Abstract
Background/Objectives: Neurodegenerative diseases represent a growing global health challenge with limited therapeutic options. Physical exercise has emerged as a promising non-pharmacological intervention with potential neuroprotective effects. This narrative review examines the mechanisms through which exercise induces neuroplasticity and their implications for neurodegenerative disease prevention. Methods: We synthesized evidence from molecular, animal, and human studies on exercise-induced neuroplasticity and neurodegenerative disease prevention through a comprehensive literature review. Results: Exercise enhances neuroplasticity through multiple pathways: (1) neurotrophic signaling (BDNF, IGF-1, VEGF), (2) neuroendocrine regulation, (3) epigenetic modifications, and (4) metabolic pathway optimization. These molecular changes support structural adaptations including hippocampal neurogenesis, enhanced synaptic plasticity, improved cerebrovascular function, and optimized brain network connectivity. Exercise directly impacts pathological features of neurodegenerative diseases by reducing protein aggregation, attenuating excitotoxicity and oxidative stress, and enhancing mitochondrial function. Clinical evidence consistently demonstrates associations between physical activity and reduced neurodegenerative risk, with intervention studies supporting causal benefits on cognitive function and brain structure. Conclusions: Exercise represents a multi-target intervention addressing several pathological mechanisms simultaneously across various neurodegenerative conditions. Its accessibility, minimal side effects, and multiple health benefits position it as a promising preventive strategy. Future research should focus on understanding individual response variability, developing sensitive biomarkers, and creating personalized exercise prescriptions for optimal neuroprotection.
1. Introduction
Neurodegenerative diseases represent a growing global health challenge, with conditions such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis affecting millions worldwide [1]. These disorders are characterized by progressive neuronal loss and functional decline, with limited therapeutic options currently available. The personal, societal, and economic burden of these conditions is substantial and expected to increase dramatically as populations age [2]. This pressing reality has intensified the search for effective preventive strategies that could modify disease trajectories or delay onset.
Physical exercise has emerged as a promising non-pharmacological intervention with potential neuroprotective effects [3]. Epidemiological evidence consistently demonstrates that physically active individuals have reduced risk of cognitive decline and neurodegenerative disease development [4]. However, the biological mechanisms underlying these associations remain incompletely understood. The concept of neuroplasticity—the brain’s capacity to adapt its structure and function in response to various stimuli—provides a framework for investigating how exercise might confer neuroprotection [5].
Neuroplasticity encompasses multiple processes occurring across molecular, cellular, and systems levels, including neurogenesis, synaptogenesis, angiogenesis, and changes in neural network connectivity [6]. Exercise has been shown to modulate these processes through various pathways involving neurotrophic factors, inflammatory mediators [7], metabolic signals, and epigenetic modifications [8]. Understanding these mechanisms is crucial for developing evidence-based exercise prescriptions and identifying potential therapeutic targets [9].
While previous reviews have examined aspects of exercise-induced neuroplasticity and neuroprotection, our approach differs in several key respects. Cotman and colleagues [3] pioneered work on growth factor cascades and inflammation in exercise-induced brain health, but advances in epigenetic and metabolic pathways have expanded this understanding considerably. More recent reviews by Voss et al. [4] and Duzel et al. [10] focused primarily on cognitive outcomes or specific brain regions, whereas our review integrates findings across molecular, structural, and functional domains with direct applications to diverse neurodegenerative conditions. Unlike previous work that has often separated mechanistic and clinical evidence, we bridge basic science with translational implications, providing an updated synthesis that encompasses the full spectrum from molecular pathways to clinical applications and future research directions.
Despite significant advances in the field, several knowledge gaps persist regarding the optimal parameters of exercise (type, intensity, duration, and frequency) [5], the durability of exercise-induced adaptations [4], and variations in individual responsiveness. Additionally, the translation of mechanistic insights from animal models to human applications presents ongoing challenges. This narrative review aims to synthesize current evidence on the mechanisms through which exercise promotes neuroplasticity and their implications for neurodegenerative disease prevention.
We will examine the molecular mediators of exercise-induced neuroplasticity, structural and functional adaptations in the brain [10], neuroimmune and inflammatory pathways, direct effects on disease-specific pathological features, and clinical evidence supporting exercise as a preventive strategy, overviewed in Figure 1. Furthermore, we will discuss translational implications for clinical practice and identify promising directions for future research. By integrating findings across these domains, this review seeks to advance our understanding of how exercise can be leveraged as a neuroprotective intervention against neurodegenerative disorders.
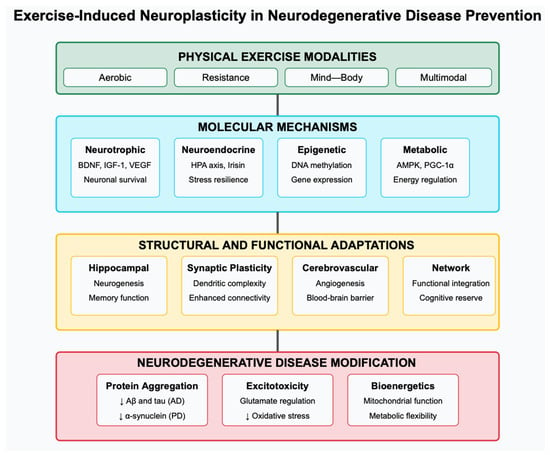
Figure 1.
Conceptual framework of exercise-induced neuroplasticity in neurodegenerative disease prevention. The framework illustrates the progression from exercise stimulus (top) through molecular mediators, structural/functional adaptations, and neuroimmune pathways (middle rows) to direct effects on neurodegenerative pathology and clinical outcomes (bottom rows). Arrows indicate the interconnections between these multiple levels of exercise-induced adaptation.
2. Molecular Mediators of Exercise-Induced Neuroplasticity
Exercise induces a complex cascade of molecular responses that drive neuroplasticity and potentially confer neuroprotection against neurodegenerative processes. These molecular mediators represent critical mechanisms through which physical activity may modify disease risk and progression. This section examines key signaling pathways activated by exercise that contribute to beneficial neuroplastic changes.
2.1. Neutrophic Factors
Brain-derived neurotrophic factor (BDNF) emerges as a central mediator in exercise-induced neuroplasticity [3,11]. Acute and chronic exercise consistently increase BDNF expression in the hippocampus and other brain regions in animal models [12]. In humans, peripheral BDNF levels rise following exercise sessions, with the magnitude of increase correlating with exercise intensity [13,14]. BDNF binds primarily to tropomyosin receptor kinase B (TrkB), activating downstream signaling pathways including phosphatidylinositol 3-kinase (PI3K)/Akt, mitogen-activated protein kinase (MAPK), and phospholipase C-γ (PLC-γ) [11]. These pathways promote neuronal survival, differentiation, and synaptic plasticity—processes particularly relevant to neurodegenerative disease prevention [15].
Other neurotrophic factors also respond to exercise stimuli. Insulin-like growth factor-1 (IGF-1) increases with exercise and can cross the blood–brain barrier to support neuronal growth and survival [16]. Notably, blocking IGF-1 signaling attenuates exercise-induced neurogenesis, suggesting its crucial role in mediating these effects [17]. Similarly, vascular endothelial growth factor (VEGF) increases with exercise and contributes to both angiogenesis and neurogenesis in the hippocampus [18]. The interactive effects between BDNF, IGF-1, and VEGF likely create a molecular environment conducive to neuroplasticity and neuroprotection [4,11].
2.2. Neuroendocrine Responses
Exercise activates the hypothalamic–pituitary–adrenal (HPA) axis, resulting in acute elevations of glucocorticoids that, when properly regulated, may enhance cognitive function and neuroplasticity [19]. Regular exercise appears to optimize HPA axis function, potentially counteracting the deleterious effects of chronic stress on brain health that have been implicated in neurodegenerative processes [20].
Exercise also stimulates the release of irisin, a myokine cleaved from fibronectin type III domain-containing protein 5 (FNDC5) in skeletal muscle [21]. Irisin can cross the blood–brain barrier and induce BDNF expression in the hippocampus, providing a direct muscle–brain communication pathway relevant to exercise-induced neuroplasticity [21,22]. Additionally, exercise-induced increases in circulating catecholamines may influence brain function through both direct and indirect mechanisms, including enhanced alertness and metabolic regulation [23].
2.3. Epigenetic Mechanisms
Emerging evidence indicates that exercise induces epigenetic modifications that regulate gene expression patterns favoring neuroplasticity [24]. DNA methylation changes in the BDNF gene promoter region occur with exercise, potentially underpinning sustained increases in BDNF expression [25]. Exercise also modulates histone modifications, particularly histone acetylation, which generally promotes a transcriptionally active chromatin state [26]. These epigenetic changes provide a mechanistic explanation for how transient exercise stimuli might lead to lasting effects on brain function and resilience against neurodegeneration [24,27].
2.4. Metabolic Signaling Pathways
Exercise activates several metabolic pathways with neuroplastic implications. AMP-activated protein kinase (AMPK), a cellular energy sensor activated during exercise, promotes mitochondrial biogenesis and metabolic efficiency in neurons [28]. Similarly, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) increases with exercise and regulates mitochondrial function while also influencing BDNF expression [29]. The exercise-induced activation of sirtuins, particularly SIRT1, may enhance neuroprotection through the deacetylation of targets like PGC-1α and Forkhead box O (FOXO) transcription factors [30].
The mammalian target of the rapamycin (mTOR) pathway, which regulates protein synthesis and cellular growth, is also modulated by exercise in ways that may support synaptic plasticity and neurogenesis [31]. Intriguingly, metabolic adaptations to exercise appear to shift brain metabolism toward enhanced efficiency and resilience, potentially countering the bioenergetic deficits observed in many neurodegenerative conditions [32,33].
Understanding these molecular mediators provides a foundation for developing targeted interventions that could simulate or enhance exercise benefits, particularly for individuals with limited exercise capacity. Furthermore, these mechanisms highlight potential biomarkers for monitoring exercise efficacy in clinical populations and suggest parameters that might optimize exercise prescription for neuroprotection.
2.5. Summary of Molecular Mechanisms
The molecular pathways activated by exercise create a coordinated response that enhances neuronal resilience and plasticity. As shown in Table 1, neurotrophic factors, particularly BDNF, serve as central mediators, while neuroendocrine responses optimize the stress response system. Epigenetic modifications provide mechanisms for sustained adaptations, and metabolic signaling enhances cellular energetics and mitochondrial function. These pathways interact synergistically, with considerable crosstalk between systems. For instance, exercise-induced AMPK activation not only improves mitochondrial function but also influences BDNF expression through PGC-1α. This molecular convergence may explain why exercise simultaneously impacts multiple pathological features of neurodegenerative diseases, offering advantages over single-target pharmacological approaches. Understanding these molecular mediators provides a foundation for developing targeted interventions and biomarkers to monitor exercise efficacy in clinical populations.

Table 1.
Molecular mediators of exercise-induced neuroplasticity. Table rows represent the four major categories of molecular mediators (Neurotrophic Factors, Neuroendocrine Responses, Epigenetic Mechanisms, and Metabolic Signaling) through which exercise induces neuroplasticity. For each category, specific key mediators are presented along with their exercise-induced effects, neuroplasticity outcomes, and relevance to neurodegenerative processes. ↑ indicates increase/upregulation.
3. Structural and Functional Adaptations
Exercise induces remarkable structural and functional adaptations in the brain that may underlie its neuroprotective effects against neurodegenerative processes. These adaptations occur across multiple time scales and brain regions, collectively enhancing neural resilience. This section examines the key exercise-induced structural and functional changes with particular relevance to neurodegenerative disease prevention.
3.1. Hippocampal Neurogenesis
One of the most well-documented effects of exercise on brain structure is enhanced adult hippocampal neurogenesis—the generation of new neurons in the dentate gyrus region of the hippocampus [34]. Rodent studies consistently demonstrate that voluntary wheel running increases neural progenitor cell proliferation, survival, and differentiation into mature neurons [34,35]. These new neurons integrate into existing circuits and contribute to hippocampal function, particularly pattern separation and memory formation [36].
The neurogenic effects of exercise appear dose-dependent, with moderate-intensity exercise typically producing optimal results [37]. Multiple mechanisms likely contribute to exercise-induced neurogenesis, including increased cerebral blood flow, enhanced neurotrophic factor signaling (particularly BDNF and IGF-1), and reduced inflammation [38,39]. While direct evidence of exercise-induced neurogenesis in humans remains challenging to obtain, indirect neuroimaging and peripheral biomarker studies support the translational relevance of these findings [40,41].
In the context of neurodegenerative diseases, enhanced hippocampal neurogenesis may provide a compensatory mechanism against neuronal loss and contribute to cognitive reserve—the brain’s resilience against pathological insults [42]. Indeed, animal models of Alzheimer’s and Parkinson’s disease show that exercise can partially rescue impaired neurogenesis associated with these conditions [43].
3.2. Synaptic Plasticity and Dendritic Remodeling
Exercise enhances synaptic plasticity—the ability of synapses to strengthen or weaken over time—across multiple brain regions [44]. Both long-term potentiation (LTP) and long-term depression (LTD), cellular correlates of learning and memory, are modulated by exercise [45]. Running increases the expression of synaptic proteins involved in neurotransmission and structural plasticity, including synaptophysin, synapsin I, and postsynaptic density protein 95 (PSD-95) [46,47].
At the structural level, exercise promotes dendritic remodeling, increasing dendritic length, complexity, and spine density in hippocampal and cortical neurons [48]. These morphological changes expand the connectivity potential of neurons and may provide structural substrates for enhanced cognitive function [49]. Notably, these adaptations occur not only during development but also throughout adulthood and even in aging, suggesting a lifelong capacity for exercise-induced structural remodeling [50].
Exercise-induced synaptic plasticity may directly counteract the synapse loss characteristic of many neurodegenerative conditions. In Alzheimer’s disease models, exercise preserves synaptic integrity despite amyloid pathology, potentially through BDNF-mediated mechanisms [51,52]. Similarly, in Parkinson’s disease models, exercise mitigates synaptic dysfunction in corticostriatal circuits through enhanced dopaminergic signaling and spine formation [53].
3.3. Cerebrovascular Adaptations
Exercise induces significant adaptations in the cerebrovascular system, with important implications for brain health and neurodegenerative disease prevention. Angiogenesis—the formation of new blood vessels—occurs in response to regular exercise, particularly in the hippocampus and motor cortex [54]. This increased vascular density improves oxygen and nutrient delivery while enhancing the clearance of metabolic waste products [55].
Beyond structural changes, exercise enhances cerebrovascular function through improved endothelial nitric oxide production, increased cerebral blood flow, and enhanced cerebrovascular reactivity [56,57]. These adaptations may protect against the vascular contributions to cognitive impairment and neurodegeneration [58]. Indeed, cerebrovascular dysfunction often precedes and contributes to neurodegenerative pathology, making exercise-induced vascular adaptations particularly relevant for disease prevention [59].
Regular physical activity also promotes blood–brain barrier (BBB) integrity, potentially reducing the infiltration of neurotoxic substances and inflammatory mediators [60]. The cerebrovascular benefits of exercise appear particularly important in aging, when vascular dysfunction becomes more prevalent and contributes to neurodegeneration [61,62].
3.4. Network Connectivity Changes
Modern neuroimaging techniques have revealed that exercise influences large-scale brain network organization and connectivity [63]. Regular physical activity enhances functional connectivity within and between networks involved in cognitive control, memory, and sensorimotor function [64,65]. These connectivity changes may reflect more efficient information processing and compensatory mechanisms that support cognitive performance despite age-related decline or pathology [66].
Exercise particularly impacts the default mode network (DMN), which shows disrupted connectivity in neurodegenerative conditions [67]. Interventional studies demonstrate that aerobic exercise can partially normalize DMN connectivity in older adults, potentially counteracting pathological network changes [68,69]. Additionally, exercise influences connectivity in the hippocampal–cortical memory system, potentially supporting memory function through enhanced network integration [70].
White matter integrity, essential for efficient communication between brain regions, also responds to exercise [71]. Regular physical activity is associated with greater white matter volume and improved microstructural properties in fiber tracts connecting frontal, temporal, and parietal regions [72,73]. These white matter adaptations may provide structural substrates for enhanced network function and cognitive reserve against neurodegenerative processes [74].
The structural and functional adaptations described above offer multiple potential mechanisms through which exercise may prevent or mitigate neurodegenerative diseases. By enhancing neurogenesis, synaptic plasticity, cerebrovascular function, and network connectivity, exercise appears to build “brain reserves” that could delay symptom onset or slow progression even in the presence of pathology. Understanding these adaptations provides a neurobiological foundation for exercise as a preventive strategy against neurodegeneration and highlights potential targets for therapeutic development.
3.5. Summary of Structural and Functional Adaptations
The structural and functional adaptations induced by exercise establish a neurobiological foundation for neuroprotection against neurodegenerative processes. Hippocampal neurogenesis, enhanced synaptic plasticity, improved cerebrovascular function, and optimized network connectivity collectively build cognitive and neural reserve that may delay pathology onset or progression. These adaptations are mutually reinforcing—neurogenesis supports memory function, synaptic plasticity enhances information processing, and vascular improvements optimize nutrient delivery and waste removal, while network changes improve brain resilience and compensatory capacity. The dynamic nature of these adaptations throughout the lifespan suggests exercise remains effective even in aging, when neurodegenerative vulnerability increases. Together, these structural and functional changes establish multiple mechanisms through which physical activity may prevent neurodegeneration, providing compelling targets for both preventive strategies and therapeutic development.
4. Neuroimmune and Inflammatory Pathways
Anti-inflammatory effects. Neuroinflammation plays a crucial role in the pathogenesis of neurodegenerative diseases, making the anti-inflammatory effects of exercise particularly relevant for disease prevention. Exercise modulates complex neuroimmune interactions that can create a more favorable environment for neuronal survival and function. This section examines how exercise influences inflammatory processes in the brain and their implications for neurodegenerative disease prevention.
4.1. Anti-Inflammatory Effects
Regular physical activity consistently demonstrates anti-inflammatory effects both peripherally and centrally [75,76]. While acute exercise transiently increases circulating pro-inflammatory cytokines, chronic exercise training leads to lower baseline levels of inflammatory markers, including C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6) [77,78]. This systemic anti-inflammatory effect may indirectly benefit the brain by reducing peripheral inflammation that can communicate with and influence central inflammatory processes [79].
In the central nervous system, exercise reduces the expression of pro-inflammatory cytokines and increases anti-inflammatory mediators in multiple brain regions, particularly the hippocampus [7,80]. This shift toward an anti-inflammatory environment may protect against the chronic low-grade neuroinflammation associated with aging and neurodegenerative conditions [81]. The exercise-induced release of muscle-derived anti-inflammatory factors, including IL-10 and IL-1 receptor antagonist (IL-1ra), may contribute to these central effects by crossing the blood–brain barrier or signaling through vagal afferents [82,83].
The anti-inflammatory effects of exercise appear particularly beneficial in the context of metabolic disorders that increase neuroinflammation and neurodegenerative risk. In animal models of obesity and diabetes, exercise attenuates hippocampal inflammation and associated cognitive deficits [84,85]. Similarly, in humans with metabolic syndrome, regular physical activity reduces systemic inflammation and may mitigate related cognitive impairment [86,87].
4.2. Microglial Phenotype Regulation
Microglia, the resident immune cells of the brain, exist on a spectrum from pro-inflammatory (M1-like) to anti-inflammatory, neuroprotective (M2-like) phenotypes [88]. Exercise appears to shift microglial activation toward the neuroprotective M2-like state characterized by enhanced phagocytic activity, increased production of anti-inflammatory cytokines, and release of neurotrophic factors [89,90].
In aged animals, exercise reverses the age-associated shift toward pro-inflammatory microglial priming, potentially through mechanisms involving CX3CL1 (fractalkine) signaling [91,92]. Similarly, in models of Alzheimer’s disease, physical activity reduces microglial activation associated with amyloid pathology while enhancing the clearance of amyloid-beta through the promotion of phagocytic activity [93,94]. This dual effect on microglia—reducing harmful inflammatory activation while enhancing beneficial clearance functions—may be particularly relevant for preventing protein aggregation-related neurodegenerative diseases [95].
Interestingly, microglial regulation through exercise may involve communication with peripheral immune cells, including regulatory T cells, which increase with regular physical activity [96]. These cells can infiltrate the b219rain under certain conditions and produce anti-inflammatory cytokines that influence microglial phenotype [97]. The interplay between peripheral and central immune responses in exercise-mediated neuroprotection represents an active area of investigation with therapeutic implications [98].
4.3. Cytokine Profiles
Exercise shifts the balance of cytokines in the brain toward an anti-inflammatory profile that supports neuroplasticity and resilience [99]. While acute exercise transiently increases IL-6, this is followed by increases in anti-inflammatory cytokines, including IL-10 and IL-1ra [27]. With regular training, baseline levels of pro-inflammatory cytokines (IL-1β, TNF-α, IL-6) decrease while anti-inflammatory mediators increase or maintain higher responsiveness [100,101].
The cytokine profile changes induced by exercise may directly influence neurogenesis, synaptic plasticity, and cognitive function [102]. For example, IL-10 promotes neuronal survival and neurogenesis, while IL-1ra blocks the negative effects of IL-1β on synaptic plasticity and memory [103]. Exercise-induced reductions in TNF-α may be particularly beneficial, as this cytokine is elevated in multiple neurodegenerative conditions and can induce neuronal apoptosis when chronically elevated [104].
The temporal dynamics of cytokine responses to exercise may be critical for understanding its benefits. The transient increases in certain inflammatory mediators during acute exercise may trigger adaptive responses, including antioxidant enzyme upregulation and stress protein expression, that enhance cellular resilience [105,106]. This “hormetic” response—a beneficial adaptation to mild, intermittent stress—may underlie some of exercise’s neuroprotective effects [107].
4.4. Blood–Brain Barrier Integrity
The blood–brain barrier (BBB) plays a crucial role in maintaining brain homeostasis and protecting neural tissue from peripheral toxins and inflammatory mediators [37]. BBB dysfunction occurs in aging and neurodegenerative diseases, potentially contributing to disease progression through increased permeability to harmful substances and immune cells [108,109].
Exercise appears to enhance BBB integrity through multiple mechanisms [110]. Regular physical activity upregulates tight junction proteins, including occludin and claudin-5, which maintain BBB structural integrity [111]. Exercise also enhances the expression of glucose transporter 1 (GLUT-1) in brain endothelial cells, potentially improving cerebral glucose metabolism while maintaining barrier function [112].
In animal models of neurodegenerative diseases, exercise attenuates BBB disruption and associated cognitive deficits [60,113]. For example, in models of Alzheimer’s disease, physical activity reduces BBB leakage associated with amyloid pathology and rescues cognitive function [114]. Similarly, in models of Parkinson’s disease, exercise prevents BBB breakdown in the substantia nigra and mitigates dopaminergic neuron loss [115].
The protective effects of exercise on BBB integrity may involve reduced oxidative stress and inflammation in cerebrovascular endothelial cells [116]. Additionally, exercise-induced increases in angiogenic factors like VEGF promote not only angiogenesis but also the recruitment of pericytes that support BBB function [117,118]. Through these mechanisms, regular physical activity may help maintain a selective, functional barrier that protects against neurodegenerative processes while allowing beneficial nutrient transport.
Understanding the neuroimmune adaptations to exercise provides insights into potential preventive mechanisms against neurodegeneration. By reducing neuroinflammation, promoting neuroprotective microglial phenotypes, optimizing cytokine profiles, and maintaining BBB integrity, exercise creates an environment conducive to neuronal health and resilience. These adaptations may be particularly important in the context of aging, when neuroinflammatory processes become more prominent and contribute to neurodegenerative vulnerability.
4.5. Summary of Neuroimmune and Inflammatory Pathways
Exercise-induced modifications to neuroimmune and inflammatory pathways create an environment conducive to neuronal survival and function while countering neuroinflammatory processes implicated in neurodegeneration. By reducing pro-inflammatory cytokine expression, shifting microglial phenotypes toward neuroprotective states, optimizing cytokine profiles, and maintaining BBB integrity, exercise addresses inflammation at multiple levels. These effects appear particularly relevant in aging, when chronic low-grade neuroinflammation becomes more prominent. The temporal dynamics of these adaptations—with transient increases in some inflammatory mediators potentially triggering beneficial hormetic responses—highlight the complex relationship between exercise intensity, frequency, and inflammatory outcomes. The integrated nature of peripheral and central immune communication suggests that exercise’s systemic anti-inflammatory effects may complement local brain adaptations, potentially establishing a neuroimmunomodulatory foundation for preventing neurodegeneration.
5. Exercise Effects on Pathological Features of Neurodegenerative Diseases
Beyond its general effects on neuroplasticity and neuroimmune function, physical exercise directly impacts specific pathological features of neurodegenerative diseases. These targeted effects may contribute substantially to exercise’s preventive potential. This section examines how exercise influences key pathological processes across major neurodegenerative conditions.
5.1. Amyloid-β and Tau Pathology
The accumulation of amyloid-β (Aβ) plaques and hyperphosphorylated tau tangles represents hallmark pathologies in Alzheimer’s disease (AD). Evidence from animal models consistently demonstrates that exercise reduces Aβ burden [52,119]. In transgenic AD mice, voluntary running decreases Aβ plaque load in the hippocampus and cortex while reducing soluble Aβ levels [94,120]. These effects occur through multiple mechanisms, including enhanced Aβ clearance, reduced Aβ production, and improved proteostasis [121].
Exercise appears to enhance Aβ clearance through several pathways. Physical activity upregulates Aβ-degrading enzymes, including neprilysin and insulin-degrading enzyme (IDE) [122,123]. Exercise also improves glymphatic clearance—the brain’s waste removal system—potentially through increased cerebral blood flow and reduced neuroinflammation [124,125]. Additionally, exercise enhances microglial phagocytosis of Aβ while reducing pro-inflammatory microglial activation that can exacerbate amyloid pathology [126].
Regarding tau pathology, regular exercise reduces tau hyperphosphorylation in several transgenic mouse models [127,128]. This effect appears to be mediated through the modulation of kinases and phosphatases that regulate the tau phosphorylation status, including glycogen synthase kinase-3β (GSK-3β) and protein phosphatase 2A (PP2A) [85,129]. Exercise also enhances autophagy—a cellular degradation system—potentially promoting the clearance of pathological tau species [130,131].
In humans, observational studies indicate that physically active individuals have lower amyloid and tau burden as measured through PET imaging and cerebrospinal fluid biomarkers [132,133]. Interventional studies show that exercise can improve cognition in patients with mild cognitive impairment and early AD, though evidence for direct effects on pathology in humans remains limited [86,134]. Nevertheless, the consistent effects observed in animal models suggest that exercise may directly impact AD pathophysiology, potentially slowing pathological progression when implemented early.
5.2. α-Synuclein Aggregation
Alpha-synuclein (α-syn) aggregation represents the primary pathological hallmark of Parkinson’s disease (PD) and other synucleinopathies. Mounting evidence suggests that exercise influences α-syn metabolism and aggregation, potentially mitigating this pathological process [135]. In rodent models of PD, forced exercise reduces α-syn aggregation in the substantia nigra and striatum while attenuating associated neuroinflammation [115,136].
The mechanisms underlying these effects likely involve enhanced proteostasis—the cellular machinery that maintains protein homeostasis. Exercise upregulates molecular chaperones, particularly heat shock proteins (HSPs), that assist in protein folding and prevent aggregation [137]. Additionally, exercise enhances autophagy-lysosomal function, a primary pathway for α-syn clearance [138]. The ubiquitin–proteasome system, another degradation pathway relevant to α-syn clearance, also shows enhanced function with regular physical activity [139].
Beyond effects on α-syn directly, exercise protects dopaminergic neurons through multiple complementary mechanisms. Physical activity increases levels of neurotrophic factors in the basal ganglia, particularly glial cell line-derived neurotrophic factor (GDNF) and BDNF, which support dopaminergic neuron survival [140,141]. Exercise also reduces oxidative stress in these neurons, potentially through the upregulation of antioxidant enzymes and improved mitochondrial function [81,142].
In PD patients, regular exercise improves motor symptoms, potentially by enhancing dopaminergic signaling and promoting compensatory mechanisms [143,144]. While direct evidence for exercise effects on α-syn pathology in humans remains limited, the convergent evidence from animal models and clinical benefits observed in patients supports exercise as a promising intervention for modifying disease progression [145,146].
5.3. Excitotoxity and Oxidative Stress
Excitotoxicity—neuronal damage resulting from excessive glutamate receptor activation—and oxidative stress represent common pathological mechanisms across multiple neurodegenerative conditions [147]. Exercise appears to mitigate both processes through complementary adaptations that enhance neuronal resilience [3,148].
Regarding excitotoxicity, exercise modulates glutamatergic signaling through multiple mechanisms. Regular physical activity normalizes glutamate receptor expression and function, particularly N-methyl-D-aspartate (NMDA) receptors implicated in excitotoxic cascades [149]. Exercise also enhances the expression of glutamate transporters in astrocytes, promoting efficient glutamate clearance from synaptic spaces [150,151]. Additionally, exercise increases GABAergic inhibitory tone in several brain regions, potentially counterbalancing excessive excitatory signaling [44].
Exercise significantly influences redox homeostasis in the brain, generally enhancing antioxidant capacity and reducing oxidative damage [152,153]. While acute exercise transiently increases reactive oxygen species (ROS) production, chronic training upregulates antioxidant enzymes, including superoxide dismutase (SOD), catalase, and glutathione peroxidase [154,155]. This adaptive response, consistent with hormetic principles, enhances cellular capacity to manage oxidative challenges [156].
Importantly, exercise reduces oxidative damage to cellular components implicated in neurodegeneration. Regular physical activity decreases lipid peroxidation, protein carbonylation, and DNA oxidation in brain regions vulnerable to neurodegeneration [51,157]. These effects appear particularly pronounced in aging, when oxidative stress increases and contributes to neurodegenerative vulnerability [158,159].
The neuroprotective effects of exercise against excitotoxicity and oxidative stress extend across multiple neurodegenerative conditions. In Huntington’s disease models, exercise reduces striatal excitotoxicity and associated neuronal loss [160,161]. In amyotrophic lateral sclerosis (ALS) models, exercise attenuates oxidative damage in motor neurons and delays symptom onset, though effects appear intensity-dependent [162,163]. These cross-cutting effects highlight exercise’s potential to address common pathological mechanisms rather than disease-specific pathologies alone.
5.4. Mitochondrial Function and Bioenergetics
Mitochondrial dysfunction represents a central feature of neurodegenerative pathogenesis, contributing to bioenergetic deficits, oxidative stress, and ultimately neuronal death [164]. Exercise robustly improves mitochondrial function and cellular bioenergetics through adaptations that enhance energy production efficiency and resilience [29,165].
Regular physical activity increases mitochondrial biogenesis—the generation of new mitochondria—in multiple brain regions [166,167]. This effect occurs primarily through the activation of PGC-1α, a transcriptional coactivator that regulates genes involved in mitochondrial replication and function [168,169]. Exercise also enhances the expression of mitochondrial enzymes in the electron transport chain, potentially improving ATP production capacity [170,171].
Beyond biogenesis, exercise improves mitochondrial quality control mechanisms that maintain a healthy mitochondrial pool. Physical activity enhances mitophagy—the selective autophagy of damaged mitochondria—preventing the accumulation of dysfunctional organelles that can generate excessive ROS and trigger cell death [172,173]. Exercise also promotes mitochondrial dynamics, facilitating the fusion and fission processes that maintain functional mitochondrial networks [174,175].
In the context of neurodegenerative diseases, exercise-induced mitochondrial adaptations may directly counter disease-specific bioenergetic deficits. In AD models, exercise improves hippocampal mitochondrial function despite amyloid pathology, potentially through enhanced calcium handling capacity and reduced oxidative damage [130,176]. In PD models, physical activity protects nigral dopaminergic neurons against mitochondrial toxins while enhancing complex I activity—a respiratory chain component often deficient in PD [177,178].
Interestingly, exercise enhances metabolic flexibility in neural tissues, potentially allowing neurons to utilize alternative fuel sources when glucose metabolism is compromised [179,180]. This adaptation may be particularly relevant in neurodegenerative conditions where cerebral glucose hypometabolism precedes clinical symptoms [181,182]. By enhancing ketone body utilization and lactate transport, exercise may provide metabolic substrates that bypass impaired glucose metabolism [12,183].
The direct effects of exercise on the pathological features of neurodegenerative diseases highlight its potential as a targeted preventive strategy. By addressing specific disease mechanisms—whether amyloid and tau pathology in AD, α-synuclein aggregation in PD, or common processes like excitotoxicity and mitochondrial dysfunction—exercise represents a multifaceted intervention that may modify disease trajectories. These mechanistic insights provide a rationale for implementing exercise early in disease courses or preventively in at-risk populations, potentially delaying pathological progression and symptom onset.
5.5. Summary of Exercise Effects on Pathological Features
The direct effects of exercise on the pathological features of neurodegenerative diseases highlight its potential as a targeted preventive strategy, as visible in Table 2. By addressing specific disease mechanisms—whether amyloid and tau pathology in AD, α-synuclein aggregation in PD, or common processes like excitotoxicity and mitochondrial dysfunction—exercise represents a multifaceted intervention that may modify disease trajectories. Unlike single-target pharmacological approaches, exercise simultaneously affects multiple pathological processes through complementary mechanisms. This multi-target effect likely contributes to exercise’s broad efficacy across diverse neurodegenerative conditions despite their distinct pathological signatures. The convergence of these mechanisms on common cellular processes, including protein homeostasis, redox balance, and energy metabolism, suggests that exercise enhances fundamental cellular resilience mechanisms. These findings provide a strong mechanistic rationale for implementing exercise early in disease trajectories or preventively in at-risk populations, potentially delaying pathological progression and symptom onset.

Table 2.
Exercise effects on neurodegenerative disorders: mechanisms and preventive potential. Table rows represent major neurodegenerative disorders and how they are affected by exercise. For each disorder, pathological features, exercise-mediated mechanisms, supporting evidence (both preclinical and clinical), and preventive implications are presented. ↓ indicates decrease/downregulation; ↑ indicates increase/upregulation.
6. Clinical and Epidemiological Evidence
The mechanistic insights described in previous sections are complemented by substantial clinical and epidemiological evidence supporting exercise as a preventive strategy against neurodegenerative diseases. These studies establish associations between physical activity and disease risk while providing insights into dose–response relationships and exercise modality considerations. This section examines the human evidence for exercise effects on neurodegenerative outcomes.
6.1. Exercise and Cognitive Outcomes
Observational studies consistently demonstrate that physically active individuals maintain better cognitive function across the lifespan compared to sedentary counterparts [184,185]. A meta-analysis of 45 studies including over 117,410 samples found that physical activity was associated with a 38% reduced risk of cognitive decline [186]. Similarly, another meta-analysis of 16 prospective studies with over 160,000 participants demonstrated that higher levels of physical activity were associated with approximately 40% reduced risk of Alzheimer’s disease [187].
The cognitive domains most consistently benefiting from exercise include executive function, processing speed, and memory—functions commonly impaired in neurodegenerative conditions [188,189]. Interestingly, these cognitive benefits appear across the spectrum from healthy aging to mild cognitive impairment (MCI) and early-stage dementia, suggesting exercise may be beneficial even after cognitive changes have begun [190,191].
Randomized controlled trials (RCTs) provide stronger evidence for causal relationships between exercise and cognitive outcomes. A meta-analysis of 39 RCTs demonstrated that aerobic exercise interventions significantly improved attention, executive function, and memory in healthy older adults [192]. In individuals with MCI, a 6-month aerobic exercise intervention improved executive function and increased hippocampal volume compared to stretching controls [193]. Even in patients with early Alzheimer’s disease, a meta-analysis of 18 RCTs found moderate positive effects of exercise on global cognition [194].
Neuroimaging studies provide additional evidence for exercise effects on brain structure and function relevant to neurodegeneration. Higher fitness levels and regular physical activity are associated with greater gray matter volume in regions vulnerable to age-related atrophy, particularly the hippocampus and prefrontal cortex [195,196]. Longitudinal intervention studies demonstrate that aerobic exercise increases hippocampal volume in healthy older adults, potentially counteracting age-related volume loss [41,197]. Similarly, exercise enhances white matter integrity and functional connectivity in networks affected by neurodegenerative processes [198,199].
6.2. Preventive Potential Across Neurodegenerative Conditions
While Alzheimer’s disease has received the most research attention, evidence suggests exercise may provide preventive benefits across multiple neurodegenerative conditions. For Parkinson’s disease (PD), prospective cohort studies demonstrate that moderate to vigorous physical activity is associated with 34–43% reduced risk of developing the condition [200]. A meta-analysis of eight prospective studies totaling 544,336 participants found that higher physical activity levels were associated with a significant reduction in PD risk (RR: 0.66; 95% CI: 0.57–0.78) [201]. These protective associations appear stronger for moderate to vigorous activity and may be more pronounced in men than women [202].
For patients already diagnosed with PD, exercise interventions improve motor symptoms, gait, balance, and quality of life [203,204]. A meta-analysis demonstrated that various exercise modalities improved UPDRS motor scores, with particularly strong effects for resistance training and tai chi [205]. Beyond motor symptoms, emerging evidence suggests exercise may improve non-motor symptoms, including cognition and depression in PD patients [206,207].
Regarding amyotrophic lateral sclerosis (ALS), the relationship with exercise appears more complex. Some studies suggest that intense physical activity or specific occupational activities may increase ALS risk, potentially through oxidative stress mechanisms [208,209]. However, moderate physical activity has not been consistently associated with increased risk, and some evidence suggests it may be protective when implemented appropriately [210,211]. The seemingly paradoxical relationship may reflect an interaction between exercise intensity, genetic susceptibility, and underlying pathophysiology [212].
For Huntington’s disease (HD), a genetic condition with complete penetrance, exercise cannot prevent disease occurrence but may delay symptom onset or slow progression. Animal studies demonstrate that exercise delays motor symptom onset, improves motor function, and reduces striatal neurodegeneration in HD models [161,213]. Limited clinical studies in pre-symptomatic and early-stage HD patients suggest exercise may improve motor function, cognitive performance, and quality of life [214,215]. These findings highlight the potential for exercise to modify disease trajectories even in conditions with strong genetic determinants.
6.3. Dose–Response Relationships
Understanding dose–response relationships is crucial for optimizing exercise prescriptions for neurodegenerative disease prevention. Current evidence suggests a non-linear relationship between exercise volume and cognitive benefits, with moderate amounts typically providing optimal effects [216,217]. A meta-analysis of prospective studies found that the largest risk reduction for cognitive decline occurred with moderate physical activity levels, with minimal additional benefit from higher volumes [218,219].
Regarding intensity, moderate-to-vigorous aerobic exercise (approximately 60–75% of maximum heart rate) appears most consistently associated with cognitive benefits and reduced neurodegenerative risk [220,221]. However, even light-intensity physical activity, such as walking, shows protective associations when performed regularly [222,223]. This suggests that incorporating regular movement throughout the day, rather than focusing solely on structured exercise sessions, may provide meaningful benefits.
Exercise frequency also influences outcomes, with most studies reporting optimal cognitive benefits with 3–5 sessions per week [223,224]. Interestingly, some evidence suggests that even one or two weekly sessions of vigorous activity may provide substantial protective benefits, supporting the concept that some exercise is significantly better than none [225,226].
Duration requirements appear to vary by intensity, with longer durations needed for lower-intensity activities to achieve comparable benefits. Most successful interventions for cognitive outcomes involve 30–60 min sessions, though accumulated shorter bouts may provide similar benefits when total volume is matched [226,227]. Importantly, longitudinal studies suggest that sustained participation over months to years is necessary for optimal neuroprotection, highlighting the importance of establishing sustainable exercise habits [228,229].
6.4. Exercise Modality Considerations
Different exercise modalities may offer complementary benefits for neurodegenerative disease prevention. Aerobic exercise most consistently demonstrates positive effects on cognitive function, hippocampal volume, and cerebral blood flow [230,231]. A meta-analysis found that aerobic exercise produced the largest effects on executive function compared to other modalities [231,232].
Resistance training shows emerging evidence for cognitive benefits, particularly for executive function and memory [233,234]. Mechanisms may involve increased production of myokines, reduced inflammation, and enhanced insulin sensitivity [46,235]. Interestingly, combined aerobic and resistance training may provide greater cognitive benefits than either modality alone, suggesting synergistic effects [236,237].
Mind–body exercises, including tai chi, yoga, and dance, demonstrate promising effects on cognitive function and motor symptoms in older adults and neurodegenerative populations [238,239]. These modalities combine physical activity with cognitive, social, and mindfulness components that may enhance neuroplasticity through multiple pathways [240,241]. A meta-analysis found that tai chi improved cognitive performance in older adults with and without cognitive impairment [241,242].
Cognitively challenging exercise forms, such as dance, tennis, or martial arts, may provide enhanced benefits through simultaneous physical and cognitive engagement [243,244]. These activities require coordination, adaptation, and executive function during movement, potentially stimulating neuroplasticity through multiple mechanisms [245,246]. This supports the concept of “dual-task” training as a particularly effective approach for neurodegenerative disease prevention [246,247].
The clinical and epidemiological evidence reviewed here supports exercise as a promising preventive strategy against neurodegenerative diseases. The consistent associations between physical activity and reduced disease risk, combined with demonstrated benefits on cognitive function and brain structure, provide a compelling rationale for implementing exercise interventions. Understanding dose–response relationships and modality considerations allows for optimizing exercise prescriptions for at-risk populations, potentially enhancing preventive efficacy.
6.5. Summary of Clinical and Epidemiological Evidence
The robust clinical and epidemiological evidence supporting exercise as a preventive strategy for neurodegenerative diseases complements the mechanistic insights from molecular and animal studies. Observational findings consistently demonstrate reduced risk across multiple neurodegenerative conditions, while intervention studies show causal benefits on cognitive function, brain structure, and specific symptomatology. The apparent dose–response relationships and modality considerations identified here inform evidence-based exercise prescriptions, suggesting that moderate-intensity, multimodal approaches may provide optimal benefit. However, the heterogeneity in study designs, populations, and outcomes highlights the need for more standardized approaches to exercise prescription and assessment. The convergent evidence across epidemiological, interventional, neuroimaging, and biomarker studies strengthens the case for exercise implementation while providing a foundation for personalized approaches tailored to specific neurodegenerative risk profiles and individual characteristics.
7. Translational Implications
Translating the extensive mechanistic and clinical evidence on exercise-induced neuroplasticity into effective preventive strategies requires careful consideration of implementation challenges and personalization approaches. This section examines the translational implications of exercise research for neurodegenerative disease prevention, focusing on practical considerations for clinical application.
7.1. Exercise Prescription Considerations
Developing evidence-based exercise prescriptions for neurodegenerative disease prevention requires balancing efficacy with adherence and safety considerations [248,249,250]. The traditional FITT-VP framework (Frequency, Intensity, Time, Type, Volume, and Progression) provides a useful structure for prescription development [249]. Based on current evidence, a general prescription for cognitive health and neurodegenerative disease prevention might include the following:
- Frequency: Approximately 3–5 days per week of aerobic activities, with additional 2–3 days of resistance training [218,251];
- Intensity: Moderate intensity (approximately 60–75% of maximum heart rate) for most sessions, with some vigorous intervals (>75% maximum heart rate) if tolerated [252,253];
- Time: Approximately 30–60 min per session, which may be accumulated in shorter bouts (10+ minutes) for those with limited capacity [253,254];
- Type: Multimodal approach combining aerobic, resistance, and motor skill components, with emphasis on cognitively engaging activities [192,255,256];
- Volume: Approximately 150 min of moderate-to-vigorous activity weekly, with additional light activity throughout the day [255];
- Progression: Gradual increases in duration before intensity, with periodic variation to maintain engagement and challenge [257,258].
However, these general guidelines require modification based on individual factors including age, baseline fitness, comorbidities, and preferences [258,259]. For older adults with mobility limitations or chronic conditions, low-impact modalities such as recumbent cycling, water-based exercises, or chair-based routines may provide accessible alternatives while still conferring cognitive benefits [220,260].
Clinical monitoring becomes particularly important for high-risk individuals, including those with cardiovascular disease, orthopedic limitations, or neurological symptoms [260]. Pre-exercise screening using validated tools such as the Physical Activity Readiness Questionnaire (PAR-Q+) can identify individuals requiring medical clearance before increasing activity levels [261,262]. For those with established neurodegenerative conditions, supervision by trained exercise physiologists or physical therapists may optimize safety and efficacy [145,263].
7.2. Personalization Approaches for At-Risk Populations
Different neurodegenerative conditions and risk profiles may benefit from tailored exercise approaches that target specific pathological mechanisms [264,265]. For individuals at risk of Alzheimer’s disease, particularly those with genetic predisposition (e.g., APOE ε4 carriers) or family history, aerobic exercise emphasizing hippocampal engagement appears most beneficial [265]. Some evidence suggests that APOE ε4 carriers may show enhanced cognitive responses to exercise interventions, potentially reflecting compensatory mechanisms or greater room for improvement [266].
For those with Parkinson’s disease risk factors or prodromal symptoms, exercises focusing on dual-task performance, balance, amplitude training, and rhythmic activities may provide targeted benefits [267]. Programs like Dance for Parkinson’s and LSVT BIG have demonstrated efficacy for symptom management and may have preventive potential when implemented early [268]. The incorporation of external cueing and attentional strategies appears particularly valuable for this population [269].
Age-specific considerations are also important, as different life stages may benefit from distinct exercise approaches [270]. In midlife (40–60 years), when neurodegenerative pathology often begins accumulating without symptoms, vigorous aerobic training and resistance exercise focusing on metabolic health may provide optimal preventive effects [271]. In contrast, older adults (65+ years) may benefit more from multimodal programs emphasizing fall prevention, social engagement, and gradual progression [272].
Personalization may extend to timing considerations, with evidence suggesting that exercise timing relative to circadian rhythms influences outcomes [273]. Morning exercise has been associated with better adherence and cognitive benefits in some populations, potentially through interactions with cortisol cycles and sleep quality [274]. However, individual chronotype variations suggest that optimal timing may differ between “morning larks” and “night owls”, highlighting another dimension for personalization [275].
Technological approaches to personalization are rapidly evolving, with wearable devices, smartphone applications, and remote monitoring systems enabling dynamic adjustment of exercise prescriptions [276]. These technologies can track adherence, physiological responses, and performance metrics to optimize exercise dose while providing motivational feedback [277]. For example, heart rate variability monitoring during exercise may help identify optimal intensity zones for neuroprotective effects while minimizing stress responses [278].
7.3. Integration with Other Lifestyle Interventions
Exercise appears to function synergistically with other lifestyle factors to enhance neuroprotection, suggesting that integrated approaches may provide optimal benefits [279]. The FINGER trial demonstrated that a multidomain intervention combining exercise, cognitive training, dietary modification, and vascular risk management improved cognitive function in at-risk older adults more effectively than single-domain approaches [280]. Similar multidomain trials including MAPT and PreDIVA have provided supportive, though somewhat mixed, evidence for this integrated approach [281].
Nutritional factors appear particularly complementary to exercise effects on brain health [282,283]. Mediterranean-style diets rich in polyphenols, omega-3 fatty acids, and antioxidants may enhance exercise-induced neuroplasticity through shared mechanisms involving BDNF signaling, reduced inflammation, and improved vascular function [284,285]. Some evidence suggests that protein consumption in close proximity to resistance exercise may enhance muscle protein synthesis and the release of myokines with neuroprotective effects [286,287].
Cognitive engagement during or proximal to exercise may also amplify neuroplastic responses [287,288]. “Exergaming” approaches combining physical activity with cognitive challenges have shown promising results for cognitive enhancement in older adults [289,290]. Similarly, performing cognitive tasks immediately following exercise may leverage the transiently enhanced plasticity induced by acute exercise, potentially strengthening learning and memory consolidation [291,292].
Sleep quality represents another important complementary factor, as exercise can improve sleep architecture, while adequate sleep enhances exercise recovery and cognitive performance [292]. Addressing sleep disturbances may therefore improve exercise adherence and efficacy for neurodegenerative prevention [293]. Conversely, excessive evening exercise may disrupt sleep in sensitive individuals, highlighting the need for personalized timing recommendations [294].
Stress management techniques, including mindfulness meditation and breathing exercises, may complement physical activity by reducing cortisol levels and neuroinflammation [295]. The combination of moderate exercise with stress reduction practices potentially provides dual benefits: exercise-induced resilience to stress and reduced chronic stress that might otherwise impair neuroplasticity [296].
7.4. Challenges in Implementation
Despite substantial evidence supporting exercise for neurodegenerative disease prevention, significant implementation challenges remain [297]. Perhaps most fundamental is the challenge of behavior change—initiating and maintaining physical activity habits in predominantly sedentary populations [298]. Behavioral economic approaches, including commitment devices, social incentives, and immediate rewards, show promise for increasing exercise adherence [299]. Additionally, motivational interviewing techniques and stages-of-change models provide frameworks for tailoring behavioral strategies to individual readiness levels [300].
Access disparities present another implementation challenge, as socioeconomic factors influence both exercise opportunities and neurodegenerative disease risk [301]. Community-based programs in accessible locations, subsidized memberships for fitness facilities, and home-based exercise options with minimal equipment requirements may help address these disparities [302]. Technological solutions such as smartphone apps and online communities may expand access, though digital literacy barriers must be considered for older populations [303].
Healthcare system integration represents a crucial implementation pathway that remains underdeveloped [304]. Exercise prescriptions remain underutilized in clinical settings due to time constraints, limited provider training, and reimbursement challenges [305]. Programs like Exercise is Medicine aim to address these barriers by standardizing assessment and prescription protocols while developing referral networks to qualified exercise professionals [306]. Expanding these initiatives may facilitate the wider implementation of exercise as preventive medicine for neurodegenerative conditions [307].
Public health messaging around exercise for brain health requires refinement to effectively motivate behavior change [308]. Messages emphasizing immediate benefits (improved mood, energy, sleep) alongside long-term neuroprotection may increase relevance across age groups [309]. Additionally, framing exercise as a positive addition rather than an obligatory burden may reduce resistance, particularly among those with negative past experiences with physical activity [310].
The translation of exercise research into effective preventive strategies for neurodegenerative diseases requires addressing these implementation challenges while refining personalization approaches. By developing targeted exercise prescriptions, integrating complementary lifestyle factors, and addressing barriers to adoption, clinicians and public health professionals can leverage the neuroplastic potential of physical activity to reduce the growing burden of neurodegenerative diseases.
7.5. Summary of Translational Implications
The translation of exercise research into effective preventive strategies requires addressing multiple challenges while capitalizing on emerging opportunities. Exercise prescription development must balance evidence for efficacy with considerations of adherence, accessibility, and safety while accounting for individual differences in age, comorbidities, and risk profiles. Personalized approaches based on neurodegenerative risk factors and genetic determinants may optimize preventive benefits, particularly when integrated with complementary lifestyle interventions. However, significant implementation barriers remain, including behavioral challenges, access disparities, healthcare system integration, and public health messaging limitations. Addressing these barriers requires coordinated efforts across clinical, public health, and policy domains. By developing targeted exercise prescriptions, advancing personalization approaches, integrating complementary lifestyle factors, and addressing implementation challenges, clinicians and public health professionals can leverage exercise’s neuroplastic potential to reduce neurodegenerative disease burden.
8. Future Research Directions
Despite significant advances in our understanding of exercise-induced neuroplasticity and its potential for neurodegenerative disease prevention, important research gaps remain. Addressing these gaps could enhance the development of evidence-based prevention strategies and optimize their implementation. This section examines promising future research directions across mechanistic, methodological, and translational domains.
8.1. Mechanistic Gaps
Several mechanistic questions require further investigation to fully understand exercise-induced neuroprotection [311]. The relative contributions of different pathways—neurotrophic, anti-inflammatory, vascular, and metabolic—to exercise-mediated benefits remain incompletely characterized [312]. Future research should employ experimental designs that selectively block or enhance specific pathways to determine their necessity and sufficiency for neuroprotective effects [313]. For example, using conditional knockouts of BDNF or its receptor TrkB in specific brain regions could help establish the causal role of this pathway in exercise-induced cognitive benefits [314].
The systemic versus local origins of exercise-induced neuroplasticity represent another important mechanistic question [315]. While peripheral factors like myokines, hepatokines, and immune mediators clearly influence brain function, their relative importance compared to direct neural activation remains unclear [316]. Research designs comparing the systemic administration of exercise-induced factors with localized brain interventions could help disentangle these mechanisms [317]. The recently identified “exercisekines” and extracellular vesicles released during physical activity represent promising targets for such investigations [318].
The temporal dynamics of exercise-induced adaptations require further characterization, particularly regarding the persistence of effects after exercise cessation [319]. Longitudinal studies with multiple time points are needed to determine how quickly different adaptations develop, their maintenance duration, and potential rebound effects [320]. This information is crucial for optimizing exercise prescription parameters, including frequency and periodization [321].
Individual variability in responsiveness to exercise represents a critical research frontier [322]. Some individuals show robust cognitive and neuroplastic responses to physical activity, while others demonstrate minimal changes despite similar training regimens [323]. Understanding the genetic, epigenetic, physiological, and psychological factors underlying this variability could inform personalized approaches [324]. Multi-omics technologies, including genomics, proteomics, and metabolomics, offer powerful tools for characterizing response heterogeneity [325].
8.2. Methological Considerations
Advancing exercise–neuroplasticity research requires methodological refinements to enhance reliability, validity, and translational potential [326]. The standardization of exercise protocols represents a fundamental need, as variability in intensity quantification, progression parameters, and supervision methods complicates cross-study comparisons [327]. Consensus guidelines for describing exercise interventions with precision would facilitate more meaningful meta-analyses and replication efforts [328].
Novel neuroimaging approaches offer promising opportunities for the non-invasive assessment of exercise effects on brain structure and function [329]. Advanced techniques including arterial spin labeling, functional connectivity analyses, diffusion kurtosis imaging, and PET imaging of neuroinflammation could provide more sensitive and specific measures of exercise-induced changes [330]. Multimodal imaging combining structural, functional, and molecular measures may be particularly informative for characterizing comprehensive brain adaptations [331].
Improved biomarker development represents another methodological priority [332]. Blood-based markers reflecting neuroplasticity, neuroinflammation, and neurodegeneration could provide accessible monitoring tools for exercise efficacy [333]. Promising candidates include exosomal microRNAs, protein panels measuring neurotrophic factors and cytokines, and metabolites reflecting brain energy metabolism [334]. The validation of these markers against direct brain measures and clinical outcomes remains essential [335].
Animal models that better recapitulate human neurodegenerative conditions could enhance mechanistic insights with greater translational relevance [336]. Emerging genetic models incorporating multiple pathological features and age-related changes may provide more clinically relevant platforms for testing exercise interventions [337]. Additionally, models allowing voluntary versus forced exercise comparisons could help disentangle stress-related confounds from direct physical activity benefits [338].
8.3. Novel Biomarkers of Exercise-Induced Neuroplasticity
Developing reliable, sensitive biomarkers of exercise-induced neuroplasticity represents a crucial research priority with significant clinical implications [339]. These biomarkers could serve multiple purposes: identifying individuals likely to benefit from specific exercise regimens, monitoring intervention efficacy, and providing early indicators of neuroprotection before clinical symptoms emerge [340].
Blood-based biomarkers offer practical advantages for longitudinal monitoring [341]. Recent advances in blood sampling techniques have enabled the detection of brain-derived extracellular vesicles carrying proteins, lipids, and microRNAs that reflect central nervous system states [342]. Exercise-responsive microRNAs, particularly those involved in BDNF regulation, synaptic plasticity, and mitochondrial biogenesis, represent promising candidates for monitoring neuroplastic responses [343]. Similarly, proteomic panels measuring multiple neurotrophic factors, inflammatory markers, and metabolic regulators may provide more comprehensive assessment than single-protein approaches [331].
Neurophysiological measures including electroencephalography (EEG) and transcranial magnetic stimulation (TMS) offer direct assessment of functional neuroplasticity with high temporal resolution [323]. Event-related potentials during cognitive tasks show sensitivity to exercise interventions and correlate with performance improvements. Similarly, TMS protocols measuring cortical excitability, intracortical inhibition, and late cortical disinhibition detect neuroplastic changes following both acute and chronic exercise. These techniques could provide more proximal measures of brain adaptation than behavioral outcomes alone [326].
Digital biomarkers derived from wearable technology and smartphone-based assessment are emerging as scalable, ecologically valid measurement approaches [341]. Continuous monitoring of gait parameters, sleep quality, heart rate variability, and daily activity patterns may capture subtle changes preceding clinical improvement. Similarly, frequent brief cognitive assessments via smartphone applications can detect trajectory changes with greater sensitivity than infrequent laboratory testing [329]. The combination of passive monitoring with active assessment offers a particularly rich characterization of exercise effects across contexts.
The integration of multiple biomarker modalities through advanced analytical approaches represents a promising direction for capturing exercise-induced neuroplasticity comprehensively [330]. Machine learning methods can identify patterns across biomarker types that predict individual responses more accurately than single measures [331]. Longitudinal modeling approaches incorporating repeated biomarker assessments may detect subtle changes and individual trajectories missed by traditional group-level analyses.
8.4. Precision Approaches to Prevention
Advancing toward precision prevention approaches represents a critical frontier in exercise research for neurodegenerative disease. Initial efforts should focus on identifying responder phenotypes—characteristic profiles of individuals who show robust neuroplastic and cognitive responses to specific exercise modalities. Large dataset analyses incorporating genetic, physiological, neuroimaging, and behavioral measures could reveal patterns predicting differential responsiveness [334]. These analyses would ideally include diverse populations across ages, comorbidity profiles, and genetic risk factors to capture heterogeneity comprehensively.
Adaptive intervention designs offer promising frameworks for developing personalized exercise prescriptions. Sequential Multiple Assignment Randomized Trial (SMART) designs allow for the systematic optimization of intervention sequences based on individual responses [219]. Similarly, Multiphase Optimization Strategy (MOST) approaches can efficiently test multiple intervention components to identify optimal combinations for specific populations [338]. These experimental paradigms could determine which exercise parameters (modality, intensity, timing) should be adjusted based on which individual characteristics.
Digital health technologies enable dynamic prescription approaches that continuously adjust based on real-time monitoring [341]. Closed-loop systems incorporating wearable sensors, smartphone-based cognitive assessment, and algorithm-driven prescription adjustments could optimize exercise parameters on an ongoing basis. These approaches might be particularly valuable for maintaining effectiveness during changing health states, motivation levels, or environmental circumstances.
The integration of exercise with other interventions through precision approaches represents another promising direction. Nutritional supplements, cognitive training, sleep optimization, and stress management might show synergistic effects with exercise in specific populations. Factorial experimental designs can efficiently test these combinations while identifying interaction effects that inform optimal multimodal regimens. For example, omega-3 supplementation might enhance exercise benefits specifically in APOE ε4 carriers, while cognitive–motor dual-tasks might provide optimal benefits for those with particular cognitive profiles [281].
The future research directions outlined here highlight the multidisciplinary nature of exercise–neuroplasticity research and its potential for advancing neurodegenerative disease prevention. By addressing mechanistic gaps, refining methodological approaches, developing sensitive biomarkers, and pursuing precision prevention strategies, researchers can enhance our understanding of exercise-induced neuroplasticity while optimizing its translation into effective preventive interventions. These advances may ultimately contribute to reducing the growing burden of neurodegenerative diseases through evidence-based, personalized exercise approaches.
9. Conclusions
Exercise-induced neuroplasticity represents a promising avenue for neurodegenerative disease prevention, supported by converging evidence from molecular, animal, and human studies. This narrative review has synthesized current knowledge across multiple domains, revealing the complex and multifaceted mechanisms through which physical activity may confer neuroprotection and enhance resilience against neurodegenerative processes.
The key contribution of this review is the synthesis of evidence across multiple domains—from molecular pathways to clinical applications—establishing exercise as a unique multi-target intervention for neurodegenerative disease prevention. By connecting mechanistic insights with practical implications, we provide a translational framework that distinguishes our approach from previous reviews that have often focused on isolated aspects of exercise effects. Our integration of recent advances in understanding epigenetic modifications, metabolic signaling, neuroimmune interactions, and precision approaches offers a comprehensive foundation for developing evidence-based, personalized exercise prescriptions tailored to specific neurodegenerative risk profiles.
The molecular mediators of exercise-induced neuroplasticity—including neurotrophic factors, neuroendocrine responses, epigenetic modifications, and metabolic signaling pathways—create a molecular environment conducive to neuronal health and function. These pathways support structural and functional adaptations, including enhanced hippocampal neurogenesis, synaptic plasticity, cerebrovascular function, and network connectivity. Meanwhile, exercise’s effects on neuroimmune and inflammatory pathways help maintain a balanced inflammatory milieu that supports neuroplasticity and opposes chronic neuroinflammation associated with neurodegenerative conditions.
Particularly compelling is exercise’s direct impact on pathological features of neurodegenerative diseases. In reducing amyloid and tau pathology, mitigating α-synuclein aggregation, attenuating excitotoxicity and oxidative stress, and enhancing mitochondrial function, physical activity appears to address multiple disease mechanisms simultaneously. This multi-target approach contrasts with most pharmacological strategies focused on single pathways and may explain exercise’s broad efficacy across conditions.
Clinical and epidemiological evidence consistently demonstrates associations between physical activity and reduced neurodegenerative disease risk, with randomized controlled trials supporting causal benefits on cognitive function and brain structure. The apparent dose–response relationships and modality considerations highlighted in this review provide a foundation for evidence-based exercise prescriptions tailored to neurodegenerative prevention.
Translating this knowledge into effective prevention strategies requires attention to personalization approaches, integration with complementary lifestyle factors, and implementation strategies that address adherence barriers. Future research should focus on filling mechanistic gaps, developing sensitive biomarkers of exercise-induced neuroplasticity, and advancing precision approaches that optimize interventions for individual characteristics and risk profiles.
Exercise represents a cost-effective, accessible intervention with minimal side effects and multiple health benefits beyond brain function. Its potential to modify neurodegenerative disease trajectories warrants increased attention in clinical practice, public health initiatives, and research prioritization. By advancing our understanding of exercise-induced neuroplasticity and optimizing its implementation, we may develop more effective strategies to reduce the growing burden of neurodegenerative diseases in our aging population.
Author Contributions
M.M., A.I., A.H., T.T., E.C. and J.B. contributed equally to this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Ethical review and approval were waived for this study due to the narrative review nature of the study.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon reasonable request to the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript: [157]
| AD | Alzheimer’s Disease; |
| ALS | Amyotrophic Lateral Sclerosis; |
| AMPK | AMP-Activated Protein Kinase; |
| Aβ | Amyloid-beta; |
| BDNF | Brain-Derived Neurotrophic Factor; |
| BBB | Blood–Brain Barrier; |
| CRP | C-reactive Protein; |
| DMN | Default Mode Network; |
| EEG | Electroencephalography; |
| FNDC5 | Fibronectin Type III Domain-Containing Protein 5; |
| FOXO | Forkhead Box O; |
| GDNF | Glial Cell Line-Derived Neurotrophic Factor; |
| GLUT-1 | Glucose Transporter 1; |
| GSK-3β | Glycogen Synthase Kinase-3β; |
| HD | Huntington’s Disease; |
| HPA | Hypothalamic–Pituitary–Adrenal; |
| HSPs | Heat Shock Proteins; |
| IDE | Insulin-Degrading Enzyme; |
| IGF-1 | Insulin-like Growth Factor-1; |
| IL-1β | Interleukin-1 beta; |
| IL-1ra | Interleukin-1 Receptor Antagonist; |
| IL-6 | Interleukin-6; |
| IL-10 | Interleukin-10; |
| LTD | Long-Term Depression; |
| LTP | Long-Term Potentiation; |
| MAPK | Mitogen-Activated Protein Kinase; |
| MCI | Mild Cognitive Impairment; |
| MOST | Multiphase Optimization Strategy; |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; |
| mTOR | Mammalian Target of Rapamycin; |
| NMDA | N-methyl-D-aspartate; |
| PAR-Q+ | Physical Activity Readiness Questionnaire; |
| PD | Parkinson’s Disease; |
| PET | Positron Emission Tomography; |
| PGC-1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-alpha; |
| P13K | Phosphatidylinositol 3-Kinase; |
| PLC-γ | Phospholipase C-gamma; |
| PP2A | Protein Phosphatase 2A; |
| PSD-95 | Postsynaptic Density Protein 95; |
| RCT | Randomized Controlled Trial; |
| ROS | Reactive Oxygen Species; |
| SIRT1 | Sirtuin 1; |
| SMART | Sequential Multiple Assignment Randomized Trial; |
| SOD | Superoxide Dismutase; |
| TMS | Transcranial Magnetic Stimulation; |
| TNF-α | Tumor Necrosis Factor-alpha; |
| TrkB | Tropomyosin Receptor Kinase B; |
| VEGF | Vascular Endothelial Growth Factor; |
| WMH | White Matter Hyperintensities; |
| α-syn | Alpha-Synuclein. |
References
- Peplow, P.V.; Martinez, B.; Gennarelli, T.A. Prevalence, Needs, Strategies, and Risk Factors for Neurodegenerative Diseases. In Neurodegenerative Diseases Biomarkers: Towards Translating Research to Clinical Practice; Peplow, P.V., Martinez, B., Gennarelli, T.A., Eds.; Springer: New York, NY, USA, 2022; pp. 3–8. [Google Scholar] [CrossRef]
- Zaib, S.; Javed, H.; Khan, I.; Jaber, F.; Sohail, A.; Zaib, Z.; Mehboob, T.; Tabassam, N.; Ogaly, H.A. Neurodegenerative Diseases: Their Onset, Epidemiology, Causes and Treatment. ChemistrySelect 2023, 8, e202300225. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.-A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef]
- Voss, M.W.; Vivar, C.; Kramer, A.F.; van Praag, H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci. 2013, 17, 525–544. [Google Scholar] [CrossRef]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of Physical Exercise on Cognitive Functioning and Wellbeing: Biological and Psychological Benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef]
- Joshua, A.M. Neuroplasticity. In Physiotherapy for Adult Neurological Conditions; Joshua, A.M., Ed.; Springer Nature: Singapore, 2022; pp. 1–30. [Google Scholar] [CrossRef]
- Spielman, L.J.; Little, J.P.; Klegeris, A. Physical activity and exercise attenuate neuroinflammation in neurological diseases. Brain Res. Bull. 2016, 125, 19–29. [Google Scholar] [CrossRef]
- Cass, S.P. Alzheimer’s Disease and Exercise: A Literature Review. Curr. Sports Med. Rep. 2017, 16, 19. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. S3), 1–72. [Google Scholar] [CrossRef]
- Duzel, E.; van Praag, H.; Sendtner, M. Can physical exercise in old age improve memory and hippocampal function? Brain 2016, 139, 662–673. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. eLife 2016, 5, e15092. [Google Scholar] [CrossRef]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Liu, C.S.; Sherman, C.; Chan, S.; Lanctôt, K.L. The Effect of Exercise Training on Resting Concentrations of Peripheral Brain-Derived Neurotrophic Factor (BDNF): A Meta-Analysis. PLoS ONE 2016, 11, e0163037. [Google Scholar] [CrossRef]
- Schmolesky, M.T.; Webb, D.L.; Hansen, R.A. The Effects of Aerobic Exercise Intensity and Duration on Levels of Brain-Derived Neurotrophic Factor in Healthy Men. J. Sports Sci. Med. 2013, 12, 502–511. [Google Scholar] [PubMed]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Trejo, J.L.; Carro, E.; Torres-Alemán, I. Circulating Insulin-Like Growth Factor I Mediates Exercise-Induced Increases in the Number of New Neurons in the Adult Hippocampus. J. Neurosci. 2001, 21, 1628–1634. [Google Scholar] [CrossRef]
- Ding, Q.; Vaynman, S.; Akhavan, M.; Ying, Z.; Gomez-Pinilla, F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 2006, 140, 823–833. [Google Scholar] [CrossRef]
- Morland, C.; Andersson, K.A.; Haugen, Ø.P.; Hadzic, A.; Kleppa, L.; Gille, A.; Rinholm, J.E.; Palibrk, V.; Diget, E.H.; Kennedy, L.H.; et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat. Commun. 2017, 8, 15557. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Nakagawa, S.; An, Y.; Ito, K.; Kitaichi, Y.; Kusumi, I. The exercise-glucocorticoid paradox: How exercise is beneficial to cognition, mood, and the brain while increasing glucocorticoid levels. Front. Neuroendocrinol. 2017, 44, 83–102. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Lee, K.; Mattson, M.P. Central Mechanisms of HPA Axis Regulation by Voluntary Exercise. Neuromol. Med. 2008, 10, 118–127. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise Induces Hippocampal BDNF through a PGC-1α/FNDC5 Pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef] [PubMed]
- McMorris, T. Developing the catecholamines hypothesis for the acute exercise-cognition interaction in humans: Lessons from animal studies. Physiol. Behav. 2016, 165, 291–299. [Google Scholar] [CrossRef]
- Fernandes, J.; Arida, R.M.; Gomez-Pinilla, F. Physical exercise as an epigenetic modulator of brain plasticity and cognition. Neurosci. Biobehav. Rev. 2017, 80, 443–456. [Google Scholar] [CrossRef]
- Ieraci, A.; Mallei, A.; Musazzi, L.; Popoli, M. Physical exercise and acute restraint stress differentially modulate hippocampal brain-derived neurotrophic factor transcripts and epigenetic mechanisms in mice. Hippocampus 2015, 25, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Elsner, V.R.; Lovatel, G.A.; Bertoldi, K.; Vanzella, C.; Santos, F.M.; Spindler, C.; de Almeida, E.F.; Nardin, P.; Siqueira, I.R. Effect of different exercise protocols on histone acetyltransferases and histone deacetylases activities in rat hippocampus. Neuroscience 2011, 192, 580–587. [Google Scholar] [CrossRef]
- Danese, A.; McEwen, B.S. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol. Behav. 2012, 106, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Marosi, K.; Mattson, M.P. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol. Metab. 2014, 25, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.L.; Murphy, E.A.; McClellan, J.L.; Carmichael, M.D.; Davis, J.M. Exercise training increases mitochondrial biogenesis in the brain. J. Appl. Physiol. 2011, 111, 1066–1071. [Google Scholar] [CrossRef]
- Huffman, D.M.; Schafer, M.J.; LeBrasseur, N.K. Energetic interventions for healthspan and resiliency with aging. Exp. Gerontol. 2016, 86, 73–83. [Google Scholar] [CrossRef]
- Pilegaard, H.; Saltin, B.; Neufer, P.D. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J. Physiol. 2003, 546, 851–858. [Google Scholar] [CrossRef]
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; de la Monte, S.; et al. Brain energy rescue: An emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633. [Google Scholar] [CrossRef]
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent metabolic switching, neuroplasticity and brain health. Nat. Rev. Neurosci. 2018, 19, 81–94. [Google Scholar] [CrossRef]
- van Praag, H.; Kempermann, G.; Gage, F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999, 2, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Nokia, M.S.; Lensu, S.; Ahtiainen, J.P.; Johansson, P.P.; Koch, L.G.; Britton, S.L.; Kainulainen, H. Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J. Physiol. 2016, 594, 1855–1873. [Google Scholar] [CrossRef]
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 2019, 24, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Okamoto, M.; Shibato, J.; Lee, M.C.; Matsui, T.; Rakwal, R.; Soya, H. Long-Term Mild, rather than Intense, Exercise Enhances Adult Hippocampal Neurogenesis and Greatly Changes the Transcriptomic Profile of the Hippocampus. PLoS ONE 2015, 10, e0128720. [Google Scholar] [CrossRef]
- Vivar, C.; Potter, M.C.; van Praag, H. All About Running: Synaptic Plasticity, Growth Factors and Adult Hippocampal Neurogenesis. In Neurogenesis and Neural Plasticity; Belzung, C., Wigmore, P., Eds.; Springer: Berlin, Heidelberg, 2013; pp. 189–210. ISBN 978-3-642-36232-3. [Google Scholar]
- Fabel, K.; Fabel, K.; Tam, B.; Kaufer, D.; Baiker, A.; Simmons, N.; Kuo, C.J.; Palmer, T.D. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci. 2003, 18, 2803–2812. [Google Scholar] [CrossRef]
- Pereira, A.C.; Huddleston, D.E.; Brickman, A.M.; Sosunov, A.A.; Hen, R.; McKhann, G.M.; Sloan, R.; Gage, F.H.; Brown, T.R.; Small, S.A. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. USA 2007, 104, 5638–5643. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Ryan, S.M.; Kelly, Á.M. Exercise as a pro-cognitive, pro-neurogenic and anti-inflammatory intervention in transgenic mouse models of Alzheimer’s disease. Ageing Res. Rev. 2016, 27, 77–92. [Google Scholar] [CrossRef]
- Yau, S.; Gil-Mohapel, J.; Christie, B.R.; So, K. Physical exercise-induced adult neurogenesis: A good strategy to prevent cognitive decline in neurodegenerative diseases? Biomed. Res. Int. 2014, 2014, 403120. [Google Scholar] [CrossRef]
- Molteni, R.; Ying, Z.; Gómez-Pinilla, F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur. J. Neurosci. 2002, 16, 1107–1116. [Google Scholar] [CrossRef]
- van Praag, H.; Christie, B.R.; Sejnowski, T.J.; Gage, F.H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 13427–13431. [Google Scholar] [CrossRef]
- Vaynman, S.; Ying, Z.; Gomez-Pinilla, F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur. J. Neurosci. 2004, 20, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Stranahan, A.M.; Khalil, D.; Gould, E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus 2007, 17, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Eadie, B.D.; Redila, V.A.; Christie, B.R. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J. Comp. Neurol. 2005, 486, 39–47. [Google Scholar] [CrossRef]
- García-Mesa, Y.; López-Ramos, J.C.; Giménez-Llort, L.; Revilla, S.; Guerra, R.; Gruart, A.; Laferla, F.M.; Cristòfol, R.; Delgado-García, J.M.; Sanfeliu, C. Physical exercise protects against Alzheimer’s disease in 3xTg-AD mice. J. Alzheimers Dis. 2011, 24, 421–454. [Google Scholar] [CrossRef] [PubMed]
- Adlard, P.A.; Perreau, V.M.; Pop, V.; Cotman, C.W. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J. Neurosci. 2005, 25, 4217–4221. [Google Scholar] [CrossRef]
- Toy, W.A.; Petzinger, G.M.; Leyshon, B.J.; Akopian, G.K.; Walsh, J.P.; Hoffman, M.V.; Vučković, M.G.; Jakowec, M.W. Treadmill exercise reverses dendritic spine loss in direct and indirect striatal medium spiny neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. Neurobiol. Dis. 2014, 63, 201–209. [Google Scholar] [CrossRef]
- Swain, R.A.; Harris, A.B.; Wiener, E.C.; Dutka, M.V.; Morris, H.D.; Theien, B.E.; Konda, S.; Engberg, K.; Lauterbur, P.C.; Greenough, W.T. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience 2003, 117, 1037–1046. [Google Scholar] [CrossRef]
- Bolduc, V.; Thorin-Trescases, N.; Thorin, E. Endothelium-dependent control of cerebrovascular functions through age: Exercise for healthy cerebrovascular aging. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H620–H633. [Google Scholar] [CrossRef]
- Barnes, J.N.; Taylor, J.L.; Kluck, B.N.; Johnson, C.P.; Joyner, M.J. Cerebrovascular reactivity is associated with maximal aerobic capacity in healthy older adults. J. Appl. Physiol. 2013, 114, 1383–1387. [Google Scholar] [CrossRef]
- Bailey, D.M.; Marley, C.J.; Brugniaux, J.V.; Hodson, D.; New, K.J.; Ogoh, S.; Ainslie, P.N. Elevated Aerobic Fitness Sustained Throughout the Adult Lifespan Is Associated With Improved Cerebral Hemodynamics. Stroke 2013, 44, 3235–3238. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; DeCarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular Contributions to Cognitive Impairment and Dementia. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- He, J.; Hsuchou, H.; He, Y.; Kastin, A.J.; Wang, Y.; Pan, W. Sleep Restriction Impairs Blood–Brain Barrier Function. J. Neurosci. 2014, 34, 14697–14706. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Chida, Y. Physical activity and risk of neurodegenerative disease: A systematic review of prospective evidence. Psychol. Med. 2009, 39, 3–11. [Google Scholar] [CrossRef]
- Brown, A.D.; McMorris, C.A.; Longman, R.S.; Leigh, R.; Hill, M.D.; Friedenreich, C.M.; Poulin, M.J. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol. Aging 2010, 31, 2047–2057. [Google Scholar] [CrossRef]
- Burdette, J.H.; Laurienti, P.J.; Espeland, M.A.; Morgan, A.R.; Telesford, Q.; Vechlekar, C.D.; Hayaska, S.; Jennings, J.J.; Katula, J.A.; Kraft, R.A.; et al. Using network science to evaluate exercise-associated brain changes in older adults. Front. Aging Neurosci. 2010, 2, 1695. [Google Scholar] [CrossRef]
- Voss, M.W.; Erickson, K.I.; Prakash, R.S.; Chaddock, L.; Malkowski, E.; Alves, H.; Kim, J.S.; Morris, K.S.; White, S.M.; Wójcicki, T.R.; et al. Functional connectivity: A source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia 2010, 48, 1394–1406. [Google Scholar] [CrossRef] [PubMed]
- Chirles, T.J.; Reiter, K.; Weiss, L.R.; Alfini, A.J.; Nielson, K.A.; Smith, J.C. Exercise Training and Functional Connectivity Changes in Mild Cognitive Impairment and Healthy Elders. J. Alzheimer Dis. 2017, 57, 845–856. [Google Scholar] [CrossRef]
- Buckner, R.L.; Sepulcre, J.; Talukdar, T.; Krienen, F.M.; Liu, H.; Hedden, T.; Andrews-Hanna, J.R.; Sperling, R.A.; Johnson, K.A. Cortical Hubs Revealed by Intrinsic Functional Connectivity: Mapping, Assessment of Stability, and Relation to Alzheimer’s Disease. J. Neurosci. 2009, 29, 1860–1873. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.W.; Weng, T.B.; Burzynska, A.Z.; Wong, C.N.; Cooke, G.E.; Clark, R.; Fanning, J.; Awick, E.; Gothe, N.P.; Olson, E.A.; et al. Fitness, but not physical activity, is related to functional integrity of brain networks associated with aging. NeuroImage 2016, 131, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Best, J.R.; Davis, J.C.; Nagamatsu, L.S.; Wang, S.; Boyd, L.A.; Hsiung, G.R.; Voss, M.W.; Eng, J.J.; Liu-Ambrose, T. Aerobic exercise promotes executive functions and impacts functional neural activity among older adults with vascular cognitive impairment. Br. J. Sports Med. 2018, 52, 184–191. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Ford, J.M. Default Mode Network Activity and Connectivity in Psychopathology. Annu. Rev. Clin. Psychol. 2012, 8, 49–76. [Google Scholar] [CrossRef]
- Sexton, C.E.; Betts, J.F.; Demnitz, N.; Dawes, H.; Ebmeier, K.P.; Johansen-Berg, H. A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. NeuroImage 2016, 131, 81–90. [Google Scholar] [CrossRef]
- Johnson, N.F.; Kim, C.; Clasey, J.L.; Bailey, A.; Gold, B.T. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. NeuroImage 2012, 59, 1514–1523. [Google Scholar] [CrossRef]
- Burzynska, A.Z.; Chaddock-Heyman, L.; Voss, M.W.; Wong, C.N.; Gothe, N.P.; Olson, E.A.; Knecht, A.; Lewis, A.; Monti, J.M.; Cooke, G.E.; et al. Physical Activity and Cardiorespiratory Fitness Are Beneficial for White Matter in Low-Fit Older Adults. PLoS ONE 2014, 9, e107413. [Google Scholar] [CrossRef]
- Tseng, B.Y.; Uh, J.; Rossetti, H.C.; Cullum, C.M.; Diaz-Arrastia, R.F.; Levine, B.D.; Lu, H.; Zhang, R. Masters athletes exhibit larger regional brain volume and better cognitive performance than sedentary older adults. J. Magn. Reson. Imaging 2013, 38, 1169–1176. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Flynn, M.G.; McFarlin, B.K.; Markofski, M.M. State of the Art Reviews: The Anti-Inflammatory Actions of Exercise Training. Am. J. Lifestyle Med. 2007, 1, 220–235. [Google Scholar] [CrossRef]
- Woods, J.A.; Wilund, K.R.; Martin, S.A.; Kistler, B.M. Exercise, Inflammation and Aging. Aging Dis. 2011, 3, 130–140. [Google Scholar]
- Petersen, A.M.W.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Kohman, R.A.; Bhattacharya, T.K.; Wojcik, E.; Rhodes, J.S. Exercise reduces activation of microglia isolated from hippocampus and brain of aged mice. J. Neuroinflammation 2013, 10, 885. [Google Scholar] [CrossRef]
- Koo, J.-H.; Jang, Y.-C.; Hwang, D.-J.; Um, H.-S.; Lee, N.-H.; Jung, J.-H.; Cho, J.-Y. Treadmill exercise produces neuroprotective effects in a murine model of Parkinson’s disease by regulating the TLR2/MyD88/NF-κB signaling pathway. Neuroscience 2017, 356, 102–113. [Google Scholar] [CrossRef]
- Jensen, C.S.; Bahl, J.M.; Østergaard, L.B.; Høgh, P.; Wermuth, L.; Heslegrave, A.; Zetterberg, H.; Heegaard, N.H.H.; Hasselbalch, S.G.; Simonsen, A.H. Exercise as a potential modulator of inflammation in patients with Alzheimer’s disease measured in cerebrospinal fluid and plasma. Exp. Gerontol. 2019, 121, 91–98. [Google Scholar] [CrossRef]
- Pedersen, B.K. Exercise-induced myokines and their role in chronic diseases. Brain Behav. Immun. 2011, 25, 811–816. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Martin, B.; Maudsley, S. Anti-Inflammatory Effects of Physical Activity in Relationship to Improved Cognitive Status in Humans and Mouse Models of Alzheimer’s Disease. Curr. Alzheimer Res. 2012, 9, 86–92. [Google Scholar] [CrossRef]
- Kang, E.-B.; Cho, J.-Y. Effect of treadmill exercise on PI3K/AKT/mTOR, autophagy, and Tau hyperphosphorylation in the cerebral cortex of NSE/htau23 transgenic mice. J. Exerc. Nutrition Biochem. 2015, 19, 199–209. [Google Scholar] [CrossRef]
- Kadoglou, N.P.; Iliadis, F.; Angelopoulou, N.; Perrea, D.; Ampatzidis, G.; Liapis, C.D.; Alevizos, M. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 837–843. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Mee-inta, O.; Zhao, Z.-W.; Kuo, Y.-M. Physical Exercise Inhibits Inflammation and Microglial Activation. Cells 2019, 8, 691. [Google Scholar] [CrossRef]
- Littlefield, A.M.; Setti, S.E.; Priester, C.; Kohman, R.A. Voluntary exercise attenuates LPS-induced reductions in neurogenesis and increases microglia expression of a proneurogenic phenotype in aged mice. J. Neuroinflammation 2015, 12, 138. [Google Scholar] [CrossRef]
- Vukovic, J.; Colditz, M.J.; Blackmore, D.G.; Ruitenberg, M.J.; Bartlett, P.F. Microglia Modulate Hippocampal Neural Precursor Activity in Response to Exercise and Aging. J. Neurosci. 2012, 32, 6435–6443. [Google Scholar] [CrossRef]
- Barrientos, R.M.; Frank, M.G.; Crysdale, N.Y.; Chapman, T.R.; Ahrendsen, J.T.; Day, H.E.W.; Campeau, S.; Watkins, L.R.; Patterson, S.L.; Maier, S.F. Little Exercise, Big Effects: Reversing Aging and Infection-Induced Memory Deficits, and Underlying Processes. J. Neurosci. 2011, 31, 11578–11586. [Google Scholar] [CrossRef]
- Nichol, K.E.; Poon, W.W.; Parachikova, A.I.; Cribbs, D.H.; Glabe, C.G.; Cotman, C.W. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J. Neuroinflammation 2008, 5, 13. [Google Scholar] [CrossRef]
- Brown, B.M.; Rainey-Smith, S.R.; Dore, V.; Peiffer, J.J.; Burnham, S.C.; Laws, S.M.; Taddei, K.; Ames, D.; Masters, C.L.; Rowe, C.C.; et al. Self-Reported Physical Activity is Associated with Tau Burden Measured by Positron Emission Tomography. J. Alzheimer Dis. 2018, 63, 1299–1305. [Google Scholar] [CrossRef]
- Wang, J.; Song, H.; Tang, X.; Yang, Y.; Vieira, V.J.; Niu, Y.; Ma, Y. Effect of exercise training intensity on murine T-regulatory cells and vaccination response. Scand. J. Med. Sci. Sports 2012, 22, 643–652. [Google Scholar] [CrossRef]
- Lowder, T.; Dugger, K.; Deshane, J.; Estell, K.; Schwiebert, L.M. Repeated bouts of aerobic exercise enhance regulatory T cell responses in a murine asthma model. Brain Behav. Immun. 2010, 24, 153–159. [Google Scholar] [CrossRef]
- Svensson, M.; Lexell, J.; Deierborg, T. Effects of Physical Exercise on Neuroinflammation, Neuroplasticity, Neurodegeneration, and Behavior: What We Can Learn From Animal Models in Clinical Settings. Neurorehabil. Neural Repair 2015, 29, 577–589. [Google Scholar] [CrossRef]
- Eyre, H.A.; Papps, E.; Baune, B.T. Treating Depression and Depression-Like Behavior with Physical Activity: An Immune Perspective. Front. Psychiatry 2013, 4, 41561. [Google Scholar] [CrossRef]
- Slusher, A.L.; Zúñiga, T.M.; Acevedo, E.O. Maximal Exercise Alters the Inflammatory Phenotype and Response of Mononuclear Cells. Med. Sci. Sports Exerc. 2018, 50, 675–683. [Google Scholar] [CrossRef]
- McAfoose, J.; Baune, B.T. Evidence for a cytokine model of cognitive function. Neurosci. Biobehav. Rev. 2009, 33, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.A. Interleukin-1β exerts a myriad of effects in the brain and in particular in the hippocampus: Analysis of some of these actions. In Vitamins & Hormones; Academic Press: Cambridge, MA, USA, 2002; Volume 64, pp. 185–219. [Google Scholar] [CrossRef]
- Clark, I.A.; Alleva, L.M.; Vissel, B. The roles of TNF in brain dysfunction and disease. Pharmacol. Ther. 2010, 128, 519–548. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Gomez-Cabrera, M.-C.; Vina, J. Exercise and Hormesis. Ann. N. Y. Acad. Sci. 2006, 1067, 425–435. [Google Scholar] [CrossRef]
- Radak, Z.; Chung, H.Y.; Goto, S. Exercise and hormesis: Oxidative stress-related adaptation for successful aging. Biogerontology 2005, 6, 71–75. [Google Scholar] [CrossRef]
- Mattson, M.P.; Leak, R.K. The hormesis principle of neuroplasticity and neuroprotection. Cell Metab. 2024, 36, 315–337. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. The Blood-Brain Barrier in Health and Chronic Neurodegenerative Disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef]
- Małkiewicz, M.A.; Szarmach, A.; Sabisz, A.; Cubała, W.J.; Szurowska, E.; Winklewski, P.J. Blood-brain barrier permeability and physical exercise. J. Neuroinflammation 2019, 16, 15. [Google Scholar] [CrossRef]
- Hamilton, N.B.; Attwell, D.; Hall, C.N. Pericyte-mediated regulation of capillary diameter: A component of neurovascular coupling in health and disease. Front. Neuroenergetics 2010, 2, 1453. [Google Scholar] [CrossRef]
- Yeh, S.-H.; Chuang, H.; Lin, L.-W.; Hsiao, C.-Y.; Eng, H.L. Regular tai chi chuan exercise enhances functional mobility and CD4CD25 regulatory T cells. Br. J. Sports Med. 2006, 40, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Erdő, F.; Denes, L.; de Lange, E. Age-associated physiological and pathological changes at the blood–brain barrier: A review. J. Cereb. Blood Flow Metab. 2017, 37, 4–24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef]
- Jang, Y.; Koo, J.-H.; Kwon, I.; Kang, E.-B.; Um, H.-S.; Soya, H.; Lee, Y.; Cho, J.-Y. Neuroprotective effects of endurance exercise against neuroinflammation in MPTP-induced Parkinson’s disease mice. Brain Res. 2017, 1655, 186–193. [Google Scholar] [CrossRef]
- Sorriento, D.; Di Vaia, E.; Iaccarino, G. Physical Exercise: A Novel Tool to Protect Mitochondrial Health. Front. Physiol. 2021, 12, 660068. [Google Scholar] [CrossRef]
- Villar-Cheda, B.; Sousa-Ribeiro, D.; Rodriguez-Pallares, J.; Rodriguez-Perez, A.I.; Guerra, M.J.; Labandeira-Garcia, J.L. Aging and Sedentarism Decrease Vascularization and VEGF Levels in the Rat Substantia Nigra. Implications for Parkinson’s Disease. J. Cereb. Blood Flow Metab. 2009, 29, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.-H.; Li, J.; Zhou, Y.; Rafols, J.A.; Clark, J.C.; Ding, Y. Cerebral Angiogenesis and Expression of Angiogenic Factors in Aging Rats after Exercise. Curr. Neurovascular Res. 2006, 3, 15–23. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, H.L.; Zhang, H.; Tong, X.J. Treadmill exercise enhances synaptic plasticity, but does not alter β-amyloid deposition in hippocampi of aged APP/PS1 transgenic mice. Neuroscience 2015, 298, 357–366. [Google Scholar] [CrossRef]
- Lin, T.-W.; Shih, Y.-H.; Chen, S.-J.; Lien, C.-H.; Chang, C.-Y.; Huang, T.-Y.; Chen, S.-H.; Jen, C.J.; Kuo, Y.-M. Running exercise delays neurodegeneration in amygdala and hippocampus of Alzheimer’s disease (APP/PS1) transgenic mice. Neurobiol. Learn. Mem. 2015, 118, 189–197. [Google Scholar] [CrossRef]
- Brown, B.M.; Peiffer, J.J.; Martins, R.N. Multiple effects of physical activity on molecular and cognitive signs of brain aging: Can exercise slow neurodegeneration and delay Alzheimer’s disease? Mol. Psychiatry 2013, 18, 864–874. [Google Scholar] [CrossRef]
- Lazarov, O.; Robinson, J.; Tang, Y.-P.; Hairston, I.S.; Korade-Mirnics, Z.; Lee, V.M.-Y.; Hersh, L.B.; Sapolsky, R.M.; Mirnics, K.; Sisodia, S.S. Environmental Enrichment Reduces Aβ Levels and Amyloid Deposition in Transgenic Mice. Cell 2005, 120, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Maesako, M.; Uemura, K.; Kubota, M.; Kuzuya, A.; Sasaki, K.; Hayashida, N.; Asada-Utsugi, M.; Watanabe, K.; Uemura, M.; Kihara, T.; et al. Exercise Is More Effective than Diet Control in Preventing High Fat Diet-induced β-Amyloid Deposition and Memory Deficit in Amyloid Precursor Protein Transgenic Mice. J. Biol. Chem. 2012, 287, 23024–23033. [Google Scholar] [CrossRef]
- Kress, B.T.; Iliff, J.J.; Xia, M.; Wang, M.; Wei, H.S.; Zeppenfeld, D.; Xie, L.; Kang, H.; Xu, Q.; Liew, J.A.; et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014, 76, 845–861. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, D.; Zhang, Q.; Liang, F.; Dai, G.; Zeng, J.; Pei, Z.; Xu, G.; Lan, Y. Voluntary Exercise Promotes Glymphatic Clearance of Amyloid Beta and Reduces the Activation of Astrocytes and Microglia in Aged Mice. Front. Mol. Neurosci. 2017, 10, 144. [Google Scholar] [CrossRef]
- Tapia-Rojas, C.; Aranguiz, F.; Varela-Nallar, L.; Inestrosa, N.C. Voluntary Running Attenuates Memory Loss, Decreases Neuropathological Changes and Induces Neurogenesis in a Mouse Model of Alzheimer’s Disease. Brain Pathol. 2016, 26, 62–74. [Google Scholar] [CrossRef]
- Leem, Y.-H.; Lim, H.-J.; Shim, S.-B.; Cho, J.-Y.; Kim, B.-S.; Han, P.-L. Repression of tau hyperphosphorylation by chronic endurance exercise in aged transgenic mouse model of tauopathies. J. Neurosci. Res. 2009, 87, 2561–2570. [Google Scholar] [CrossRef]
- Belarbi, K.; Burnouf, S.; Fernandez-Gomez, F.-J.; Laurent, C.; Lestavel, S.; Figeac, M.; Sultan, A.; Troquier, L.; Leboucher, A.; Caillierez, R.; et al. Beneficial effects of exercise in a transgenic mouse model of Alzheimer’s disease-like Tau pathology. Neurobiol. Dis. 2011, 43, 486–494. [Google Scholar] [CrossRef]
- Um, H.S.; Kang, E.B.; Leem, Y.H.; Cho, I.H.; Yang, C.H.; Chae, K.R.; Hwang, D.Y.; Cho, J.Y. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer’s disease in an NSE/APPsw-transgenic model. Int. J. Mol. Med. 2008, 22, 529–539. [Google Scholar] [CrossRef]
- Marques-Aleixo, I.; Santos-Alves, E.; Balça, M.M.; Rizo-Roca, D.; Moreira, P.I.; Oliveira, P.J.; Magalhães, J.; Ascensão, A. Physical exercise improves brain cortex and cerebellum mitochondrial bioenergetics and alters apoptotic, dynamic and auto(mito)phagy markers. Neuroscience 2015, 301, 480–495. [Google Scholar] [CrossRef]
- He, C.; Sumpter, R., Jr.; Levine, B. Exercise induces autophagy in peripheral tissues and in the brain. Autophagy 2012, 8, 1548–1551. [Google Scholar] [CrossRef]
- Liang, K.Y.; Mintun, M.A.; Fagan, A.M.; Goate, A.M.; Bugg, J.M.; Holtzman, D.M.; Morris, J.C.; Head, D. Exercise and Alzheimer’s disease biomarkers in cognitively normal older adults. Ann. Neurol. 2010, 68, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.M.; Peiffer, J.J.; Taddei, K.; Lui, J.K.; Laws, S.M.; Gupta, V.B.; Taddei, T.; Ward, V.K.; Rodrigues, M.A.; Burnham, S.; et al. Physical activity and amyloid-β plasma and brain levels: Results from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Mol. Psychiatry 2013, 18, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Valera, E.; Masliah, E. Combination therapies: The next logical Step for the treatment of synucleinopathies? Mov. Disord. 2016, 31, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Kuo, T.-T.; Kao, J.-H.; Huang, E.Y.-K.; Hsieh, T.-H.; Chou, Y.-C.; Hoffer, B.J. Exercise Ameliorates Motor Deficits and Improves Dopaminergic Functions in the Rat Hemi-Parkinson’s Model. Sci. Rep. 2018, 8, 3973. [Google Scholar] [CrossRef] [PubMed]
- Patki, G.; Lau, Y.-S. Impact of exercise on mitochondrial transcription factor expression and damage in the striatum of a chronic mouse model of Parkinson’s disease. Neurosci. Lett. 2011, 505, 268–272. [Google Scholar] [CrossRef]
- Mazzulli, J.R.; Zunke, F.; Isacson, O.; Studer, L.; Krainc, D. α-Synuclein–induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc. Natl. Acad. Sci. USA 2016, 113, 1931–1936. [Google Scholar] [CrossRef]
- Real, C.C.; Ferreira, A.F.B.; Chaves-Kirsten, G.P.; Torrão, A.S.; Pires, R.S.; Britto, L.R.G. BDNF receptor blockade hinders the beneficial effects of exercise in a rat model of Parkinson’s disease. Neuroscience 2013, 237, 118–129. [Google Scholar] [CrossRef]
- Cohen, A.D.; Tillerson, J.L.; Smith, A.D.; Schallert, T.; Zigmond, M.J. Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: Possible role of GDNF. J. Neurochem. 2003, 85, 299–305. [Google Scholar] [CrossRef]
- Tuon, T.; Valvassori, S.S.; Lopes-borges, J.; Luciano, T.; Trom, C.B.; Silva, L.A.; Quevedo, J.; Souza, C.T.; Lira, F.S.; Pinho, R.A. Physical training exerts neuroprotective effects in the regulation of neurochemical factors in an animal model of Parkinson’s disease. Neuroscience 2012, 227, 305–312. [Google Scholar] [CrossRef]
- Fisher, B.E.; Li, Q.; Nacca, A.; Salem, G.J.; Song, J.; Yip, J.; Hui, J.S.; Jakowec, M.W.; Petzinger, G.M. Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson’s disease. NeuroReport 2013, 24, 509. [Google Scholar] [CrossRef]
- van der Kolk, N.M.; deVries, N.M.; Kessels, R.P.C.; Joosten, H.; Zwinderman, A.H.; Post, B.; Bloem, B.R. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: A double-blind, randomised controlled trial. Lancet Neurol. 2019, 18, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Chen, W.; Liu, X.; Qiao, D.; Zhou, F.-M. Exercise-Induced Neuroprotection of the Nigrostriatal Dopamine System in Parkinson’s Disease. Front. Aging Neurosci. 2017, 9, 358. [Google Scholar] [CrossRef]
- Lau, A.; Tymianski, M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. Eur. J. Physiol. 2010, 460, 525–542. [Google Scholar] [CrossRef]
- Aguiar, A.S.; Speck, A.E.; Prediger, R.D.S.; Kapczinski, F.; Pinho, R.A. Downhill training upregulates mice hippocampal and striatal brain-derived neurotrophic factor levels. J. Neural Transm. 2008, 115, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.O.; Mantese, C.E.; Porciuncula, L.O.; Ghisleni, G.; Vinade, L.; Souza, D.O.; Portela, L.V. Exercise affects glutamate receptors in postsynaptic densities from cortical mice brain. Brain Res. 2005, 1065, 20–25. [Google Scholar] [CrossRef]
- Mota, B.C.; Pereira, L.; Souza, M.A.; Silva, L.F.A.; Magni, D.V.; Ferreira, A.P.O.; Oliveira, M.S.; Furian, A.F.; Mazzardo-Martins, L.; da Silva, M.D.; et al. Exercise Pre-conditioning Reduces Brain Inflammation and Protects against Toxicity Induced by Traumatic Brain Injury: Behavioral and Neurochemical Approach. Neurotox. Res. 2012, 21, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Lee, S.-H.; Kim, K.-T.; Zeng, X.; Han, I. TRPV4: A Sensor for Homeostasis and Pathological Events in the CNS. Mol. Neurobiol. 2018, 55, 8695–8708. [Google Scholar] [CrossRef]
- Navarro, A.; Gomez, C.; López-Cepero, J.M.; Boveris, A. Beneficial effects of moderate exercise on mice aging: Survival, behavior, oxidative stress, and mitochondrial electron transfer. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R505–R511. [Google Scholar] [CrossRef]
- Radak, Z.; Marton, O.; Nagy, E.; Koltai, E.; Goto, S. The complex role of physical exercise and reactive oxygen species on brain. J. Sport Health Sci. 2013, 2, 87–93. [Google Scholar] [CrossRef]
- De Souza, C.T.; Frederico, M.J.S.; Da Luz, G.; Cintra, D.E.; Ropelle, E.R.; Pauli, J.R.; Velloso, L.A. Acute exercise reduces hepatic glucose production through inhibition of the Foxo1/HNF-4α pathway in insulin resistant mice. J. Physiol. 2010, 588, 2239–2253. [Google Scholar] [CrossRef]
- Marosi, K.; Bori, Z.; Hart, N.; Sárga, L.; Koltai, E.; Radák, Z.; Nyakas, C. Long-term exercise treatment reduces oxidative stress in the hippocampus of aging rats. Neuroscience 2012, 226, 21–28. [Google Scholar] [CrossRef]
- Radak, Z.; Chung, H.Y.; Koltai, E.; Taylor, A.W.; Goto, S. Exercise, oxidative stress and hormesis. Ageing Res. Rev. 2008, 7, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Asha Devi, S. Aging Brain: Prevention of Oxidative Stress by Vitamin E and Exercise. Sci. World J. 2009, 9, 387376. [Google Scholar] [CrossRef]
- Poon, H.F.; Calabrese, V.; Scapagnini, G.; Butterfield, D.A. Free radicals and brain aging. Clin. Geriatr. Med. 2004, 20, 329–359. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.J.; Busse, M.; Openshaw, R.; Rosser, A.E.; Dunnett, S.B.; Brooks, S.P. Exercise attenuates neuropathology and has greater benefit on cognitive than motor deficits in the R6/1 Huntington’s disease mouse model. Exp. Neurol. 2013, 248, 457–469. [Google Scholar] [CrossRef]
- Deforges, S.; Branchu, J.; Biondi, O.; Grondard, C.; Pariset, C.; Lécolle, S.; Lopes, P.; Vidal, P.-P.; Chanoine, C.; Charbonnier, F. Motoneuron survival is promoted by specific exercise in a mouse model of amyotrophic lateral sclerosis. J. Physiol. 2009, 587, 3561–3572. [Google Scholar] [CrossRef]
- Carreras, I.; Yuruker, S.; Aytan, N.; Hossain, L.; Choi, J.-K.; Jenkins, B.G.; Kowall, N.W.; Dedeoglu, A. Moderate exercise delays the motor performance decline in a transgenic model of ALS. Brain Res. 2010, 1313, 192–201. [Google Scholar] [CrossRef]
- Reddy, P.H.; Reddy, T.P. Mitochondria as a Therapeutic Target for Aging and Neurodegenerative Diseases. Curr. Alzheimer Res. 2011, 8, 393–409. [Google Scholar] [CrossRef]
- Marques-Aleixo, I.; Oliveira, P.J.; Moreira, P.I.; Magalhães, J.; Ascensão, A. Physical exercise as a possible strategy for brain protection: Evidence from mitochondrial-mediated mechanisms. Prog. Neurobiol. 2012, 99, 149–162. [Google Scholar] [CrossRef]
- Bayod, S.; del Valle, J.; Canudas, A.M.; Lalanza, J.F.; Sanchez-Roige, S.; Camins, A.; Escorihuela, R.M.; Pallàs, M. Long-term treadmill exercise induces neuroprotective molecular changes in rat brain. J. Appl. Physiol. 2011, 111, 1380–1390. [Google Scholar] [CrossRef]
- Reznick, R.M.; Shulman, G.I. The role of AMP-activated protein kinase in mitochondrial biogenesis. J. Physiol. 2006, 574, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Bayod, S.; del Valle, J.; Lalanza, J.F.; Sanchez-Roige, S.; de Luxán-Delgado, B.; Coto-Montes, A.; Canudas, A.M.; Camins, A.; Escorihuela, R.M.; Pallàs, M. Long-term physical exercise induces changes in sirtuin 1 pathway and oxidative parameters in adult rat tissues. Exp. Gerontol. 2012, 47, 925–935. [Google Scholar] [CrossRef]
- Gusdon, A.M.; Callio, J.; Distefano, G.; O’Doherty, R.M.; Goodpaster, B.H.; Coen, P.M.; Chu, C.T. Exercise increases mitochondrial complex I activity and DRP1 expression in the brains of aged mice. Exp. Gerontol. 2017, 90, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, Y.; Zhang, P.; Sha, H.; Jia, J.; Hu, Y.; Zhu, J. Exercise induces mitochondrial biogenesis after brain ischemia in rats. Neuroscience 2012, 205, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Lira, V.A.; Greene, N.P. Exercise Training-Induced Regulation of Mitochondrial Quality. Exerc. Sport Sci. Rev. 2012, 40, 159. [Google Scholar] [CrossRef]
- Barrientos, A.; Moraes, C.T. Titrating the Effects of Mitochondrial Complex I Impairment in the Cell Physiology. J. Biol. Chem. 1999, 274, 16188–16197. [Google Scholar] [CrossRef]
- Lionaki, E.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Mitochondria, autophagy and age-associated neurodegenerative diseases: New insights into a complex interplay. Biochim. Et Biophys. Acta Bioenerg. 2015, 1847, 1412–1423. [Google Scholar] [CrossRef]
- Bo, H.; Zhang, Y.; Ji, L.L. Redefining the role of mitochondria in exercise: A dynamic remodeling. Ann. New York Acad. Sci. 2010, 1201, 121–128. [Google Scholar] [CrossRef]
- Cheng, A.; Wan, R.; Yang, J.-L.; Kamimura, N.; Son, T.G.; Ouyang, X.; Luo, Y.; Okun, E.; Mattson, M.P. Involvement of PGC-1α in the formation and maintenance of neuronal dendritic spines. Nat. Commun. 2012, 3, 1250. [Google Scholar] [CrossRef]
- Goes, A.T.R.; Souza, L.C.; Filho, C.B.; Del Fabbro, L.; De Gomes, M.G.; Boeira, S.P.; Jesse, C.R. Neuroprotective effects of swimming training in a mouse model of Parkinson’s disease induced by 6-hydroxydopamine. Neuroscience 2014, 256, 61–71. [Google Scholar] [CrossRef]
- Lau, Y.-S.; Patki, G.; Das-Panja, K.; Le, W.-D.; Ahmad, S.O. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson’s disease with moderate neurodegeneration. Eur. J. Neurosci. 2011, 33, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Dienel, G.A. Brain Lactate Metabolism: The Discoveries and the Controversies. J. Cereb. Blood Flow Metab. 2012, 32, 1107–1138. [Google Scholar] [CrossRef]
- Cunnane, S.C.; Courchesne-Loyer, A.; St-Pierre, V.; Vandenberghe, C.; Pierotti, T.; Fortier, M.; Croteau, E.; Castellano, C.-A. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2016, 1367, 12–20. [Google Scholar] [CrossRef]
- Mosconi, L.; Mistur, R.; Switalski, R.; Tsui, W.H.; Glodzik, L.; Li, Y.; Pirraglia, E.; De Santi, S.; Reisberg, B.; Wisniewski, T.; et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.; Nugent, S.; Roy, M.; Courchesne-Loyer, A.; Croteau, E.; Tremblay, S.; Castellano, A.; Pifferi, F.; Bocti, C.; Paquet, N.; et al. Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition 2011, 27, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Magistretti, P.J.; Allaman, I. Lactate in the brain: From metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef]
- Blondell, S.J.; Hammersley-Mather, R.; Veerman, J.L. Does physical activity prevent cognitive decline and dementia?: A systematic review and meta-analysis of longitudinal studies. BMC Public Health 2014, 14, 510. [Google Scholar] [CrossRef]
- Sofi, F.; Valecchi, D.; Bacci, D.; Abbate, R.; Gensini, G.F.; Casini, A.; Macchi, C. Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. J. Intern. Med. 2011, 269, 107–117. [Google Scholar] [CrossRef]
- Guure, C.B.; Ibrahim, N.A.; Adam, M.B.; Said, S.M. Impact of Physical Activity on Cognitive Decline, Dementia, and Its Subtypes: Meta-Analysis of Prospective Studies. BioMed Res. Int. 2017, 2017, 9016924. [Google Scholar] [CrossRef]
- Santos-Lozano, A.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Quindós-Rubial, M.; Fiuza-Luces, C.; Cristi-Montero, C.; Emanuele, E.; Garatachea, N.; Lucia, A. Physical Activity and Alzheimer Disease: A Protective Association. Mayo Clin. Proc. 2016, 91, 999–1020. [Google Scholar] [CrossRef]
- Prakash, R.S.; Voss, M.W.; Erickson, K.I.; Kramer, A.F. Physical activity and cognitive vitality. Annu. Rev. Psychol. 2015, 66, 769–797. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.J.; Blumenthal, J.A.; Hoffman, B.M.; Cooper, H.; Strauman, T.A.; Welsh-Bohmer, K.; Browndyke, J.N.; Sherwood, A. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosom. Med. 2010, 72, 239–252. [Google Scholar] [CrossRef]
- Öhman, H.; Savikko, N.; Strandberg, T.E.; Pitkälä, K.H. Effect of physical exercise on cognitive performance in older adults with mild cognitive impairment or dementia: A systematic review. Dement. Geriatr. Cogn. Disord. 2014, 38, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Lautenschlager, N.T.; Cox, K.L.; Flicker, L.; Foster, J.K.; van Bockxmeer, F.M.; Xiao, J.; Greenop, K.R.; Almeida, O.P. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: A randomized trial. JAMA 2008, 300, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2018, 52, 154–160. [Google Scholar] [CrossRef]
- Baker, L.D.; Frank, L.L.; Foster-Schubert, K.; Green, P.S.; Wilkinson, C.W.; McTiernan, A.; Plymate, S.R.; Fishel, M.A.; Watson, G.S.; Cholerton, B.A.; et al. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch. Neurol. 2010, 67, 71–79. [Google Scholar] [CrossRef]
- Groot, C.; Hooghiemstra, A.M.; Raijmakers, P.G.H.M.; van Berckel, B.N.M.; Scheltens, P.; Scherder, E.J.A.; van der Flier, W.M.; Ossenkoppele, R. The effect of physical activity on cognitive function in patients with dementia: A meta-analysis of randomized control trials. Ageing Res. Rev. 2016, 25, 13–23. [Google Scholar] [CrossRef]
- Bugg, J.M.; Head, D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol Aging 2011, 32, 506–514. [Google Scholar] [CrossRef]
- Erickson, K.I.; Leckie, R.L.; Weinstein, A.M. Physical activity, fitness, and gray matter volume. Neurobiol Aging 2014, 35 (Suppl. 2), S20–S28. [Google Scholar] [CrossRef]
- ten Brinke, L.F.; Bolandzadeh, N.; Nagamatsu, L.S.; Hsu, C.L.; Davis, J.C.; Miran-Khan, K.; Liu-Ambrose, T. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: A 6-month randomised controlled trial. Br. J. Sports Med. 2015, 49, 248–254. [Google Scholar] [CrossRef]
- Voss, M.W.; Heo, S.; Prakash, R.S.; Erickson, K.I.; Alves, H.; Chaddock, L.; Szabo, A.N.; Mailey, E.L.; Wójcicki, T.R.; White, S.M.; et al. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: Results of a one-year exercise intervention. Hum. Brain Mapp. 2013, 34, 2972–2985. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.W.; Prakash, R.S.; Erickson, K.I.; Basak, C.; Chaddock, L.; Kim, J.S.; Alves, H.; Heo, S.; Szabo, A.N.; White, S.M.; et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front. Aging Neurosci. 2010, 2, 32. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lagerros, Y.T.; Bellocco, R.; Adami, H.-O.; Fang, F.; Pedersen, N.L.; Wirdefeldt, K. Physical activity and risk of Parkinson’s disease in the Swedish National March Cohort. Brain 2014, 138 Pt 2, 269–275. [Google Scholar] [CrossRef]
- Xu, Q.; Park, Y.; Huang, X.; Hollenbeck, A.; Blair, A.; Schatzkin, A.; Chen, H. Physical activities and future risk of Parkinson disease. Neurology 2010, 75, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Han, D.; Cheng, Q.; Zhang, P.; Zhao, C.; Min, J.; Wang, F. Association of Levels of Physical Activity With Risk of Parkinson Disease: A Systematic Review and Meta-analysis. JAMA Netw. Open 2018, 1, e182421. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.M.; Schwarzschild, M.A.; Hernán, M.A.; Ascherio, A. Physical activity and the risk of Parkinson disease. Neurology 2005, 64, 664–669. [Google Scholar] [CrossRef]
- Shu, H.-F.; Yang, T.; Yu, S.-X.; Huang, H.-D.; Jiang, L.-L.; Gu, J.-W.; Kuang, Y.-Q. Aerobic exercise for Parkinson’s disease: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2014, 9, e100503. [Google Scholar] [CrossRef]
- Mak, M.K.; Wong-Yu, I.S.; Shen, X.; Chung, C.L. Long-term effects of exercise and physical therapy in people with Parkinson disease. Nat. Rev. Neurol. 2017, 13, 689–703. [Google Scholar] [CrossRef]
- Uhrbrand, A.; Stenager, E.; Pedersen, M.S.; Dalgas, U. Parkinson’s disease and intensive exercise therapy--a systematic review and meta-analysis of randomized controlled trials. J. Neurol. Sci. 2015, 353, 9–19. [Google Scholar] [CrossRef]
- Wu, P.-L.; Lee, M.; Huang, T.-T. Effectiveness of physical activity on patients with depression and Parkinson’s disease: A systematic review. PLoS ONE 2017, 12, e0181515. [Google Scholar] [CrossRef]
- David, F.J.; Robichaud, J.A.; Leurgans, S.E.; Poon, C.; Kohrt, W.M.; Goldman, J.G.; Comella, C.L.; Vaillancourt, D.E.; Corcos, D.M. Exercise improves cognition in Parkinson’s disease: The PRET-PD randomized, clinical trial. Mov. Disord. 2015, 30, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Harwood, C.A.; McDermott, C.J.; Shaw, P.J. Physical activity as an exogenous risk factor in motor neuron disease (MND): A review of the evidence. Amyotroph. Lateral Scler. 2009, 10, 191–204. [Google Scholar] [CrossRef]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Longinetti, E.; Mariosa, D.; Larsson, H.; Almqvist, C.; Lichtenstein, P.; Ye, W.; Fang, F. Physical and cognitive fitness in young adulthood and risk of amyotrophic lateral sclerosis at an early age. Eur. J. Neurol. 2017, 24, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Gallo, V.; Vanacore, N.; Bueno-de-Mesquita, H.B.; Vermeulen, R.; Brayne, C.; Pearce, N.; Wark, P.A.; Ward, H.A.; Ferrari, P.; Jenab, M.; et al. Physical activity and risk of Amyotrophic Lateral Sclerosis in a prospective cohort study. Eur. J. Epidemiol. 2016, 31, 255–266. [Google Scholar] [CrossRef]
- Belbasis, L.; Bellou, V.; Evangelou, E. Environmental Risk Factors and Amyotrophic Lateral Sclerosis: An Umbrella Review and Critical Assessment of Current Evidence from Systematic Reviews and Meta-Analyses of Observational Studies. Neuroepidemiology 2016, 46, 96–105. [Google Scholar] [CrossRef]
- Yuen, E.Y.; Liu, W.; Karatsoreos, I.N.; Feng, J.; McEwen, B.S.; Yan, Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc. Natl. Acad. Sci. USA 2009, 106, 14075–14079. [Google Scholar] [CrossRef]
- Quinn, L.; Hamana, K.; Kelson, M.; Dawes, H.; Collett, J.; Townson, J.; Roos, R.; van der Plas, A.A.; Reilmann, R.; Frich, J.C.; et al. A randomized, controlled trial of a multi-modal exercise intervention in Huntington’s disease. Parkinsonism Relat. Disord. 2016, 31, 46–52. [Google Scholar] [CrossRef]
- Fritz, N.E.; Rao, A.K.; Kegelmeyer, D.; Kloos, A.; Busse, M.; Hartel, L.; Carrier, J.; Quinn, L. Physical Therapy and Exercise Interventions in Huntington’s Disease: A Mixed Methods Systematic Review. J. Huntingt. Dis. 2017, 6, 217–235. [Google Scholar] [CrossRef]
- Gomes-Osman, J.; Cabral, D.F.; Morris, T.P.; McInerney, K.; Cahalin, L.P.; Rundek, T.; Oliveira, A.; Pascual-Leone, A. Exercise for cognitive brain health in aging: A systematic review for an evaluation of dose. Neurol. Clin. Pract. 2018, 8, 257–265. [Google Scholar] [CrossRef]
- Erickson, K.I.; Hillman, C.; Stillman, C.M.; Ballard, R.M.; Bloodgood, B.; Conroy, D.E.; Macko, R.; Marquez, D.X.; Petruzzello, S.J.; Powell, K.E.; et al. Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Med. Sci. Sports Exerc. 2019, 51, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Sardahaee, F.S.; Anderssen, S.; Ballard, C. Alzheimer’s Society Systematic Review group Is physical activity a potential preventive factor for vascular dementia? A systematic review. Aging Ment. Health 2010, 14, 386–395. [Google Scholar] [CrossRef]
- Sanders, L.M.J.; Hortobágyi, T.; la Bastide-van Gemert, S.; van der Zee, E.A.; van Heuvelen, M.J.G. Dose-response relationship between exercise and cognitive function in older adults with and without cognitive impairment: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0210036. [Google Scholar] [CrossRef]
- Panza, G.A.; Taylor, B.A.; MacDonald, H.V.; Johnson, B.T.; Zaleski, A.L.; Livingston, J.; Thompson, P.D.; Pescatello, L.S. Can Exercise Improve Cognitive Symptoms of Alzheimer’s Disease? J. Am. Geriatr. Soc. 2018, 66, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Weuve, J.; Kang, J.H.; Manson, J.E.; Breteler, M.M.B.; Ware, J.H.; Grodstein, F. Physical activity, including walking, and cognitive function in older women. JAMA 2004, 292, 1454–1461. [Google Scholar] [CrossRef]
- Stubbs, B.; Chen, L.-J.; Chang, C.-Y.; Sun, W.-J.; Ku, P.-W. Accelerometer-assessed light physical activity is protective of future cognitive ability: A longitudinal study among community dwelling older adults. Exp. Gerontol. 2017, 91, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.; Marshall, S.J.; Patterson, R.E.; Marinac, C.R.; Natarajan, L.; Rosenberg, D.; Wasilenko, K.; Crist, K. Objectively measured physical activity is related to cognitive function in older adults. J. Am. Geriatr. Soc. 2013, 61, 1927–1931. [Google Scholar] [CrossRef]
- O’Donovan, G.; Lee, I.-M.; Hamer, M.; Stamatakis, E. Association of “Weekend Warrior” and Other Leisure Time Physical Activity Patterns With Risks for All-Cause, Cardiovascular Disease, and Cancer Mortality. JAMA Intern. Med. 2017, 177, 335–342. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Edwards, M.K.; Frith, E. Potential avenues for exercise to activate episodic memory-related pathways: A narrative review. Eur. J. Neurosci. 2017, 46, 2067–2077. [Google Scholar] [CrossRef]
- Tarumi, T.; Rossetti, H.; Thomas, B.P.; Harris, T.; Tseng, B.Y.; Turner, M.; Wang, C.; German, Z.; Martin-Cook, K.; Stowe, A.M.; et al. Exercise Training in Amnestic Mild Cognitive Impairment: A One-Year Randomized Controlled Trial. J. Alzheimers Dis. 2019, 71, 421–433. [Google Scholar] [CrossRef]
- Sobol, N.A.; Hoffmann, K.; Frederiksen, K.S.; Vogel, A.; Vestergaard, K.; Brændgaard, H.; Gottrup, H.; Lolk, A.; Wermuth, L.; Jakobsen, S.; et al. Effect of aerobic exercise on physical performance in patients with Alzheimer’s disease. Alzheimers Dement. 2016, 12, 1207–1215. [Google Scholar] [CrossRef]
- Chapman, S.B.; Aslan, S.; Spence, J.S.; DeFina, L.F.; Keebler, M.W.; Didehbani, N.; Lu, H. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front. Aging Neurosci. 2013, 5, 75. [Google Scholar] [CrossRef]
- Colcombe, S.J.; Erickson, K.I.; Scalf, P.E.; Kim, J.S.; Prakash, R.; McAuley, E.; Elavsky, S.; Marquez, D.X.; Hu, L.; Kramer, A.F. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1166–1170. [Google Scholar] [CrossRef]
- Barha, C.K.; Davis, J.C.; Falck, R.S.; Nagamatsu, L.S.; Liu-Ambrose, T. Sex differences in exercise efficacy to improve cognition: A systematic review and meta-analysis of randomized controlled trials in older humans. Front. Neuroendocrinol. 2017, 46, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Fiatarone Singh, M.A.; Gates, N.; Saigal, N.; Wilson, G.C.; Meiklejohn, J.; Brodaty, H.; Wen, W.; Singh, N.; Baune, B.T.; Suo, C.; et al. The Study of Mental and Resistance Training (SMART) study—Resistance training and/or cognitive training in mild cognitive impairment: A randomized, double-blind, double-sham controlled trial. J. Am. Med. Dir. Assoc. 2014, 15, 873–880. [Google Scholar] [CrossRef]
- Liu-Ambrose, T.; Nagamatsu, L.S.; Graf, P.; Beattie, B.L.; Ashe, M.C.; Handy, T.C. Resistance training and executive functions: A 12-month randomized controlled trial. Arch. Intern. Med. 2010, 170, 170–178. [Google Scholar] [CrossRef]
- Herold, F.; Törpel, A.; Schega, L.; Müller, N.G. Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements—A systematic review. Eur. Rev. Aging Phys. Act. 2019, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Colcombe, S.; Kramer, A.F. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol. Sci. 2003, 14, 125–130. [Google Scholar] [CrossRef]
- Wayne, P.M.; Walsh, J.N.; Taylor-Piliae, R.E.; Wells, R.E.; Papp, K.V.; Donovan, N.J.; Yeh, G.Y. Effect of tai chi on cognitive performance in older adults: Systematic review and meta-analysis. J. Am. Geriatr. Soc. 2014, 62, 25–39. [Google Scholar] [CrossRef]
- Gu, Q.; Wu, S.-J.; Zheng, Y.; Zhang, Y.; Liu, C.; Hou, J.-C.; Zhang, K.; Fang, X.-M. Tai Chi Exercise for Patients with Chronic Heart Failure: A Meta-analysis of Randomized Controlled Trials. Am. J. Phys. Med. Rehabil. 2017, 96, 706–716. [Google Scholar] [CrossRef]
- Burzynska, A.Z.; Jiao, Y.; Knecht, A.M.; Fanning, J.; Awick, E.A.; Chen, T.; Gothe, N.; Voss, M.W.; McAuley, E.; Kramer, A.F. White Matter Integrity Declined Over 6-Months, but Dance Intervention Improved Integrity of the Fornix of Older Adults. Front. Aging Neurosci. 2017, 9, 59. [Google Scholar] [CrossRef]
- Zou, L.; Loprinzi, P.D.; Yeung, A.S.; Zeng, N.; Huang, T. The Beneficial Effects of Mind-Body Exercises for People With Mild Cognitive Impairment: A Systematic Review With Meta-analysis. Arch. Phys. Med. Rehabil. 2019, 100, 1556–1573. [Google Scholar] [CrossRef]
- Wu, C.; Yi, Q.; Zheng, X.; Cui, S.; Chen, B.; Lu, L.; Tang, C. Effects of Mind-Body Exercises on Cognitive Function in Older Adults: A Meta-Analysis. J. Am. Geriatr. Soc. 2019, 67, 749–758. [Google Scholar] [CrossRef]
- Kattenstroth, J.-C.; Kalisch, T.; Holt, S.; Tegenthoff, M.; Dinse, H.R. Six months of dance intervention enhances postural, sensorimotor, and cognitive performance in elderly without affecting cardio-respiratory functions. Front. Aging Neurosci. 2013, 5, 5. [Google Scholar] [CrossRef]
- Voelcker-Rehage, C.; Godde, B.; Staudinger, U.M. Cardiovascular and coordination training differentially improve cognitive performance and neural processing in older adults. Front. Hum. Neurosci. 2011, 5, 26. [Google Scholar] [CrossRef]
- Bherer, L.; Erickson, K.I.; Liu-Ambrose, T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J. Aging Res. 2013, 2013, 657508. [Google Scholar] [CrossRef]
- Moon, A.; Jang, S.; Kim, J.-H.; Jang, S. Risk of falls or fall-related injuries associated with potentially inappropriate medication use among older adults with dementia. BMC Geriatr. 2024, 24, 699. [Google Scholar] [CrossRef]
- Zubala, A.; MacGillivray, S.; Frost, H.; Kroll, T.; Skelton, D.A.; Gavine, A.; Gray, N.M.; Toma, M.; Morris, J. Promotion of physical activity interventions for community dwelling older adults: A systematic review of reviews. PLoS ONE 2017, 12, e0180902. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P.; American College of Sports Medicine American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Hoffmann, K.; Sobol, N.A.; Frederiksen, K.S.; Beyer, N.; Vogel, A.; Vestergaard, K.; Brændgaard, H.; Gottrup, H.; Lolk, A.; Wermuth, L.; et al. Moderate-to-High Intensity Physical Exercise in Patients with Alzheimer’s Disease: A Randomized Controlled Trial. J. Alzheimers Dis. 2016, 50, 443–453. [Google Scholar] [CrossRef]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Després, J.-P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, A.; Fenesi, B.; Paolucci, E.; Heisz, J.J. The effects of aerobic exercise intensity on memory in older adults. Appl. Physiol. Nutr. Metab. 2020, 45, 591–600. [Google Scholar] [CrossRef]
- Murphy, M.H.; Lahart, I.; Carlin, A.; Murtagh, E. The Effects of Continuous Compared to Accumulated Exercise on Health: A Meta-Analytic Review. Sports Med. 2019, 49, 1585–1607. [Google Scholar] [CrossRef]
- Bamidis, P.D.; Vivas, A.B.; Styliadis, C.; Frantzidis, C.; Klados, M.; Schlee, W.; Siountas, A.; Papageorgiou, S.G. A review of physical and cognitive interventions in aging. Neurosci. Biobehav. Rev. 2014, 44, 206–220. [Google Scholar] [CrossRef]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, W.; Vogel, T.; Schmitt, E.; Kaltenbach, G.; Geny, B.; Lang, P.O. Health benefits of aerobic training programs in adults aged 70 and over: A systematic review. Arch. Gerontol. Geriatr. 2017, 69, 110–127. [Google Scholar] [CrossRef] [PubMed]
- Falck, R.S.; Davis, J.C.; Best, J.R.; Crockett, R.A.; Liu-Ambrose, T. Impact of exercise training on physical and cognitive function among older adults: A systematic review and meta-analysis. Neurobiol. Aging 2019, 79, 119–130. [Google Scholar] [CrossRef]
- Ahlskog, J.E. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology 2011, 77, 288–294. [Google Scholar] [CrossRef]
- Sherrington, C.; Fairhall, N.J.; Wallbank, G.K.; Tiedemann, A.; Michaleff, Z.A.; Howard, K.; Clemson, L.; Hopewell, S.; Lamb, S.E. Exercise for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2019, 1, CD012424. [Google Scholar] [CrossRef]
- Riebe, D.; Franklin, B.A.; Thompson, P.D.; Garber, C.E.; Whitfield, G.P.; Magal, M.; Pescatello, L.S. Updating ACSM’s Recommendations for Exercise Preparticipation Health Screening. Med. Sci. Sports Exerc. 2015, 47, 2473–2479. [Google Scholar] [CrossRef]
- Bredin, S.S.D.; Gledhill, N.; Jamnik, V.K.; Warburton, D.E.R. PAR-Q+ and ePARmed-X+: New risk stratification and physical activity clearance strategy for physicians and patients alike. Can. Fam. Physician 2013, 59, 273–277. [Google Scholar]
- Yu, F.; Vock, D.M.; Zhang, L.; Salisbury, D.; Nelson, N.W.; Chow, L.S.; Smith, G.; Barclay, T.R.; Dysken, M.; Wyman, J.F. Cognitive Effects of Aerobic Exercise in Alzheimer’s Disease: A Pilot Randomized Controlled Trial. J. Alzheimers Dis. 2021, 80, 233–244. [Google Scholar] [CrossRef]
- McGough, E.L.; Lin, S.-Y.; Belza, B.; Becofsky, K.M.; Jones, D.L.; Liu, M.; Wilcox, S.; Logsdon, R.G. A Scoping Review of Physical Performance Outcome Measures Used in Exercise Interventions for Older Adults With Alzheimer Disease and Related Dementias. J. Geriatr. Phys. Ther. 2019, 42, 28–47. [Google Scholar] [CrossRef]
- King, L.A.; Wilhelm, J.; Chen, Y.; Blehm, R.; Nutt, J.; Chen, Z.; Serdar, A.; Horak, F.B. Effects of Group, Individual, and Home Exercise in Persons With Parkinson Disease: A Randomized Clinical Trial. J. Neurol. Phys. Ther. 2015, 39, 204–212. [Google Scholar] [CrossRef]
- Head, D.; Bugg, J.M.; Goate, A.M.; Fagan, A.M.; Mintun, M.A.; Benzinger, T.; Holtzman, D.M.; Morris, J.C. Exercise Engagement as a Moderator of the Effects of APOE Genotype on Amyloid Deposition. Arch. Neurol. 2012, 69, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Deeny, S.P.; Poeppel, D.; Zimmerman, J.B.; Roth, S.M.; Brandauer, J.; Witkowski, S.; Hearn, J.W.; Ludlow, A.T.; Contreras-Vidal, J.L.; Brandt, J.; et al. Exercise, APOE, and working memory: MEG and behavioral evidence for benefit of exercise in epsilon4 carriers. Biol. Psychol. 2008, 78, 179–187. [Google Scholar] [CrossRef]
- Petzinger, G.M.; Fisher, B.E.; McEwen, S.; Beeler, J.A.; Walsh, J.P.; Jakowec, M.W. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol. 2013, 12, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, J.; Morris, M.E.; Bhriain, O.N.; Saunders, J.; Clifford, A.M. Dance for people with Parkinson disease: What is the evidence telling us? Arch. Phys. Med. Rehabil. 2015, 96, 141–153. [Google Scholar] [CrossRef]
- Ghai, S.; Ghai, I.; Schmitz, G.; Effenberg, A.O. Effect of rhythmic auditory cueing on parkinsonian gait: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 506. [Google Scholar] [CrossRef]
- Kennedy, G.; Hardman, R.J.; Macpherson, H.; Scholey, A.B.; Pipingas, A. How Does Exercise Reduce the Rate of Age-Associated Cognitive Decline? A Review of Potential Mechanisms. J. Alzheimers Dis. 2017, 55, 1–18. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, C.; Michaleff, Z.A.; Fairhall, N.; Paul, S.S.; Tiedemann, A.; Whitney, J.; Cumming, R.G.; Herbert, R.D.; Close, J.C.T.; Lord, S.R. Exercise to prevent falls in older adults: An updated systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 1750–1758. [Google Scholar] [CrossRef]
- Thomas, R.; Beck, M.M.; Lind, R.R.; Korsgaard Johnsen, L.; Geertsen, S.S.; Christiansen, L.; Ritz, C.; Roig, M.; Lundbye-Jensen, J. Acute Exercise and Motor Memory Consolidation: The Role of Exercise Timing. Neural Plast. 2016, 2016, 6205452. [Google Scholar] [CrossRef] [PubMed]
- Stutz, J.; Eiholzer, R.; Spengler, C.M. Effects of Evening Exercise on Sleep in Healthy Participants: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Vitale, J.A.; Weydahl, A. Chronotype, Physical Activity, and Sport Performance: A Systematic Review. Sports Med. 2017, 47, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Skjæret, N.; Nawaz, A.; Morat, T.; Schoene, D.; Helbostad, J.L.; Vereijken, B. Exercise and rehabilitation delivered through exergames in older adults: An integrative review of technologies, safety and efficacy. Int. J. Med. Inform. 2016, 85, 1–16. [Google Scholar] [CrossRef]
- Chen, F.-T.; Etnier, J.L.; Chan, K.-H.; Chiu, P.-K.; Hung, T.-M.; Chang, Y.-K. Effects of Exercise Training Interventions on Executive Function in Older Adults: A Systematic Review and Meta-Analysis. Sports Med. 2020, 50, 1451–1467. [Google Scholar] [CrossRef]
- Ding, D.; Lawson, K.D.; Kolbe-Alexander, T.L.; Finkelstein, E.A.; Katzmarzyk, P.T.; van Mechelen, W.; Pratt, M. Lancet Physical Activity Series 2 Executive Committee The economic burden of physical inactivity: A global analysis of major non-communicable diseases. Lancet 2016, 388, 1311–1324. [Google Scholar] [CrossRef]
- Kivipelto, M.; Mangialasche, F.; Ngandu, T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 653–666. [Google Scholar] [CrossRef]
- Andrieu, S.; Guyonnet, S.; Coley, N.; Cantet, C.; Bonnefoy, M.; Bordes, S.; Bories, L.; Cufi, M.-N.; Dantoine, T.; Dartigues, J.-F.; et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): A randomised, placebo-controlled trial. Lancet Neurol. 2017, 16, 377–389. [Google Scholar] [CrossRef]
- Flanagan, E.; Lamport, D.; Brennan, L.; Burnet, P.; Calabrese, V.; Cunnane, S.C.; de Wilde, M.C.; Dye, L.; Farrimond, J.A.; Emerson Lombardo, N.; et al. Nutrition and the ageing brain: Moving towards clinical applications. Ageing Res. Rev. 2020, 62, 101079. [Google Scholar] [CrossRef]
- Cassilhas, R.C.; Lee, K.S.; Fernandes, J.; Oliveira, M.G.M.; Tufik, S.; Meeusen, R.; de Mello, M.T. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience 2012, 202, 309–317. [Google Scholar] [CrossRef]
- Vauzour, D.; Camprubi-Robles, M.; Miquel-Kergoat, S.; Andres-Lacueva, C.; Bánáti, D.; Barberger-Gateau, P.; Bowman, G.L.; Caberlotto, L.; Clarke, R.; Hogervorst, E.; et al. Nutrition for the ageing brain: Towards evidence for an optimal diet. Ageing Res. Rev. 2017, 35, 222–240. [Google Scholar] [CrossRef]
- El Khoury, D.; Anderson, G.H. Recent advances in dietary proteins and lipid metabolism. Curr. Opin. Lipidol. 2013, 24, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Tipton, K.D.; Aarsland, A.; Wolf, S.E.; Wolfe, R.R. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am. J. Physiol. 1997, 273, E99–E107. [Google Scholar] [CrossRef]
- Stanmore, E.; Stubbs, B.; Vancampfort, D.; de Bruin, E.D.; Firth, J. The effect of active video games on cognitive functioning in clinical and non-clinical populations: A meta-analysis of randomized controlled trials. Neurosci. Biobehav. Rev. 2017, 78, 34–43. [Google Scholar] [CrossRef]
- Mura, G.; Carta, M.G.; Sancassiani, F.; Machado, S.; Prosperini, L. Active exergames to improve cognitive functioning in neurological disabilities: A systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2018, 54, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Frith, E. A brief primer on the mediational role of BDNF in the exercise-memory link. Clin. Physiol. Funct. Imaging 2019, 39, 9–14. [Google Scholar] [CrossRef]
- Roig, M.; Nordbrandt, S.; Geertsen, S.S.; Nielsen, J.B. The effects of cardiovascular exercise on human memory: A review with meta-analysis. Neurosci. Biobehav. Rev. 2013, 37, 1645–1666. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, B.A.; Neufeld, E.V.; Boland, D.M.; Martin, J.L.; Cooper, C.B. Interrelationship between Sleep and Exercise: A Systematic Review. Adv. Prev. Med. 2017, 2017, 1364387. [Google Scholar] [CrossRef]
- Kredlow, M.A.; Capozzoli, M.C.; Hearon, B.A.; Calkins, A.W.; Otto, M.W. The effects of physical activity on sleep: A meta-analytic review. J. Behav. Med. 2015, 38, 427–449. [Google Scholar] [CrossRef] [PubMed]
- Vitale, J.A.; Bonato, M.; La Torre, A.; Banfi, G. Heart Rate Variability in Sport Performance: Do Time of Day and Chronotype Play A Role? J. Clin. Med. 2019, 8, 723. [Google Scholar] [CrossRef]
- Puterman, E.; Weiss, J.; Lin, J.; Schilf, S.; Slusher, A.L.; Johansen, K.L.; Epel, E.S. Aerobic exercise lengthens telomeres and reduces stress in family caregivers: A randomized controlled trial—Curt Richter Award Paper 2018. Psychoneuroendocrinology 2018, 98, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Dhabhar, F.S. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef]
- Malm, C.; Jakobsson, J.; Isaksson, A. Physical Activity and Sports-Real Health Benefits: A Review with Insight into the Public Health of Sweden. Sports 2019, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Sansano-Nadal, O.; Giné-Garriga, M.; Brach, J.S.; Wert, D.M.; Jerez-Roig, J.; Guerra-Balic, M.; Oviedo, G.; Fortuño, J.; Gómara-Toldrà, N.; Soto-Bagaria, L.; et al. Exercise-Based Interventions to Enhance Long-Term Sustainability of Physical Activity in Older Adults: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Int. J. Environ. Res. Public Health 2019, 16, 2527. [Google Scholar] [CrossRef]
- Fredrix, M.; McSharry, J.; Flannery, C.; Dinneen, S.; Byrne, M. Goal-setting in diabetes self-management: A systematic review and meta-analysis examining content and effectiveness of goal-setting interventions. Psychol. Health 2018, 33, 955–977. [Google Scholar] [CrossRef]
- Frith, E.; Loprinzi, P.D. Physical activity is associated with higher cognitive function among adults at risk for Alzheimer’s disease. Complement Ther. Med. 2018, 36, 46–49. [Google Scholar] [CrossRef]
- Reis, R.S.; Salvo, D.; Ogilvie, D.; Lambert, E.V.; Goenka, S.; Brownson, R.C. Lancet Physical Activity Series 2 Executive Committee Scaling up physical activity interventions worldwide: Stepping up to larger and smarter approaches to get people moving. Lancet 2016, 388, 1337–1348. [Google Scholar] [CrossRef]
- Chen, Y.-R.R.; Schulz, P.J. The Effect of Information Communication Technology Interventions on Reducing Social Isolation in the Elderly: A Systematic Review. J. Med. Internet Res. 2016, 18, e18. [Google Scholar] [CrossRef]
- Khan, K.M.; Weiler, R.; Blair, S.N. Prescribing exercise in primary care. BMJ 2011, 343, d4141. [Google Scholar] [CrossRef] [PubMed]
- Sallis, R.; Franklin, B.; Joy, L.; Ross, R.; Sabgir, D.; Stone, J. Strategies for promoting physical activity in clinical practice. Prog. Cardiovasc. Dis. 2015, 57, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Thornton, J.S.; Frémont, P.; Khan, K.; Poirier, P.; Fowles, J.; Wells, G.D.; Frankovich, R.J. Physical activity prescription: A critical opportunity to address a modifiable risk factor for the prevention and management of chronic disease: A position statement by the Canadian Academy of Sport and Exercise Medicine. Br. J. Sports Med. 2016, 50, 1109–1114. [Google Scholar] [CrossRef]
- Nilsson, M.H.; Iwarsson, S.; Thordardottir, B.; Haak, M. Barriers and Facilitators for Participation in People with Parkinson’s Disease. J. Park. Dis. 2015, 5, 983–992. [Google Scholar] [CrossRef]
- Latimer-Cheung, A.E.; Rhodes, R.E.; Kho, M.E.; Tomasone, J.R.; Gainforth, H.L.; Kowalski, K.; Nasuti, G.; Perrier, M.-J.; Duggan, M. Canadian Physical Activity Guidelines Messaging Recommendation Workgroup Evidence-informed recommendations for constructing and disseminating messages supplementing the new Canadian Physical Activity Guidelines. BMC Public Health 2013, 13, 419. [Google Scholar] [CrossRef]
- Knox, E.C.L.; Musson, H.; Adams, E.J. Knowledge of physical activity recommendations in adults employed in England: Associations with individual and workplace-related predictors. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 69. [Google Scholar] [CrossRef]
- Berry, T.R.; McCarville, R.E.; Rhodes, R.E. Getting to know the competition: A content analysis of publicly and corporate funded physical activity advertisements. J. Health Commun. 2008, 13, 169–180. [Google Scholar] [CrossRef]
- Cotman, C.W.; Engesser-Cesar, C. Exercise enhances and protects brain function. Exerc. Sport Sci. Rev. 2002, 30, 75–79. [Google Scholar] [CrossRef]
- Tari, A.R.; Norevik, C.S.; Scrimgeour, N.R.; Kobro-Flatmoen, A.; Storm-Mathisen, J.; Bergersen, L.H.; Wrann, C.D.; Selbæk, G.; Kivipelto, M.; Moreira, J.B.N.; et al. Are the neuroprotective effects of exercise training systemically mediated? Prog. Cardiovasc. Dis. 2019, 62, 94–101. [Google Scholar] [CrossRef]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef]
- Brigadski, T.; Leßmann, V. The physiology of regulated BDNF release. Cell Tissue Res. 2020, 382, 15–45. [Google Scholar] [CrossRef]
- Pedersen, B.K. Physical activity and muscle-brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.M.; Fan, X.; Bieri, G.; Smith, L.K.; Sanchez-Diaz, C.I.; Schroer, A.B.; Gontier, G.; Casaletto, K.B.; Kramer, J.H.; Williams, K.E.; et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science 2020, 369, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.Y.; Becke, A.; Berron, D.; Becker, B.; Sah, N.; Benoni, G.; Janke, E.; Lubejko, S.T.; Greig, N.H.; Mattison, J.A.; et al. Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function. Cell Metab. 2016, 24, 332–340. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Cassilhas, R.C.; Tufik, S.; de Mello, M.T. Physical exercise, neuroplasticity, spatial learning and memory. Cell Mol. Life Sci. 2016, 73, 975–983. [Google Scholar] [CrossRef]
- Raichlen, D.A.; Alexander, G.E. Adaptive Capacity: An Evolutionary Neuroscience Model Linking Exercise, Cognition, and Brain Health. Trends Neurosci. 2017, 40, 408–421. [Google Scholar] [CrossRef]
- Walsh, J.J.; Scribbans, T.D.; Bentley, R.F.; Kellawan, J.M.; Gurd, B.; Tschakovsky, M.E. Neurotrophic growth factor responses to lower body resistance training in older adults. Appl. Physiol. Nutr. Metab. 2016, 41, 315–323. [Google Scholar] [CrossRef]
- Etnier, J.L.; Wideman, L.; Labban, J.D.; Piepmeier, A.T.; Pendleton, D.M.; Dvorak, K.K.; Becofsky, K. The Effects of Acute Exercise on Memory and Brain-Derived Neurotrophic Factor (BDNF). J. Sport Exerc. Psychol. 2016, 38, 331–340. [Google Scholar] [CrossRef]
- Barha, C.K.; Galea, L.A.; Nagamatsu, L.S.; Erickson, K.I.; Liu-Ambrose, T. Personalising exercise recommendations for brain health: Considerations and future directions. Br. J. Sports Med. 2017, 51, 636–639. [Google Scholar] [CrossRef]
- Contrepois, K.; Wu, S.; Moneghetti, K.J.; Hornburg, D.; Ahadi, S.; Tsai, M.-S.; Metwally, A.A.; Wei, E.; Lee-McMullen, B.; Quijada, J.V.; et al. Molecular Choreography of Acute Exercise. Cell 2020, 181, 1112–1130.e16. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.; Baktir, M.A.; Srivatsan, M.; Salehi, A. Neuroprotective effects of physical activity on the brain: A closer look at trophic factor signaling. Front. Cell Neurosci. 2014, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Herold, F.; Müller, P.; Gronwald, T.; Müller, N.G. Dose-Response Matters!—A Perspective on the Exercise Prescription in Exercise-Cognition Research. Front. Psychol. 2019, 10, 2338. [Google Scholar] [CrossRef] [PubMed]
- Ammann, B.C.; Knols, R.H.; Baschung, P.; de Bie, R.A.; de Bruin, E.D. Application of principles of exercise training in sub-acute and chronic stroke survivors: A systematic review. BMC Neurol. 2014, 14, 167. [Google Scholar] [CrossRef]
- Firth, J.; Stubbs, B.; Vancampfort, D.; Schuch, F.; Lagopoulos, J.; Rosenbaum, S.; Ward, P.B. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. Neuroimage 2018, 166, 230–238. [Google Scholar] [CrossRef]
- Thomas, B.P.; Tarumi, T.; Sheng, M.; Tseng, B.; Womack, K.B.; Cullum, C.M.; Rypma, B.; Zhang, R.; Lu, H. Brain Perfusion Change in Patients with Mild Cognitive Impairment After 12 Months of Aerobic Exercise Training. J. Alzheimers Dis. 2020, 75, 617–631. [Google Scholar] [CrossRef]
- Voss, M.W.; Nagamatsu, L.S.; Liu-Ambrose, T.; Kramer, A.F. Exercise, brain, and cognition across the life span. J. Appl. Physiol. 2011, 111, 1505–1513. [Google Scholar] [CrossRef]
- Gjevestad, G.O.; Holven, K.B.; Ulven, S.M. Effects of Exercise on Gene Expression of Inflammatory Markers in Human Peripheral Blood Cells: A Systematic Review. Curr. Cardiovasc. Risk Rep. 2015, 9, 34. [Google Scholar] [CrossRef]
- Janelidze, S.; Mattsson, N.; Palmqvist, S.; Smith, R.; Beach, T.G.; Serrano, G.E.; Chai, X.; Proctor, N.K.; Eichenlaub, U.; Zetterberg, H.; et al. Plasma P-tau181 in Alzheimer’s disease: Relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med. 2020, 26, 379–386. [Google Scholar] [CrossRef]
- Kanfer, R.; Frese, M.; Johnson, R.E. Motivation related to work: A century of progress. J. Appl. Psychol. 2017, 102, 338–355. [Google Scholar] [CrossRef]
- Mattsson, N.; Andreasson, U.; Zetterberg, H.; Blennow, K. Alzheimer’s Disease Neuroimaging Initiative Association of Plasma Neurofilament Light With Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol. 2017, 74, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Mckee, A.C.; Daneshvar, D.H. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Lezi, E.; Burns, J.M.; Swerdlow, R.H. Effect of high-intensity exercise on aged mouse brain mitochondria, neurogenesis, and inflammation. Neurobiol. Aging 2014, 35, 2574–2583. [Google Scholar] [CrossRef]
- Voss, M.W.; Soto, C.; Yoo, S.; Sodoma, M.; Vivar, C.; van Praag, H. Exercise and Hippocampal Memory Systems. Trends Cogn. Sci. 2019, 23, 318–333. [Google Scholar] [CrossRef]
- Pieramico, V.; Esposito, R.; Sensi, F.; Cilli, F.; Mantini, D.; Mattei, P.A.; Frazzini, V.; Ciavardelli, D.; Gatta, V.; Ferretti, A.; et al. Combination training in aging individuals modifies functional connectivity and cognition, and is potentially affected by dopamine-related genes. PLoS ONE 2012, 7, e43901. [Google Scholar] [CrossRef]
- Rasmussen, P.; Brassard, P.; Adser, H.; Pedersen, M.V.; Leick, L.; Hart, E.; Secher, N.H.; Pedersen, B.K.; Pilegaard, H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 2009, 94, 1062–1069. [Google Scholar] [CrossRef]
- Mustapic, M.; Eitan, E.; Werner, J.K.; Berkowitz, S.T.; Lazaropoulos, M.P.; Tran, J.; Goetzl, E.J.; Kapogiannis, D. Plasma Extracellular Vesicles Enriched for Neuronal Origin: A Potential Window into Brain Pathologic Processes. Front. Neurosci. 2017, 11, 278. [Google Scholar] [CrossRef]
- Baggish, A.L.; Park, J.; Min, P.-K.; Isaacs, S.; Parker, B.A.; Thompson, P.D.; Troyanos, C.; D’Hemecourt, P.; Dyer, S.; Thiel, M.; et al. Rapid upregulation and clearance of distinct circulating microRNAs after prolonged aerobic exercise. J. Appl. Physiol. 2014, 116, 522–531. [Google Scholar] [CrossRef]
- Basso, J.C.; Suzuki, W.A. The Effects of Acute Exercise on Mood, Cognition, Neurophysiology, and Neurochemical Pathways: A Review. Brain Plast. 2017, 2, 127–152. [Google Scholar] [CrossRef]
- Hübner, L.; Godde, B.; Voelcker-Rehage, C. Acute Exercise as an Intervention to Trigger Motor Performance and EEG Beta Activity in Older Adults. Neural Plast. 2018, 2018, 4756785. [Google Scholar] [CrossRef]
- Singh, A.M.; Neva, J.L.; Staines, W.R. Acute exercise enhances the response to paired associative stimulation-induced plasticity in the primary motor cortex. Exp. Brain Res. 2014, 232, 3675–3685. [Google Scholar] [CrossRef] [PubMed]
- Moraes, H.; Deslandes, A.; Silveira, H.; Ribeiro, P.; Cagy, M.; Piedade, R.; Pompeu, F.; Laks, J. The effect of acute effort on EEG in healthy young and elderly subjects. Eur. J. Appl. Physiol. 2011, 111, 67–75. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Glidden, A.M.; Holloway, M.R.; Birbeck, G.L.; Schwamm, L.H. Teleneurology and mobile technologies: The future of neurological care. Nat. Rev. Neurol. 2018, 14, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Muurling, M.; de Boer, C.; Kozak, R.; Religa, D.; Koychev, I.; Verheij, H.; Nies, V.J.M.; Duyndam, A.; Sood, M.; Fröhlich, H.; et al. Remote monitoring technologies in Alzheimer’s disease: Design of the RADAR-AD study. Alzheimers Res. Ther. 2021, 13, 89. [Google Scholar] [CrossRef]
- Bot, B.M.; Suver, C.; Neto, E.C.; Kellen, M.; Klein, A.; Bare, C.; Doerr, M.; Pratap, A.; Wilbanks, J.; Dorsey, E.R.; et al. The mPower study, Parkinson disease mobile data collected using ResearchKit. Sci. Data 2016, 3, 160011. [Google Scholar] [CrossRef]
- Intzandt, B.; Black, S.E.; Lanctôt, K.L.; Herrmann, N.; Oh, P.; Middleton, L.E. Is Cardiac Rehabilitation Exercise Feasible for People with Mild Cognitive Impairment? Can. Geriatr. J. 2015, 18, 65–72. [Google Scholar] [CrossRef][Green Version]
- Kivipelto, M.; Ngandu, T.; Laatikainen, T.; Winblad, B.; Soininen, H.; Tuomilehto, J. Risk score for the prediction of dementia risk in 20 years among middle aged people: A longitudinal, population-based study. Lancet Neurol. 2006, 5, 735–741. [Google Scholar] [CrossRef]
- McLaren, L.; Sumar, N.; Barberio, A.M.; Trieu, K.; Lorenzetti, D.L.; Tarasuk, V.; Webster, J.; Campbell, N.R. Population-level interventions in government jurisdictions for dietary sodium reduction. Cochrane Database Syst. Rev. 2016, 9, CD010166. [Google Scholar] [CrossRef]
- Kivipelto, M.; Mangialasche, F.; Snyder, H.M.; Allegri, R.; Andrieu, S.; Arai, H.; Baker, L.; Belleville, S.; Brodaty, H.; Brucki, S.M.; et al. World-Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimers Dement. 2020, 16, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, K.S.; Cooper, C.; Frisoni, G.B.; Frölich, L.; Georges, J.; Kramberger, M.G.; Nilsson, C.; Passmore, P.; Mantoan Ritter, L.; Religa, D.; et al. A European Academy of Neurology guideline on medical management issues in dementia. Eur. J. Neurol. 2020, 27, 1805–1820. [Google Scholar] [CrossRef]
- Almirall, D.; Nahum-Shani, I.; Sherwood, N.E.; Murphy, S.A. Introduction to SMART designs for the development of adaptive interventions: With application to weight loss research. Transl. Behav. Med. 2014, 4, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Klasnja, P.; Hekler, E.B.; Shiffman, S.; Boruvka, A.; Almirall, D.; Tewari, A.; Murphy, S.A. Microrandomized trials: An experimental design for developing just-in-time adaptive interventions. Health Psychol. 2015, 34S, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.M.; Baker, T.B.; Mermelstein, R.J.; Piper, M.E.; Jorenby, D.E.; Smith, S.S.; Christiansen, B.A.; Schlam, T.R.; Cook, J.W.; Fiore, M.C. The multiphase optimization strategy for engineering effective tobacco use interventions. Ann. Behav. Med. 2011, 41, 208–226. [Google Scholar] [CrossRef]
- Stetler, C.B.; Legro, M.W.; Wallace, C.M.; Bowman, C.; Guihan, M.; Hagedorn, H.; Kimmel, B.; Sharp, N.D.; Smith, J.L. The role of formative evaluation in implementation research and the QUERI experience. J. Gen. Intern. Med. 2006, 21 (Suppl. 2), S1-8. [Google Scholar] [CrossRef]
- Wong, C.-M.; Lai, H.-K.; Ou, C.-Q.; Ho, S.-Y.; Chan, K.-P.; Thach, T.-Q.; Yang, L.; Chau, Y.-K.; Lam, T.-H.; Hedley, A.J.; et al. Is exercise protective against influenza-associated mortality? PLoS ONE 2008, 3, e2108. [Google Scholar] [CrossRef]
- Kourtis, L.C.; Regele, O.B.; Wright, J.M.; Jones, G.B. Digital biomarkers for Alzheimer’s disease: The mobile/ wearable devices opportunity. NPJ Digit. Med. 2019, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Andrieu, S.; Coley, N.; Lovestone, S.; Aisen, P.S.; Vellas, B. Prevention of sporadic Alzheimer’s disease: Lessons learned from clinical trials and future directions. Lancet Neurol. 2015, 14, 926–944. [Google Scholar] [CrossRef]
- Rosenberg, A.; Ngandu, T.; Rusanen, M.; Antikainen, R.; Bäckman, L.; Havulinna, S.; Hänninen, T.; Laatikainen, T.; Lehtisalo, J.; Levälahti, E.; et al. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: The FINGER trial. Alzheimers Dement. 2018, 14, 263–270. [Google Scholar] [CrossRef]
- Tait, J.L.; Duckham, R.L.; Milte, C.M.; Main, L.C.; Daly, R.M. Influence of Sequential vs. Simultaneous Dual-Task Exercise Training on Cognitive Function in Older Adults. Front. Aging Neurosci. 2017, 9, 368. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).