The Pierpaoli’s Herbarium MBMP: A Historical Window into Marine Biodiversity of the Ionian Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Irma Pierpaoli
2.2. MBMP Herbarium
2.3. Assessment of Biodiversity Changes
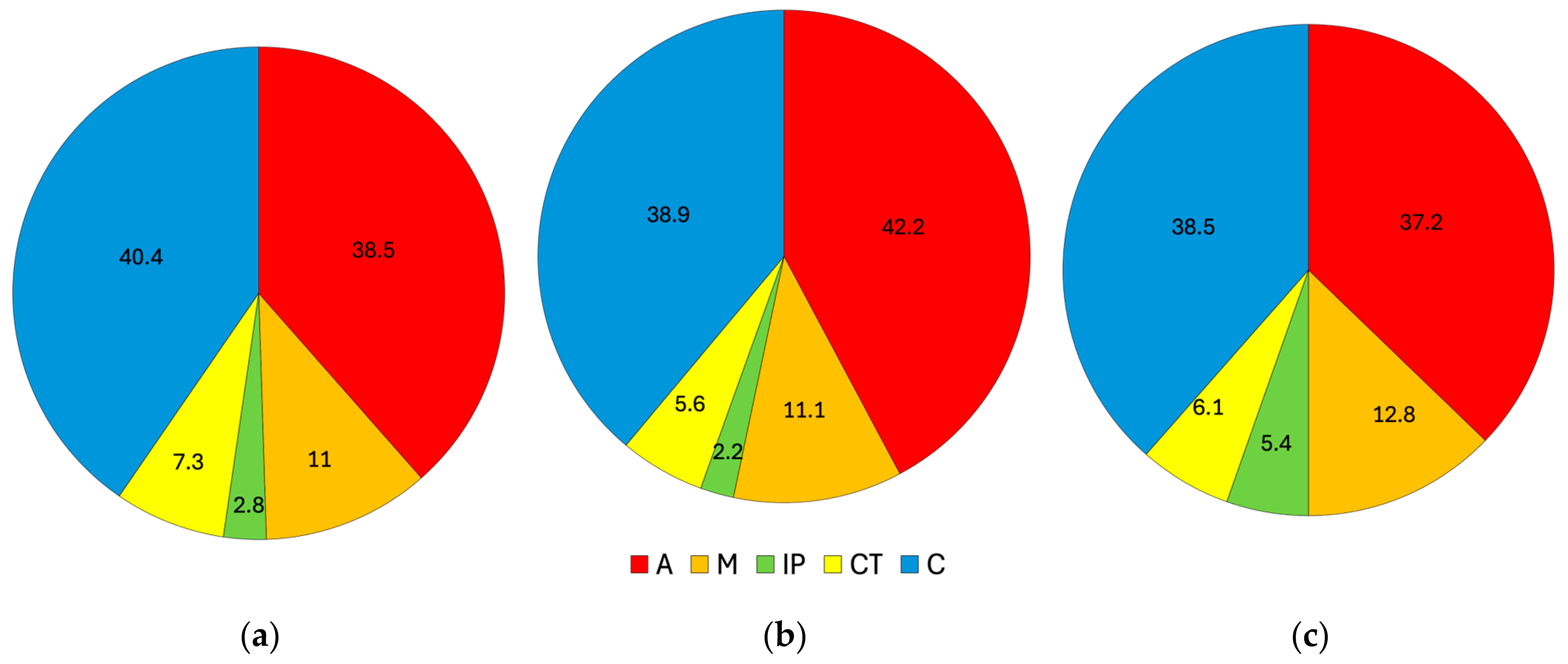
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MBMP | Algario Irma Pierpaoli, Museo di Biologia Marina, Porto Cesareo (Lecce), Italy |
| CNR | Consiglio Nazionale delle Ricerche |
| NRRP | National Recovery and Resilience Plan |
| ITINERIS | Italian INtegrated Environmental Research Infrastructure System |
| DiSSCo | Distributed System of Scientific Collections |
| NGS | Next-Generation Sequencing |
| MIDS | Minimum Information about a Digital Specimen |
| NIS | Non-Indigenous Species |
| TAR | Herbarium Istituto Sperimentale Talassografico “A. Cerruti”, Taranto, Italy |
| FAIR | Findable, Accessible, Interoperable, Reusable |
| GBIF | Global Biodiversity Information Facility |
References
- Cristofolini, G. Origin and evolution of herbaria in the sixteenth century. Rend. Fis. Acc. Lincei 2024, 35, 63–75. [Google Scholar] [CrossRef]
- Mannino, A.M.; Armeli Minicante, S.; Rodríguez-Prieto, C. Phycological Herbaria as a useful tool to monitor long-term changes of macroalgae diversity: Some case studies from the Mediterranean Sea. Diversity 2020, 12, 309. [Google Scholar] [CrossRef]
- Heberling, J.M.; Prather, L.A.; Tonsor, S.J. The changing uses of herbarium data in an era of global change: An overview using Automated Content Analysis. BioScience 2019, 69, 812–822. [Google Scholar] [CrossRef]
- Rouhan, G.; Gaudeul, M. Plant taxonomy: A historical perspective, current challenges, and perspectives. In Molecular Plant Taxonomy. Methods in Molecular Biology; Besse, P., Ed.; Humana: New York, NY, USA, 2020; Volume 2222, pp. 1–38. [Google Scholar] [CrossRef]
- Manoylov, K.M. Taxonomic identification of algae (morphological and molecular): Species concepts, methodologies, and their implications for ecological bioassessment. J. Phycol. 2014, 50, 409–424. [Google Scholar] [CrossRef]
- Marín-Rodulfo, M.; Rondinel-Mendoza, K.V.; Martín-Girela, I.; Cañadas, E.M.; Lorite, J. Old meets new: Innovative and evolving uses of herbaria over time as revealed by a literature review. Plants People Planet 2024, 6, 1261–1271. [Google Scholar] [CrossRef]
- Kates, H.R.; Doby, J.R.; Siniscalchi, C.M.; LaFrance, R.; Soltis, D.E.; Soltis, P.S.; Guralnick, R.P.; Folk, R.A. The effects of herbarium specimen characteristics on short-read NGS sequencing success in nearly 8000 specimens: Old, degraded samples have lower DNA yields but consistent sequencing success. Front. Plant Sci. 2021, 12, 669064. [Google Scholar] [CrossRef] [PubMed]
- Flannery, M.C. The road to herbaria: Teaching and learning about biology, aesthetics, and the history of botany. J. Biosci. 2023, 48, 58. [Google Scholar] [CrossRef]
- Davis, C.C. The herbarium of the future. Trends Ecol. Evol. 2023, 38, 412–423. [Google Scholar] [CrossRef]
- Merrill, E.D. On the utility of field labels in herbarium practise. Science 1916, 44, 664–670. [Google Scholar] [CrossRef]
- Haston, E.; Chapman, C. MIDS: The digitisation standard for Natural Science collections. Biodivers. Inf. Sci. Stand. 2022, 6, e94604. [Google Scholar] [CrossRef]
- Shweta, S.; Dwivedi, A.; Subramaniam, B.; Kaushik, S.; Sahu, N. Herbaria: A valuable resource of the time treasured historic plant specimens with boundless research potential for environmental sustainability. Environ. Dev. Sustain. 2024. [Google Scholar] [CrossRef]
- Schramm, W. Factors influencing seaweed responses to eutrophication: Some results from EU-project EUMAC. In Sixteenth International Seaweed Symposium. Developments in Hydrobiology; Kain, J.M., Brown, M.T., Lahaye, M., Eds.; Springer: Dordrecht, Germany, 1999; Volume 137, pp. 583–592. [Google Scholar] [CrossRef]
- Johnston, E.L.; Dafforn, K.A.; Clark, G.F.; Rius, M.; Floerl, O. How anthropogenic activities affect the establishment and spread of non-indigenous species post-arrival. In Oceanography and Marine Biology. An Annual Review; Hawkins, S.J., Evans, A.J., Dale, A.C., Firth, L.B., Hughes, D.J., Smith, I.P., Eds.; CRC Press: Boca Raton, FL, USA, 2017; Volume 55, pp. 2–33. [Google Scholar]
- Wilkinson, M.; Tittley, I. The marine algae of Elie, Scotland: A re-assessment. Bot. Mar. 1979, 22, 249–256. [Google Scholar] [CrossRef]
- Furnari, G.; Cormaci, M.; Serio, D. Catalogue of the benthic marine macroalgae of the Italian coast of the Adriatic Sea. Bocconea 1999, 12, 5–214. [Google Scholar]
- Herbert, R.J.H.; Ma, L.; Marston, A.; Farnham, W.F.; Tittley, I.; Cornes, R.C. The calcareous brown alga Padina pavonica in southern Britain: Population change and tenacity over 300 years. Mar. Biol. 2016, 163, 46. [Google Scholar] [CrossRef]
- Husa, V.; Steen, H.; Sjøtun, K. Historical changes in macroalgal communities in Hardangerfjord (Norway). Mar. Biol. Res. 2014, 10, 226–240. [Google Scholar] [CrossRef]
- Robuchon, M.; Lamy, D.; Kervran, L.; Dennetiere, B.; Julliard, R.; Le Gall, L. Dinard Herbarium: A source of information to infer temporal changes in seaweed communities? Cryptog. Algol. 2016, 37, 47–60. [Google Scholar] [CrossRef]
- Petrocelli, A.; Cecere, E.; Rubino, F. Successions of phytobenthos species in a Mediterranean transitional water system: The importance of long term observations. Nat. Conserv. 2019, 34, 217–246. [Google Scholar] [CrossRef]
- Cecere, E.; Saracino, O.D. The Irma Pierpaoli (1891–1967) herbarium of the Stazione di Biologia Marina of Porto Cesareo. In The Italian Phycological Patrimony; Abdelahad, N., Ed.; Officine Grafiche Borgia I.G.E.A.: Roma, Italy, 1999; p. 42. [Google Scholar]
- Rindi, F.; Gavio, B.; Diaz, P.; Di Camillo, C.G. Long-term changes in the benthic macroalgal flora of a coastal area affected by urban impacts (Conero Riviera, Mediterranean Sea). Biodivers. Conserv. 2020, 29, 2275–2295. [Google Scholar] [CrossRef]
- Cecere, E.; Cormaci, M.; Furnari, G. The marine algae of Mar Piccolo, Taranto (southern Italy): A re-assessment. Bot. Mar. 1991, 34, 221–227. [Google Scholar] [CrossRef]
- Petrocelli, A.; Wolf, M.A.; Sciuto, K.; Sfriso, A.; Rubino, F.; Ricci, P.; Cecere, E. Long-term data prove useful to keep track of non-indigenous seaweed fate. Front. Environ. Sci. 2023, 11, 1075458. [Google Scholar] [CrossRef]
- Cecere, E. Irma Pierpaoli, un’antesignana nell’insegnamento e nella ricerca scientifica. In Il Pitagora di Taranto. Un Secolo di Presenza sul Territorio; Terzulli, F., Ed.; Tipografia La Due Mari: Taranto, Italy, 2011; pp. 339–352. [Google Scholar]
- Cecere, E.; Saracino, O.; Petrocelli, A. Sui primi studi delle macroalghe marine bentoniche del litorale marchigiano. Biol. Mar. Mediterr. 2002, 9, 517–518. [Google Scholar]
- Rindi, F.; Bellanti, G.; Annibaldi, A.; Accoroni, S. Reconstructing historical changes in the macroalgal vegetation of a central Mediterranean coastal area based on herbarium collections. Diversity 2024, 16, 741. [Google Scholar] [CrossRef]
- De Tullio, M. Irma, Vittoria, Lucrezia: Appunti per una botanica al femminile. In Scienziati di Puglia; De Ceglie, F.P., Ed.; Mario Adda Editore: Bari, Italy, 2007; p. 511. [Google Scholar]
- Miglietta, A.M. Pierpaoli, Irma. In Dizionario Biografico Degli Italiani; Istituto dell’Enciclopedia Italiana: Roma, Italy, 2015; Volume 83, Available online: http://www.treccani.it/enciclopedia/irma-pierpaoli_%28Dizionario-Biografico%29/ (accessed on 30 July 2025).
- Papa, L.; Cecere, E.; Petrocelli, A.; Spada, L. Digitization of the marine Herbarium “TAR” to increase biodiversity knowledge. Diversity 2025, 17, 641. [Google Scholar] [CrossRef]
- Pierpaoli, I. Prima contribuzione allo studio della alghe nel Golfo di Taranto. Riv. Biol. 1923, 5–6, 1–19. [Google Scholar]
- Pierpaoli, I. L’epifitismo nelle alghe. Note sugli ambienti tarantino e anconetano. Thalass. Jonica 1959, 2, 46–51. [Google Scholar]
- Pierpaoli, I. Microfotografie di alghe del Golfo di Taranto. Thalass. Jonica 1960, 3, 100–106. [Google Scholar]
- Cecere, E.; Saracino, O.D.; Fanelli, M.; Petrocelli, A. Presence of a drifting algal bed in the Mar Piccolo basin, Taranto (Ionian Sea, Southern Italy). J. Appl. Phycol. 1992, 4, 323–327. [Google Scholar] [CrossRef]
- Cecere, E.; Cormaci, M.; Furnari, G.; Petrocelli, A.; Saracino, O.; Serio, D. Benthic algal flora of Cheradi Islands (Gulf of Taranto, Mediterranean Sea). Nova Hedwig. 1996, 62, 191–214. [Google Scholar] [CrossRef]
- Furnari, G.; Giaccone, G.; Cormaci, M.; Alongi, G.; Catra, M.; Nisi, A.; Serio, D. Macrophytobenthos. Biol. Mar. Mediterr. 2010, 17 (Suppl. 1), 801–828. [Google Scholar]
- Gall, E.A.; Le Duff, M.; Sauriau, P.G.; De Casamajor, M.N.; Gevaert, F.; Poisson, E.; Hacquebart, P.; Joncourt, Y.; Barillé, A.-L.; Buchet, R.; et al. Implementation of a new index to assess intertidal seaweed communities as bioindicators for the European Water Framework Directory. Ecol. Indic. 2016, 60, 162–173. [Google Scholar] [CrossRef]
- Barrientos, S.; Barreiro, R.; Cremades, J.; Piñeiro-Corbeira, C. Setting the basis for a long-term monitoring network of intertidal seaweed assemblages in northwest Spain. Mar. Environ. Res. 2020, 160, 105039. [Google Scholar] [CrossRef] [PubMed]
- Wernberg, T.; Russell, B.D.; Thomsen, M.S.; Gurgel, C.F.D.; Bradshaw, C.J.; Poloczanska, E.S.; Connell, S.D. Seaweed communities in retreat from ocean warming. Curr. Biol. 2011, 21, 1828–1832. [Google Scholar] [CrossRef] [PubMed]
- Sales, M.; Ballesteros, E. Shallow Cystoseira (Fucales: Ochrophyta) assemblages thriving in sheltered areas from Menorca (NW Mediterranean): Relationships with environmental factors and anthropogenic pressures. Estuar. Coast. Shelf Sci. 2009, 84, 476–482. [Google Scholar] [CrossRef]
- Sales, M.; Cebrian, E.; Tomas, F.; Ballesteros, E. Pollution impacts and recovery potential in three species of the genus Cystoseira (Fucales, Heterokontophyta). Estuar. Coast. Shelf Sci. 2011, 92, 347–357. [Google Scholar] [CrossRef]
- Cecere, E.; Fanelli, G.; Petrocelli, A.; Saracino, O.D. Changes in seaweed biodiversity of the Gargano coast (Adriatic Sea, Mediterranean Sea). In Mediterranean Ecosystems; Faranda, F.M., Guglielmo, L., Spezie, G., Eds.; Springer: Milan, Italy, 2001; pp. 347–351. [Google Scholar]
- Benedetti-Cecchi, L.; Pannacciulli, F.; Bulleri, F.; Moschella, P.S.; Airoldi, L.; Relini, G.; Cinelli, F. Predicting the consequences of anthropogenic disturbance: Large-scale effects of loss of canopy algae on rocky shores. Mar. Ecol. Prog. Ser. 2001, 214, 137–150. [Google Scholar] [CrossRef]
- Piñeiro-Corbeira, C.; Barrientos, S.; Provera, I.; García, M.E.; Díaz-Tapia, P.; Peña, V.; Bárbara, I.; Barreiro, R. Kelp forests collapse reduces understorey seaweed β-diversity. Ann. Bot. 2024, 133, 93–104. [Google Scholar] [CrossRef]
- Cecere, E.; Cormaci, M.; Furnari, G.; Tursi, A.; Caciorgna, O. Phytocenoses in the Mar Piccolo in Taranto (Ionian Sea, Southern Italy): Mesolittoral level and infralittoral fringe. Rapp. Comm. Int. Mer Médit. 1988, 31, 2. [Google Scholar]
- Devlin, M.; Brodie, J. Nutrients and eutrophication. In Marine Pollution—Monitoring, Management and Mitigation; Springer Textbooks in Earth Sciences, Geography and Environment; Reichelt-Brushett, A., Ed.; Springer: Berlin, Germany, 2023; pp. 75–100. [Google Scholar] [CrossRef]
- Alabiso, G.; Cecere, E.; Petrocelli, A.; Ricci, P. Ammonium uptake by Gracilaria dura (Rhodophyta, Gracilariales) from the Mar Piccolo of Taranto. Biol. Mar. Mediterr. 2007, 14, 116–117. [Google Scholar]
- Alabiso, G.; Ricci, P.; Belmonte, M.; Petrocelli, A.; Cecere, E. Ammonium uptake by Gracilaria gracilis (Stackhouse) Steentoft, Irvine et Farnham (Gracilariales, Rhodophyta) from the Mar Piccolo of Taranto. Biol. Mar. Mediterr. 2009, 16, 246–247. [Google Scholar]
- Coelho, S.M.; Rijstenbil, J.W.; Brown, M.T. Impacts of anthropogenic stresses on the early development stages of seaweeds. J. Aquat. Ecosyst. Stress Recov. 2000, 7, 317–333. [Google Scholar] [CrossRef]
- Cecere, E.; Petrocelli, A.; Verlaque, M. Vegetative reproduction by multicellular propagules in Rhodophyta: An overview. Mar. Ecol. 2011, 32, 419–437. [Google Scholar] [CrossRef]
- Eriksson, B.K.; Johansson, G. Effects of sedimentation on macroalgae: Species-specific responses are related to reproductive traits. Oecologia 2005, 143, 438–448. [Google Scholar] [CrossRef]
- Cecere, E.; Petrocelli, A. The Mar Piccolo of Taranto. In Flora and Vegetation of the Italian Transitional Water Systems; Cecere, E., Petrocelli, A., Izzo, G., Sfriso, A., Eds.; CORILA, Stampa “Multigraf”: Venice, Italy, 2009; pp. 195–227. [Google Scholar]
- Pastore, M. Mar Piccolo; Nuova Editrice Apulia: Martina Franca, Italy, 1993; pp. 1–163. [Google Scholar]
- Alabiso, G.; Cannalire, M.; Ghionda, D.; Milillo, M.; Leone, G.; Caciorgna, O. Particulate matter and chemical-physical conditions of an inner sea: The Mar Piccolo in Taranto. A new statistical approach. Mar. Chem. 1997, 58, 373–388. [Google Scholar] [CrossRef]
- Alabiso, G.; Giacomini, M.; Milillo, M.; Ricci, P. The Taranto sea system: 8 years of chemical-physical measurements. Biol. Mar. Mediterr. 2005, 12, 369–373. [Google Scholar]
- Dominik, J.; Leoni, S.; Cassin, D.; Guarneri, I.; Bellucci, L.G.; Zonta, R. Eutrophication history and organic carbon burial rate recorded in sediment cores from the Mar Piccolo of Taranto (Italy). Environ. Sci. Pollut. Res. 2023, 30, 56713–56730. [Google Scholar] [CrossRef]
- Kralj, M.; De Vittor, C.; Comici, C.; Relitti, F.; Auriemma, R.; Alabiso, G.; Del Negro, P. Recent evolution of the physical-chemical characteristics of a Site of National Interest—The Mar Piccolo of Taranto (Ionian Sea)—And changes over the last 20 years. Environ. Sci. Pollut. Res. 2016, 23, 12675–12690. [Google Scholar] [CrossRef] [PubMed]
- Basson, P.W.; Hardy, J.T.; Lakkis, V. Ecology of marine macroalgae in relation to pollution along the coast of Lebanon. Acta Adriat. 1976, 18, 307–325. [Google Scholar]
- Cotton, A.D. On the increase of Colpomenia sinuosa in Britain. Bull. Misc. Informat. Kew 1911, 1911, 153–157. [Google Scholar] [CrossRef]
- Airoldi, L. The effects of sedimentation on rocky coast assemblages. In Oceanography and Marine Biology: An Annual Review, 1st ed.; Gibson, R.N., Atkinson, R.J.A., Eds.; CRC Press: London, UK, 2003; Volume 41, pp. 161–236. [Google Scholar]
- Flórez-Leiva, L.; Rangel-Campo, A.; Díaz-Ruiz, M.; Venera-Pontón, D.E.; Díaz-Pulido, G. Effects of sedimentation on the recruitment of the macroalgae Dictyota spp. and Lobophora variegata: An experimental study in the Tayrona National Natural Park, Colombian Caribbean. Bol. Investig. Mar. Cost. 2010, 39, 41–56. [Google Scholar]
- Airoldi, L. Roles of disturbance, sediment stress, and substratum retention on spatial dominance in algal turf. Ecology 1998, 79, 2759–2770. [Google Scholar] [CrossRef]
- Prado, P.; Alcoverro, T.; Romero, J. Seasonal response of Posidonia oceanica epiphyte assemblages to nutrient increase. Mar. Ecol. Progr. Ser. 2008, 359, 89–98. [Google Scholar] [CrossRef]
- Tsioli, S.; Papathanasiou, V.; Rizouli, A.; Kosmidou, M.; Katsaros, C.; Papastergiadou, E.; Küpper, F.C.; Orfanidis, S. Diversity and composition of algal epiphytes on the Mediterranean seagrass Cymodocea nodosa: A scale-based study. Bot. Mar. 2021, 64, 101–118. [Google Scholar] [CrossRef]
- Otero-Schmitt, J.; Pérez-Cirera, J.L. Epiphytism on Cystoseira (Fucales, Phaeophyta) from the Atlantic coast of northwest Spain. Bot. Mar. 1996, 39, 445–465. [Google Scholar] [CrossRef]
- Mačić, V.; Svirčev, Z. Macroepiphytes on Cystoseira species (Phaeophyceae) on the coast of Montenegro. Fresenius Environ. Bull. 2014, 23, 29–34. [Google Scholar] [CrossRef]
- Tokida, J. Marine algae epiphytic on Laminariales plants. Bull. Fac. Fish. Hokkaido Univ. 1960, 11, 73–105. [Google Scholar]
- Bjærke, M.R.; Fredriksen, S. Epiphytic macroalgae on the introduced brown seaweed Sargassum muticum (Yendo) Fensholt (Phaeophyceae) in Norway. Sarsia 2003, 88, 353–364. [Google Scholar] [CrossRef]
- Petrocelli, A.; Cecere, E.; Verlaque, M. Alien marine macrophytes in transitional water systems: New entries and reappearances in a Mediterranean coastal basin. BioInvasions Rec. 2013, 2, 177–184. [Google Scholar] [CrossRef]
- Cormaci, M.; Furnari, G.; Alongi, G. Marine benthic flora of the Mediterranean Sea: Chlorophyta. Bull. Accad. Gioenia Sci. Nat. 2014, 47, FP11–FP436. [Google Scholar]
- Shepherd, S.A.; Watson, J.E.; Womersley, H.B.S.; Carey, J.M. Long-term changes in macroalgal assemblages after increased sedimentation and turbidity in Western Port, Victoria, Australia. Bot. Mar. 2009, 52, 195–206. [Google Scholar] [CrossRef]
- Cecere, E.; Alabiso, G.; Carlucci, R.; Petrocelli, A.; Verlaque, M. Fate of two invasive or potentially invasive alien seaweeds in a central Mediterranean transitional water system: Failure and success. Bot. Mar. 2016, 59, 451–462. [Google Scholar] [CrossRef]
- Eggert, A. Seaweed responses to temperature. In Seaweed Biology: Novel Insights into Ecophysiology, Ecology and Utilization; Wiencke, C., Bischof, K., Eds.; Springer: Berlin, Germany, 2012; Volume 219, pp. 47–66. [Google Scholar] [CrossRef]
- Darling, J.A.; Carlton, J.T. A framework for understanding marine cosmopolitanism in the Anthropocene. Front. Mar. Sci. 2018, 5, 293. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.A.; Buosi, A.; Juhmani, A.S.F.; Sfriso, A. Shellfish import and hull fouling as vectors for new red algal introductions in the Venice Lagoon. Estuar. Coast. Shelf Sci. 2018, 215, 30–38. [Google Scholar] [CrossRef]
- Perrone, C.; Cecere, E. Two solieriacean algae new to the Mediterranean: Agardhiella subulata and Solieria filiformis (Rhodophyta, Gigartinales). J. Phycol. 1994, 30, 98–108. [Google Scholar] [CrossRef]
- Cecere, E. Sulla presenza nel Golfo di Taranto di una specie nuova per il Mediterraneo: Solieria filiformis (Kützing) Gabrielson (Rhodophyta, Gigartinales). Oebalia 1990, 16, 629–631. [Google Scholar]
- Zanolla, M.; Andreakis, N. Towards an integrative phylogeography of invasive marine seaweeds, based on multiple lines of evidence. In Seaweed Phylogeography; Hu, Z.M., Fraser, C., Eds.; Springer: Dordrecht, Germany, 2016; pp. 187–207. [Google Scholar] [CrossRef]
- Gargiulo, G.M.; De Masi, F.; Tripodi, G. Structure and reproduction of Gracilaria longa sp. nov. (Rhodophyta, Gigartinales) from the Mediterranean Sea. Giorn. Bot. Ital. 1987, 121, 247–257. [Google Scholar] [CrossRef]
- Bebber, D.P.; Carine, M.A.; Wood, J.R.; Wortley, A.H.; Harris, D.J.; Prance, G.T.; Davidse, G.; Paige, J.; Pennington, T.D.; Robson, N.K.B.; et al. Herbaria are a major frontier for species discovery. Proc. Natl. Acad. Sci. USA 2010, 107, 22169–22171. [Google Scholar] [CrossRef]
- Orfanidis, S.; Rindi, F.; Cebrian, E.; Fraschetti, S.; Nasto, I.; Taskin, E.; Bianchelli, S.; Papathanasiou, V.; Kosmidou, M.; Caragnano, A.; et al. Effects of natural and anthropogenic stressors on Fucalean brown seaweeds across different spatial scales in the Mediterranean Sea. Front. Mar. Sci. 2021, 8, 658417. [Google Scholar] [CrossRef]
- Thibaut, T.; Blanfune, A.; Verlaque, M.; Boudouresque, C.F.; Ruitton, S. The Sargassum conundrum: Very rare, threatened or locally extinct in the NW Mediterranean and still lacking protection. Hydrobiologia 2016, 781, 3–23. [Google Scholar] [CrossRef]
- Cacabelos, E.; Martins, G.M.; Thompson, R.; Prestes, A.C.L.; Azevedo, J.M.N.; Neto, A.I. Factors limiting the establishment of canopy–forming algae on artificial structures. Estuar. Coast. Shelf Sci. 2016, 181, 277–283. [Google Scholar] [CrossRef]
- Heberling, J.M. Herbaria as big data sources of plant traits. Int. J. Pl. Sci. 2022, 183, 87–118. [Google Scholar] [CrossRef]
- Harley, C.D.; Anderson, K.M.; Demes, K.W.; Jorve, J.P.; Kordas, R.L.; Coyle, T.A.; Graham, M.H. Effects of climate change on global seaweed communities. J. Phycol. 2012, 48, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Cecere, E.; Saracino, O.D.; Petrocelli, A. Propagules of Alsidium corallinum (Rhodomelaceae, Rhodophyta). Bot. Mar. 2002, 45, 580–585. [Google Scholar] [CrossRef]
- Manzano, S.; Julier, A.C.M. How FAIR are plant sciences in the twenty-first century? The pressing need for reproducibility in plant ecology and evolution. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2021, 288, 20202597. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrocelli, A.; Cecere, E.; Spada, L.; Papa, L. The Pierpaoli’s Herbarium MBMP: A Historical Window into Marine Biodiversity of the Ionian Sea. Phycology 2025, 5, 67. https://doi.org/10.3390/phycology5040067
Petrocelli A, Cecere E, Spada L, Papa L. The Pierpaoli’s Herbarium MBMP: A Historical Window into Marine Biodiversity of the Ionian Sea. Phycology. 2025; 5(4):67. https://doi.org/10.3390/phycology5040067
Chicago/Turabian StylePetrocelli, Antonella, Ester Cecere, Lucia Spada, and Loredana Papa. 2025. "The Pierpaoli’s Herbarium MBMP: A Historical Window into Marine Biodiversity of the Ionian Sea" Phycology 5, no. 4: 67. https://doi.org/10.3390/phycology5040067
APA StylePetrocelli, A., Cecere, E., Spada, L., & Papa, L. (2025). The Pierpaoli’s Herbarium MBMP: A Historical Window into Marine Biodiversity of the Ionian Sea. Phycology, 5(4), 67. https://doi.org/10.3390/phycology5040067






