localized extirpation of top invertebrate predators. The rocky intertidal zones of all three ecoregions share many similar marine taxa that make up the biological compositions of the communities: for example, the brown algae Colpomenia and Macrocystis, the red algae Lithothamnion, Corallina, Pterosiphonia, and Porphyra, and several species of the genus Ulva constitute a considerable portion of the intertidal zones of all three ecoregions [79]. Marine gastropods (Tegula, Lesson 1833 and Fissurella Bruguiere 1789, Muricidae 1815) occupy different niche habitats, while periwinkles (Littorinidae) occupy similar niche habitats in all three ecoregions (Figure 5). A coordinated effort to analyze the community compositions in these ecoregions could provide a roadmap for predicting effects on the biodiversity in the ecosystem following extreme heatwave events [116].

Figure 5.
Marine intertidal taxa representative of high intertidal communities found in the northeastern and southeastern Pacific ecoregions. Each taxa represents a functional group similarity among ecoregions. Clockwise from top left: the brown alga Colpomenia sinuosa in the northern Humboldtian ecoregion near Lima, Peru; Colpomenia peregrina in the NAPF ecoregion in Sitka Sound, Alaska; the periwinkle Echinolittorina peruviana in a small tidepool in the Humboldtian ecoregion; Littorina sitkana in a tidepool in Sitka Sound; the green alga Ulva among red algae (center right of photo); canopy-forming Ulva rigida on mussel assemblages in the Humboldtian ecoregion.
4. Discussion and Future Directions
The intertidal zone is unlike any other ecosystem on Earth in terms of the plants, animals, and algae that have adapted to living in the harshest of conditions that this biome has to offer. Measures of diversity and analysis of the physiological adaptations that regulate tolerances and thresholds to this harsh environment, i.e., nutrient inputs, temperature, desiccation, pollution, etc., have been the impetus for research in many regions of the Pacific. First and foremost, a census of marine life in poorly studied or unstudied ecosystems is vital to precisely describe the location, abundance, and range for any given species and is fundamental for the very comprehensive analysis of the physiological and environmental adaptations that accompany disturbance. There are many examples in the published literature that describe the ranges of rocky intertidal species along the Chilean coast, which are often accompanied by the assumption that conspecifics co-occur in unexplored areas of the Peruvian coast. An abundance of reference guides, identification books and dichotomous keys have been published on northeastern Pacific intertidal flora and fauna; however, the majority of real research on the systems that support these communities has only been carried out in the central Pacific Northwest (California, Oregon and Washington). Although more attention has recently been drawn to these understudied rocky intertidal areas in the eastern Pacific, more work must be carried out to accomplish a full understanding of the various hierarchical structures and the processes that drive richness and abundance along the coastline.
The inherent features and rugged accessibility of the intertidal zone have formed an historical and present-day significance in the culture of coastal-dwelling humans that cannot be understated. Intertidal communities continue to provide food, habitat, and sanctuary for a large diversity of living organisms on a global scale. The trans-regional connection among marine intertidal communities is not a new concept in marine community biology and has been previously explored for the pre-glacial distributions of molluscs and leathery kelp species in Europe and South Africa [132]. As illustrated throughout this review paper, Southeast Alaskan and Peruvian intertidal ecosystems share similar taxa, functional group diversity and community structure; therefore, what we have learned from the dynamics of intertidal hierarchies and the resilience of Peruvian intertidal communities following acute sea surface anomalies from El Niño will serve to lend understanding on the effect of chronic and acute marine heatwaves on boreal rocky intertidal communities in the near future [25,115]. As has been demonstrated in the scientific literature covered here, several decades of research orchestrated by researchers and groups such as MARINe provide proof that continuous census work is essential for maintaining the integrity of marine protected areas in the northeast Pacific. Census work from these thoroughly investigated regions of the Pacific coast serves as a benchmark for intertidal census in poorly studied or unstudied regions, and in fact, an increased interest in intertidal ecology is underway in Peru and Southeast Alaska at the time of writing this.
There exists a strong history of intertidal research in many areas of the eastern Pacific, and clearly, more can be learned from studying the effects of sea surface temperature, upwelling, and nutrient availability on the functional group and trophic structure of biota associated with mussels. One of the major questions is, how will the diversity and ecological functioning of intertidal communities change in the context of climate change but also other key stressors including the arrival of alien species, sea level rise and pollution [133]? Patterns of zonation may change following El Niño or other climatic disturbances; therefore, Engle [39] recommends a re-survey of transects at a minimum of every five years in order to provide a continuum of census data in the intertidal communities under study. Significant results in terms of analyzing patterns of zonation can only be increased with the collection of robust data, and by increasing the frequency of surveys on larger temporal scales more information will be available for determining how intertidal communities change over time. Increasing the frequency of monitoring of commercially exploited invertebrates, algae, and juvenile fish as well as expanding monitoring protocols to include shallow subtidal habitats will also provide a metric on the efficacy of marine protected areas, or at least provide support for establishing new ones. Several measures in beta diversity for analyzing sample data to provide a coherent picture of species turnover should be considered. In their analysis of multi-scale turnover diversity of intertidal systems in Japan, Okuda et al. [13] defined diversity as the probability of encountering the same species among samples using Simpson’s Diversity Index; however, their method provided many small-scale assessments of beta diversity versus an assessment of transitional species turnover throughout a bioregional continuum. Broitman et al. [31] have recognized variations in patterns of functional groups associated with trophic-level abundances and strength of upwelling in rocky intertidal assemblages, and this could be assessed on a smaller scale using temporal-scale intertidal seawater temperature measurements coinciding with intertidal community surveys. Such a treatment design may shed light on the causes of observed changes in intertidal community trophic structure, particularly following El Niño or marine heatwave events. Furthermore, it is important to remember that a temporal survey design may reveal new or previously unreported species, such as the polychaete (cf.) Boccardia wellingtonensis collected by Wilbur et al. [99] in varying abundance over the course of a three-year survey at Reserva Punta San Juan (PSJ) in the RNSIIPG.
We propose a broadened scope of census that encompasses species turnover and diversity among the NAPF, GUAY, and Humboldtian ecoregions at a minimum of fifteen marine intertidal sites that are unique in terms of research effort. In particular, we parse the Humboldtian ecoregion into two sub-ecoregions according to the proximity of the shoreline to upwelling; for example, the “Humboldtian Wide Shelf ecoregion (HWS)” is between 12° S and 13° S and the “Humboldtian Narrow Shelf ecoregion (HNS)” is south of 13° S [130,134]. The sites under consideration are Pirate’s Cove, Kayak Island, Whale Park, and Kresta Point in the NAPF ecoregion, Playa Acapulco, Los Organos, El Nuro, and Cabo Blanco in the GUAY ecoregion, Playa Ensenada, Playa Farallones, and Playa Palmeras in the HWS ecoregion, and Reserva Punta San Juan, Playa Siete Huecos, and Antofagasta in the HNS ecoregion (Table 2). These sites are appropriate for carrying out a census of invertebrate and algal communities using sample-based biodiversity surveys and measures of similarity that provide coherent patterns of zonation on small, medium, and large spatial scales. The steps following this census and diversity analysis should include smaller-scale measurements of the characteristics of mussel matrix complexity (i.e., mussel biomass, layering and bed depth, age class) in an effort to understand the underlying factors of diversity in the algal and invertebrate communities associated with mussel assemblages in these intertidal systems [94,99]. Future directions in research should integrate DNA analysis, nutrient inputs, chlorophyll abundance, and the physical attributes of the local water bodies (nutrients, temperature and salinity) to explore the relationships between wave exposure, upwelling, plankton availability and the growth and complexity of mussel assemblages. Additionally, assessments of localized upwelling events can be explored using nearshore and offshore sea surface temperatures from in situ and satellite imagery as a means to examine environmental effects on biological composition of the communities [11,31]. Given that the intertidal zone has played such an important role in human history that continues to this day, filling these gaps in our knowledge will certainly provide the necessary tools for predicting the magnitude of impacts from marine heatwaves and oils spills, and for broad-scale protection of this valuable ecosystems from pollution and climate change.

Table 2.
The ecoregions (left column), site names with abbreviations (middle column), and decimal coordinates (right column) proposed for a trans-regional census and comparative analysis of functional structure in intertidal ecosystems in the eastern Pacific.
5. Conclusions
Marine intertidal ecosystems have increasingly received research attention throughout much of the Pacific; however, many bioregional gaps in poorly studied or unstudied areas in the northern and southern hemispheres remain. A proposal to use census regions that have been poorly studied or unstudied should not be controversial, considering that the methods used previously have enriched our knowledge and understanding of the biological compositions and physiological adaptations of species in well-researched intertidal communities. By taking census of the intertidal biological communities within these regions, we are equipped to ground-truth the range and abundance of any given species, to correct any reporting bias that may exist, and to provide a factual basis for our assessments of spatial distributions and patterns of diversity and richness on broad regional scales.
Author Contributions
Conceptualization, L.W.; methodology, L.W.; validation, L.W.; resources, L.W.; data curation, L.W.; writing—original draft preparation, L.W.; writing—review and editing, L.W., V.L., B.I.-E. and F.C.K.; visualization, L.W.; supervision, F.C.K.; project administration, L.W.; funding acquisition, L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially supported by the University of Aberdeen, School of Biological Sciences. We would also like to thank the MASTS pooling initiative (The Marine Alliance for Science and Technology for Scotland). MASTS is funded by the Scottish Funding Council (grant reference HR09011) and contributing institutions.
Acknowledgments
We would like to express our sincerest thanks to Henry Larsen (Tlingit and Haida) for his knowledge and contributions regarding the importance of the intertidal zone to indigenous coastal people of Southeast Alaska. We are grateful to Susana Cárdenas Alayza from the Center for Environmental Sustainability of the Universidad de Cayetano Heredia in Lima Peru for her generous assistance with navigating research logistics at Reserva Punta San Juan, and to Shaleyla Kalez of EcoOceanica for her contribution in the northern subtropical region of Peru.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Helmuth, B.; Miezkowska, N.; Moore, P.; Hawkins, S.J. Living on the Edge of Two Changing Worlds: Forecasting the Responses of Rocky Intertidal Ecosystems to Climate Change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 373–404. [Google Scholar] [CrossRef]
- Pandori, L.L.M.; Sorte, C.J.B. Spatial and Temporal Scales of Exposure and Sensitivity Drive Mortality Risk Patterns across Life Stages. Ecosphere 2021, 12, e03552. [Google Scholar] [CrossRef]
- Fenberg, P.B.; Menge, B.A. Interactions in the Marine Benthos; Cambridge University Press: Cambridge, UK, 2019; ISBN 978-1-108-23579-2. [Google Scholar]
- Szathmary, L.P.; Helmuth, B.; Wethey, D.S. Climate Change in the Rocky Intertidal Zone: Predicting and Measuring the Body Temperature of a Keystone Predator. Mar. Ecol. Prog. Ser. 2009, 374, 46–56. [Google Scholar] [CrossRef]
- Coletti, H.A.; Bodkin, J.L.; Monson, D.H.; Ballachey, B.E.; Dean, T.A. Detecting and Inferring Cause of Change in an Alaska Nearshore Marine Ecosystem. Ecosphere 2016, 7, e01489. [Google Scholar] [CrossRef]
- Menge, B.A.; Close, S.L.; Hacker, S.D.; Nielsen, K.J.; Chan, F. Biogeography of Macrophyte Productivity: Effects of Oceanic and Climatic Regimes across Spatiotemporal Scales. Limnol. Oceanogr. 2021, 66, 711–726. [Google Scholar] [CrossRef]
- Tarazona, J.; Espinoza, R.; Solís, M.; Arntz, W. Crecimiento y Producción Somática de La Concha de Abanico (Argopecten purpuratus) En Bahía Independencia, Pisco (Perú) Comparados Entre Eventos El Niño y La Niña. Rev. Biol. Mar. Oceanogr. 2007, 42, 275–285. [Google Scholar] [CrossRef]
- Konar, B.; Iken, K.; Cruz-Motta, J.J.; Benedetti-Cecchi, L.; Knowlton, A.; Pohle, G.; Miloslavich, P.; Edwards, M.; Kimani, E.; Riosmena-Rodriguez, R.; et al. Current Patterns of Macroalgal Diversity and Biomass in Northern Hemisphere Rocky Shores. PLoS ONE 2010, 5, e13195. [Google Scholar] [CrossRef] [PubMed]
- Moi, D.A.; García-Ríos, R.; Hong, Z.; Daquila, B.V.; Mormul, R.P. Intermediate Disturbance Hypothesis in Ecology: A Literature Review. Ann. Zool. Fenn. 2020, 57, 67–78. [Google Scholar] [CrossRef]
- Valqui, J.; Ibañez-Erquiaga, B.; Pacheco, A.S.; Wilbur, L.; Ochoa, D.; Cardich, J.; Pérez-Huaranga, M.; Salas-Gismondi, R.; Pérez, A.; Indacochea, A.; et al. Changes in Rocky Intertidal Communities after the 2015 and 2017 El Niño Events along the Peruvian Coast. Estuar. Coast. Shelf Sci. 2021, 250, 107142. [Google Scholar] [CrossRef]
- Ibanez-Erquiaga, B.; Pacheco, A.S.; Rivadeneira, M.M.; Tejada, C.L. Biogeographical Zonation of Rocky Intertidal Communities along the Coast of Peru (3.5-13.5 S Southeast Pacific). PLoS ONE 2018, 13, e0208244. [Google Scholar] [CrossRef]
- Kraan, C.; Aarts, G.; Piersma, T.; Dormann, C.F. Temporal Variability of Ecological Niches: A Study on Intertidal Macrobenthic Fauna. Oikos 2013, 122, 754–760. [Google Scholar] [CrossRef]
- Okuda, T.; Noda, T.; Yamamoto, T.; Ito, N.; Nakaoka, M. Latitudinal Gradient of Species Diversity: Multi-Scale Variability in Rocky Intertidal Sessile Assemblages along the Northwestern Pacific Coast. Popul. Ecol. 2004, 46, 159–170. [Google Scholar] [CrossRef]
- Thyrring, J.; Peck, L.S. Global Gradients in Intertidal Species Richness and Functional Groups. eLife 2021, 10, e64541. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, E. Large-Scale Species-Richness Gradients in the Atlantic Ocean. Proc. Biol. Sci. 2002, 269, 1715–1720. [Google Scholar] [CrossRef]
- Hillebrand, H. On the Generality of the Latitudinal Diversity Gradient. Am. Nat. 2004, 163, 192–211. [Google Scholar] [CrossRef] [PubMed]
- Lambshead, P.J.; Tietjen, J.; Ferrero, T.; Jensen, P. Latitudinal Diversity Gradients in the Deep Sea with Special Reference to North Atlantic Nematodes. Mar. Ecol. Prog. Ser. 2000, 194, 159–167. [Google Scholar] [CrossRef]
- Bridges, A.E.H.; Barnes, D.K.A.; Bell, J.B.; Ross, R.E.; Howell, K.L. Depth and Latitudinal Gradients of Diversity in Seamount Benthic Communities. J. Biogeogr. 2022, 49, 904–915. [Google Scholar] [CrossRef]
- Cruz-Motta, J.J.; Miloslavich, P.; Guerra-Castro, E.; Hernández-Agreda, A.; Herrera, C.; Barros, F.; Navarrete, S.A.; Sepúlveda, R.D.; Glasby, T.M.; Bigatti, G.; et al. Latitudinal Patterns of Species Diversity on South American Rocky Shores: Local Processes Lead to Contrasting Trends in Regional and Local Species Diversity. J. Biogeogr. 2020, 47, 1966–1979. [Google Scholar] [CrossRef]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Shaw, D.G.; Bader, H.R. Environmental Science in a Legal Context: The Exxon Valdez Experience. Ambio 1996, 25, 430–434. [Google Scholar]
- Ray, G.C.; Grassle, J.F. Marine Biological Diversity: A Scientific Program to Help Conserve Marine Biological Diversity Is Urgently Required. BioScience 1991, 41, 453–458. [Google Scholar] [CrossRef]
- Menegotto, A.; Rangel, T.F. Mapping Knowledge Gaps in Marine Diversity Reveals a Latitudinal Gradient of Missing Species Richness. Nat. Commun. 2018, 9, 4713. [Google Scholar] [CrossRef] [PubMed]
- Menge, B.A. Community Regulation: Under What Conditions Are Bottom-up Factors Important on Rocky Shores? Ecology 1992, 73, 755–765. [Google Scholar] [CrossRef]
- Menge, B.A.; Sutherland, J.P. Community Regulation: Variation in Disturbance, Competition, and Predation in Relation to Environmental Stress and Recruitment. Am. Nat. 1987, 130, 730–757. [Google Scholar] [CrossRef]
- Scrosati, R.; Heaven, C. Spatial Trends in Community Richness, Diversity, and Evenness across Rocky Intertidal Environmental Stress Gradients in Eastern Canada. Mar. Ecol. Prog. Ser. 2007, 342, 1–14. [Google Scholar] [CrossRef]
- Scrosati, R.; van Genne, B.; Heaven, C.S.; Watt, C.A. Species Richness and Diversity in Different Functional Groups across Environmental Stress Gradients: A Model for Marine Rocky Shores. Ecography 2011, 34, 151–161. [Google Scholar] [CrossRef]
- Miner, C.M.; Burnaford, J.L.; Ammann, K.; Becker, B.H.; Fradkin, S.C.; Ostermann-Kelm, S.; Smith, J.R.; Whitaker, S.G.; Raimondi, P.T. Latitudinal Variation in Long-Term Stability of North American Rocky Intertidal Communities. J. Anim. Ecol. 2021, 90, 2077–2093. [Google Scholar] [CrossRef] [PubMed]
- Claisse, J.T.; Blanchette, C.A.; Dugan, J.E.; Williams, J.P.; Freiwald, J.; Pondella II, D.J.; Schooler, N.K.; Hubbard, D.M.; Davis, K.; Zahn, L.A.; et al. Biogeographic Patterns of Communities across Diverse Marine Ecosystems in Southern California. Mar. Ecol. 2018, 39, e12453. [Google Scholar] [CrossRef]
- Blanchette, C.A.; Melissa Miner, C.; Raimondi, P.T.; Lohse, D.; Heady, K.E.K.; Broitman, B.R. Biogeographical Patterns of Rocky Intertidal Communities along the Pacific Coast of North America. J. Biogeogr. 2008, 35, 1593–1607. [Google Scholar] [CrossRef]
- Broitman, B.R.; Navarrete, S.A.; Smith, F.; Gaines, S.D. Geographic Variation of Southeastern Pacific Intertidal Communities. Mar. Ecol. Prog. Ser. 2001, 224, 21–34. [Google Scholar] [CrossRef]
- López-Rojas, V.I.; Flores-Garza, R.; García-Ibáñez, S.; Ruiz-Campos, G.; Flores-Rodríguez, P.; Violante-González, J.; Torreblanca-Ramírez, C. New Records of Bivalves in the Mexican Pacific Transitional Zone. Biodiversity 2020, 21, 150–164. [Google Scholar] [CrossRef]
- Ens, N.J.; Harvey, B.; Davies, M.M.; Thomson, H.M.; Meyers, K.J.; Yakimishyn, J.; Lee, L.C.; McCord, M.E.; Gerwing, T.G. The Green Wave: Reviewing the Environmental Impacts of the Invasive European Green Crab (Carcinus maenas) and Potential Management Approaches. Environ. Rev. 2022, 30, 306–322. [Google Scholar] [CrossRef]
- Hewson, I.; Button, J.B.; Gudenkauf, B.M.; Miner, B.; Newton, A.L.; Gaydos, J.K.; Wynne, J.; Groves, C.L.; Hendler, G.; Murray, M.; et al. Densovirus Associated with Sea-Star Wasting Disease and Mass Mortality. Proc. Natl. Acad. Sci. USA 2014, 111, 17278–17283. [Google Scholar] [CrossRef]
- Fukuyama, A.K.; Shigenaka, G.; Coats, D.A. Status of Intertidal Infaunal Communities Following the Exxon Valdez Oil Spill in Prince William Sound, Alaska. Mar. Pollut. Bull. 2014, 84, 56–69. [Google Scholar] [CrossRef]
- Driskell, W.; Fukyama, A.; Houghton, J.; Lees, D.; Shigenaka, G.; Mearns, A. Impacts on Intertidal Fauna: Exxon Valdez Oil Spill and Cleanup. Int. Oil Spill Conf. Proc. 1993, 1, 355–362. [Google Scholar] [CrossRef]
- Wolfe, D.; Krahn, M.; Casillas, E.; Scott, K., Jr.; Lunz, J.; Payne, J.; Thompson, T. Toxicity of Intertidal and Subtidal Sediments Contaminated by the Exxon Valdez Oil Spill. In American Fisheries Society Symposium; 1993; Available online: https://www.researchgate.net/publication/349537444_Toxicity_of_Intertidal_and_Subtidal_Sediments_Contaminated_by_the_Exxon_Valdez_Oil_Spill (accessed on 25 December 2023).
- Short, J.W.; Maselko, J.M.; Lindeberg, M.R.; Harris, P.M.; Rice, S.D. Vertical Distribution and Probability of Encountering Intertidal Exxon Valdez Oil on Shorelines of Three Embayments within Prince William Sound, Alaska. Environ. Sci. Technol. 2006, 40, 3723–3729. [Google Scholar] [CrossRef]
- Engle, J.M. Unified Monitoring Protocols for the Multi-Agency Rocky Intertidal Network; U.S. Department of the Interior Minerals Management Service Pacific OCS Region, Marine Science Institute, University of California: Santa Barbara, CA, USA, 2008; Volume 1. [Google Scholar]
- DeVogelaere, A.P.; Foster, M.S. Damage and Recovery in Intertidal Fucus Gardneri Assemblages Following the “Exxon Valdez” Oil Spill. Mar. Ecol. Prog. Ser. Oldendorf 1994, 106, 263–271. [Google Scholar] [CrossRef]
- Folke, C.; Carpenter, S.; Walker, B.; Scheffer, M.; Elmqvist, T.; Gunderson, L.; Holling, C.S. Regime Shifts, Resilience, and Biodiversity in Ecosystem Management. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 557–581. [Google Scholar] [CrossRef]
- Van Diggelen, A.D.; Worden, S.E.; Frimodig, A.J.; Wertz, S.P. California’s Lessons Learned and Recommendations for Effective Marine Protected Area Network Management. Mar. Policy 2022, 137, 104928. [Google Scholar] [CrossRef]
- Driskell, W.B.; Ruesink, J.L.; Lees, D.C.; Houghton, J.P.; Lindstrom, S.C. Long-Term Signal of Disturbance: Fucus gardneri after the Exxon Valdez Oil Spill. Ecol. Appl. 2001, 11, 815–827. [Google Scholar] [CrossRef]
- Colwell, R.K.; Chao, A.; Gotelli, N.J.; Lin, S.-Y.; Mao, C.X.; Chazdon, R.L.; Longino, J.T. Models and Estimators Linking Individual-Based and Sample-Based Rarefaction, Extrapolation and Comparison of Assemblages. J. Plant Ecol. 2012, 5, 3–21. [Google Scholar] [CrossRef]
- Chao, A.; Chazdon, R.L.; Colwell, R.K.; Shen, T.-J. A New Statistical Approach for Assessing Similarity of Species Composition with Incidence and Abundance Data: A New Statistical Approach for Assessing Similarity. Ecol. Lett. 2005, 8, 148–159. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and Extrapolation with Hill Numbers: A Framework for Sampling and Estimation in Species Diversity Studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Chao, A. Estimating the Population Size for Capture-Recapture Data with Unequal Catchability. Biometrics 1987, 43, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Steneck, R.S.; Watling, L. Feeding Capabilities and Limitation of Herbivorous molluscs: A Functional Group Approach. Mar. Biol. 1982, 68, 299–319. [Google Scholar] [CrossRef]
- Steneck, R.S.; Dethier, M.N. A Functional Group Approach to the Structure of Algal-Dominated Communities. Oikos 1994, 69, 476–498. [Google Scholar] [CrossRef]
- Steneck, R.S.; Vavrinec, J.; Leland, A.V. Accelerating Trophic-Level Dysfunction in Kelp Forest Ecosystems of the Western North Atlantic. Ecosystems 2004, 7, 323–332. [Google Scholar] [CrossRef]
- Bosman, A.L.; Hockey, P.A.R.; Siegfried, W.R. The Influence of Coastal Upwelling on the Functional Structure of Rocky Intertidal Communities. Oecologia 1987, 72, 226–232. [Google Scholar] [CrossRef]
- Castilla, J.C.; Paine, R.T. Predation and Community Organization on Eastern Pacific, Temperate Zone, Rocky Intertidal Shores. Rev. Chil. Hist. Nat. 1987, 60, 131–151. [Google Scholar]
- Ricotta, C.; Pavoine, S.; Bacaro, G.; Acosta, A.T.R. Functional Rarefaction for Species Abundance Data. Methods Ecol. Evol. 2012, 3, 519–525. [Google Scholar] [CrossRef]
- Saeedi, H.; Costello, M.J.; Warren, D.; Brandt, A. Latitudinal and Bathymetrical Species Richness Patterns in the NW Pacific and Adjacent Arctic Ocean. Sci. Rep. 2019, 9, 9303. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, C.; Saeedi, H.; Costello, M.J. Bimodality of Latitudinal Gradients in Marine Species Richness. Trends Ecol. Evol. 2016, 31, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Fenberg, P.; Menge, B.; Raimondi, P.; Rivadeneira, M. Biogeographic Structure of the Northeastern Pacific Rocky Intertidal: The Role of Upwelling and Dispersal to Drive Patterns. Ecography 2014, 38, 83–95. [Google Scholar] [CrossRef]
- Grace, J.B. The Factors Controlling Species Density in Herbaceous Plant Communities: An Assessment. Perspect. Plant Ecol. Evol. Syst. 1999, 2, 1–28. [Google Scholar] [CrossRef]
- Svensson, J.R.; Lindegarth, M.; Jonsson, P.R.; Pavia, H. Disturbance–Diversity Models: What Do They Really Predict and How Are They Tested? Proc. R. Soc. B Biol. Sci. 2012, 279, 2163–2170. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples, 2013. Available online: http://purl.oclc.org/estimates (accessed on 25 December 2023).
- Fisher, R.A.; Corbet, A.S.; Williams, C.B. The Relation Between the Number of Species and the Number of Individuals in a Random Sample of an Animal Population. J. Anim. Ecol. 1943, 12, 42. [Google Scholar] [CrossRef]
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Gotelli, N.; Colwell, R. Estimating Species Richness. In Frontiers in Measuring Biodiversity; Oxford University Press: Oxford, UK, 2011; Volume 12, pp. 39–54. Available online: https://www.researchgate.net/publication/236734446_Estimating_species_richness (accessed on 25 December 2023).
- Steneck, R.S. Herbivory on Coral Reefs: A Synthesis. In Proceedings of the 6th International Coral Reef Symposium, Townsville, Australia, 8–12 August 1988; Volume 1, pp. 37–49. [Google Scholar]
- Dawson, E.Y. A Review of the Ecology, Distribution, and Affinities of the Benthic Flora. Syst. Zool. 1960, 9, 93–100. [Google Scholar] [CrossRef]
- UC Santa Barbara, 1970; Santa Barbara Oil Pollution, 1969 Display: A Study of the Biological Effects of the Oil Spill Which Occurred at Santa Barbara, California in 1969 (No. 15080DZR 11/70); Water pollution control research series; U.S. Department of the Interior, Federal Water Quality Control Administration, University of California: Santa Barbara, CA, USA, 1970; p. 49. 53p.
- Wilbur, L.; Gkafas, G.; Saradopoulou, J.; Louca, V.; Exadactylos, A.; Küpper, F.C. Alaskan Fucus Beds as a Hitherto-Unconsidered Nursery Habitat for Herring. Under review 2024. [Google Scholar]
- Thornton, T.F.; Butler, V.; Funk, F.; Moss, M.; Hebert, J.; Elder, T. Herring Synthesis: Documenting and Modeling Herring Spawning Areas within Socio-Ecological Systems over Time in the Southeastern Gulf of Alaska; North Pacific Research Board: Anchorage, AK, USA, 2010; p. 591. [Google Scholar]
- Miner, C.M.; Burnaford, J.L.; Ambrose, R.F.; Antrim, L.; Bohlmann, H.; Blanchette, C.A.; Engle, J.M.; Fradkin, S.C.; Gaddam, R.; Harley, C.D.G.; et al. Large-Scale Impacts of Sea Star Wasting Disease (SSWD) on Intertidal Sea Stars and Implications for Recovery. PLoS ONE 2018, 13, e0192870. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.A.; Lunecke, K.M.; Lépez, M.I. The Response of an Intertidal Concholepas concholepas (Gastropoda) Population to Protection from Man in Southern Chile and the Effects on Benthic Sessile Assemblages. Oikos 1986, 46, 359–364. [Google Scholar] [CrossRef]
- Dethier, M.; Duggins, D. Variation in Strong Interactions in the Intertidal Zone along a Geographical Gradient: A Washington-Alaska Comparison. Mar. Ecol. Prog. Ser. 1988, 50, 97–105. [Google Scholar] [CrossRef]
- Mogollón, R.; Arellano, C.; Villegas, P.; Espinoza-Morriberón, D.; Tam, J. REPSOL Oil Spill off Central Perú in January 2022: A Modeling Case Study. Mar. Pollut. Bull. 2023, 194, 115282. [Google Scholar] [CrossRef] [PubMed]
- Marks-Dauenhauer, N.; Dauenhauer, R. Haa Shuká, Our Ancestors, 1st ed.; University of Washington Press: Washington, DC, USA, 1987; Volume 1. [Google Scholar]
- Edwards, K.; Lafferty, A.; Marks, J.; Pegues, J.; Sarabia, H.; Colley, B.; Katzeek, D.; White, F.; Leer, J. Dictionary of Tlingit; Sealaska Heritage Institute: Juneau, AK, USA, 2011. [Google Scholar]
- Chicoine, D.; Rojas, C. Shell Resources and Maritime Economy at Caylán, Coastal Ancash, Peru. J. Isl. Coast. Archaeol. 2013, 8, 336–360. [Google Scholar] [CrossRef]
- López de la Lama, R.; de la Puente, S.; Sueiro, J.C.; Chan, K.M.A. Reconnecting with the Past and Anticipating the Future: A Review of Fisheries-Derived Cultural Ecosystem Services in Pre-Hispanic Peru. People Nat. 2021, 3, 129–147. [Google Scholar] [CrossRef]
- Benson, E.P. The Worlds of the Moche on the North Coast of Peru; University of Texas Press: Austin, TX, USA, 2021; ISBN 978-0-292-73760-0. [Google Scholar]
- Weinberg, C.; Osborn, J.; Espino Huaman, R. Marine Shellfish Exploitation as a Means of Reducing Vulnerability to Resource Uncertainty in Southern Coastal Peru (200 BCE–150 CE). Holocene 2022, 32, 1503–1517. [Google Scholar] [CrossRef]
- Torres Silva, E.; Macalupú Rosado, J. Bancos naturales de Donax obesulus en el litoral de la Región Piura, Perú. In Natural Banks of Donax Obesulus in the Coast of the Piura Region, Peru. 2014. Available online: https://repositorio.imarpe.gob.pe/handle/20.500.12958/3156 (accessed on 25 December 2023).
- Pulido Capurro, V.; Arana Bustamante, C.; Olivera Carhuaz, E.; Riveros, J.C.; Pulido Capurro, V.; Arana Bustamante, C.; Olivera Carhuaz, E.; Riveros, J.C. El Derrame de Petróleo En El Terminal 2 de La Refinería La Pampilla y Sus Efectos En La Biodiversidad de Las Costas Del Litoral Marino, Perú. Arnaldoa 2022, 29, 71–88. [Google Scholar]
- Tasso, V.; Haddad, M.E.; Assadi, C.; Canales, R.; Aguirre, L.; Vélez-Zuazo, X. Macrobenthic Fauna from an Upwelling Coastal Area of Peru (Warm Temperate South-Eastern Pacific Province -Humboldtian Ecoregion). Biodivers. Data J. 2018, 6, e28937. [Google Scholar] [CrossRef]
- Lannoo, M. Leopold’s Shack and Ricketts’s Lab: The Emergence of Environmentalism; University of California Press: Oakland, CA, USA, 2010; ISBN 978-0-520-26478-6. [Google Scholar]
- Bustamente, R.H.; Branch, G.M.; Eekhout, S.; Robertson, B.; Zoutendyk, P.; Schleyer, M.; Dye, A.; Hanekom, N.; Jurd, M.; McQuaid, C.; et al. Gradients of Intertidal Primary Productivitiy around the Coast of South Africa and Their Relationships with Consumer Biomass. Oecologia 1994, 102, 189–201. [Google Scholar] [CrossRef]
- Lathlean, J.A.; McWilliam, R.A.; Ayre, D.J.; Minchinton, T.E. Biogeographical Patterns of Rocky Shore Community Structure in South-East Australia: Effects of Oceanographic Conditions and Heat Stress. J. Biogeogr. 2015, 42, 1538–1552. [Google Scholar] [CrossRef]
- Ishida, K.; Tachibana, M.; Yao, Y.; Wada, Y.; Noda, T. The Impact of Marine Heatwaves on Rocky Intertidal Communities: Evidence of Accumulative Carryover Effects of Marine Heatwaves. Front. Mar. Sci. 2023, 10, 1146148. [Google Scholar] [CrossRef]
- Konar, B.; Iken, K.; Edwards, M. Depth-Stratified Community Zonation Patterns on Gulf of Alaska Rocky Shores. Mar. Ecol. 2008, 30, 63–73. [Google Scholar] [CrossRef]
- Grout, J.A.; Levings, C.D. Effects of Acid Mine Drainage from an Abandoned Copper Mine, Britannia Mines, Howe Sound, British Columbia, Canada, on Transplanted Blue Mussels (Mytilus edulis). Mar. Environ. Res. 2001, 51, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Helmuth, B.A. Intertidal Mussel Microclimates: Predicting the Body Temperature of a Sessile Invertebrate. Ecol. Monogr. 1998, 68, 51–74. [Google Scholar] [CrossRef]
- Helmuth, B.; Harley, C.D.G.; Halpin, P.M.; O’donnell, M.; Hofmann, G.E.; Blanchette, C.A. Climate Change and Latitudinal Patterns of Intertidal Thermal Stress. Science 2002, 298, 1015–1017. [Google Scholar] [CrossRef]
- Guiñez, R. Castilla A Tridimensional Self-Thinning Model for Multilayered Intertidal Mussels. Am. Soc. Nat. 1999, 154, 341–357. [Google Scholar]
- Elsberry, L.A.; Bracken, M.E.S. Functional Redundancy Buffers Mobile Invertebrates against the Loss of Foundation Species on Rocky Shores. Mar. Ecol. Prog. Ser. 2021, 673, 43–54. [Google Scholar] [CrossRef]
- Prado, L.; Castilla, J.C. The Bioengineer Perumytilus purpuratus (Mollusca: bivalvia) in Central Chile: Biodiversity, Habitat Structural Complexity and Environmental Heterogeneity. J. Mar. Biol. Assoc. UK 2006, 86, 417–421. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Nishihira, M. Islands of Mytilus as a Habitat for Small Intertidal Animals: Effect of Island Size on Community Structure. Mar. Ecol. Prog. Ser. 1985, 25, 71–81. [Google Scholar] [CrossRef]
- Wilbur, L.; Küpper, F.C.; Louca, V. Algal Cover as a Driver of Diversity in Communities Associated with Mussel Assemblages across Eastern Pacific Ecoregions. Mar. Ecol. 2024, 18, e12785. [Google Scholar] [CrossRef]
- Hawkins, S.J.; Hartnoll, R.G. Factors Determining the Upper Limits of Intertidal Canopy Forming Algae. Mar. Ecol. Prog. Ser. 1985, 20, 265–271. [Google Scholar] [CrossRef]
- Alvarado, J.L.; Castilla, J.C. Tridimensional Matrices of Mussels Perumytilus purpuratus on Intertidal Platforms with Varying Wave Forces in Central Chile. Mar. Ecol. Prog. Ser. 1996, 133, 133–141. [Google Scholar] [CrossRef]
- Wijsman, J.W.M.; Troost, K.; Fang, J.; Roncarati, A. Global Production of Marine Bivalves. Trends and Challenges. In Goods and Services of Marine Bivalves; Smaal, A.C., Ferreira, J.G., Grant, J., Petersen, J.K., Strand, Ø., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 7–26. ISBN 978-3-319-96776-9. [Google Scholar]
- Tsuchiya, M.; Nishihira, M. Islands of Mytilus Edulis as a Habitat for Small Intertidal Animals: Effect of Mytilus Age Structure on the Species Composition of the Associated Fauna and Community Organization. Mar. Ecol. Prog. Ser. 1986, 31, 171–178. [Google Scholar] [CrossRef]
- Wilbur, L.; Küpper, F.C.; Katsiaras, N.; Louca, V. Predicting Diversity in Benthic Macro-Scale Communities Associated with Mussel Matrices in Three Pacific Ecoregions. Mar. Ecol. 2023, 44, e12741. [Google Scholar] [CrossRef]
- Harley, C.G.D.; Helmuth, B. Local and Regional Scale Effects of Wave Exposure, Thermal Stress, and Absolute versus Effective Shore Level on Patterns of Intertidal Zonation. Limnol. Oceanogr. 2003, 48, 1498–1508. [Google Scholar] [CrossRef]
- Wilson, J.; Matejusova, I.; McIntosh, R.E.; Carboni, S.; Bekaert, M. New Diagnostic SNP Molecular Markers for the Mytilus Species Complex. PLoS ONE 2018, 13, e0200654. [Google Scholar] [CrossRef] [PubMed]
- Hilbish, T.J.; Carson, E.W.; Plante, J.R.; Weaver, L.A.; Gilg, M.R. Distribution of Mytilus edulis, M. Galloprovincialis, and Their Hybrids in Open-Coast Populations of Mussels in Southwestern England. Mar. Biol. 2002, 2002, 137–142. [Google Scholar] [CrossRef]
- Wenne, R.; Bach, L.; Zbawicka, M.; Strand, J.; McDonald, J.H. A First Report on Coexistence and Hybridization of Mytilus trossulus and M. Edulis Mussels in Greenland. Polar Biol. 2016, 39, 343–355. [Google Scholar] [CrossRef]
- Zuykov, M.; Anderson, J.; Archambault, P.; Dufresne, F.; Pelletier, E. Mytilus Trossulus and Hybrid (M. Edulis-M. Trossulus)—New Hosts Organisms for Pathogenic Microalgae Coccomyxa Sp. from the Estuary and Northwestern Gulf of St. Lawrence, Canada. J. Invertebr. Pathol. 2018, 153, 145–146. [Google Scholar] [CrossRef]
- Tsuchiya, M. Mass Mortality in a Population of the Mussel Mytilus edulis L. Caused by High Temperature on Rocky Shores. J. Exp. Mar. Biol. Ecol. 1983, 66, 101–111. [Google Scholar] [CrossRef]
- Fisheries, N. Tide to Table Profile: Alaska Shellfish Farms|NOAA Fisheries. Available online: https://www.fisheries.noaa.gov/feature-story/tide-table-profile-alaska-shellfish-farms (accessed on 25 December 2023).
- Buschbaum, C.; Dittmann, S.; Hong, J.-S.; Hwang, I.-S.; Strasser, M.; Thiel, M.; Valdivia, N.; Yoon, S.-P.; Reise, K. Mytilid Mussels: Global Habitat Engineers in Coastal Sediments. Helgol. Mar. Res. 2009, 63, 47–58. [Google Scholar] [CrossRef]
- Firstater, F.N.; Hildago, F.J.; Lomovasky, B.J.; Ramos, E.; Gamero, P.; Iribarne, O. Habitat Structure Is More Important than Nutrient Supply in Odifying Mussel Bed Assemblage in an Upwelling Area of the Peruvian Coast. Helgol. Mar. Res. 2010, 65, 187–196. [Google Scholar] [CrossRef]
- Anderson, M.J.; Tolimieri, N.; Millar, R.B. Beta Diversity of Demersal Fish Assemblages in the North-Eastern Pacific: Interactions of Latitude and Depth. PLoS ONE 2013, 8, e57918. [Google Scholar] [CrossRef] [PubMed]
- Free, C.M.; Anderson, S.C.; Hellmers, E.A.; Muhling, B.A.; Navarro, M.O.; Richerson, K.; Rogers, L.A.; Satterthwaite, W.H.; Thompson, A.R.; Burt, J.M.; et al. Impact of the 2014–2016 Marine Heatwave on US and Canada West Coast Fisheries: Surprises and Lessons from Key Case Studies. Fish Fish. 2023, 24, 652–674. [Google Scholar] [CrossRef]
- Alheit, J.; Niquen, M. Regime Shifts in the Humboldt Current Ecosystem. Prog. Oceanogr. 2004, 60, 201–222. [Google Scholar] [CrossRef]
- Carrasco, S.; Santander, H. The El Nino Event and Its Influence on the Zooplankton off Peru. J. Geophys. Res. 1987, 92, 14405–14410. [Google Scholar] [CrossRef]
- Lonhart, S.I.; Tupen, J.W. New Range Records of 12 Marine Invertebrates: The Role of El Nino and Other Mechanisms in Southern and Central California. Bull. South. Calif. Acad. Sci. 2001, 100, 238–249. [Google Scholar]
- Figueroa, J.; Guillermo-Hinojosa, E.; Álvarez, A.; Ipanaque, M.; Hernández, W.; Valdivia, L. Línea Base Biológica Terrestre y Marina de La Reserva Nacional Sistema de Islas, Islotes y Puntas Guaneras; Capítulo 1—Caracterización de La Fauna: Aves, Mamíferos y Reptiles; Islote Don Martín: Lima, Peru, 2019; pp. 9–111. [Google Scholar]
- Spiecker, B.J.; Menge, B.A. El Niño and Marine Heatwaves: Ecological Impacts on Oregon Rocky Intertidal Kelp Communities at Local to Regional Scales. Ecol. Monogr. 2022, 92, e1504. [Google Scholar] [CrossRef]
- Overland, J.E. Causes of the Record-Breaking Pacific Northwest Heatwave, Late June 2021. Atmosphere 2021, 12, 1434. [Google Scholar] [CrossRef]
- Traiger, S.B.; Bodkin, J.L.; Coletti, H.A.; Ballachey, B.; Dean, T.; Esler, D.; Iken, K.; Konar, B.; Lindeberg, M.R.; Monson, D.; et al. Evidence of Increased Mussel Abundance Related to the Pacific Marine Heatwave and Sea Star Wasting. Mar. Ecol. 2022, 43, e12715. [Google Scholar] [CrossRef]
- Di Lorenzo, E.; Mantua, N. Multi-Year Persistence of the 2014/15 North Pacific Marine Heatwave. Nat. Clim. Change 2016, 6, 1042–1047. [Google Scholar] [CrossRef]
- Welch, H.; Savoca, M.S.; Brodie, S.; Jacox, M.G.; Muhling, B.A.; Clay, T.A.; Cimino, M.A.; Benson, S.R.; Block, B.A.; Conners, M.G.; et al. Impacts of Marine Heatwaves on Top Predator Distributions Are Variable but Predictable. Nat. Commun. 2023, 14, 5188. [Google Scholar] [CrossRef] [PubMed]
- Wilbur, L.; Küpper, F.C.; Louca, V. An Assessment of Seasonal Upwelling with Emphasis on Intertidal Temperatures and Shifts in the Abundances of Taxa Associated with Mussel Assemblages at Reserva Punta San Juan (Part of the RNSIIPG Network in Peru) Following the Coastal El Niño of 2017. Manuscript in Preparation for Submission to Estuarine, Coastal and Shelf Science 2023. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4744044 (accessed on 25 December 2023).
- Laufkötter, C.; Zscheischler, J.; Frölicher, T.L. High-Impact Marine Heatwaves Attributable to Human-Induced Global Warming. Science 2020, 369, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- Stabeno, P.J.; Bond, N.A.; Hermann, A.J.; Kachel, N.B.; Mordy, C.W.; Overland, J.E. Meteorology and Oceanography of the Northern Gulf of Alaska. Cont. Shelf Res. 2004, 24, 859–897. [Google Scholar] [CrossRef]
- Qiu, B. Kuroshio and Oyashio Currents. In Encyclopedia of Ocean Sciences; Elsevier: Amsterdam, The Netherlands, 2001; pp. 1413–1425. ISBN 978-0-12-227430-5. [Google Scholar]
- Kaufman, D.S.; Manley, W.F. Pleistocene Maximum and Late Wiscosinan Glacier Extenst across Alaska, USA. Available online: http://akatlas.geology.buffalo.edu/ (accessed on 22 May 2020).
- Larsen, C.F.; Motyka, R.J.; Freymueller, J.T.; Ivins, E.R. Rapid Viscoelastic Uplift in Southeast Alaska Caused by Post-Little Ice Age Glacial Retreat. Earth Planet. Sci. Lett. 2005, 237, 548–560. [Google Scholar] [CrossRef]
- Larsen, C.F.; Motyka, R.J.; Freymueller, J.T.; Echelmeyer, K.A.; Ivins, E.R. Rapid Uplift of Southern Alaska Caused by Recent Ice Loss. Geophys. J. Int. 2004, 158, 1118–1133. [Google Scholar] [CrossRef]
- Pye, K.; Tsoar, H. Aeolian Sand and Sand Dunes; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-85909-3. [Google Scholar]
- Zuta, S.; Guillén, O. Oceanografía de las aguas costeras del Perú. Bol. Inst. Mar Perú 1970, 2, 157–324. [Google Scholar]
- Silva, N.; Rojas, N.; Fedele, A. Water Masses in the Humboldt Current System: Properties, Distribution, and the Nitrate Deficit as a Chemical Water Mass Tracer for Equatorial Subsurface Water off Chile. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2009, 56, 1004–1020. [Google Scholar] [CrossRef]
- Strub, P.T.; Mesías, J.M.; Montecino, V.; Rutlant, J.; Salinas, S. Coastal Ocean Circulation off Western South America. In The Sea; John Wiley and Sons: Hoboken, NJ, USA,, 1998; Volume 11, pp. 273–313. [Google Scholar]
- Wyrtki, K. Equatorial Currents in the Pacific 1950 to 1970 and Their Relations to the Trade Winds. J. Phys. Oceanogr. 1974, 4, 372–380. [Google Scholar] [CrossRef]
- Vermeij, G.J. Trans-Equatorial Connections between Biotas in the Temperate Eastern Atlantic. Mar. Biol. 1992, 112, 343–348. [Google Scholar] [CrossRef]
- Küpper, F.C.; Kamenos, N.A. The Future of Marine Biodiversity and Marine Ecosystem Functioning in UK Coastal and Trritorial Waters (Including UK Overseas Territories)—With an Emphasis on Marine Macrophyte Communities. Bot. Mar. 2018, 61, 521–535. [Google Scholar] [CrossRef]
- Camus, P.A. Biogeografía Marina de Chile Continental. Rev. Chil. Hist. Nat. 2001, 74, 587–617. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).