Abstract
Nutritional status plays a vital role in regulating ovary activity. This regulation is mediated by the hypothalamus–pituitary–gonad axis and by effects exerted directly on the ovary. Therefore, to achieve the best reproductive performance, it is essential to know how the nutritional status affects the secretion of GnRH, gonadotrophins, and sex steroid hormones. Adequate body reserves and energy balance are critical for optimal reproductive performance in sheep and goats. However, over- or under-conditioned animals experience issues like extended anestrus, irregular ovarian cycles, and reduced conception. Body condition scoring allows for the evaluation of the relationships between adiposity, nutritional status, and fertility. Acute feed deficits briefly stimulate processes, but chronic restrictions suppress pulsatile LH release, disrupting ovarian function. The process of follicle development is a very complex one which involves intricate interactions between the pituitary gonadotrophins and metabolic hormones as well as between the locally produced factors by the ovarian somatic and germ cells including the IGF system and the TGF-β superfamily members. Genotype and nutrition are factors that have an impact on follicular development, and seasonal factors are also involved. This review will give a brief overview on how the body condition can be evaluated and the effects of nutrition on the hypothalamus–pituitary–gonad axis and ovarian activity, which are responsible for reproductive regulation. This paper presents a clear and reasonable summary of the pathway that runs from the nutritional status of small ruminants to ovarian activity through the hypothalamic–pituitary–gonadal axis. This review summarizes methods for body condition evaluation in small ruminants and evidence regarding acute versus prolonged nutritional impacts on the hypothalamic–pituitary–gonadal axis and ovarian activity controlling reproduction.
1. Introduction
Adequate body reserves and the plane of nutrition are well established as foundational for securing optimal reproductive efficiency and fertility outcomes in sheep and goats [1,2,3,4,5]. Females carrying excessive or insufficient fat and muscle cover exhibit perturbed patterns of cyclicity, attenuated estrus expression, reduced ovulation quotas, elevated embryonic loss risk, extended postpartum anestrus, and overall lowered fecundity from weakened pregnancy rates and survival, impacting productivity [3,4,5,6,7]. The reproductive potential can be enhanced with metabolic inputs and dynamic body conditions modulated by dietary intake factors, representing a key determinant in controlling this achievement.
BCS remains one of the most important strategies for assessing the adiposity level, a critical nutritional status that should always be measured in animals. This offers the opportunity to proactively implement nutritional interventions to correct discrepancies [3,8]. Within the realm of BMI, a five-point scale is typically utilized, with 1 assigned to patients who are very thin and 5 to those who are overweight. This scoring system is, among many other things, a reflection of the carcass duration and the total energy reserves [4,5,6,7,8,9].
The latest research shows BCS can be vital when regulating reproductive functions. The secretion of gonadotropins by the hypothalamic–pituitary axis is mediated by the BCS, which regulates ovarian function. This is achieved through a signaling mechanism. Moreover, the invisible effect of BCS works on intraovarian nutrient sensor pathways essential for folliculogenesis, good-quality oocytes, ovulation after mating, and corpus luteum function after mating [5,7,8,9,10]. Changes in body composition are known to be a cause of a reduction in the pulsation of LH, which is supported through metabolism hormones including leptin, insulin, and IGF-1, which are required to activate hypothalamic generators of GnRH [1,7]. LH is therefore the substance that theca and granulosa cells need to produce steroid, which is required for successful ovulation and secretory formation and maintenance of the corpus luteum [7,9]. Alongside that, there is a manner by which metabolic hormone ovarian actions are complimented by leptin, insulin, and IGF-1 for the process of follicular maturation.
Nutritional insufficiency generates reproductive dysfunction through interrelated endocrine and metabolic channels that compromise fertility. Short-term deficits may initially activate portions of the hypothalamic–pituitary–gonadal (HPG) axis before chronic restrictions that override transient stimulus effects [11,12]. Timing mediates outcomes across the production calendar from gestation to lactation [1,13]. This review summarizes the current evidence regarding body condition relationships with fertility, practical scoring methods enabling assessment, and approaches targeting improved productivity through strategic dietary interventions around critical reproductive periods in sheep and goat production.
2. Evaluating Body Condition
2.1. Body Condition Scoring
The body condition score (BCS) plays a very important role in subjective assessment, which lets us know how extensive the fat reserves in the animal body are and how much muscle the animal has. It is measured on a five-point scale that ranges from 1 (emaciated) to 5 (obesity), and is the standardized scale for conducting studies [3,5]. The best-suited body composition would be centered on a mark of 3, while anything above 4 signifies over-conditioning, and anything below two indicates severe malnourishment. This is an effect of the fact that the reliability of near-infrared technology has been confirmed through carcass fat measurements [3,5]. They are comparable to kidney knob depth, confirming the strong correlation with real tissue reserves.
BCS assessments are especially critical before substantial events in the breeding process, such as mating, late gestation, and parturition, when the routine of animals’ nutrient needs and the body’s use of resources are significantly changed. In addition, seasonal assessment is a tool that is also used to measure the effect of environmental factors on the condition of an animal; for example, the constriction period during winter or summer may result in a loss of conditioning if the pasture quality is poor and supplementation is not carried out [2,4].
Moreover, the age and the reproductive status of the animals is also important to consider when grading the BCS. The females of animals with a large body mass, for example cows, goats, and those that are lactating or those that are pregnant, have high nutritional requirements, and providing extra nutrition can help keep them in good physical condition [3,9]. Feeding the right amount of nutrients at specific times and making necessary changes to nutritional programs are the significant factors that must be considered to achieve an ideal balance in order to support wellness, growth, and reproductive function in animals [6,14]. Monitoring livestock from the time they are growing is essential, especially during harsh weather conditions that can lead to pasture losses and animals’ poor health. In the same sense, it is during winter that an animal can face food shortages, which may lead to additional energy expenditure as they try to regulate their body temperatures [2,15]. This can be made worse without adequate nutrition support given to the animal.
Making changes to animal diets based on these checks is very important. This means that the feeding plan must align with particular food shortages or excess, and this is enabled by checking often [16]. Farmers can make their animals grow, reproduce, and stay healthy by adjusting the diet they eat. This method ensures that the correct conditions are achieved through constantly changing certain aspects and emphasizes how important being flexible is to ensure a farm with animals succeeds.
2.2. Relationship of BCS with Fertility
Extensive evidence across sheep and goat breeds demonstrates that females entering mating periods in optimal physical condition and adiposity exhibit improved fertility outcomes, including heightened conception and twinning rates and larger subsequent litter sizes and weaning weights [4,5,6,7,9]. Well-nourished ewes also display elevated ovulation quotas up to 200–300% of those of poorly conditioned cohorts [8,14]. Flushing management before breeding poorly nourished ewes amplifies these responses by rapidly elevating score thresholds through concentrated energy supplementation in the weeks preceding the introduction of rams [8,14]. Recent research supports these findings, indicating that ewes with a BCS below optimal levels face reproductive inefficiencies. For instance, fertility rates and reproductive rates are positively correlated with BCS, peaking at a score of around 3.0 to 3.5. Beyond this point, no further reproductive benefits are observed with increased BCS1 [2,8,10,14,17]. Moreover, maintaining ewes at a BCS below 3.3 can lead to an approximate 20% decrease in the reproductive rate compared to those at or above this threshold.
Prolonged underfeeding markedly suppresses pituitary gonadotropin secretion required to adequately support ovarian folliculogenesis, oocyte maturation, and corpus luteum formation and function following mating. The direct effects of poor reserves include smaller subordinate follicles with compromised steroidogenic capacity and inadequate luteolysis that further constrains fertility [8,10,14,17]. Therefore, a dynamic body energy balance exerts significant control over the internal reproductive schedule through interconnected endocrine, metabolic, and reproductive system pathways governing optimal fertility. Carefully managing conditions via exogenous inputs aligned with physiological state spares endogenous protein losses while stabilizing neuroendocrine profiles, securing prolificacy.
These connected pathways show how the body’s energy and ability to reproduce are closely related in a complex way. Matching external factors with the body’s state can change the balance of hormones and metabolic components that matter for health. This is important for small ruminants. This effective form of care improves fertility and lowers the risk of bad impacts on the hormone system [18,19]. Essentially, it is a complete plan that understands how external factors and actions internally work together. So, controlling how the body uses energy is crucial in achieving and maintaining an excellent reproductive plan internally. It helps to protect newborns from any problems. In this regard, a comprehensive approach to animal care that includes both external environmental factors in addition to internal physiological processes is essential for improving fertility as well as preventing the negative impacts on the endocrine system. Through efficient energy utilization, the producers can ensure the achievement and maintenance of a reliable reproduction program that enhances fertility rates, and, at the same time, protects the health of newborns [20,21]. Properly managing animals holistically is crucial for ensuring that the animals are well fed, reproduction is regular, and their stress is kept at a minimum, which will in turn lead to the success of the breeding program and the good animal welfare of the herd.
3. Metabolism Regulation of Gonadotropin Secretion
As a lower net priority for nutrient partitioning, the reproductive neuroendocrine axis exhibits sensitivity to fluctuations in energy availability, as signaled by adipose hormones like leptin, insulin, ghrelin, adiponectin, and IGF-1 [11,13,15,22,23,24]. Fed versus fasted states prompt counter-regulatory appetite and metabolic centers within the hypothalamus to stimulate or suppress downstream reproductive function accordingly [13,23]. For example, short-term fasting imposes a negative energy balance, mildly elevating GnRH and subsequent LH secretion, attributed to certain orexigenic neuropeptide drivers like neuropeptide Y (NPY) overriding the anorexigenic inhibition of GnRH neurons as peripheral insulin and leptin levels decline [13,15,22]. This initial activation may temporarily promote follicular development and ovulation capacity [17,22,25,26,27].
The reproductive neuroendocrine axis, in particular, is a system which is highly sensitive to the proper energy balance at the level of the body, and is influenced by several hormones synthesized and secreted by fat tissue including leptin, insulin, ghrelin, adiponectin, and IGF-1 (Figure 1). These are the hormones that the hypothalamus looks upon to provide the relevant signals in order to make the required alterations to the reproductive functions depending on the availability of energy at that moment [15,17]. During the state of fasting, energy sources are limited, which results in the rise of GnRH and LH secretions, which are mild in nature [5,7]. This is partly because of the role of orexigenic neuropeptides such as neuropeptide Y (NPY) that can stimulate GnRH neurons and thus eliminate the suppression of peripheral insulin and leptin, causing hypothalamic–pituitary–gonadal axis dysfunction [22,27]. These receptors activate a temporary process for the development of follicles and the possibility of ovulation, thereby facilitating egg formation even during an energy deficit.
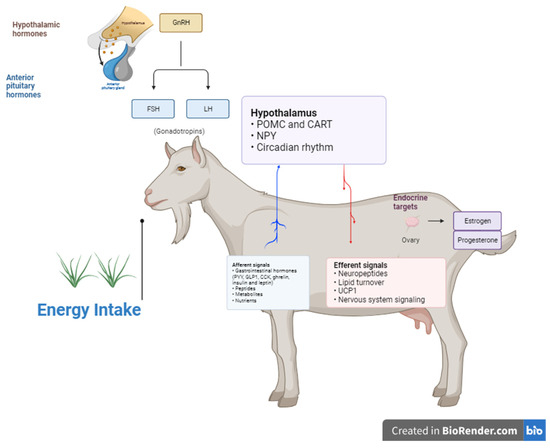
Figure 1.
Schematic of hypothalamic integration of signals from appetite/metabolic centers and peripheral hormones controlling GnRH neuron activity and downstream pituitary gonadotropin secretion [13,15].
However, if more severe or prolonged undernutrition resulting in substantive fat loss persists, this will consistently inhibit the pulsatile LH output required to initiate puberty, achieve ovulation, and maintain adult ovarian cycling [8,13,22]. Key metabolic signals like leptin not only directly excite GnRH neurons but also serve as permissive gatekeepers allowing maturation—without which, reproductive activation may remain largely suppressed regardless of acute stimuli [15,22]. Declining insulin, IGF-1, ghrelin, and adiponectin reduce gonadotropin increase through additional pathways [13,17]. For example, adiponectin inhibits GnRH secretion in cultured hypothalamic fragments, which impairs pituitary LH release [23].
With an ongoing negative energy balance, attenuated metabolic regulators like leptin, insulin, IGF-1, ghrelin, and adiponectin reduce GnRH neurons responsiveness to excitatory inputs like kisspeptin [15,23,28]. Simultaneously, unrestrained Y neuropeptide (NPY) orexigenic drive overrides declining temporary signals, exacerbating chronic inhibitory tone suppressing GnRH pulse generators [15,26]. Downstream, the resultant inadequate amplitude and frequency of LH pulses fail to adequately mature responsive antral follicles, transition dominant structures to periovulatory estrogenic competence, or rescue the corpus luteum to maintain progesterone production after ovulation [10,13].
These ovarian deficiencies combine to profoundly disrupt fertility through missed or discrete estrus events, anovulation, short luteal phases post mating, and early embryonic losses attributed to inadequate hormonal support [13,25]. Furthermore, more studies have helped to explain this adaptive system through a more intricate neuroendocrine feedback loop. NPY, for example, has been known to act as a dual factor in reproductive function, being a stimulator and inhibitor depending on the context of metabolic areas [23,26]. Although short-duration fasting can boost reproductive function through the aforementioned actions, a long-term excessive negative energy balance may lead to reproductive system suppression (Table 1), demonstrating the priorities of the body to maintain survival rather than reproduction in the period of energy deficit [23,26]. Therefore, while transient metabolic challenges may intermittently activate portions of the reproductive axis, persistent nutritional insufficiency suppresses pulsatile LH drive underlying regular ovarian cycling and pregnancy success in sheep and goats [11,12]. Dietary restrictions’ precise duration and physiological timing mediate reproductive responses based on the specific energetically challenged state—gestation, lactation, or seasonal transitions [23,29]. For example, nutrient deficits from early pregnancy may impact placental development, fetal organ maturation, and offspring growth, whereas later deficiencies could acutely disrupt ovarian function at the first estrus post pregnancy [11,13,23].

Table 1.
Summary of key hormonal regulators of GnRH/LH secretion and effects of negative energy balance [13,14,23,25].
4. Nutrition and Ovarian Function
4.1. Direct Metabolic Effects on Folliculogenesis
Independent of reducing systemic LH availability from suppressed hypothalamic–pituitary signaling, variations in body energy reserves and tissue fuel metabolism also directly influence ovarian follicle recruitment, selection, growth, maturation, and steroidogenic capacity through local autocrine, paracrine, and endocrine signals [10,13,24]. For example, underfed ewes exhibit smaller antral follicle diameters before the preovulatory LH surge, accompanied by constrained intracellular maturation markers like reduced CYP19A1 aromatase enzyme expression impairing estradiol biosynthesis required for behavioral estrus display and ovulation [12,27]. Normal follicular estrogen production provides positive feedback, triggering the LH surge. Impaired steroidogenesis contributes to anovulation and infertility in nutritionally compromised females [24].
Key metabolic hormones, including insulin, IGF-1, leptin, adiponectin, and Growth Hormone (GH), synergize with gonadotropins to enable continued follicle development under marginal systemic LH or nutritional conditions [12,13,24]. Leptin, insulin, ghrelin, and adiponectin receptors densely populate all ovarian compartments in sheep and cattle [12,28,29,30]. Ligand binding stimulates proliferation, differentiation, and sex steroid output by granulosa and theca cells comprising recruited follicles [12,28,29,30]. IGF-1 further amplifies Follicular-Stimulating Hormone (FSH) and LH initiation of growth and steroidogenesis while also preserving oocyte developmental competence [24,30]. Via these complementary signals, optimal follicle health and function depend on adequate energy reserves and balanced metabolic homeostasis [24,30]. Resource losses amplify reproductive dysfunction through compounding ovarian defects.
4.2. Additional Ovarian Impacts
Attenuated follicular growth and steroid synthetic capacity reduce ovulatory rates and subsequent corpus luteum quality with additional consequences, including lower luteal-phase progesterone production and greater chances of early embryo/fetal mortality in nutrient-restricted females [4,13,14,28]. In vitro culture studies demonstrate similar dysfunction, with hindered luteinization progression and progesterone output from granulosa cells deprived of metabolic support even in the presence of LH [24,28]. The last several years have ushered in significant progress in the field of nutrition as its contribution to ovarian function has become more evident. This process has generated several very important findings about how follicles develop. Research has demonstrated that lipid metabolism is a crucial aspect of oocyte maturation since lipid metabolites supply the cell with energy, steroid hormone precursors, and agents that trigger meiosis resumption [8,15,24,31]. Through these concatenated deficiencies spanning folliculogenesis, ovulation, and luteal function, a negative energy balance constrains the establishment and maintenance of pregnancy required for maximal fertility [4,13,14]. Dire impacts on oocyte quality also decrease developmental competence and increase early embryonic loss [13,28]. Monitoring external indicators like BCS helps time nutritional interventions protecting developing follicles and corpora lutea while also retaining productivity in sheep and goats.
Nevertheless, the disproportionate state of matters, especially in fat-rich environments, are the direct factor of the fallacy of follicular cells. On the other hand, studies show that moderate intensity may have the potential to increase the number of the total follicles and of the primary follicles as well (Table 2), which underlines the role of daily routines that include physical activity in supporting follicular health [28,31]. In addition, the HPO axis is important in the hierarchical organization of the processes of follicle maturation to ovulation through its hormonal regulation [8,10,14,17,25,26,27]. These findings have shown the importance of a set-back approach in dietary and exercise habits for the sake of optimum ovary function and point to the probable new paths of improving fertility treatment in the context of metabolic challenges [18,31].

Table 2.
Adverse effects of poor body condition and undernutrition on key events in the ovarian reproductive cycle [4,14,24,28].
5. Improving Fertility through Body Condition Management
5.1. Target Condition Scores
Achieving optimal BCS targets before key reproductive periods allows small ruminants to maximize their fertility potential. The recommended minimum thresholds are 2.5–3.0 [1,4,15,28]. However, aiming for BCS 3.0–3.5 is suggested for highly prolific genotypes carrying twins and triplets to support greater anticipated fetal and subsequent offspring nutrient demands [4,13]. Higher adiposity reserves improve conception chances, litter sizes, milk output, and lamb growth via HPG axis amplification and intraovarian mechanisms [1,4,13,28].
Furthermore, the impact of a good BCS on reproductive success goes beyond impregnation. This helps for the all-round health and life of small ruminants. Also, if a ewe or doe is kept in good shape, it can better handle the body’s strain during birth. This makes it easier to enter milk-maker mode after giving birth [12]. In the real world, in terms of the ways used to manage, reach, and keep the best physical condition, a complete plan includes what animals eat, watching their health, and quick actions when required [28]. Goats eating well with the right food and energy helps them achieve and maintain good physical fitness. Checking the body condition score (BCS) often and changing feeding schedules if needed helps to manage animals’ fitness and the growth of small ruminants.
The optimal assessment of body condition scores (BCSs) is one of the major approaches to boosting the fertility of small ruminants. For that reason, the optimal BCS (body condition score) is obtained right before the critical reproductive periods in order to obtain the best possible fertility outcome. The recommended BCS is 2.5–3.0 for breeds that are highly prolific, especially those that carry twins and triplets. In such cases, a BCS of 3.0–3.5 would be optimum to fulfill the increased fetal and offspring nutritional demands [12,32]. For females, fat stores have multiple roles from increased conception rates, to larger litter sizes, as well as better milk production and lamb growth [16,24,32,33]. The effects of these benefits arise as a result of the HPG axis activation and intragranular processes, which promote reproductive functions.
In the course of the latest research, some new strategies have been discovered, aimed at attaining higher fertility in small ruminants through BCS management. A supplementary approach, such as targeted nutritional supplementation as a potential option can bring about significant changes in reproductive traits [12,34]. To be more specific, as replacement for nutritional deficiencies or improving the overall health status of the male, supplements have been seen to better the breeding condition, increase the number of embryos for larger litters, reduce early embryo loss and provide better postnatal nutrition and growth [18,33,34].
5.2. Strategic Dietary Adjustments
Nutrition management across the annual production calendar targets key periods of elevated requirements or vulnerability to body reserve loss. Strategic intervention optimizes energy balance, avoiding BCS extremes that impair fertility. The post-weaning through pre-breeding interval is an important nutritional transition, as females recover previous lactation expenditures while also preparing to reinitiate ovarian cycles [1,22]. Protein and adipose catabolism on declining pasture quality conditions can cause a negative energy balance and precipitate substantial BCS losses by breeding [22]. Modest supplementation with digestible fiber and high-protein pellets helps compensate for seasonal nutritional gaps, providing glucogenic and lipogenic substrates that conserve endogenous reserves critical for restarting reproductive cycles [1,10,22]. Recent research has continued to look into the perception of flushing as well as its impacts on the reproductive system, mostly the hypothalamic–pituitary–gonadal (HPG) axis in animals. Flushing, which comprises increasing the nutritional intake above maintenance levels shortly before and after breeding, aims to enhance body condition, and hence, reproductive outcomes [21,34]. Further studies have demonstrated that this practice could increase the ovulation rate and improve embryo survival, probably due to the improved energy availability, which supports the HPG axis during the critical stages of reproduction [35]. At the center of this planned performance is the increased help from a hormone called gonadotropin-releasing hormone (GnRH). This brain chemical is crucial in letting the body know it is ready for pregnancy. So, the ovaries step in and bring more ovary follicles into play. The axis becomes even stronger as it makes more of a hormone called luteinizing hormone (LH). This is very important for the process of ovulation. It does this by connecting hormone changes with resource availability. This makes sure things go well for creating a new life. Its stimulation primes the HPG axis in anticipation of sufficient resources for subsequent fetal and offspring priorities. It drives heightened GnRH support, the recruitment of larger ovarian follicle cohorts, LH secretion, increased ovulation quotas, and greater potential litter sizes [7,13,15].
Dietary adjustments are strategic for maintaining good shape and health as well as for the need to reproduce. In particular, the period post lactation and the re-establishment of ovarian cycles, which takes place during the time between weaning and pre-breeding, is the interval when nourishment should be sufficient to recharge and restore the body after the lactation process [21,36]. Furthermore, the intake of additional protein and energy-dense feedstuffs can prevent a negative caloric balance and safeguard from severe BCS decline, which might have negative effects on the reproductive function. Thus, these preventive strategies are of great importance for maintaining the health and productivity of the herd during the breeding period as well as beyond this period.
5.3. Requirements during Pregnancy and Lactation
While flushing strategies briefly stimulate fertility, care must be taken to avoid over-conditioning, which can also constrain prolificacy [1,4,11]. Requirements during early pregnancy are modest but increase exponentially during late gestation proportional to the litter size [22,24]. Gradually elevating provisions prevents sudden BCS spikes while ensuring sufficient nutrient flow to fetoplacental units [1,22,24], supporting fast fetus growth. The peri-parturient interval spanning late gestation through early lactation represents the peak annual nutritional demand [1,12,22]. The synthesis of colostrum and milk depletes glucose stores, with production surpassing the energy intake capacity [12]. Steaming up, enhancing energy and protein levels, in the diet is advised from the last third of pregnancy to the peak of lactation. Supplementing digestible fiber and bypass proteins spares labile tissue catabolism, supporting mammary development while promptly re-attaining good physical condition to resume ovarian cycling post weaning [1,12,22]. Additionally, strategic adjustments are advised during mid-lactation as females approach rebreeding targets. Additional grain or high-quality pasture prevents over-mobilization when entering subsequent anestrus while restoring adiposity for the next reproductive round [1,22].
5.4. Nutritional Programming of Fertility
In addition to direct impacts on the reproductive axis, the maternal nutritional status during the critical developmental window shapes long-term metabolic and physiological trajectories in offspring through a phenomenon known as fetal programming [28,29,30,37]. Modest to severe maternal underfeeding from early gestation onwards affects growth patterns, appetite regulation, and reproductive maturity in offspring via complex epigenetic changes that tune gene expression [28,29,30,37]. In goats, maternal nutritional restriction has been shown to impact offspring’s metabolic responses, which can influence their reproductive capabilities. For example, nutrient-restricted offspring experience altered glucose metabolism as well as insulin sensitivity, possibly affecting their fertility in addition to reproductive performance later in life. These changes underline the significance of adequate maternal nutrition during gestation for the long-term health and reproductive success of goat offspring [21,36]. High gestational glucocorticoid exposure may mediate these outcomes by altering the tissue glucocorticoid sensitivity in offspring [28]. Suboptimal maternal protein or micronutrient intake also decreases follicle reserves and ovarian responsiveness to gonadotropins in daughters [37].
Recent studies have provided insights into the adaptive mechanisms that occur in response to placental insufficiency, a condition that can compromise fetal development [18,29]. However, persistent maternal underfeeding through mid–late gestation has greater detrimental impacts than temporary early deficits, permanently modifying the structure and function of regulating appetite circuitries and metabolic tissues involved in nutrient partitioning decisions [28,29,30,37]. Lasting reproductive consequences reflect complex tradeoffs that balance limited resources. This compensatory response, while crucial for survival, may have downstream effects on other systems, potentially impacting the reproductive capabilities later in life [35]. While the precise genomic mechanisms remain unclear, altered DNA methylation patterns in the promoter regions of implicated genes suggest broad shifts in epigenetic control [28,29,37]. These heritable modifications persistently alter phenotypic expression without any alterations to the underlying DNA sequence [36]. Further research should clarify maternal signals and offspring pathways to design interventions that mitigate negative fetal programming caused by a poor gestational diet.
5.5. Practical Feeding Recommendations
Incorporating current scientific evidence into practical feeding programs that strategically target periods of elevated requirements or vulnerability enables optimal dynamic body condition management, ensuring maximal fertility from small ruminant operations. The general minimum score thresholds are 2.5–3.0 for maiden ewe lambs and mature dry females during anestrus, while a score of 3.0–3.5 should be aimed for in highly prolific multiparous breeds [1,4,15]. Pre-breeding flushing procedures raise levels over 2 weeks to add 0.5–1.0 condition units, driving heightened ovulation rates, uterine milk production, and subsequent litter sizes [7,13,15]. Maintaining a steady nutritional intake during gestation is crucial for supporting the significant increase in fetal growth that occurs in the later stages, while also preventing the extremes of adiposity [3,15,30]. Finally, supplementary bypass proteins and digestible fiber during early lactation spare excessive glucose loss from labile tissue catabolism [1,12,22].
A streamlined feeding cycle blueprint is important for strategically increasing intake for flushing, adjusting rations throughout pregnancy, and supporting lactation to maintain appropriate body reserves for crucial events while avoiding protracted deficits or sudden gains that damage fertility. Further improvements may be added according to the available feedstuffs, facility infrastructure, and budget concerns. In response to changing reproductive priorities, the proactive nutritional modification of the body condition score protects productivity, animal health, and farm profitability.
As animal farming changes, it is essential to keep making things better. This helps the farm be more productive, keeps animals healthy, and makes sure the farm is successful. A big thing to consider in this always-changing place is ensuring we have many different types of food [18]. Alongside nutrition management, ruminants’ health and productivity are ensured. Producers need to scrutinize feeding strategies regularly, as well as adjust them accordingly to meet requirements, and consider the quality and obtainability of feed resources. Such modifications may consist of changing the feed composition, adding supplements, and/or the alternative sourcing of nutrients to ensure optimal animal health [20,34,38].
Also, setting up the environment is very important for the animals’ well-being and happiness. Updating and improving building designs can help improve birth results [18]. Having good housing, letting the air flow properly, and managing the waste well are all important things that affect how healthy livestock can be as well as how much they reproduce. Farmers need to keep updated about changes in building plans. They should consider making their land more modern to meet the latest rules and best ways of doing things.
When trying to improve things, farmers should carefully check how much it will cost and if it is worth doing. This means there is a need to find a balance between using money on things that will help in the future and dealing with current financial issues [18]. Working with farming experts who know about food and money can help farmers develop plans that fit their budget limits and future aims.
On the farm, what matters for achieving newborns in livestock changes all the time because of the different requirements, genes, and external factors. Changing what we eat is becoming the main plan used to deal with the changing goals of small ruminants. Keeping an animal’s body weight in check helps them stay fit for successful reproduction [18]. Changing the food consumed can help control their body weight and ensure they stay healthy for reproduction [39]. Checking the health of animals often helps farmers fix problems quickly. This keeps their animals safe and makes them produce more.
6. Materials, Methods, and Key Results
This review synthesized research regarding the effects of the body condition score and nutritional status on reproductive outcomes in goats. Studies were systematically identified using online scientific databases. Combinations of the following search terms were used: body condition score, body condition, nutrition, diet, metabolism, reproduction, fertility, goats. The specific details extracted included animal species, breed(s), sample sizes, female physiological state, dietary intervention details, duration of nutritional changes, reproductive targets analyzed, quantitative impacts on fertility outcomes, pertinent endocrine and physiological outcomes, statistics and author conclusions, and study limitations.
The extracted results were compiled and analyzed to identify trends related to nutrition and reproduction. The key parameters assessed across the included works were the numbers exhibiting estrus or conceiving relative to controls, circulating concentrations of metabolic hormones, timing of postpartum estrus resumption, ovulation rates, and embryo/fetal loss percentages. Overall, consistent evidence demonstrates that body energy reserves and balance substantially impact the reproductive capacity in small ruminants through both the metabolic modulation of gonadotropin output and direct effects on the ovary [1,2,3,4,5]. During a negative energy balance, declining metabolic signals from leptin, insulin, IGF-1, and other fatty hormones increase appetite-stimulating neuropeptide Y, suppressing GnRH and subsequent LH pulse secretion [5,7,9]. Reduced LH, leptin, and IGF-1 directly decrease ovarian follicle growth, steroid output, ovulation rates, and corpus luteum function, which are critical for fertility [5,6,7,8,9,10,14].
In mature females, poor physical condition lengthens the postpartum anestrus interval before reproductive cycling resumes [4,7]. Among growing ewe lambs, underfeeding prevents the initiation of ovarian function [10]. In active breeding seasons, nutritional disruption reduces the likelihood of conception, increasing embryo loss [5,14]. Brief, moderate deficits may temporarily stimulate reproductive activation through some pathways, but persistent reserve shortfalls consistently suppress fertility measures [2,6,7,8,9]. Strategic flushing and dietary adjustments to maintain optimum condition scores counteract these declines, improving outcomes [8,13,15,22]. Therefore, while some genotype and species variation occur, the evidence demonstrates body reserves and energy balance significantly control key aspects of reproductive physiology in grazing goats. Regular monitoring and appropriate nutritional interventions remain vital for securing optimal reproductive efficiency.
Along with careful cleaning and changes in food, other important things are needed to keep grazing goats in the best health possible. This also helps improve how well they give birth [34]. A key thing is taking care of where animals eat grass. Managing grassland well is important to make sure animals have good food all the time. For example, using rotational grazing can help keep the pasture good by preventing too much eating from happening and letting it grow back.
Also, special health programs are very important for helping with small ruminants’ reproductive health. It is important to deal with any health problems quickly. When trying to make reproduction better, genetic factors should also be considered [35]. Picking out good traits for fertility and adaptability in animals can help create a strong group that breeds well [16].
Changes in weather and seasons can significantly affect how sheep or goats reproduce. Knowing and changing how to manage certain aspects for different seasons is important for improving reproduction [36]. For example, changing when animals breed to match times when there is plenty of food can help increase small ruminant births and their chances of survival [31]. Adding technology and data-based methods to reproductive management can provide helpful information. Tools like body condition scoring and technologies such as ultrasound allow farmers to check how healthy their animals are. This lets them make important choices about caring for these creatures [34]. Data analysis can spot trends and patterns. This lets managers adjust what they do before problems happen.
7. Conclusions
While genotype specifics may introduce additional variation, the available evidence demonstrates that body energy reserves substantially impact the reproductive capacity in grazing sheep and goats with both acute signals of positive status and chronic deficits capable of stimulating or suppressing reproductive neuroendocrine activity at the hypothalamic and pituitary tiers. Independent ovarian actions of metabolic factors also modulate follicle dynamics, steroidogenesis, and subsequent corpus luteum function following mating. Characterizing the precise durations of nutritional challenges inducing temporary activation versus sustained suppression across the diverse physiological phases remains an active area of investigation to further refine practical feeding guidance promoting optimal fertility outcomes. Scoring and monitoring conditions relative to informing strategic dietary adjustments will continue to provide simple but powerful tools for stabilizing and enhancing reproductive performance within small ruminant production systems.
Author Contributions
Conceptualization, R.V. and H.Q.; methodology, A.S.C. and H.Q.; investigation, R.V. and H.Q.; writing—original draft preparation, A.S.C. and F.S.; writing—review and editing, A.S.C., F.S., R.V. and H.Q.; supervision, F.S., R.V. and H.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing does not apply to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Al-Sabbagh, T.A. The effect of ewe feeding level prepartum on colostrum production and lamb performance of Najdi sheep. Small Rumin. Res. 2009, 81, 139–143. [Google Scholar] [CrossRef]
- Backholer, K.; Smith, J.T.; Rao, A.; Pereira, A.; Iqbal, J.; Ogawa, S.; Li, Q.; Clarke, I.J. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology 2010, 151, 2233–2243. [Google Scholar] [CrossRef] [PubMed]
- Brooks, K.; Burns, G.; Spencer, T.E. Conceptus elongation in ruminants: Roles of Progesterone, prostaglandin, interferon tau, and cortisol. J. Anim. Sci. Biotechnol. 2014, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.C.; Alves, B.R.; Williams, G.L. Neuroendocrine signaling pathways and the nutritional control of puberty in heifers. Anim. Reprod. 2018, 15 (Suppl. S1), 868. [Google Scholar] [CrossRef]
- Childs, G.V.; Odle, A.K.; MacNicol, M.C.; MacNicol, A.M. The importance of leptin to reproduction. Endocrinology 2021, 162, bqaa204. [Google Scholar] [CrossRef] [PubMed]
- Chowen, J.A.; Argente-Arizón, P.; Freire-Regatillo, A.; Argente, J. Sex differences in the neuroendocrine control of metabolism and the implication of astrocytes. Front. Neuroendocrinol. 2018, 48, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.F.; Navarrete, F.; Carrasco, A.; Dorado, J.; Saravia, F. Effect of bST administration on plasma concentrations of IGF-I and follicular dynamics and ovulation during the interovulatory cycle of sheep and goats. Theriogenology 2019, 123, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Scaramuzzi, R.J.; Reverchon, M. The effect of nutrition and metabolic status on the development of follicles, oocytes and embryos in ruminants. Animal 2014, 8, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Diaz, D.; Rosiles, R.J.; Urias-Castro, C.J.; Rodriguez-Gaxiola, M.A.; Gaxiola, S.M.; Montero-Pardo, A. Systematic review and meta-analysis of the efficacy of reproductive management practices used to induce resumption of ovarian cyclical activity in anestrous does. Prev. Vet. Med. 2019, 169, 104709. [Google Scholar] [CrossRef] [PubMed]
- Farrell, B. Goat Nutrition: Bailey’s Bit about Nutrition. Midwestern BioAg. Available online: www.midwesternbioag.com/goat-nutrition-baileys-bit-about-nutrition/ (accessed on 11 October 2021).
- Kenyon, P.R.; Blair, H.T.; Jenkinson, C.M.; Morris, S.T.; Mackenzie, D.D.; Peterson, S.W. The effect of ewe size and nutritional regimen beginning in early pregnancy on ewe and lamb performance to weaning. N. Z. J. Agric. Res. 2014, 57, 152–166. [Google Scholar] [CrossRef]
- Livadas, S.; Chrousos, G.P. Molecular and environmental mechanisms regulating puberty initiation: An integrated approach. Front. Endocrinol. 2019, 10, 828. [Google Scholar] [CrossRef]
- Hudson, A.D.; Kauffman, A.S. Metabolic actions of kisspeptin signaling: Effects on body weight, energy expenditure, and feeding. Pharmacol. Ther. 2022, 231, 107974. [Google Scholar] [CrossRef] [PubMed]
- Fthenakis, G.C.; Arsenos, G.; Brozos, C.; Fragkou, I.A.; Giadinis, N.D.; Giannenas, I. Health management of ewes during pregnancy. Anim. Reprod. Sci. 2012, 130, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.W.; Elias, C.F. Neuroanatomical framework of the metabolic control of reproduction. Physiol. Rev. 2018, 98, 2349–2380. [Google Scholar] [CrossRef] [PubMed]
- Misztal, T.; Hasiec, M.; Szlis, M.; Tomaszewska-Zaremba, D.; Marciniak, E. Stimulatory effect of dopamine derivative, salsolinol, on pulsatile luteinizing hormone secretion in seasonally anestrous sheep: Focus on dopamine, kisspeptin and gonadotropin-releasing hormone. Anim. Reprod. Sci. 2019, 208, 106102. [Google Scholar] [CrossRef] [PubMed]
- Gindri, P.; de Ávila Castro, N.; Mion, B.; Gasperin, B.G.; Pegoraro, L.M.; Rincón, J.A.; Vieira, A.D.; Pradieé, J.; Pfeifer, L.F.; Corrêa, M.N.; et al. Intrafollicular lipopolysaccharide injection delays ovulation in cows. Anim. Reprod. Sci. 2019, 211, 106226. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; Kimura, K. Regulation of gonadotropin secretion by monitoring energy availability. Reprod. Med. Biol. 2015, 14, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Norambuena, M.C.; Silva, M.; Urra, F.; Ulloa-Leal, C.; Fernández, A.; Adams, G.P. Effects of nutritional restriction on metabolic, endocrine, and ovarian function in llamas (Lama glama). Domest. Anim. Endocrinol. 2013, 45, 94–102. [Google Scholar] [CrossRef]
- Ntallaris, T.; Humblot, P.; Båge, R.; Sjunnesson, Y.; Dupont, J.; Berglund, B. Effect of energy balance profiles on metabolic and reproductive response in Holstein and Swedish Red cows. Theriogenology 2017, 90, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Tur, İ.; Dínç, D.A.; Semacan, A. Protein based flushing related blood urea nitrogen effects on ovarian response, embryo recovery and embryo quality in superovulated ewes. Theriogenology 2017, 98, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Halmemies-Beauchet-Filleau, A.; Rinne, M.; Lamminen, M.; Mapato, C.; Ampapon, T.; Wanapat, M.; Vanhatalo, A. Alternative and novel feeds for ruminants: Nutritive value, product quality and environmental aspects. Animal 2018, 12, S295–S309. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, F.B.; de Souza, J.C.; Campos, J.P.; Faria, L.R.; da Silva, D.R.; Ascari, I.J.; de Lima, R.R.; Furusho-Garcia, I.F.; de Paula Nogueira, G.; Alves, N.G. Ovarian follicular development, hormonal and metabolic profile in prepubertal ewe lambs with moderate dietary restriction and lipid supplementation. Anim. Reprod. Sci. 2019, 204, 152–164. [Google Scholar] [CrossRef]
- Leroy, J.L.; Langbeen, A.; Van Hoeck, V.; Bols, P.E. Metabolism and reproduction, the battle for nutrients. Adv. Dairy Technol. 2010, 22, 25–34. [Google Scholar]
- Grazul-Bilska, A.T.; Bass, C.S.; Kaminski, S.L.; Ebel, K.K.; Leke, E.; Thammasiri, J.; Kraisoon, A.; Navanukraw, C.; Holst, M.; Shelton, M.; et al. Effects of plane of nutrition and arginine on ovarian follicles in non-pregnant sheep: Cell proliferation, and expression of endothelial nitric oxide and its receptor. Acta Histochem. 2019, 121, 189–197. [Google Scholar] [CrossRef]
- Grazul-Bilska, A.T.; Dorsam, S.T.; Reyaz, A.; Valkov, V.; Bass, C.S.; Kaminski, S.L.; Redmer, D.A. Follicle-stimulating hormone receptors expression in ovine corpora lutea during luteal phase: Effect of nutritional plane and follicle-stimulating hormone treatment. Domest. Anim. Endocrinol. 2020, 71, 106391. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; Duan, C.H.; Hao, Q.H.; Liu, Y.Q.; Li, T.; Zhang, Y.J. Effect of short-term nutritional supplementation on hormone concentrations in ovarian follicular fluid and steroid regulating gene mRNA abundances in granulosa cells of ewes. Anim. Reprod. Sci. 2019, 211, 106208. [Google Scholar] [CrossRef] [PubMed]
- Manfredi-Lozano, M.; Roa, J.; Tena-Sempere, M. Connecting metabolism and gonadal function: Novel central neuropeptide pathways involved in the metabolic control of puberty and fertility. Front. Neuroendocrinol. 2018, 48, 37–49. [Google Scholar] [CrossRef]
- Maidin, M.S.; Blackberry, M.A.; Milton, J.T.; Hawken, P.A.; Martin, G.B. Nutritional supplements, leptin, insulin and progesterone in female australian cashmere goats. APCBEE Procedia 2014, 8, 299–304. [Google Scholar] [CrossRef][Green Version]
- McGrath, J.; Duval, S.M.; Tamassia, L.F.; Kindermann, M.; Stemmler, R.T.; de Gouvea, V.N.; Acedo, T.S.; Immig, I.; Williams, S.N.; Celi, P. Nutritional strategies in ruminants: A lifetime approach. Res. Vet. Sci. 2018, 116, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Pinos, H.; Carrillo, B.; Diaz, F.; Chowen, J.A.; Collado, P. Differential vulnerability to adverse nutritional conditions in male and female rats: Modulatory role of estradiol during development. Front. Neuroendocrinol. 2018, 48, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Nestor, C.C.; Merkley, C.M.; Lehman, M.N.; Hileman, S.M.; Goodman, R.L. KNDy neurons as the GnRH pulse generator: Recent studies in ruminants. Peptides 2023, 164, 171005. [Google Scholar] [CrossRef] [PubMed]
- Melcangi, R.C.; Panzica, G.C. Neuroactive steroids and metabolic axis. Front. Neuroendocrinol. 2018, 48, 1–2. [Google Scholar] [CrossRef]
- Prathap, P.; Chauhan, S.S.; Leury, B.J.; Cottrell, J.J.; Dunshea, F.R. Towards sustainable livestock production: Estimation of methane emissions and dietary interventions for mitigation. Sustainability 2021, 13, 6081. [Google Scholar] [CrossRef]
- Zsarnovszky, A.; Kiss, D.; Jocsak, G.; Nemeth, G.; Toth, I.; Horvath, T.L. Thyroid hormone-and estrogen receptor interactions with natural ligands and endocrine disruptors in the cerebellum. Front. Neuroendocrinol. 2018, 48, 23–36. [Google Scholar] [CrossRef]
- Wang, J.; Sun, L.; Zhang, T.; Zhou, H.; Xu, J.; Lou, Y. Under starved conditions the expression pattern of hypothalamic Kisspeptin/GPR54 and role of kisspeptin-10 in restoration of LH secretion in prepubertal ewes. J. Anim. Plant Sci. 2014, 24, 1003–1007. [Google Scholar]
- Marciniak, E.; Górski, K.; Hasiec, M.; Misztal, T. Hypothalamic-pituitary GnRH/LH axis activity is affected by salsolinol in sheep during lactation: Effects of intracerebroventricular infusions of salsolinol and its antagonizing analogue. Theriogenology 2016, 86, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Quennell, J.H.; Mulligan, A.C.; Tups, A.; Liu, X.; Phipps, S.J.; Kemp, C.J. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology 2009, 150, 2805–2812. [Google Scholar] [CrossRef] [PubMed]
- Schuermann, Y.; Welsford, G.E.; Nitschmann, E.; Wykes, L.; Duggavathi, R. Association between pre-breeding metabolic profiles and reproductive performance in heifers and lactating dairy cows. Theriogenology 2019, 131, 79–88. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).