Simple Summary
Cattle production in the Brazilian Amazon is increasingly threatened by climate change. Rising temperatures, humidity, and extreme weather affect feed quality, water availability, and disease risks. This review systematically analyzes the impact of global warming on nutritional deficiencies, metabolic disorders, and infectious diseases in cattle in the Amazon biome. By integrating evidence from global and regional studies, we provide a framework to understand vulnerabilities and outline adaptation strategies. These include genetic selection for heat tolerance, climate-smart husbandry practices, improved nutritional management, and disease surveillance. The findings emphasize the urgent need for policies that integrate animal health, food security, and One Health perspectives to ensure sustainable cattle production in the Amazon.
Abstract
Climate change poses significant challenges to livestock, particularly in tropical regions. The Amazon biome, which hosts one of the world’s largest cattle populations, faces growing risks of nutritional, metabolic, and infectious diseases driven by heat stress (HS) and environmental instability. This systematic review synthesizes evidence from primary studies, international reports (IPCC, FAO), and peer-reviewed literature on cattle physiology, disease dynamics, and climate adaptation. HS reduces feed intake, disrupts endocrine–metabolic homeostasis, and suppresses immunity, increasing susceptibility to metabolic, deficiency and infectious diseases. Breed-specific immune responses offer opportunities for genetic and management-based adaptation. Socio-economic impacts disproportionately affect smallholders, linking livestock health to food security and poverty. Ensuring sustainable cattle production in the Amazon will require climate-smart strategies integrating nutrition, genetics, reproduction, and health management, supported by policies that align adaptation and mitigation. Future research should prioritize immune-metabolic biomarkers, periparturient disease monitoring, and genomic tools for thermotolerance.
1. Introduction
Climate change represents one of the most significant and multifactorial challenges to livestock production worldwide, particularly in tropical systems where elevated temperatures, high humidity, intense rainfall, prolonged droughts, and, in rare but increasingly documented events, sudden cold fronts or freezing conditions act synergistically to disrupt animal health and productivity []. The Brazilian Amazon biome, which now hosts over 93 million cattle, accounting for nearly 40% of the national herd, has become one of the regions where the impacts of climatic instability on livestock are most pronounced. Recent governmental and FAO-linked assessments indicate that up to 70–80% of Amazonian cattle experience some degree of climate-related physiological stress annually, with effects varying seasonally according to temperature–humidity fluctuations, hydrological extremes, and forage availability [].
Importantly, climate change in the Amazon is not restricted to heat stress (HS). The region exhibits marked intra-annual climatic variability, with severe droughts typically occurring from August to November, followed by intense and prolonged rainfall from December to May. These extreme oscillations modify pasture composition, reduce nutrient density, impair rumen function, and promote pathogen proliferation []. Drought conditions intensify plant lignification, increase the risk of toxic plant ingestion, and drastically reduce water availability, while the rainy season exacerbates flooding, soil saturation, and the proliferation of waterborne and vector-borne pathogens []. Together, these cyclic pressures threaten the long-term sustainability of cattle production systems in this highly sensitive biome [].
Physiological responses to HS and other climate stressors include reductions in dry matter intake, altered rumen fermentation, endocrine–metabolic shifts, oxidative stress, and marked impairments in immune function [,]. These disruptions collectively predispose cattle to metabolic diseases, mineral deficiencies, and increased susceptibility to infectious and parasitic agents. Recent studies emphasize that immune-metabolic responses differ significantly between breeds exposed to tropical climates, reinforcing the need for genetic and management-based adaptation strategies capable of enhancing resilience under Amazonian environmental conditions [].
Infectious diseases are profoundly shaped by climate variability. Rising temperatures and humidity expand the ecological niches of pathogens and their vectors, while flooding events during the December–May rainy season create ideal reservoirs for leptospirosis and fasciolosis [,,]. Conversely, the August–November drought period favors the accumulation of toxic metabolites in forages, increases the ingestion of hazardous plants, and reduces water quality and availability, all of which compromise immune defenses. Vector-borne diseases, including trypanosomiasis and tick-borne infections, are projected to intensify with warming trends, extended wet seasons, and the expansion of vector habitats, paralleling patterns observed in other tropical livestock systems [,,]. These epidemiological changes pose increasing threats to cattle health, production efficiency, and regional food security.
Beyond the biological impacts, climate change exerts substantial socio-economic burdens on Amazonian smallholders, who depend heavily on cattle production for subsistence, income generation, and local food supply. Reduced pasture quality during droughts, infrastructure damage during floods, elevated disease incidence, and increased production costs directly undermine household resilience and exacerbate rural poverty []. These consequences resonate across the broader food system, linking animal health, human nutrition, ecosystem function, and socio-economic stability under the One Health framework [].
The objective of this systematic review is to critically analyze the effects of increasing global temperatures and associated climate stressors on cattle health in the Amazon biome, with emphasis on nutritional deficiencies, metabolic disorders, and infectious diseases. By synthesizing physiological, epidemiological, and socio-economic evidence, this review provides insights into vulnerabilities, adaptation strategies, and research priorities essential for ensuring sustainable cattle production in a rapidly warming and climatically unstable region.
2. Materials and Methods
This review was conducted as a systematic narrative review, integrating a broad evidence synthesis with structured methodological elements adapted from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) guidelines []. A comprehensive literature search was performed in the PubMed, Scopus, Web of Science, and SciELO databases, covering publications up to September 2025. The Boolean string used was: (“cattle” OR “bovine” OR “livestock”) AND (“climate change” OR “global warming” OR “heat stress”) AND (“nutritional deficiency” OR “metabolic disease” OR “infectious disease” OR “Amazon biome”). The search was complemented by manual screening of reference lists from relevant review articles, technical reports, and international agency documents (Food and Agriculture Organization-FAO, Intergovernmental Panel on Climate Change-IPCC, United States Global Change Research Program-USGCRP).
Eligibility criteria included peer-reviewed publications, technical reports, and official documents addressing the impacts of climate change on cattle health, production, and disease dynamics, with emphasis on tropical and Amazonian contexts. Both experimental and observational studies were considered, provided they described physiological mechanisms, epidemiological trends, nutritional effects, or adaptation strategies. Only documents in English, Portuguese, or Spanish were included. Conference abstracts, theses, non-peer-reviewed reports, and opinion pieces were excluded.
The initial search retrieved 124 records. After duplicate removal, 99 remained. Following title and abstract screening, 87 were assessed for full-text eligibility. Finally, 81 documents met all inclusion criteria and were incorporated into the synthesis. Two reviewers independently screened records, and disagreements were resolved through consensus. Data extracted from each study included study design, geographic focus, cattle breed or system, climate variables analyzed, health outcomes (nutritional, metabolic, or infectious), and proposed adaptation or mitigation strategies.
The results were summarized descriptively and organized into thematic categories corresponding to the major sections of this review. Where applicable, tables and figures were employed to enhance comparability and clarity across studies. The overall screening and selection process is illustrated in the PRISMA flow diagram (Figure 1).
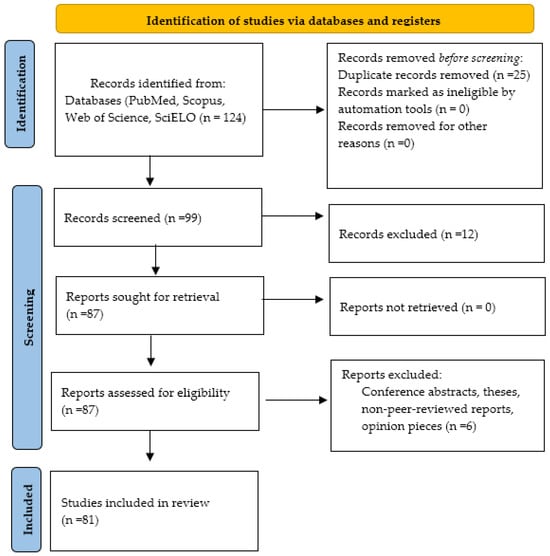
Figure 1.
PRISMA flow diagram summarizing the screening and selection process of studies included in this systematic narrative review. From 124 records initially identified, 99 remained after duplicate removal, 87 were assessed for full-text eligibility, and 81 were included in the final synthesis.
3. Climate Change and Cattle Production: A Global Perspective
3.1. Livestock as Victims and Drivers
Livestock production systems play a dual role in the context of climate change: they are both highly vulnerable to climatic shifts and significant contributors to global greenhouse gas (GHG) emissions [,]. Approximately 14.5% of global greenhouse gas (GHG) emissions are attributable to livestock, of which 44% corresponds to methane (CH4) primarily from enteric fermentation in ruminants, 27% to nitrous oxide (N2O) from manure and fertilized soils, and 29% to carbon dioxide (CO2) from land-use change, feed production, and energy consumption []. Manure management, deforestation for pasture expansion, and feed production further exacerbate the sector’s carbon footprint [,]. At the same time, livestock production is directly constrained by the very climate impacts it helps drive, including rising ambient temperatures, humidity, and the increased frequency of extreme weather events [,].
The Amazon biome exemplifies this paradox. Cattle in this region are exposed to elevated thermal loads, frequent flooding, and prolonged droughts, all of which impair productivity [,]. HS reduces feed intake and milk yield, compromises growth, and elevates disease susceptibility, thereby diminishing economic returns and threatening food security [,]. This interplay highlights livestock’s position as both victim and driver of climate change, necessitating systemic adaptation and mitigation measures [].
The biological impacts of HS in cattle extend beyond productivity losses. Physiological adjustments include respiratory alkalosis, increased rectal temperature, altered endocrine responses such as increased prolactin and reduced thyroid hormone activity, and shifts in metabolic partitioning [,]. Such changes result in decreased fertility, increased rates of abortion, and heightened vulnerability to infectious diseases []. These physiological responses, while adaptive in the short term, contribute to long-term production losses that threaten the sustainability of cattle production systems in the tropics [,].
3.2. Evidence from IPCC and FAO Reports
The IPCC has underscored that even with global warming limited to 1.5 °C, livestock systems, particularly in tropical regions, face significant vulnerabilities []. These include reductions in pasture productivity, increased prevalence of vector-borne diseases, and heightened risks of nutritional deficiencies due to altered forage quality [,]. The 1.5 °C threshold is not a safe limit for livestock; instead, it represents a critical point at which adaptation strategies become essential [].
The FAO emphasizes the cascading impacts of climate change on food security, animal health, and socio-economic stability []. Livestock are central to rural livelihoods, particularly in low- and middle-income countries where cattle serve as both an economic and nutritional asset []. In the Amazon biome, smallholder farmers are disproportionately affected, as they lack the infrastructure and resources to implement adaptive strategies such as advanced cooling systems or feed supplementation. This exacerbates vulnerability and deepens inequalities in global food systems [,].
FAO reports further highlight that climate change affects not only animal productivity but also the nutritional quality of livestock-derived products. Declines in protein content, changes in MUFA (monounsaturated fatty acid) and PUFA (polyunsaturated fatty acid), and reductions in micronutrients such as iron, zinc, sodium, and potassium have been documented under heat-stressed production systems [,]. These changes have direct implications for human nutrition and public health, reinforcing the One Health perspective that links animal health, environmental change, and human well-being []. The Temperature Humidity Index (THI) in the Amazon Biome typically ranges between 72 and 88, with the average value around 75–80 in most areas. These values indicate conditions ranging from comfortable to moderate heat stress, and occasionally severe stress in some regions or at certain times of the day [].
3.3. Multi-Hazard Risks
One of the defining characteristics of climate change impacts on livestock is the convergence of multiple stressors. Heat, floods, and droughts interact to create complex hazard profiles that amplify disease risks and undermine production [,]. For instance, flooding events increase the risk of leptospirosis and fasciolosis by expanding aquatic habitats for intermediate hosts [,,], while droughts intensify the risk of plant intoxications and nutritional deficiencies by reducing pasture availability and altering forage composition [,].
These multi-hazard conditions have significant implications for disease ecology. Higher temperatures and humidity extend the survival of many pathogens in the environment, while also enhancing vector development rates [,,,]. Simultaneously, HS compromises the immune system of cattle, reducing their ability to mount effective responses against infections [,,,]. The combined effect is an increased incidence and severity of infectious diseases, with direct consequences for productivity and animal welfare [,].
From a systems perspective, multi-hazard risks also extend to the supply chain. Infrastructure damage from floods disrupts the transport of feed and veterinary supplies, while prolonged droughts reduce water availability for both animals and forage crops []. This creates feedback loops in which climate extremes exacerbate resource scarcity, leading to heightened competition and increased vulnerability of rural communities.
Moreover, the convergence of hazards accelerates pasture degradation, particularly in fragile ecosystems such as the Amazon []. Deforestation and land-use change for cattle production already exert pressure on biodiversity, and climate extremes intensify this degradation []. As pastures degrade, invasive plant species proliferate, reducing forage quality and availability []. This directly impacts cattle nutrition and predisposes animals to metabolic diseases and intoxications [].
The multi-hazard risks associated with climate change necessitate integrated management strategies. These include climate-smart agricultural practices, enhanced disease surveillance, and the development of early warning systems that account for the interaction of multiple hazards []. In tropical regions such as the Amazon, where the effects of climate change are amplified, the implementation of these strategies is urgent to safeguard both animal health and human livelihoods [].
4. Vulnerabilities of the Amazon Biome
4.1. Heat Stress and Physiology
HS is one of the most pressing challenges for cattle production in the Amazon biome, where elevated temperatures and humidity frequently surpass the thresholds of thermoneutrality [,,]. In the eastern Amazon, where annual mean temperatures surpass 28 °C and relative humidity remains > 80%, the physiological threshold for thermoneutrality is exceeded for most of the year, making Amazonian cattle chronically heat-stressed. Empirical studies in Pará and Maranhão reveal sustained elevation in rectal temperature and respiration rate in zebu and crossbred dairy cows compared to similar systems in subtropical Brazil []. These findings demonstrate that HS in the Amazon is more persistent and cumulative than in other biomes, leading to metabolic exhaustion and reduced reproductive performance.
HS disrupts thermoregulation, forcing cattle to activate physiological mechanisms such as increased respiration rate, sweating, and peripheral vasodilation to dissipate excess body heat [,]. These adjustments demand significant energy resources, which reduces the energy available for production and reproduction [,,].
Studies in Holsteins and Jerseys demonstrate breed-specific immune remodeling under HS, with Holsteins showing increased circulating B cells and reduced monocytes, while Jerseys exhibit different leukocyte shifts [,]. Such findings highlight the importance of genetic background in defining resilience to HS and indicate opportunities for targeted breeding strategies [,,]. In addition to immune alterations, HS induces hormonal imbalances, including elevated prolactin and cortisol levels and altered insulin sensitivity, which compromise metabolic homeostasis [,,].
Heat stress (HS) also directly impairs reproductive performance, leading to reduced fertility, increased early embryonic loss, and prolonged calving intervals [,]. These outcomes arise from disrupted endocrine signaling and compromised follicular development under elevated thermal load []. Importantly, periparturient cows exposed to HS exhibit markedly higher vulnerability to transition-period metabolic and infectious diseases, including milk fever (hypocalcemia), mastitis, ketosis, fatty liver syndrome, and subclinical or clinical metabolic imbalances that weaken immunity during the critical pre- and postpartum windows. HS exacerbates negative energy balance, reduces dry matter intake, and impairs calcium homeostasis, thereby predisposing cows to retained placenta, metritis, and other postpartum uterine disorders that further compromise productivity and health [,].
4.2. Nutritional Vulnerabilities
The Amazon biome is characterized by diverse but fragile pasture ecosystems, which are highly sensitive to climatic extremes [,]. Under elevated temperatures and drought, forage digestibility declines due to increased lignification, reducing nutrient availability for cattle [,]. This predisposes animals to energy imbalances and ruminal dysfunction, contributing to metabolic disorders such as acidosis [].
Mineral imbalances are widely documented in the Amazon biome, where pastures commonly exhibit deficiencies not only in selenium, zinc, and copper but also in calcium and phosphorus due to the naturally acidic, highly weathered, and nutrient-leached soils characteristic of the region []. These macro- and trace minerals play central roles in skeletal development, metabolic regulation, antioxidant defense, and immune competence; thus, their scarcity under climate-stressed conditions exacerbates cattle susceptibility to metabolic disturbances and infectious diseases [,,,,]. Selenium deficiency has been associated with increased incidence of mastitis, retained placenta, and impaired antioxidant capacity, while low zinc and copper levels compromise epithelial integrity, reproductive performance, and innate immune responses [,,]. Additionally, the poor and oxidized soils of the Amazon contribute to reduced carotenoid availability, predisposing cattle to vitamin A and E deficiencies, which further impair vision, fertility, and oxidative balance. These nutritional constraints intensify during drought periods, when pasture biomass declines, and during heavy rainfall events, when excessive leaching reduces mineral and vitamin content, collectively amplifying the physiological stress faced by Amazonian herds [].
Pasture degradation further amplifies nutritional vulnerabilities in Amazonian cattle systems. Climate extremes accelerate soil erosion and biodiversity loss, favoring the spread of invasive or low-nutrient plant species that diminish the nutritional value of forage and alter botanical composition [,]. During drought periods, when forage availability sharply declines, cattle are more likely to ingest plants containing antinutritional factors such as saponins, oxalates, nitrates, tannins, and cyanogenic glycosides, which are ordinarily avoided under normal grazing conditions. These compounds can induce a range of toxic syndromes, including ruminal irritation, hypocalcemia, oxalate nephrosis, methemoglobinemia, hepatic damage, and impaired nutrient absorption, thereby increasing morbidity and reducing productive performance [,,,]. In the Amazon biome, prolonged dry seasons intensify this risk by concentrating these compounds in stressed plants and by forcing cattle to consume species such as Brachiaria spp. (saponins), Amaranthus spp. (oxalates and nitrates), and Manihot spp. (cyanogenic glycosides) when preferred forages are unavailable. Consequently, drought-associated plant intoxications have become an important cause of illness and mortality in Amazonian herds, complicating diagnostic and veterinary management and magnifying the impacts of climate variability on livestock health [,,,]
4.3. Extreme Weather and Disease Ecology
Extreme weather events, particularly floods and prolonged rainy seasons, create ideal conditions for the proliferation of pathogens and vectors [,,]. Standing water promotes the persistence of Leptospira spp., increasing the risk of leptospirosis outbreaks in cattle and posing zoonotic threats to human populations [,]. Similarly, fasciolosis prevalence rises in flooded pastures due to the expansion of snail intermediate hosts [].
Droughts, conversely, increase the risk of intoxication from toxic plants and predispose animals to dehydration and metabolic stress [,]. Reduced water availability impairs feed intake and digestion, contributing to nutritional deficiencies and weakening immune defenses [,]. This dual exposure to floods and drought illustrates the multi-hazard nature of climate risks in the Amazon [].
Trypanosomiasis represents another major threat in Amazonian systems, where vectors such as tabanids proliferate under warm and humid conditions. Climate variability influences vector abundance and seasonality, altering transmission dynamics and disease prevalence. Similarly, parasitic infections such as Haemonchus contortus and Fasciola hepatica intensify under wetter conditions, while tick-borne pathogens expand with warming temperatures [,,].
The interplay between climate variability and disease ecology creates significant challenges for herd health management. HS-induced immunosuppression, combined with increased pathogen exposure during floods and droughts, results in higher morbidity and mortality. This convergence of stressors emphasizes the urgent need for integrated disease surveillance and climate-adapted veterinary interventions [,].
5. Metabolic and Deficiency Diseases
5.1. Rumen Disorders
Heat stress reduces dry matter intake (DMI) and saliva buffering capacity, conditions that predispose cattle to ruminal fermentation disturbances and metabolic diseases [,,]. HS also triggers a well-characterized endocrine imbalance, marked by increases in circulating cortisol, insulin, and prolactin, reflecting heightened stress response, altered glucose insulin dynamics, and reduced mammary gland apoptosis, respectively [,,]. Concurrently, key anabolic and reproductive hormones, including triiodothyronine (T3), thyroxine (T4), estrogen, luteinizing hormone (LH), and progesterone, decrease substantially under thermal load, contributing to suppressed metabolic rate, impaired ovarian function, and reduced reproductive efficiency [,]. These hormonal shifts collectively prioritize thermoregulation over productivity, leading to decreased rumen motility, impaired fermentation efficiency, increased oxidative stress, and greater susceptibility to metabolic disorders in both beef and dairy cattle [,].
HS-induced reductions in DMI are closely linked with shifts in ruminal microbiota, including decreases in cellulolytic bacteria and increases in lactic acid-producing species, which exacerbate acidosis risk [,]. The accumulation of volatile fatty acids and lactic acid by Enterobacter asburiae reduces rumen pH, impairing fiber digestion and nutrient absorption [,]. This cascade contributes to subacute ruminal acidosis (SARA), which is increasingly reported under tropical climatic conditions [,]. In Amazonian production systems, prolonged drought followed by intense rainfall alters pasture composition, promoting lignified forages with reduced digestibility []. These cycles favor SARA, particularly where feed supplementation is limited. Field observations in Pará and Maranhão demonstrate that heat and humidity synergistically increase rumen fermentation instability, exacerbating metabolic acidosis during the transition between dry and wet seasons [].
Cattle suffering from SARA under HS conditions also exhibit marked systemic inflammation, characterized by increases in acute-phase proteins and well-defined oxidative stress biomarkers such as malondialdehyde (MDA), reactive oxygen species (ROS), thiobarbituric acid reactive substances (TBARS), decreased total antioxidant capacity (TAC), reduced glutathione (GSH), and alterations in antioxidant enzymes including superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) [,,]. The combined rise in lipid peroxidation markers and suppression of antioxidant defenses reflects a state of chronic oxidative imbalance. This pro-inflammatory and oxidative environment not only reduces production efficiency, impairing rumen function, nutrient utilization, and metabolic stability, but also predisposes animals to secondary disorders such as laminitis, liver abscesses, impaired immune responsiveness, and greater susceptibility to infectious diseases [,,,].
Nutritional interventions, including the supplementation of rumen buffers and yeast culture (Saccharomyces cerevisiae), have been shown to mitigate the adverse effects of HS on rumen fermentation [,]. However, the efficacy of such interventions depends on consistent feed availability, which is often compromised in Amazonian systems during extreme weather events [,].
5.2. Mineral and Vitamin Deficiencies
Reduced pasture nutrient density under elevated temperatures and drought increases the risk of trace mineral deficiencies [,]. Selenium, zinc, and copper are particularly critical for cattle health, as they play essential roles in antioxidant defense, immune competence, and reproductive performance [,]. Selenium deficiency, for instance, is associated with retained placenta, mastitis, and impaired fertility [,,].
Zinc deficiency compromises keratin formation in hooves and teat canals, predisposing cattle to lameness and mastitis [,]. Copper deficiency impairs erythropoiesis and bone metabolism, leading to anemia and growth retardation [,]. These deficiencies are common in the Amazon biome due to soil leaching and low mineral content in native pastures [,].
Vitamins A and E are also frequently deficient under climate-stressed conditions. Vitamin A deficiency arises from reduced beta-carotene content in drought-stressed forages, impairing epithelial integrity and immune function [,]. Vitamin E deficiency, on the other hand, increases susceptibility to oxidative stress, further compounding the negative impacts of HS [,].
Nutritional strategies to mitigate these deficiencies involve a combination of balanced mineral supplementation, including phosphorus, calcium, sodium, copper, selenium, and zinc, delivered through fortified mineral mixes, protein-energy supplements, strategic salt licks, and parenteral injectable formulations when necessary []. In regions with severe soil nutrient depletion, such as large areas of the Amazon biome, year-round access to mineral-vitamin supplementation is essential to maintain rumen function, immune competence, and reproductive performance. Additional strategies include the use of protein-energy concentrates during the dry season, corrective supplementation of fat-soluble vitamins (A, D, and E), and buffering agents that help stabilize rumen pH under HS-induced hyporexia. However, the adoption of these practices is frequently limited by the extensive nature of Amazonian production systems, long distances between paddocks, seasonal flooding, and logistical constraints that hinder the consistent delivery and monitoring of supplementation programs [,].
5.3. Plant Intoxications
Drought-stressed forages elevate the concentration of secondary plant metabolites such as alkaloids, nitrates, and cyanogenic glycosides, predisposing cattle to poisoning syndromes [,]. In the Amazon, plant intoxications are particularly problematic during prolonged dry seasons when feed scarcity forces animals to consume otherwise avoided toxic plants [].
Common intoxications include those caused by Brachiaria spp., which produce steroidal saponins leading to hepatotoxicity and photosensitization []. Other toxic species, such as Crotalaria spp., contain pyrrolizidine alkaloids that cause chronic liver damage [,]. Cases of nitrate poisoning are also reported during drought conditions, when nitrate accumulates in stressed forage crops [,].
The clinical outcomes of plant intoxications vary from reduced productivity to acute mortality, depending on toxin dose and exposure duration [,]. Subclinical cases often go undiagnosed but contribute to reduced growth rates and reproductive inefficiency [].
Management strategies for plant intoxications involve pasture diversification, rotational grazing, and provision of safe feed alternatives during critical periods [,,,]. However, the rapid pace of climate change increases the unpredictability of toxic plant proliferation, making preventive strategies more complex [,].
Early detection through clinical surveillance and postmortem diagnostics is critical to reducing economic losses from intoxications []. Research on the toxicodynamics of Amazonian plants under climate stress remains limited, highlighting the need for region-specific investigations [,].
6. Infectious Diseases and Climate Change
6.1. Bacterial and Mycotic Diseases
HS weakens immune defenses by altering leukocyte dynamics and reducing the efficiency of the innate immune response, thereby predisposing cattle to bacterial infections such as mastitis and enteric diseases [,,]. Elevated temperature-humidity index (THI) conditions are strongly correlated with increased somatic cell counts in milk, reflecting subclinical mastitis prevalence in tropical dairies [,,,]. Moreover, feed contamination with fungi during humid conditions heightens the risk of mycotoxicosis, further impairing immunity [,,].
Leptospirosis is particularly sensitive to climatic fluctuations, as flood conditions expand aquatic reservoirs that harbor pathogenic Leptospira spp. [,]. Outbreaks of leptospirosis have been consistently reported following extreme rainfall and flooding events, underscoring the role of climate-driven hydrological changes in shaping bacterial disease epidemiology []. In the Amazon biome, recurrent flooding during the rainy season maintains leptospiral reservoirs in aquatic environments, as documented along the Tocantins and Marajó floodplains []. These hydrological extremes, coupled with high organic matter in soils, favor bacterial persistence and re-infection cycles distinct from drier biomes [,].
In addition to leptospirosis, listeriosis and salmonellosis risks are exacerbated by HS and inadequate feed storage during extreme weather [,]. Rising humidity supports fungal growth in silages, promoting Listeria multiplication, while warm, moist conditions increase Salmonella contamination in feed and water sources [,]. These interactions highlight the complex interplay between climate, feed hygiene, and bacterial disease risk [,].
Mycotic infections, particularly aspergillosis, are expected to increase under warming conditions due to the proliferation of spores in poorly stored feed and bedding materials [,,]. Fungal mastitis, caused by Candida spp. and Aspergillus spp., is increasingly documented in tropical systems experiencing prolonged heat and humidity []. Such cases compromise milk quality and animal health, resulting in significant economic losses [,,]. Likewise, in the Amazon biome, the prolonged humidity exceeding 80% supports fungal proliferation in stored feed, explaining the high regional incidence of mycotoxicosis and fungal mastitis [,,].
The immunosuppressive effects of mycotoxins, particularly aflatoxins and ochratoxins, compound bacterial disease risks by weakening host defenses []. These toxins, prevalent in improperly stored grains under humid conditions, reduce lymphocyte proliferation and impair antibody responses, increasing susceptibility to opportunistic pathogens [,]. This interplay is particularly concerning in extensive systems with limited access to quality-controlled feed [,].
6.2. Viral and Vector-Borne Diseases
Climatic changes also influence the epidemiology of viral diseases, including foot-and-mouth disease (FMD), which persists longer in humid environments where viral stability is maintained [,]. Rising temperatures and increased rainfall expand habitats for insect vectors, facilitating arboviral transmission cycles []. Bluetongue virus (BTV), transmitted by Culicoides midges, exemplifies this climate-sensitive vector-borne disease [,].
African evidence highlights the expansion of Rift Valley fever and other arboviruses under warming scenarios, with rainfall-driven mosquito population booms acting as critical transmission drivers [,,]. These findings provide parallels for the Amazon, where vector dynamics are strongly influenced by precipitation and temperature fluctuations [,].
Vector-borne viral diseases are further exacerbated by altered host-vector interactions. Higher temperatures shorten the extrinsic incubation period of viruses within vectors, increasing transmission efficiency [,,]. This effect has been demonstrated for both BTV and West Nile virus, highlighting the broader implications of climate change on arbovirus epidemiology [,].
Foot-and-mouth disease (FMD), a major threat to cattle production in South America, is also influenced by climate variability. High humidity enhances viral survival in the environment, while increased cattle movement during droughts elevates transmission opportunities [,]. Consequently, climate change complicates eradication and control strategies by introducing variability into disease dynamics [,].
6.3. Parasitic Diseases
Parasitic diseases are profoundly affected by climatic changes, with warming favoring the proliferation of helminths and protozoa [,]. Haemonchus contortus, a blood-sucking nematode, thrives under warm and humid conditions, leading to higher infection intensities in cattle [,]. This parasite, already prevalent in the Amazon, poses increasing risks under projected climate scenarios [].
Fasciolosis, caused by Fasciola hepatica, is closely linked to rainfall and the presence of intermediate snail hosts [,,]. In the Amazon biome, heavy rains and flooding expand snail habitats, resulting in higher fasciolosis incidence. The disease causes chronic weight loss, anemia, and reduced productivity, significantly affecting herd profitability [,,].
Trypanosomiasis, transmitted by biting flies, is another parasitic disease projected to expand under climate change. Rising temperatures and altered rainfall patterns support vector survival and proliferation, increasing trypanosome transmission [,,]. This disease has been reported with growing frequency in tropical South America, highlighting the importance of climate-adapted surveillance [,].
Tick infestations, particularly by Rhipicephalus microplus, are expected to increase in prevalence and geographic range under climate warming [,,]. Higher temperatures accelerate tick development rates, reduce generation intervals, and enhance reproductive success. This leads to greater tick burdens and higher transmission of tick-borne pathogens such as babesiosis and anaplasmosis [,,,,].
Amazonian cattle are particularly vulnerable to tick-borne diseases, as extensive grazing systems provide abundant host-vector interactions [,,,]. Babesiosis, caused by Babesia bovis and Babesia bigemina, is strongly influenced by vector abundance, with outbreaks peaking during hot and humid conditions [,,,,,]. Similarly, anaplasmosis incidence increases under HS, reflecting synergistic effects of immunosuppression and high tick loads [,].
Emerging parasitic diseases, including neosporosis and cryptosporidiosis, may also intensify under climate change due to altered host-environment interactions. Cryptosporidiosis, for example, proliferates in warm and moist conditions, contaminating water supplies and threatening both animal and human health [,]. These trends underscore the One Health implications of parasitic diseases in the Amazon under a changing climate [,,].
7. Socio-Economic Dimensions and Food Security
Smallholders in the Amazon face disproportionate risks from climate change. Water scarcity, pasture degradation, and disease expansion undermine food security and rural incomes, creating a cycle of poverty and vulnerability [,,]. These stressors are magnified by limited access to technological innovations and veterinary infrastructure, which restricts the ability of small producers to adapt effectively [,,].
Rural livelihoods in the Amazon biome are highly dependent on cattle production, both as a direct source of income and as a nutritional resource for protein supply [,]. Climate variability reduces productivity and increases mortality, threatening food security and exacerbating social inequalities [,,]. Studies demonstrate that climate-induced losses in livestock productivity directly correlate with reduced household income, further reinforcing cycles of rural poverty [,].
Pasture degradation represents one of the most pressing socio-economic issues. Rising temperatures and unpredictable rainfall accelerate soil nutrient depletion and promote invasive species, thereby reducing the carrying capacity of grazing systems [,,]. In turn, this leads to increased costs for supplementary feed, further straining the economic resilience of smallholders [].
The expansion of infectious and parasitic diseases under climate change scenarios imposes heavy economic burdens. Veterinary costs rise significantly during outbreaks, and productivity losses due to reduced milk yield, weight gain, or fertility compromise profitability [,]. For smallholders, even minor disease outbreaks can have devastating consequences, forcing herd reduction or sale of productive animals at low market value [,,].
From a One Health perspective, the socio-economic vulnerabilities of Amazonian cattle production also impact human health. Reduced access to safe animal products and declining nutritional quality of milk and meat under HS conditions jeopardize food security for rural populations [,,]. Moreover, zoonotic disease spillovers become more likely as climate change reshapes pathogen ecology, creating risks for human communities in close contact with livestock [,,].
Climate change also affects gender dynamics and social equity in rural areas. Women, often responsible for small-scale dairy and livestock management, are disproportionately affected by increased workloads due to water and feed scarcity [,]. This gendered impact highlights the need for socially inclusive adaptation policies [,]. Furthermore, youth outmigration from rural communities, exacerbated by economic instability, reduces the labor force necessary to sustain livestock systems [].
Adaptive strategies to protect rural livelihoods must include the promotion of climate-smart agriculture, access to financial credit, and investment in disease surveillance [,,]. Policy frameworks that integrate animal health, environmental conservation, and rural development are essential for building resilience [,]. Regional cooperation, including the involvement of governmental and non-governmental organizations, can provide technological support and capacity building to ensure that smallholders are not left behind [,].
Finally, the intersection of climate change, livestock health, and food security in the Amazon underscores the urgency of coordinated action. Addressing socio-economic vulnerabilities requires systemic approaches that link adaptation and mitigation strategies to broader goals of poverty reduction, nutritional security, and sustainable development [,,]. The challenges faced by Amazonian cattle producers serve as a critical reminder of the global interconnectedness of climate, health, and socio-economic systems [,,].
8. Mitigation and Adaptation Strategies
8.1. Climate-Smart Husbandry
Climate-smart husbandry practices are essential to safeguard cattle production in the Amazon biome under climate change scenarios. Basic strategies include the provision of shading, improved ventilation, sprinkling systems, and abundant water sources to mitigate the effects of HS [,,,,,,]. Forage diversification, including the introduction of drought-tolerant and heat-resistant species, increases pasture resilience to climate variability [,]. Manure management technologies such as biodigesters reduce methane emissions while providing renewable energy sources for smallholders [,]. These interventions not only improve animal welfare but also reduce greenhouse gas emissions, creating dual benefits for production and climate mitigation (Figure 2 and Figure 3) [].
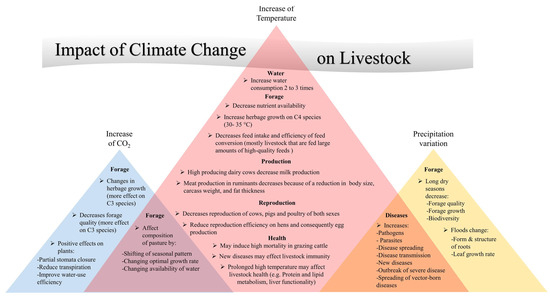
Figure 2.
Impacts of Climate Change on Livestock [].
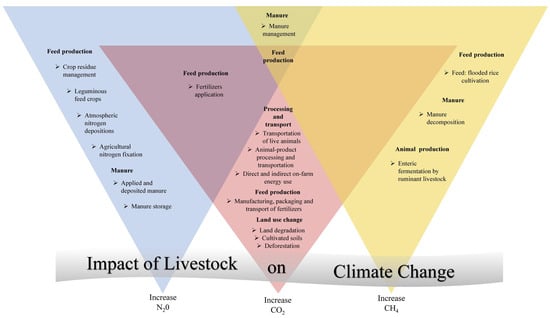
Figure 3.
Impacts of Livestock on Climate Change [].
The adoption of silvopastoral systems, which integrate trees, forage crops, and livestock, represents a promising adaptation strategy. These systems enhance soil fertility, provide shade for animals, and increase carbon sequestration [,,,,]. They also diversify farm income and reduce the reliance on monoculture pastures, which are highly vulnerable to climatic extremes []. Evidence from tropical systems shows that silvopastoral practices improve milk yield and reduce HS indicators in cattle [,,,].
Water management remains a cornerstone of adaptation. In the Amazon, where seasonal droughts are becoming more frequent, the construction of water reservoirs, rainwater harvesting systems, and efficient irrigation technologies ensures reliable access to water for both animals and forage crops [,,]. Improving water efficiency reduces stress on local ecosystems and helps sustain cattle productivity under unpredictable rainfall patterns [].
8.2. Reproductive Health and Fertility
Climate change has significant impacts on bovine reproduction. HS increases the prevalence of anestrus, retained placenta, and repeat breeding, which reduce reproductive efficiency [,,,]. High temperatures disrupt hormonal balance, impair ovarian function, and negatively affect conception rates [,]. The implementation of cooling strategies during the peripartum period, including shaded maternity pens and evaporative cooling, improves fertility outcomes and reduces postpartum disorders [,].
Nutritional interventions are critical for maintaining reproductive health under HS conditions. The supplementation of antioxidants such as selenium and vitamin E mitigates oxidative stress and enhances immune responses in periparturient cows [,,]. Balanced transition diets are essential to mitigate the negative energy balance and rumen instability that characterize the periparturient period under climate stress, improving uterine involution, metabolic homeostasis, and ovulation efficiency [,]. In heat-stressed cows, precise nutritional management should prioritize adequate dietary energy density, highly digestible fiber, and controlled fermentable carbohydrate supply to stabilize rumen pH and reduce metabolic disorders []. In the Amazon biome, where soils are intrinsically poor and forage-derived deficiencies of Ca, P, Na, Zn, Cu, and Se are common, targeted mineral supplementation is critical to sustain immune competence, reproductive performance, and thermoregulatory efficiency []. Antioxidant vitamins A and E help counteract oxidative damage and immune suppression induced by heat and humid conditions, while vitamin C contributes to modulating cortisol responses during thermal stress; biotin improves epithelial and hoof integrity, particularly relevant in periods of fungal contamination of forages []. Functional additives such as probiotics, prebiotics, and synbiotics enhance rumen microbial balance, nutrient digestibility, and epithelial resilience, supporting cows exposed to high THI and seasonal pasture limitations [,]. Together, these nutritional strategies provide a scientifically grounded framework to increase metabolic, reproductive, and immunological resilience of Amazonian cattle facing intensifying climate variability.
Reproductive technologies also play a role in adaptation. Artificial insemination (AI) with semen from heat-tolerant breeds, embryo transfer, and synchronization protocols allow producers to optimize breeding schedules during periods of reduced thermal stress []. These technologies, combined with improved management practices, help maintain reproductive performance in increasingly challenging environments [,,,].
8.3. Genetic and Genomic Selection
Genetic selection for heat tolerance and disease resistance offers long-term solutions for climate adaptation in cattle. Genomic tools such as genome-wide association studies (GWAS) and reaction-norm models identify thermotolerance alleles, including the slick-hair gene and adaptive traits inherited from Bos indicus breeds [,,,,,,,]. These traits reduce heat load and improve resilience without compromising productivity [,,,,]. Incorporating adaptive genetics into crossbreeding programs enhances herd resilience in the Amazon [,,,].
Recent advances in genomic prediction allow the simultaneous selection for productivity and resilience traits. Genomic estimated breeding values (GEBVs) integrate large datasets to identify animals best suited for tropical environments [,]. This enables more efficient genetic improvement and reduces the time required to achieve climate-adapted herds [,,]. Furthermore, the integration of genomic data with precision livestock technologies supports real-time monitoring of adaptive traits [,,].
Breed conservation also represents an important adaptation strategy. Locally adapted Amazonian and indigenous breeds possess traits that confer resilience to parasites, nutritional stress, and heat load []. Preserving these genetic resources and incorporating them into breeding programs ensures the long-term sustainability of cattle production in the biome [,]. Collaborative programs between research institutions and producers are needed to accelerate the adoption of genomic innovations [].
8.4. Policy and Systemic Approaches
Beyond farm-level strategies, systemic approaches are necessary to align livestock production with climate adaptation and mitigation goals. Policies promoting climate-smart agriculture, financial incentives for low-emission technologies, and investment in veterinary infrastructure are critical for scaling adaptation strategies [,]. Regional and international cooperation is essential to facilitate knowledge exchange and capacity building, particularly in the Amazon, where smallholders remain highly vulnerable [,].
Integrating cattle production into broader climate policies supports the achievement of Sustainable Development Goals (SDGs). By reducing emissions intensity and enhancing resilience, the livestock sector contributes to goals related to zero hunger, climate action, and poverty reduction [,,]. Ensuring that adaptation measures are socially inclusive, with attention to gender and youth dynamics, strengthens the equity and sustainability of interventions [,].
9. Future Perspectives
Future strategies for strengthening cattle production in the Amazon biome must integrate technological innovation, reproductive management, and climate-informed nutritional planning to mitigate the increasingly severe impacts of heat, drought, flooding, and infectious disease dynamics. Advances in omics technologies, including nutrigenomics, metabolomics, transcriptomics, and proteomics, offer powerful tools for identifying early immune-metabolic disruptions in cattle exposed to thermal and hydrological stressors [,,,]. These approaches allow investigators to characterize molecular pathways associated with thermotolerance, metabolic instability, and disease susceptibility, enabling the development of precision nutritional and management strategies tailored to the Amazonian climate. Integrating such biomarkers into monitoring frameworks will improve the capacity to predict vulnerability during extreme weather events and guide rapid intervention strategies aimed at preserving productivity [,,].
Climate-intelligence platforms based on Geographic Information Systems (GIS) and remote sensing represent another essential component of future adaptation efforts. These technologies can monitor pasture biomass, soil moisture, water availability, and spatial disease risk, providing producers with near-real-time information to anticipate forage scarcity, manage grazing intensity, and identify environmental conditions that favor pathogen proliferation [,,]. When linked to disease surveillance data, GIS-based early-warning systems can support targeted interventions for leptospirosis, fasciolosis, trypanosomiasis, and tick-borne infections, which are expected to intensify under projected warming and altered rainfall patterns [,,,,,]. Such integrative climate-risk dashboards designed specifically for Amazonian cattle systems would enhance decision-making at farm and regional scales.
Nutritional and reproductive management must also be adapted to the Amazon’s pronounced seasonal variability. The August–November dry period, characterized by high thermal load, declining pasture quality, and reduced water availability, requires the expansion of drought-tolerant forage species such as Brachiaria brizantha cv. Marandu, Panicum maximum cvs. Mombaça and Massai, and Brachiaria humidicola, as well as leguminous species like Stylosanthes spp., to improve protein supply. Forage banks based on sugarcane, elephant grass, or cactus pear can buffer nutritional deficits during critical months and support rumen stability under climatic stress [,]. During the December–May rainy season, when flooding increases risks for fasciolosis and leptospirosis, nutritional strategies should include mineral supplementation and improved feed hygiene to reduce exposure to waterborne pathogens [,].
A climate-informed periparturient (transition-cow) strategy is equally essential. Heat stress exacerbates the risks of metabolic diseases, reproductive failure, mastitis, and retained placenta during the transition period [,,,]. Balanced diets with adequate energy density, improved mineral profiles, and enhanced antioxidant support during the dry season can reduce postpartum complications. Coupling nutritional interventions with cooling strategies, reproductive biotechnologies, and endocrine–metabolic monitoring will contribute to maintaining fertility and minimizing production losses in Amazonian herds [,,,,].
Genomic innovation will play a decisive role in building long-term resilience. Genome-wide association studies (GWAS) and genomic estimated breeding values (GEBVs) can guide the selection of animals with superior thermotolerance, immune responsiveness, and disease resistance, accelerating genetic progress toward climate-resilient herds [,,,,,]. Incorporating adaptive traits from locally adapted Amazonian cattle is crucial for maintaining biodiversity while improving resilience to metabolic and infectious challenges [,,,,,,,].
Finally, the socio-economic dimension must remain central. Smallholders who constitute the majority of Amazonian producers are disproportionately affected by climate extremes due to limited access to credit, veterinary assistance, and climate-smart technologies [,,,]. Future initiatives must include capacity building, targeted financial support, incentives for adopting silvopastoral systems, and training in adaptive management. Strengthening these community-level strategies will not only enhance herd resilience but also support rural livelihoods and contribute to broader One Health objectives linking animal, human, and ecosystem wellbeing [,,,].
10. Conclusions
Climate change is reshaping cattle production in the Amazon biome through a complex interaction of thermal stress, hydrological instability, nutritional challenges, and altered infectious disease dynamics. The region’s cattle, exposed annually to extreme seasonal variations, experience physiologic strain that reduces productivity, compromises immune competence, and heightens vulnerability to metabolic disorders and climate-sensitive pathogens. Evidence synthesized in this review demonstrates that the Amazonian production environment is becoming increasingly unpredictable, pushing herds toward physiological thresholds that compromise long-term sustainability.
Overall, the collective findings indicate that adaptation will depend on a deeper understanding of climate-driven metabolic responses, improved reproductive management under thermal stress, and integrated strategies that safeguard both animal performance and producer livelihoods. The Amazon’s unique socio-ecological context demands solutions that strengthen resilience across nutritional, genetic, health, and environmental dimensions. Ensuring sustainable cattle production under these conditions is a central challenge for the coming decades.
Author Contributions
Conceptualization, F.M.S. and J.B.d.S.; methodology, F.M.S.; formal analysis, F.M.S. and J.B.d.S.; data curation, F.M.S. and J.B.d.S.; writing—original draft preparation, F.M.S. and J.B.d.S.; writing—review and editing, F.M.S.; supervision, F.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors are grateful to CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), FAPESPA (Fundação Amazônia de Amparo a Estudos e Pesquisas do Estado do Pará), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Conflicts of Interest
The author declares no conflicts of interest.
References
- Food and Agriculture Organization of the United Nations (FAO). Climate Change and Food Security: Risks and Responses; FAO: Rome, Italy, 2016; Available online: https://openknowledge.fao.org/handle/20.500.14283/i5188e (accessed on 12 October 2025).
- IPCC. Global Warming of 1.5 °C: An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Thornton, P.K.; van de Steeg, J.; Notenbaert, A.; Herrero, M. The Impacts of Climate Change on Livestock and Livestock Systems in Developing Countries: A Review of What We Know and What We Need to Know. Agric. Syst. 2009, 101, 113–127. [Google Scholar] [CrossRef]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock—A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013; p. 139. Available online: https://www.fao.org/4/i3437e/i3437e.pdf (accessed on 12 October 2025).
- Melillo, M.J.; McGuire, A.D.; Kicklighter, D.W.; Moore, B.; Vorosmarty, C.J.; Schloss, A.L. Global climate change and terrestrial net primary production. Nature 1993, 363, 234–240. [Google Scholar] [CrossRef]
- De Rensis, F.; Garcia-Ispierto, I.; Lopez-Gatius, F. Seasonal Heat Stress: Clinical Implications and Hormone Treatments for the Fertility of Dairy Cows. Theriogenology 2015, 84, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.J. Reproductive Physiology of the Heat-Stressed Dairy Cow: Implications for Fertility and Assisted Reproduction. Anim. Reprod. 2019, 16, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Polsky, L.; von Keyserlingk, M.A.G. Invited Review: Effects of Heat Stress on Dairy Cattle Welfare. J. Dairy Sci. 2017, 100, 8645–8657. [Google Scholar] [CrossRef]
- Nardone, A.; Ronchi, B.; Lacetera, N.; Ranieri, M.S.; Bernabucci, U. Effects of Climate Changes on Animal Production and Sustainability of Livestock Systems. Livest. Sci. 2010, 130, 57–69. [Google Scholar] [CrossRef]
- Fox, N.J.; White, P.C.L.; McClean, C.J.; Marion, G.; Evans, A.; Hutchings, M.R. Predicting Impacts of Climate Change on Fasciola hepatica Risk. PLoS ONE 2011, 6, e16126. [Google Scholar] [CrossRef]
- Caminade, C.; Van Dijk, J.; Baylis, M.; Williams, D. Modelling Recent and Future Climatic Suitability for Fasciola hepatica in Europe. Geospat. Health 2015, 9, 301–308. [Google Scholar] [CrossRef]
- Marques, R.V.; Krüger, R.F.; Peterson, A.T.; de Melo, L.F.; Vicenzi, N.; Jiménez-García, D. Climate Change Implications for the Distribution of the Babesiosis and Anaplasmosis Tick Vector, Rhipicephalus (Boophilus) microplus. Vet. Res. 2020, 51, 81. [Google Scholar] [CrossRef]
- Ogden, N.H.; Ben Beard, C.; Ginsberg, H.S.; Tsao, J. Possible Effects of Climate Change on Ixodid Ticks and the Pathogens They Transmit: Predictions and Observations. J. Med. Entomol. 2021, 58, 1536–1545. [Google Scholar] [CrossRef]
- Nuttall, P.A. Climate Change Impacts on Ticks and Tick-Borne Infections. Biologia 2022, 77, 1503–1515. [Google Scholar] [CrossRef]
- Godde, C.M.; Mason-D’cRoz, D.; Mayberry, D.; Thornton, P.; Herrero, M. Impacts of Climate Change on the Livestock Food Supply Chain; A Review of the Evidence. Glob. Food Secur. 2021, 28, 100488. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; Chou, R.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- da Silva, W.C.; Printes, O.V.N.; Lima, D.O.; da Silva, É.B.R.; Dos Santos, M.R.P.; Camargo Júnior, R.N.C.; Barbosa, A.V.C.; da Silva, J.A.R.; e Silva, A.G.M.; Silva, L.K.X.; et al. Evaluation of the Temperature and Humidity Index to Support the Implementation of a Rearing System for Ruminants in the Western Amazon. Front. Vet. Sci. 2023, 14, 1198678. [Google Scholar] [CrossRef]
- Giannone, C.; Bovo, M.; Ceccarelli, M.; Torreggiani, D.; Tassinari, P. Review of the Heat Stress-Induced Responses in Dairy Cattle. Animals 2023, 13, 3451. [Google Scholar] [CrossRef]
- Chen, L.; Thorup, V.M.; Kudahl, A.B.; Østergaard, S. Effects of Heat Stress on Feed Intake, Milk Yield, Milk Composition, and Feed Efficiency in Dairy Cows: A Meta-analysis. J. Dairy Sci. 2024, 107, 3207–3218. [Google Scholar] [CrossRef]
- De Rensis, F.; Scaramuzzi, R.J. Heat Stress and Seasonal Effects on Reproduction in the Dairy Cow—A Review. Theriogenology 2003, 60, 1139–1151. [Google Scholar] [CrossRef]
- Purse, B.V.; Mellor, P.S.; Rogers, D.J.; Samuel, A.R.; Mertens, P.P.C.; Baylis, M. Climate Change and the Recent Emergence of Bluetongue in Europe. Nat. Rev. Microbiol. 2005, 3, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Aparecido, L.E.O.; Lorençone, J.A.; Lorençone, P.A.; Torsoni, G.B.; Moraes, J.R.d.S.C.d.; de Meneses, K.C. Bioclimatic Zoning for Dairy Cows in Brazil by Statistical Modeling. J. Sci. Food Agric. 2022, 102, 6048–6063. [Google Scholar] [CrossRef]
- Sousa, A.C.; de Sousa, A.M.; Corrêa, W.C.; Marques, J.I.; de Meneses, K.C.; Pandorfi, H.; da Silva, T.G.F.; da Silva, J.L.B.; da Silva, M.V.; Machado, N.A.F. Bioclimatic Zoning and Climate Change Impacts on Dairy Farming in Maranhão (Brazil). Animals 2025, 15, 1646. [Google Scholar] [CrossRef]
- Messeri, A.; Mancini, M.; Bozzi, R.; Parrini, S.; Sirtori, F.; Morabito, M.; Crisci, A.; Messeri, G.; Ortolani, A.; Gozzini, B.; et al. Temperature-humidity Index Monitoring During Two Summer Seasons in Dairy Cow Sheds in Mugello (Tuscany). Int. J. Biometeorol. 2023, 67, 1555–1567. [Google Scholar] [CrossRef]
- Jo, J.H.; Nejad, J.G.; Peng, D.-Q.; Kim, H.-R.; Kim, S.-H.; Lee, H.-G. Characterization of Short-Term Heat Stress in Holstein Dairy Cows Using Altered Indicators of Metabolomics, Blood Parameters, Milk MicroRNA-216 and Characteristics. Animals 2021, 11, 722. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sharma, A.; Joy, A.; Dunshea, F.R.; Chauhan, S.S. The Impact of Heat Stress on Immune Status of Dairy Cattle and Strategies to Ameliorate the Negative Effects. Animals 2022, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- Garner, J.B.; Douglas, M.L.; Williams, S.R.O.; Wales, W.J.; Marett, L.C.; Nguyen, T.T.T.; Reich, C.M.; Hayes, B.J. Genomic Selection Improves Heat Tolerance in Dairy Cattle. Sci. Rep. 2016, 6, 34114. [Google Scholar] [CrossRef]
- McWhorter, T.M.; Sargolzaei, M.; Sattler, C.; Utt, M.D.; Tsuruta, S.; Misztal, I.; Lourenco, D. Single-step Genomic Predictions for Heat Tolerance of Production Yields in US Holsteins and Jerseys. J. Dairy Sci. 2023, 106, 7861–7879. [Google Scholar] [CrossRef]
- Cheruiyot, E.K.; Haile-Mariam, M.; Cocks, B.G.; Pryce, J.E. Improving Genomic Selection for Heat Tolerance in Dairy Cattle: Current Opportunities and Future Directions. Front. Genet. 2022, 13, 894067. [Google Scholar] [CrossRef]
- Muaz, K.; Riaz, M.; de Oliveira, C.A.F.; Akhtar, S.; Ali, S.W.; Nadeem, H.; Park, S.; Balasubramanian, B. Aflatoxin M1 in Milk and Dairy Products: Global Occurrence and Decontamination Methods. Toxin Rev. 2022, 41, 588–605. [Google Scholar] [CrossRef]
- Menta, P.R.; Machado, V.; Piñeiro, J.; Thatcher, W.; Santos, J.; Vieira-Neto, A. Heat Stress During the Transition Period is Associated with Impaired Production, Reproduction, and Survival in Dairy Cows. J. Dairy Sci. 2022, 105, 4474–4489. [Google Scholar] [CrossRef]
- Molinari, P.C.C.; Davidson, B.D.; Laporta, J.; Dahl, G.E.; Sheldon, I.M.; Bromfield, J.J. Prepartum Heat Stress in Dairy Cows Increases Postpartum Inflammatory Responses in Blood of Lactating Dairy Cows. J. Dairy Sci. 2023, 106, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, E.; Cattaneo, L.; Piccioli-Cappelli, F.; Mezzetti, M.; Minuti, A. International Symposium on Ruminant Physiology: The Immunometabolism of Transition Dairy Cows from Dry-off to Early Lactation-Lights and Shadows. J. Dairy Sci. 2025, 108, 7662–7674. [Google Scholar] [CrossRef]
- Gomes, F.J.; Pedreira, B.C.; Santos, P.M.; Bosi, C.; Lulu, J.; Pedreira, C.G. Microclimate Effects on canopy characteristics of shaded palisadegrass pastures in a silvopastoral system in the Amazon biome of central Brazil. Eur. J. Agron. 2020, 115, 126029. [Google Scholar] [CrossRef]
- Lehmann, J.; Kern, D.; German, L.; Mccann, J.; Martins, G.C.; Moreira, A. Soil Fertility and Production Potential. In Amazonian Dark Earths; Lehmann, J., Kern, D.C., Glaser, B., Wodos, W.I., Eds.; Springer: Dordrecht, The Netherlands, 2003. [Google Scholar] [CrossRef]
- Lemes, A.P.; Garcia, A.R.; Pezzopane, J.R.M.; Brandão, F.Z.; Watanabe, Y.F.; Cooke, R.F.; Sponchiado, M.; de Paz, C.C.P.; Camplesi, A.C.; Binelli, M.; et al. Silvopastoral System is an Alternative to Improve Animal Welfare and Productive Performance in Meat Production Systems. Sci. Rep. 2021, 11, 14092. [Google Scholar] [CrossRef]
- Mezzetti, M.; Cattaneo, L.; Passamonti, M.M.; Lopreiato, V.; Minuti, A.; Trevisi, E. The Transition Period Updated: A Review of the New Insights into the Adaptation of Dairy Cows to the New Lactation. Dairy 2021, 2, 617–636. [Google Scholar] [CrossRef]
- Sanou, C.L.; Agodzo, S.K.; Balima, L.H.; Bessah, E.; Antwi-Agyei, P.; Traoré, K. Influence of Climate Change on Livestock Diseases Occurrence in Burkina Faso, West Africa. Int. J. Biometeorol. 2025, 12, 1–15. [Google Scholar] [CrossRef]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Woznicki, S.A. Climate Change and Livestock: Impacts, Adaptation, and Mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Modabbernia, G.; Meshgi, B.; Kinsley, A.C. Climatic Variations and Fasciola: A Review of Impacts Across the Parasite Life Cycle. Parasitol. Res. 2024, 123, 300. [Google Scholar] [CrossRef]
- Rakib, M.R.H.; Marion, G.; Davidson, R.S.; White, P.C.L.; Hutchings, M.R. Effect of Heat Stress on Udder Health of Dairy Cows. J. Dairy Res. 2020, 87, 315–321. [Google Scholar] [CrossRef]
- Fox, N.J.; Marion, G.; Davidson, R.S.; White, P.C.L.; Hutchings, M.R. Livestock Helminths in a Changing Climate: Approaches and Restrictions to Meaningful Predictions. Animals 2012, 2, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.P.; de Sousa, F.C.; da Silva, A.L.; Schultz, É.B.; Londoño, R.I.V.; de Souza, P.A.R. Heat Stress in Dairy Cows: Impacts, Identification and Mitigation. Animals 2025, 15, 249. [Google Scholar] [CrossRef] [PubMed]
- Matera, R.; Cotticelli, A.; Carpio, M.G.; Biffani, S.; Iannacone, F.; Salzano, A.; Neglia, G. Relationship among Production Traits, Somatic Cell Score and THI. Ital. J. Anim. Sci. 2022, 21, 1026–1036. [Google Scholar] [CrossRef]
- Hudson, A.R.; McGregor, B.L.; Shults, P.; England, M.; Silbernagel, C.; Mayo, C.; Carpenter, M.; Sherman, T.J.; Cohnstaedt, L.W. Culicoides-Borne Orbivirus Epidemiology in a Changing Climate: A Review. J. Med. Entomol. 2023, 60, 1221–1239. [Google Scholar] [CrossRef]
- Hammami, H.; Bormann, J.; M’hAmdi, N.; Montaldo, H.; Gengler, N. Evaluation of heat stress effects on production traits and somatic cell score of Holsteins in a temperate environment. J. Dairy Sci. 2013, 96, 1844–1855. [Google Scholar] [CrossRef]
- Bokharaeian, M.; Toghdory, A.; Ghoorchi, T.; Nejad, J.G.; Esfahani, I.J. Quantitative Associations between Season, Month, and Temperature-Humidity Index with Milk Yield, Composition, Somatic Cell Counts, and Microbial Load: A Comprehensive Study across Ten Dairy Farms over an Annual Cycle. Animals 2023, 13, 3205. [Google Scholar] [CrossRef] [PubMed]
- Toghdory, A.; Ghoorchi, T.; Asadi, M.; Bokharaeian, M.; Najafi, M.; Nejad, J.G. Effects of Environmental Temperature and Humidity on Milk Composition, Microbial Load, and Somatic Cells in Milk of Holstein Dairy Cows in the Northeast Regions of Iran. Animals 2022, 12, 2484. [Google Scholar] [CrossRef]
- Jiang, Y.; Ogunade, I.M.; Vyas, D.; Adesogan, A.T. Aflatoxin in Dairy Cows: Toxicity, Occurrence in Feedstuffs and Mitigation Strategies. Toxins 2021, 13, 283. [Google Scholar] [CrossRef] [PubMed]
- Abril, A.G.; Villa, T.G.; Barros-Velázquez, J.; Cañas, B.; Sánchez-Pérez, A.; Calo-Mata, P.; Carrera, M. Staphylococcus aureus Exotoxins and Their Detection in the Dairy Industry and Mastitis. Toxins 2020, 12, 537. [Google Scholar] [CrossRef]
- Zentai, A.; Jóźwiak, Á.; Süth, M.; Farkas, Z. Carry-Over of Aflatoxin B1 from Feed to Cow Milk—A Review. Toxins 2023, 15, 195. [Google Scholar] [CrossRef] [PubMed]
- Samy, A.M.; Peterson, A.T. Climate Change Influences on the Global Potential Distribution of Bluetongue Virus. PLoS ONE 2016, 11, e0150489. [Google Scholar] [CrossRef]
- Sanders, C.J.; Shortall, C.R.; England, M.; Harrington, R.; Purse, B.; Burgin, L.; Carpenter, S.; Gubbins, S. Long-Term Shifts in the Seasonal Abundance of Adult Culicoides Biting Midges and Their Impact on Potential Arbovirus Outbreaks. J. Appl. Ecol. 2019, 56, 1649–1660. [Google Scholar] [CrossRef]
- Navarro, D.A.M.; Huere, H.R.; Buendia, R.V.; Rojas, M.; Chunga, W.A.; Gutierrez, E.V.; Abarca, W.V.; Gerónimo, H.R.; Altamiranda-Saavedra, M. Would Climate Change Influence the Potential Distribution and Ecological Niche of Bluetongue Virus and Its Main Vector in Peru? Viruses 2023, 15, 892. [Google Scholar] [CrossRef]
- Wang, H.H.; Grant, W.E.; Teel, P.D.; Hamer, S.A. Quantitative Models of Rhipicephalus (Boophilus) Ticks: Historical Review and Synthesis. Ecosphere 2017, 8, e01942. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Mallón, A.R.; Bermúdez, S.; de la Fuente, J.; Domingos, A.; García, M.P.E.; Labruna, M.B.; Merino, O.; Mosqueda, J.; Nava, S.; et al. One Health Approach to Identify Research Needs on Rhipicephalus microplus in the Americans. Pathogens 2022, 11, 1180. [Google Scholar] [CrossRef]
- Hossain, D.; Rahman, N.; Karim, M.R.; Bristi, S.Z.T.; Uddin, N.; Uddin, A.H.M.M. Climate Resilient Livestock Production System in Tropical and Subtropical Countries. In Climate-Resilient Agriculture; Hasanuzzaman, M., Ed.; Springer: Cham, Germany, 2023; Volume 1. [Google Scholar] [CrossRef]
- World Health Organization. Climate Change and Health Vulnerability and Adaptation Assessment; World Health Organization: Geneva, Switzerland, 2021; Available online: https://iris.who.int/server/api/core/bitstreams/6edda8d7-451e-4edc-b096-c84d76e1355b/content (accessed on 12 October 2025).
- Nam, K.T.; Choi, N.; Na, Y.; Choi, Y. Effect of the Temperature–Humidity Index on the Productivity of Dairy Cows and the Correlation between the Temperature–Humidity Index and Rumen Temperature Using a Rumen Sensor. Animals 2024, 14, 2848. [Google Scholar] [CrossRef] [PubMed]
- Reis, N.S.; Ferreira, I.C.; Mazocco, L.A.; Souza, A.C.B.; Pinho, G.A.S.; Neto, Á.M.d.F.; Malaquias, J.V.; Macena, F.A.; Muller, A.G.; Martins, C.F.; et al. Shade Modifies Behavioral and Physiological Responses of Low to Medium Production Dairy Cows at Pasture in an Integrated Crop-Livestock-Forest System. Animals 2021, 11, 2411. [Google Scholar] [CrossRef]
- Pezzopane, J.R.M.; Nicodemo, M.L.F.; Bosi, C.; Garcia, A.R.; Lulu, J. Animal Thermal Comfort Indexes in Silvopastoril Systems with Different Tree Arrangements. J. Thermal Biol. 2019, 79, 103–111. [Google Scholar] [CrossRef]
- Magalhães, C.A.S.; Zolin, C.A.; Lulu, J.; Lopes, L.B.; Furtini, I.V.; Vendrusculo, L.G.; Zaiatz, A.P.; Pedreira, B.C.; Pezzopane, J.R.M. Improvement of Thermal Comfort Indices in Agroforestry Systems in the Southern Brazilian Amazon. J. Therm. Biol. 2020, 91, 102636. [Google Scholar] [CrossRef]
- da Silva, W.C.; da Silva, J.A.R.; da Silva, É.B.R.; Barbosa, A.V.C.; Sousa, C.E.L.; de Carvalho, K.C.; dos Santos, M.R.P.; Neves, K.A.L.; Martorano, L.G.; Júnior, R.N.C.C.; et al. Characterization of Thermal Patterns Using Infrared Thermography and Thermolytic Responses of Cattle Reared in Three Different Systems during the Transition Period in the Eastern Amazon, Brazil. Animals 2023, 13, 2735. [Google Scholar] [CrossRef]
- da Silva, W.C.; da Silva, J.A.R.; Martorano, L.G.; da Silva, É.B.R.; de Carvalho, K.C.; Sousa, C.E.L.; Neves, K.A.L.; Júnior, R.N.C.C.; Belo, T.S.; de Santos, A.G.S.; et al. Thermal Comfort of Nelore Cattle (Bos indicus) Managed in Silvopastoral and Traditional Systems Associated with Rumination in a Humid Tropical Environment in the Eastern Amazon, Brazil. Vet. Sci. 2024, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, S.J.; Edwards, J.P.; Shirley, A.K.; Clark, C.E.F.; Schütz, K.E.; Verhoek, K.J.; Jago, J.G. Heat stress amelioration for pasture-based dairy cattle: Challenges and opportunities. Anim. Front. 2025, 15, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Woodward, S.J.R.; Edwards, J.; Verhoek, K.; Jago, J. Identifying and Predicting Heat Stress Events for Grazing Dairy Cows Using Rumen Temperature Boluses. JDS Commun. 2024, 5, 431–435. [Google Scholar] [CrossRef]
- Werlen, G.; Jain, R.; Jacinto, E. MTOR Signaling and Metabolism in Early T Cell Development. Genes 2021, 12, 728. [Google Scholar] [CrossRef]
- Pryce, J.E.; Haile-Mariam, M. Symposium review: Genomic selection for reducing environmental impact and adapting to climate change. J. Dairy Sci. 2020, 103, 5366–5375. [Google Scholar] [CrossRef] [PubMed]
- Macciotta, N.P.P.; Biffani, S.; Bernabucci, U.; Lacetera, N.; Vitali, A.; Ajmone-Marsan, P.; Nardone, A. Derivation and Genome-wide Association Study of a Principal Component-based Measure of Heat Tolerance in Dairy Cattle. J. Dairy Sci. 2017, 100, 4683–4697. [Google Scholar] [CrossRef]
- Bohlouli, M.; Halli, K.; Yin, T.; Gengler, N.; König, S. Genome-Wide Associations for Heat Stress Response Suggest Potential Candidate Genes Underlying Milk Fatty Acid Composition in Dairy Cattle. J. Dairy Sci. 2022, 105, 3323–3340. [Google Scholar] [CrossRef]
- Luo, H.; Hu, L.; Brito, L.F.; Dou, J.; Sammad, A.; Chang, Y.; Ma, L.; Guo, G.; Liu, L.; Zhai, L.; et al. Weighted single-step GWAS and RNA sequencing Reveals Key Candidate Genes Associated with Physiological Indicators of Heat Stress in Holstein Cattle. J. Anim. Sci. Biotechnol. 2022, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Dikmen, S.; Khan, F.A.; Huson, H.J.; Sonstegard, T.S.; Moss, J.I.; Dahl, G.E.; Hansen, P.J. The SLICK Hair Locus Derived from Senepol Cattle Confers Thermotolerance to Intensively Managed Lactating Holstein Cows. J. Dairy Sci. 2014, 97, 5508–5520. [Google Scholar] [CrossRef] [PubMed]
- Sosa, F.; Carmickle, A.T.; Jiménez-Cabán, E.; Ortega, M.S.; Dikmen, S.; Negrón-Pérez, V.; Jannaman, E.A.; Baktula, A.; Rincon, G.; Larson, C.C.; et al. Inheritance of the SLICK1 Allele of PRLR in Cattle. Anim. Genet. 2021, 52, 624–629. [Google Scholar] [CrossRef]
- Littlejohn, M.; Henty, K.M.; Tiplady, K.; Johnson, T.; Harland, C.; Lopdell, T.; Sherlock, R.G.; Li, W.; Lukefahr, S.D.; Shanks, B.C.; et al. Functionally reciprocal mutations of the prolactin signalling pathway define hairy and slick cattle. Nat. Commun. 2014, 5, 5861. [Google Scholar] [CrossRef]
- Zayas, G.A.; Dikmen, S.; Mateescu, R.G.; Hansen, P.J. Maintaining Breed Integrity: Successful Introgression of the SLICK1 Allele into the Holstein Breed. J. Hered. 2025, 116, 216–224. [Google Scholar] [CrossRef]
- Cuellar, C.J.; Amaral, T.F.; Rodriguez-Villamil, P.; Ongaratto, F.; Martinez, D.O.; Labrecque, R.; Losano, J.D.d.A.; Estrada-Cortés, E.; Bostrom, J.R.; Martins, K.; et al. Consequences of gene editing of PRLR on thermotolerance, growth, and male reproduction in cattle. FASEB Bioadv. 2024, 6, 223–234. [Google Scholar] [CrossRef]
- Martin-Collado, D. Are Farmers Motivated to Select for Heat Tolerance? Linking Attitudinal Factors, Perceived Climate Change Impacts, and Social Trust to Farmers Breeding Desires. J. Dairy Sci. 2024, 107, 2156–2174. [Google Scholar] [CrossRef]
- Santos, S.G.C.G.; Saraiva, E.P.; Neto, S.G.; Maia, M.I.L.; Lees, A.M.; Sejian, V.; Maia, A.S.C.; de Medeiros, G.R.; Fonsêca, V.d.F.C. Heat tolerance, Thermal Equilibrium and Environmental Management Strategies for Dairy Cows Living in Intertropical Regions. Front. Vet. Sci. 2022, 9, 982781. [Google Scholar] [CrossRef]
- Ainsworth, J.A.W.; Kingwell, R.S.; Pannell, D.J. Pasture Shade and Farm Management Effects on Cow Productivity in the Tropics. Agric. Ecosyst. Environ. 2012, 155, 105–110. [Google Scholar] [CrossRef]
- Becker, C.A.; Collier, R.J.; Stone, A.E. Invited Review: Physiological and Behavioral Effects of Heat Stress in Dairy Cows. J. Dairy Sci. 2020, 103, 6751–6770. [Google Scholar] [CrossRef] [PubMed]
- Lemal, P.; Tran, M.-N.; Atashi, H.; Schroyen, M.; Gengler, N. Adding Behavior Traits to Select for Heat Tolerance in Dairy Cattl. JDS Commun. 2024, 5, 368–373. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).