Simple Summary
Oxidative and heat stress constitute a harmful dyad for the sheep meat industry, as they induce cellular damage, inflammation, and immunosuppression, thereby negatively impacting the health, welfare, reproductive performance, growth rate, and meat quality of these animals. In warm climates, summer heat stress elicits physiological and metabolic changes associated with acclimatization and/or adaptation in sheep, which in turn stimulates oxidative stress by overproducing reactive oxygen species and reducing antioxidant defenses. In heat-stressed hair sheep, limited attention has been paid to identifying the specific factors associated with oxidative stress biomarkers, which is essential for establishing appropriate heat stress mitigation strategies for sheep farms. This study aimed to determine the contribution of climatic, physiological, metabolic, and productive factors to the oxidant-antioxidant balance in hair lambs intensively fattened under outdoor heat stress in a desert region. Our results indicate that the oxidant-antioxidant imbalance in heat-stressed hair lambs was mainly linked to climatic and physiological factors, with negligible effects from changes in energy metabolism and growth performance.
Abstract
Oxidative stress impairs the productivity and welfare of heat-stressed hair sheep; however, the key factors contributing to its occurrence remain unclear. Twenty-two Dorper × Katahdin ewe lambs weighing 23.5 ± 2.8 kg and experiencing outdoor heat stress in a desert region were used to establish the contribution of climatic variables, physiological responses, metabolism, and feedlot performance to the oxidant-antioxidant imbalance. Pearson’s correlation, principal component analysis, and multiple linear regression were applied to the datasets. Oxidative stress biomarkers showed positive relationships with average and minimum climatic variables, morning rectal temperature, serum triglycerides and insulin, and feed efficiency. Still, these biomarkers were negatively correlated with maximum ambient temperature (Te) and temperature-humidity index (THI), morning and afternoon respiratory rate (RR), total protein, and feed intake. While total oxidant capacity and fat oxidation were mainly associated with decreased maximum Te, protein oxidation was primarily associated with increased morning RR. Total antioxidant capacity was favored by a reduction in maximum THI and oxidative stress index (OSI) by an increase in maximum relative humidity. With minimal contribution (≤6%), protein and fat oxidation were also explained by increased serum insulin and feed intake, respectively, whereas OSI was explained by increased weight gain. Overall, the presence of oxidative stress in feedlot hair sheep experiencing outdoor heat stress was regulated by a combination of climatic conditions, morning RR, and, to a lesser extent, productive performance.
1. Introduction
Under current sheep meat production scenarios, where the goal is to maximize the expression of growth potential in intensive systems, fattening lambs present high metabolic activity and energy demand in the muscle, cardiac, and hepatic tissues [1]. This scenario leads to an overflow of free radicals that exceeds antioxidant system’s ability to neutralize them, resulting in a pro-oxidant state and oxidative stress [2]. At the cellular level, this situation alters homeostasis by oxidizing lipid, protein, and DNA, which negatively affects immune function, nutrient metabolism, health, welfare, and consequently, growth rate [2,3]. Furthermore, global warming is expanding the areas with hot climates, where summers are characterized by high environmental temperatures (Te) which induce heat stress (HS) in feedlot sheep [1]. HS represents a pathophysiological state in which sheep respond to adverse hot conditions through physiological, biochemical, and behavioral changes [4]. Thus, sheep under HS prioritize thermoregulatory processes over productive ones [5,6]. These adjustments are positively related to the overproduction of reactive oxygen species (ROS) and insufficient antioxidant defenses, whereby HS is considered an exogenous etiological factor for oxidative stress in farm animals, including sheep [7,8]. In this context, both oxidative stress and HS adversely affect the sheep meat industry in warm regions of the world.
Native sheep breeds from hot and harsh environments are considered valuable genetic resources because of their resilience and adaptability [9]. Most hair sheep are meat breeds widely distributed in warm climates owing to their rusticity, thermotolerance, and low reproductive seasonality, which guarantee constant mutton production throughout the year in these hostile environments [6,10]. Despite this, hair sheep exhibit physiological and metabolic thermoregulatory adjustments in response to HS to avoid hyperthermia [11]. Among these adjustments are increased respiratory rate (RR), redistribution of blood flow to peripheral tissues, adaptive heterothermy, reduction in metabolic heat production through a slight decrease in thyroid activity and altered insulin-regulated postabsorptive metabolism to improve insulin sensitivity and cortisol resistance [12]. All these adjustments are reflected, not only in the feedlot with a slow growth rate and lower feed efficiency [13], but also in high ROS production [8]. Hair sheep cells also demonstrate a remarkable ability to produce heat shock proteins [14], which play a crucial role in mitigating oxidative stress damage and ensuring cellular survival under hyperthermic conditions [3]. Consequently, oxidative stress in heat-stressed hair sheep is a latent problem that compromises their welfare and productive efficiency when the outdoor Te exceeds 30 °C [8,15]. It is important to note that the degree of physiological and metabolic adjustments in response to HS varies among sheep breeds depending on their thermotolerance [2]. Therefore, the contribution of these adjustments, including those related to climate, to the occurrence of oxidative stress in sheep exposed to HS may differ among genotypes.
To date, high Te has been linked to the presence of oxidative stress in heat-stressed hair sheep [15]. In adapted and non-adapted sheep to HS, both acclimatization and growth adjustments have been associated with changes in oxidative stress biomarkers [8,16,17,18]. Thus, a strong positive correlation of the temperature-humidity index (THI) with the oxidative stress index (OSI) and protein oxidation, as well as between serum malondialdehyde concentration (MDA) and RR, has been documented for crossbred wool sheep [17]. In Merino × Poll Dorset lambs, increased respiratory evaporation was associated with higher hydrogen peroxide concentrations and consequently a pro-oxidant status [18]. In lambs, weight gain is also positively correlated with ROS synthesis due to increased cellular metabolic activity [19]. Zhang et al. [20] did not report changes in total antioxidant capacity (TAC) and glutathione, despite the perturbations observed in physiological variables and blood gas balance when Poll Dorset × Merino × Border Leicester thermotolerant ewe lambs were exposed to short-duration heat waves (28–38 °C) in a climatic chamber. Sonadi Corriedale sheep (native breed from India) exhibited elevated serum cortisol concentrations, TAC, superoxide dismutase, catalase, lipid peroxidation, and glutathione in response to high Te and THI in summer [21]. Heat-stressed sheep activate endocrine and metabolic mechanisms that decrease endogenous heat production and ensure energy availability [12,22], while also increasing ROS production through accelerated aerobic and anaerobic pathways [16].
Factors contributing to increased oxidative stress in heat-stressed hair sheep have not been comprehensively determined. Therefore, it is essential to comprehend the biological mechanisms associated with impaired oxidative status during HS, which, in turn, could aid in establishing HS mitigation strategies based on the antioxidant supply. It was hypothesized that the oxidative stress level exhibited by heat-stressed hair lambs is associated with factors such as climatic conditions, physiological and metabolic adjustments, and growth rate. Therefore, this study aimed to establish the contribution of climatic variables, physiological responses, metabolism, and feedlot performance to the oxidant-antioxidant imbalance of hair breed lambs exposed to HS conditions in a desert region.
2. Materials and Methods
2.1. Study Site
The study was conducted during the second half of summer (i.e., August and September) at the Sheep Experimental Unit of the Institute of Agricultural Sciences, Autonomous University of Baja California. The study site is located in the Mexicali Valley (32°24′ N and 115°22′ W) where a hot desert climate prevails, with an annual rainfall of 85 mm and high Te in summer (>40 °C) [23].
2.2. Animals and Management
Lambs were handled according to the procedures described within the guidelines established in the Mexican Official Standard NOM-062-ZOO-1999 (Technical specifications for the production, care, and use of laboratory animals) [24], and the Federation Animal Science Society [25]. The UABC Ethics Committee approved and supervised all experimental procedures (letter number: 047/2024-1).
The study was conducted with female lambs selected from a group of 40 Dorper × Katahdin lambs that were weaned at 3 months of age and received prophylactic management. They were housed by body size in two pens immediately after weaning. Each lamb received 0.4 mL ivermectin (Iverfull®, Aranda Laboratory, Mexico City, Mexico), 0.5 mL ADE vitamins (Vigantol ADE Fuerte®, Elanco Animal Health Laboratory, Zapopan, Jalisco, Mexico), and 2.5 mL clostridium vaccine (Bovimune® Clostri 10, LaPisa Laboratory, La Piedad, Michoacan, Mexico). The feeding consisted of a fattening diet formulated with 70% concentrate and 30% forage (metabolizable energy = 2.8 Mcal/kg; crude protein = 17%). At 4 months of age, lambs were weighed individually, and 22 of them were selected based on their relatively homogeneous live body weights (BW = 23.5 ± 2.8 kg) ability to consume feed, and good health. Each ewe lamb represented an experimental unit and was housed in 2-m2 individual pens for 55 days; the first 15 d were used to adapt them to the pen and diet, and the following 40 days to collect data on growth performance, climatic variables, and blood analyte concentrations. Each pen had a dirt floor, cyclonic mesh walls, a galvanized sheet shade at a height of 2.5 m, a feed bunk, and a watering trough. The diet was formulated to meet nutritional requirements according to the NRC [26] and was offered ad libitum twice a day (0600 and 1800 h) along with clean, fresh water. Diet samples were collected weekly, dried in a forced-air oven at 60 °C for 24 h, and ground. These were then mixed to obtain two subsamples, which were chemically analyzed using standardized methods [27,28]. Dietary metabolizable energy (ME) was calculated using formulas [29]. The ingredients and chemical composition of the mixed diet are listed in Table 1. The health status of the animals was visually monitored daily by a veterinarian, who did not report sick animals; therefore, no animals were excluded, and the study variables were measured in all of them.

Table 1.
Diet composition and nutrient analysis of experimental diets offered to heat-stressed female lambs during the experimental period.
2.3. Data Collection
Daily climatic conditions were measured using a thermohydrometer device (Thermotracker, Higro, Culiacán, Sinaloa, Mexico), which was placed in the middle of the study area and programmed to record Te and relative humidity (RH) every 20 min. The Te and RH data were used to calculate the THI using the following formula [30]: THI = Te − [(0.31 − (0.31 × [RH/100])) × (Te − 14.4)]. For all climatic variables, daily maximum, minimum, and mean values were averaged separately to characterize the environmental conditions of the study area.
In order to minimize the impact of animal handling on the collected data, the study variables were measured in a specific sequence: first the variables that did not require animal handling (RR), then the variables with the greatest sensitivity to handling (rectal temperature [RT] and blood analytes), and finally, those with the least sensitivity (feedlot growth). All measurements of physiological variables and serum analyte concentrations were performed on experimental days 1, 20, and 40, obtaining 66 observations/variable. Physiological response data included morning (0500 h) and afternoon (1700 h) RR and RT. First, RR was quantified by counting the number of intercostal movements for 30 s, which was multiplied by two to obtain the number of breaths per minute (bpm). Then, RT was measured by inserting a digital thermometer (Delta Track, Pleasanton, CA, USA) into the rectum.
After recording physiological variables in the morning and before feeding, blood samples were collected by jugular venipuncture into 10 mL red cap Vacutainer tubes. All samples were centrifuged within the first hour of collection at 3500× g for 15 min at 10 °C. The serum was then placed in 2 mL vials and frozen at −20 °C until its use in the determination of analyte concentrations associated with energy and protein metabolism, as well as the oxidant-antioxidant balance. Serum metabolites (i.e., glucose, cholesterol, triglycerides, total protein, and urea nitrogen [BUN]) were analyzed using a liquid-phase blood auto-analyzer (EasyVet; KrontroLab, Morelia, Mich., México), while hormones (i.e., triiodothyronine [T3], thyroxine [T4], cortisol, and insulin) were analyzed using a fully automated ELISA analyzer (Thunderbolt, Gold Standard Diagnostics, California, USA) and commercial kits (Monobind Inc., Lake Forest, CA, USA). The intra- and inter-assay coefficients of variation (CV) were 5.4 and 6.7% for T3, 1.6 and 6.1% for T4, 6.4 and 7.0% for cortisol, and 4.9 and 5.6% for insulin, respectively. In addition, oxidative stress biomarkers were measured using spectrophotometry (Bio-Tek Instruments, Inc., Winooski, VT, USA) following the methods of chloramine-T [31], N-methyl-2-phenyl-indole [32], o-dianisidine (3,3′-dimethoxybenzidine) [33], and ABTS radical cation [34] for serum determination of advanced oxidation protein products (AOPP), MDA, total oxidant capacity (TOC), and TAC, respectively. The OSI was calculated as the TOC/TAC ratio [35].
Feedlot performance data included initial and final BW, average daily gain (ADG), feed intake, and feed efficiency. Ewe lambs were individually weighed using a static scale before morning feeding at the beginning and end of the 40 d feeding period, and this information was used to calculate ADG ([final—initial BW]/40 d). The daily amount of feed offered and refused per animal was also recorded to calculate the daily feed intake and feed efficiency (ADG/feed intake).
2.4. Statistical Analysis
All statistical procedures were conducted using the SAS software (SAS Inst. Inc., Cary, NC, USA; version 9.4). First, the Shapiro–Wilk normality test was applied to all variables using the PROC UNIVARIATE procedure. Then, a descriptive statistical analysis was performed using the PROC MEANS procedure. Pearson’s correlation analysis, using the PROC CORR procedure, was performed to identify the relationship levels of oxidative stress biomarkers with climatic variables, physiological constants, serum metabolite and hormone concentrations, and feedlot traits. Correlation coefficients (r) were considered significant at p ≤ 0.05 and were classified as low (r < 0.35), moderate (0.36 < r < 0.67), or strong (r > 0.68). Additionally, principal component (PC) analysis was performed using all measured parameters with the PROC PRINCOMP procedure. Only the first two PC with eigenvalues ≥ 1 were plotted [36]. Finally, multiple linear regression (MLR) analysis was performed using the PROC REG procedure to develop exploratory models that specifically identified the factors associated with each oxidative stress biomarker. Therefore, biomarkers were dependent variables, whereas climatic, physiological, and metabolic variables, as well as feedlot traits, were explanatory variables. The STEPWISE regression and Mallow’s Cp criteria were used to select the variables included in the final models. In each MLR model developed, residual analysis was applied to identify and eliminate observations that fell outside the critical values of two or more indicators (i.e., RS Student, Leverage, DFITS, COV RATIO, and DFBETAS). Coefficient of determination (R2), adjusted coefficient of determination (R2adj), root mean square error (RMSE), variance inflation factor (VIF), Durbin-Watson test (DW), and lack-of-fit test (LF) were considered to evaluate the models’ goodness of fit. The individual contribution of the variables selected in the final models was reported according to partial R2 and R2adj, which were calculated from the type III sum of squares.
3. Results
Descriptive statistics of climatic variables are presented in Table 2. The mean values ± standard deviation for Te, RH, and THI were 33.5 ± 0.8 °C, 52.5 ± 4.5%, and 30.3 ± 1.0 units, respectively. The CV in these variables ranged from 2.6 to 19.0%, and the lowest and highest CV were average Te and minimum RH, respectively.

Table 2.
Descriptive statistics of the climatic variables recorded during the experimental period.
Descriptive statistics for physiological, metabolic, and performance variables are presented in Table 3. Regarding physiological responses, female lambs in the morning had an average RR of 105 ± 19 bpm and average RT of 39.7 ± 0.2 °C, and these values increased in the afternoon by 65.2 bpm and 0.5 °C, respectively. Average serum values of metabolic analyte concentrations ranged from 0.9 ± 0.5 ng/mL (insulin) to 82.5 ± 7.2 mg/dL (glucose). Except for triglycerides (CV = 49.2%), hormone concentrations had higher CV (>35 vs. <25%) than serum metabolite concentrations. In feedlot variables, ADG, feed intake, and feed efficiency averaged 0.2 ± 0.1 kg, 1.1 ± 0.2 kg, and 0.2 ± 0.1 kg/kg, respectively. The variation for feed efficiency and ADG was 50%, while that for feed intake was only 18.2%. Oxidative stress biomarkers exhibited average values of 6.9 μmol/L, 1.5 μmol/L, 6.3 μmol Eq H2O/L, 1.2 μmol Eq Trolox/L, and 0.6 arbitrary units for AOPP, MDA, TOC, TAC, and OSI, respectively. All these biomarkers showed high CV ranging from 33.3 (OSI) to 66.7% (TAC).

Table 3.
Descriptive statistics of physiological responses, metabolic analyte concentrations, feedlot performance, and oxidative stress biomarkers of hair female lambs subjected to severe heat stress.
Correlation results between oxidative stress biomarkers and the rest of study variables are shown in Table 4. Overall, a relationship (p < 0.05; −0.85 ≤ r ≤ 0.79) existed between the oxidative stress biomarkers and climatic variables, which was positive, particularly with the average and minimum values of Te, RH, and THI, as well as with the maximum RH. Maximum Te and THI, and morning RR (p < 0.05; −0.62 ≤ r ≤ −0.28), negatively correlated with all oxidative stress biomarkers. In particular, MDA, TOC, and TAC were negatively correlated (p < 0.05; −0.48 ≤ r ≤ −0.24) with afternoon RR and positively correlated with morning RT (p < 0.05; 0.29 ≤ r ≤ 0.34). As in the afternoon RT (p < 0.05), no biomarker was correlated (p > 0.05) with serum glucose, cholesterol, BUN, cortisol, T3, and T4 concentrations. In contrast, serum insulin concentrations were positively correlated (p < 0.01; 0.35 ≤ r ≤ 0.76) with all oxidative stress biomarkers. Triglycerides were positively correlated (p < 0.05; 0.28 ≤ r ≤ 0.43) with AOPP, MDA, TOC, and TAC, whereas total protein was negatively correlated (p < 0.01; −0.52 ≤ r ≤ −0.32) with the same biomarkers. In the case of feedlot performance variables, ADG was correlated (p < 0.05) only with TAC (r = −0.30) and OSI (r = 0.27), whereas feed intake had a negative correlation (p < 0.01; −0.55 ≤ r ≤ −0.38) with AOPP, MDA, TOC, and TAC but not with OSI (p > 0.05). Feed efficiency was correlated (p < 0.05) only with AOPP, TOC, and OSI, and these relationships were positive and low (0.24 ≤ r ≤ 0.33).

Table 4.
Pearson correlations (r) among oxidative stress biomarkers and climatic conditions, physiological variables, metabolic analytes, and feedlot variables of hair female lambs subjected to severe heat stress.
Results of the PC analysis are shown in Figure 1. Two PCs were selected, which explained 50% of the total variance, with 40.7% attributed to PC1 and 9.3% to PC2. PC1 was mainly loaded (−0.27 ≥ r ≤ 0.28) with all climatic variables, TOC, morning RR, serum insulin, and feed intake, whereas PC2 was loaded (−0.38 ≥ r ≤ 0.44) with ADG, feed efficiency, morning and afternoon RT, triglycerides, OSI, and TAC. Within PC1, TOC, MDA, and AOPP showed a positive association with serum insulin and the average and minimum values of climatic variables; however, these same oxidative stress biomarkers exhibited a negative association with maximum Te and THI, morning RR, feed intake, and total protein. Within PC2, there was a positive association between OSI and ADG, feed efficiency, and T3. In addition, TAC was positively associated with morning and afternoon RT and triglycerides and negatively associated with feedlot growth, OSI, BUN, and T3.
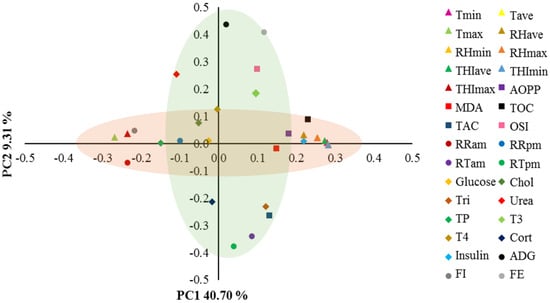
Figure 1.
Principal components (PC) analysis for climatic conditions, oxidative stress, physiological variables, metabolic analyte concentrations, and feedlot performance of hair female lambs subjected to severe heat stress. Tmin = Minimum temperature, Tave = Average temperature, Tmax = Maximum temperature, RHmin = Minimum relative humidity, RHave = Average relative humidity, RHmax = Maximum relative humidity, THImin = Minimum temperature-humidity index, THIave = Average temperature-humidity index, THImax = Maximum temperature-humidity index, AOPP = Advanced oxidation protein products, MDA = Malondialdehyde, TOC = Total oxidant capacity, TAC = Total antioxidant capacity, OSI = Oxidative stress index, RRam = Morning respiratory rate, RRpm = Afternoon respiratory rate, RTam = Morning rectal temperature, RTpm = Afternoon rectal temperature, Chol = Cholesterol, Tri = Triglycerides, TP = Total protein, T3 = Triiodothyronine, T4 = Thyroxine, Cort = Cortisol, ADG = Average daily gain, FI = Feed intake, and FE = Feed efficiency.
The MLR models developed to explain the observed changes in oxidative stress biomarkers are shown in Table 5 and Table 6. Models explained (p > 0.01) 51, 43, 76, 52, and 17% of the total observed variation in AOPP, MDA, TOC, TAC, and OSI, respectively, with climatic variables explaining the variation (p < 0.01; 13–73%) in most of these models (except AOPP).

Table 5.
Multiple linear regression equations developed to identify specific factors linked to oxidative stress biomarkers in hair ewe lambs subjected to severe heat stress.

Table 6.
Partial contribution of climatic conditions, physiological responses, metabolic analyte concentration, and feedlot performance in the prediction of oxidative stress biomarkers in hair female lambs subjected to severe heat stress.
The AOPP variation was explained by the morning RR (46%) and insulin concentration (6%), while the MDA variation was entirely attributed to maximum Te and the TAC variation to maximum THI (Table 6). Most of the variations observed in TOC and OSI were attributed to maximum Te (73%) and THI (13%), respectively. With minimal contribution (3–4%), feed intake and ADG helped to explain the variation in TAC and TOC, respectively. Overall, there was an increase in the AOPP and TAC biomarkers by reducing the morning RR and maximum THI, respectively. In contrast, MDA and TOC increased in response to a decrease in the maximum Te. OSI increased with increasing maximum RH.
4. Discussion
According to the climatic conditions, female sheep were exposed to extremely severe HS conditions as the average Te exceeded the upper limit of the thermoneutral zone (15–30 °C) [6], and the average THI was higher than 25.6 units [30]. As a result of these extreme hot environmental conditions, ewe lambs increased their body heat load, reaching afternoon RT above the normal physiological range (i.e., 38.3–39.9 °C) [22], followed by the activation of thermoregulation mechanisms (i.e., high RR) [10], impaired feedlot performance (i.e., low ADG and feed efficiency) [13], and oxidant-antioxidant imbalance [8]. Heat-stressed hair lambs increase their respiratory evaporation to dissipate the excess body heat load and make metabolic adjustments to decrease endogenous heat production, maintain energy availability in key organs, reduce catabolic activity, and keep growth as a non-priority event [6,12]. Remarkably, there are no reference values established for oxidative stress biomarkers in hair breed sheep, but our values are similar than those previously reported for finishing hair lambs under HS [37,38]. Note that enzymatic antioxidant biomarkers were not measured in the present study, and only CAT results were available, which partially limits the interpretation of some findings. Overall, both average values and variations in the data recorded for physiological, metabolic, and growth variables coincided with those reported for hair lambs experiencing high summer Te in the same study region [12,13,39].
In study, heat-stressed hair lambs modified their oxidative stress balance due to changes related to climatic conditions and growth, as well as physiological and metabolic acclimatization adjustments, which supports the hypothesis initially proposed. Specifically, the changes observed in oxidative stress biomarkers were consistently more related to climatic variables, followed by RR, feed intake, insulin, triglycerides, and total protein. This confirms, along with previous studies [38,40], that environmental HS conditions are one of the main predisposing factors to trigger oxidative stress in hair sheep, even though they are highly thermotolerant [10]. In addition, the findings suggest that our female lambs could have activated cellular mechanisms for ensuring cell survival under extreme HS conditions.
There is little evidence in the literature regarding the degree of relationship between oxidative stress markers and physiological, metabolic, and growth adjustments in sheep of any genotype under HS conditions. Belhadj et al. [17] conducted a meta-analysis of studies on heat-stressed wool sheep and found a strong positive relationship between protein oxidation (i.e., AOPP) and OSI with mean THI and RT but not with RR, which positively correlated with fat oxidation (i.e., MDA). In growing wild Soay ewe lambs, weight gain showed a moderate positive correlation with MDA [19], but not with protein oxidation, superoxide dismutase (SOD), or CAT [41]. In addition, fat oxidation has been negatively correlated with serum insulin and T4 concentrations, likewise positively with SOD, serum insulin, thyroid hormones, and glucose in multiparous ewes raised in a thermoneutral environment [42]. However, a more recent study did not report any relationship between fat and protein oxidation markers with metabolic hormone concentrations in Karayaka sheep exposed to transport and altitude stress for 5 h [43]. Our results, which align with some previously reported findings, showed that both TOC (strong) and TAC (moderate) in heat-stressed hair ewe lambs were primarily associated with fluctuations in Te and THI. Their prooxidant status was also strongly related to increased circulating insulin concentrations and moderately correlated with increased triglyceride levels, as well as reductions in RR, total serum protein, and feed intake. The sheep TAC had the same positive and negative relationships with physiological, metabolic, and growth variables as the TOC; however, only some of them were moderate (i.e., triglycerides, insulin, and feed intake), and the rest were low. This situation led to a persistent oxidant-antioxidant imbalance, resulting in increased oxidation of fat and protein. For its part, growth rate was weakly correlated with increased OSI and reduced TAC, which was not reflected in changes in fat and protein oxidation, as indicated in the literature [19].
While the correlation analysis suggests that fat and protein oxidation status, TOC, TAC, and OSI are largely related to similar climatic, physiological, metabolic, and growth factors, the PC and MLR analyses identified distinct variables associated with each oxidative stress biomarker. This confirms that both direct and indirect factors may be associated with the development of cellular oxidative stress in hair sheep exposed to outdoor HS [8,44]. Thus, most of the individual correlations identified in the Pearson correlation analysis can be related to collinearity. Our regression models validated this supposition by integrating only one or two explanatory variables from the available set to explain the variations observed in each oxidative stress marker. It is essential to note that all equations were based on the assumptions of normality, linearity, independence, and absence of collinearity.
Regression analysis indicated that environmental fluctuations in maximum Te and THI were the primary factors predisposing heat-stressed hair sheep to oxidative stress. Specifically, a reduction in maximum Te was associated with an increase in total prooxidant activity (R2adj partial = 73%) and fat oxidation (R2adj partial = 43%), whereas a decrease in maximum THI enhanced antioxidant activity (R2adj partial = 52%). These findings are partially contrary to others already published, which indicate that heat-stressed sheep exhibit ROS overproduction and elevated TOC and TAC levels as Te increases [5,8]. This suggests the activation of cellular pathways that confer thermotolerance to hair sheep with increasing Te values. Cells from hair sheep exposed to extreme Te (>40 °C) produce five times more HSP-70 protein than cells from sheep breeds intolerant to hot environments [14], whereby they could exhibit reduced oxidation of mitochondrial proteins and membrane lipids [3,14,37]. Therefore, the increased production of heat shock proteins is an advantage to mitigate cellular prooxidant activity under extremely high Te, and this could lead to a negative association between maximum Te and TOC or MDA levels. Conversely, the antioxidant enzymatic activity in sheep subjected to HS depends on the duration and severity of the thermal insult [2,40]. In this context, moderate HS facilitates its activation, whereas it is diminished under chronic and severe HS conditions [40]. This response could explain the negative association between TAC and maximum THI. Therefore, both TOC and TAC of heat-stressed hair sheep are highly dependent on daily extreme weather conditions due to the activation of HSP-linked cellular cytoprotective mechanisms.
According to the TOC equation, feed intake was also a predisposing factor for pro-oxidant status; however, this factor had a minimal contribution compared to maximum Te (R2adj partial = 3 vs. 73%). Similarly, a study conducted in lactating goats showed that high summer Te, rather than pasture-concentrate intake, promoted higher ROS levels [45]. Therefore, the positive association between TOC and feed intake could be due to an accelerated metabolic activity and increased heat production resulting from greater nutrient availability in high-grain diets [7], such as the diet consumed by the lambs in the present study. Another possible cause is the starch content of the diet (62% wheat grain), which could have contributed to a slight decrease in rumen pH. Rumen acidosis is associated with increased prooxidant activity in ruminants [46]. Unfortunately, the mineral, vitamin, and phytochemical contents of the diet were not analyzed, and some of these compounds are exogenous sources of antioxidants. Consequently, it is highly likely that the oxidant-antioxidant balance in female lambs can be influenced, at least slightly, by the diet and is not an exclusive effect of HS.
AOPP is an oxidative stress biomarker that has gained significant relevance in recent years, as oxidized proteins have considerably long half-lives, resulting in a suitable marker of oxidative damage in animals [47]. This study suggests that hair female lambs fattened under an outdoor HS environment exhibit increased protein oxidation primarily by reducing morning RR (R2adj partial = 37%) and, to a lesser extent, by increasing serum insulin (R2adj partial = 5%). The negative association between protein oxidation and morning RR is unclear because previous studies have identified increased pulmonary ventilation as an important factor for ROS overproduction (hydrogen peroxide, H2O2) and increased AOPP concentrations in heat-stressed sheep [17,18]. However, in the study area, hair lambs with higher nocturnal RR tend to have fewer breaths in the morning by activating adaptive heterothermy [6]. Notably, AOPP is recognized as a proinflammatory oxidant product that increases with hyperventilation [7]. Therefore, one plausible explanation could be that lambs experienced acute inflammatory processes in their airways and diminished their antioxidant capacity upon waking, probably due to nocturnal pulmonary hyperventilation. This condition could potentially lead to a negative association between protein oxidation and the morning RR.
In contrast, pancreatic β-cells play a crucial role in regulating energy metabolism in hepatic, lipid, and muscle tissues by synthesizing and releasing insulin. Despite their high metabolic activity, these cells are particularly susceptible to oxidative stress due to their weak antioxidant defenses [48]. Consequently, excessive ROS production can impair the proper functioning of the endoplasmic reticulum in β-cells, which is essential for protein synthesis [49]. This situation suggests that increased protein oxidation associated with insulin release may be linked to the susceptibility of β-cells to oxidative stress.
Ultimately, increased maximum RH and growth rate were identified as predisposing factors for OSI in hair female lambs. This response can be attributed to the fact that, in sheep suffering from HS, elevated RH diminishes the effectiveness of evaporative thermoregulation [50], whereas increased weight gain enhances their cellular metabolic activity [19]. Together, these processes favor a prooxidant state over an antioxidant one. It should be noted that maximum RH and ADG poorly explained the observed variation (17%) in the OSI, suggesting that there are other factors of greater importance associated with the OSI than those included in the present study. Therefore, future studies should incorporate more explanatory variables related to the amplitude of climatic variables, hematological profile, electrolytes, and animal behaviors.
Overall, our results demonstrate that climatic conditions, physiological acclimatization, metabolism, and growth rate define oxidative stress in heat-stressed hair lambs. Thus, implementing strategies to manage these factors may contribute to mitigating ROS overproduction. In outdoor conditions, it is not possible to control the weather; however, the use of some physical tools, such as shades fans, and natural barriers (i.e., trees and shrub belts), may reduce body heat load in sheep and, consequently, the need to activate physiological and metabolic adjustments to thermoregulate [1,22]. Moreover, it has been demonstrated that in thermoneutral [19] and hot conditions (as observed in this study), the accelerated growth rate of feedlot lambs leads to an increase in ROS production. Note that hair sheep breeds maintain their feed intake despite hot conditions [26]. Therefore, in addition to meeting energy and macronutrient requirements, the use of additives such as natural antioxidants, minerals, electrolytes, prebiotics, and probiotics can strengthen the antioxidant defense of hair sheep under HS [2,7,51].
5. Conclusions
Feedlot hair lambs experienced an oxidant-antioxidant imbalance in response to severe summer heat stress in a desert region. This imbalance was primarily associated with climatic and physiological factors, with minor contributions from alterations in energy metabolism and growth performance. Notably, the daily maximum Te and morning RR emerged as the predominant factors associated with prooxidant status, whereas the daily maximum THI was the primary factor influencing antioxidant status. No preponderant factor was fully identified for OSI. Overall, heat-stressed hair lambs exhibited elevated lipid oxidation and TOC as daily maximum Te decreased, along with increased protein oxidation by reducing their morning RR. Furthermore, their TAC was enhanced by a reduction in daily maximum THI values.
Author Contributions
Conceptualization, U.M.-C. and L.A.-R.; methodology, U.M.-C., L.A.-R., M.d.l.Á.L.-B. and J.A.R.-J.; software, R.D.-M. and P.L.-N.; validation, K.M.V.-G. and M.M.; formal analysis, K.M.V.-G. and R.V.-P.; investigation, K.M.V.-G. and M.d.l.Á.L.-B.; data curation, K.M.V.-G. and R.V.-P.; writing—original draft preparation, U.M.-C., K.M.V.-G. and M.d.l.Á.L.-B.; writing—review and editing, L.A.-R., R.V.-P., J.A.R.-J., M.M., C.A.M.-H., R.D.-M. and P.L.-N.; visualization, U.M.-C., M.M. and C.A.M.-H.; supervision, M.d.l.Á.L.-B., U.M.-C. and L.A.-R.; project administration, U.M.-C.; funding acquisition, U.M.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Autonomous University of Baja California within the framework of the 24th Internal Call for Funding of Research Projects (2024–2025; grant number: 200/5/C/47/24). The funding institution merely assessed the relevance, innovation, social contribution, and committed outcomes of the original protocol submitted by the first author, without suggesting any changes.
Institutional Review Board Statement
The managements of sheep were conducted in accordance with the Mexican Official Standard NOM-062-ZOO-1999, and the guidelines established by the Federation of Animal Science Society. The Institutional Ethics Committee of the Autonomous University of Baja California also approved all procedures on 7 February 2024, under Letter Number 047/2024-1.
Informed Consent Statement
No applicable.
Data Availability Statement
The datasets used in this research are available from the corresponding author upon reasonable request.
Acknowledgments
The second author thanks the Mexican Secretariat of Science, Humanities, Technology and Innovation (formerly known as CONACYT) for the maintenance scholarship granted during her Doctoral studies in Agricultural Sciences, where she developed a thesis work using part of the results presented here. All authors also acknowledge Porfirio Nicolás López and Romario Saavedra, graduate students at the Institute of Agricultural Sciences, for their valuable support during the field phase.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Te | Environmental temperature |
| HS | Heat stress |
| ROS | Reactive oxygen species |
| THI | Temperature-humidity index |
| OSI | Oxidative stress index |
| MDA | Malondialdehyde |
| RR | Respiratory rate |
| BW | Body weight |
| DM | Dry matter |
| RH | Relative humidity |
| RT | Rectal temperature |
| BUN | Urea nitrogen |
| T3 | Triiodothyronine hormone |
| T4 | Thyroxine hormone |
| CV | Coefficient of variation |
| AOPP | Advanced oxidation protein products |
| TOC | Total oxidant capacity |
| TAC | Total antioxidant capacity |
| ADG | Average daily gain |
| r | Pearson correlation coefficient |
| PC | Principal components |
| MLR | Multiple linear regression |
| R2 | Coefficient of determination |
| R2adj | Adjusted coefficient of determination |
| RMSE | Root mean square error |
| VIF | Variance inflation factor |
| DW | Durbin-Watson parameter |
| LF | Lack of fit |
References
- Ngcobo, J.N.; Egerszegi, I.; Nephawe, K.A. Recent Advances in Understanding the Impact of Environmental Heat Stress on Sheep Production and Reproductive Performance: A Subtropical Climate Perspective. Climate 2025, 13, 130. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Kiani, A.; Santhiravel, S.; Holman, B.W.B.; Lauridsen, C.; Dunshea, F.R. The Importance of Dietary Antioxidants on Oxidative Stress, Meat and Milk Production, and Their Preservative Aspects in Farm Animals: Antioxidant Action, Animal Health, and Product Quality—Invited Review. Animals 2022, 12, 3279. [Google Scholar] [CrossRef]
- Yaghoobpour, T.; Sheikhi, Z.; Nazifi, S. The Impact of Stress in Domestic Animals: Roles of Heat Shock Proteins and Acute-Phase Proteins. Vet. Res. Commun. 2025, 49, 258. [Google Scholar] [CrossRef] [PubMed]
- Tüfekci, H.; Sejian, V. Stress Factors and Their Effects on Productivity in Sheep. Animals 2023, 13, 2769. [Google Scholar] [CrossRef] [PubMed]
- McManus, C.M.; Lucci, C.M.; Maranhão, A.Q.; Pimentel, D.; Pimentel, F.; Rezende Paiva, S. Response to Heat Stress for Small Ruminants: Physiological and Genetic Aspects. Livest. Sci. 2022, 263, 105028. [Google Scholar] [CrossRef]
- Vicente Pérez, R.; Macías Cruz, U.; Avendaño Reyes, L.; Correa Calderón, A.; López Baca, M.D.l.Á.; Lara Rivera, A.L. Impacto Del Estrés Por Calor En La Producción de Ovinos de Pelo. Revisión. Rev. Mex. Cienc. Pecu. 2020, 11, 205–222. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Rashamol, V.P.; Bagath, M.; Sejian, V.; Dunshea, F.R. Impacts of Heat Stress on Immune Responses and Oxidative Stress in Farm Animals and Nutritional Strategies for Amelioration. Int. J. Biometeorol. 2021, 65, 1231–1244. [Google Scholar] [CrossRef] [PubMed]
- Nicolás-López, P.; Macías-Cruz, U.; Correa-Calderón, A.; Mellado-Bosque, M.; Díaz-Molina, R.; Avendaño-Reyes, L. Ajustes Asociados a La Aclimatación y Estrés Oxidativo En Ovinos Bajo Estrés Calórico: Una Revisión. ITEA-Inf. Tec. Econ. Agrar. 2021, 117, 494–512. [Google Scholar] [CrossRef]
- Wanjala, G.; Bagi, Z.; Gavojdian, D.; Badaoui, B.; Astuti, P.K.; Mizeranschi, A.; Ilisiu, E.; Ohran, H.; Juhas, E.P.; Loukovitis, D.; et al. Genetic Diversity and Adaptability of Native Sheep Breeds from Different Climatic Zones. Sci. Rep. 2025, 15, 14143. [Google Scholar] [CrossRef]
- McManus, C.M.; Faria, D.A.; Lucci, C.M.; Louvandini, H.; Pereira, S.A.; Paiva, S.R. Heat Stress Effects on Sheep: Are Hair Sheep More Heat Resistant? Theriogenology 2020, 155, 157–167. [Google Scholar] [CrossRef]
- Macías-Cruz, U.; Gastélum, M.A.; Álvarez, F.D.; Correa, A.; Díaz, R.; Meza-Herrera, C.A.; Mellado, M.; Avendaño-Reyes, L. Effects of Summer Heat Stress on Physiological Variables, Ovulation and Progesterone Secretion in Pelibuey Ewes under Natural Outdoor Conditions in an Arid Region. Anim. Sci. J. 2016, 87, 354–360. [Google Scholar] [CrossRef]
- Nicolás-López, P.; Macías-Cruz, U.; Mellado, M.; Correa-Calderón, A.; Meza-Herrera, C.A.; Avendaño-Reyes, L. Growth Performance and Changes in Physiological, Metabolic and Hematological Parameters Due to Outdoor Heat Stress in Hair Breed Male Lambs Finished in Feedlot. Int. J. Biometeorol. 2021, 65, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Macías-Cruz, U.; Saavedra, O.R.; Correa-Calderón, A.; Mellado, M.; Torrentera, N.G.; Chay-Canul, A.; López-Baca, M.A.; Avendaño-Reyes, L. Feedlot Growth, Carcass Characteristics and Meat Quality of Hair Breed Male Lambs Exposed to Seasonal Heat Stress (Winter vs. Summer) in an Arid Climate. Meat Sci. 2020, 169, 108202. [Google Scholar] [CrossRef]
- Romero, R.D.; Montero Pardo, A.; Montaldo, H.H.; Rodríguez, A.D.; Hernández Cerón, J. Differences in Body Temperature, Cell Viability, and HSP-70 Concentrations between Pelibuey and Suffolk Sheep under Heat Stress. Trop. Anim. Health Prod. 2013, 45, 1691–1696. [Google Scholar] [CrossRef] [PubMed]
- Valadez-García, K.M.; Avendaño-Reyes, L.; Díaz-Molina, R.; Mellado, M.; Meza-Herrera, C.A.; Correa-Calderón, A.; Macías-Cruz, U. Free Ferulic Acid Supplementation of Heat-Stressed Hair Ewe Lambs: Oxidative Status, Feedlot Performance, Carcass Traits and Meat Quality. Meat Sci. 2021, 173, 108395. [Google Scholar] [CrossRef]
- Akbarian, A.; Michiels, J.; Degroote, J.; Majdeddin, M.; Golian, A.; De Smet, S. Association between Heat Stress and Oxidative Stress in Poultry; Mitochondrial Dysfunction and Dietary Interventions with Phytochemicals. J. Anim. Sci. Biotechnol. 2016, 7, 37. [Google Scholar] [CrossRef]
- Belhadj Slimen, I.; Chniter, M.; Najar, T.; Ghram, A. Meta-Analysis of Some Physiologic, Metabolic and Oxidative Responses of Sheep Exposed to Environmental Heat Stress. Livest. Sci. 2019, 229, 179–187. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Celi, P.; Leury, B.; Liu, F.; Dunshea, F.R. Exhaled Breath Condensate Hydrogen Peroxide Concentration, a Novel Biomarker for Assessment of Oxidative Stress in Sheep during Heat Stress. Anim. Prod. Sci. 2016, 56, 1105–1112. [Google Scholar] [CrossRef]
- Nussey, D.H.; Pemberton, J.M.; Pilkington, J.G.; Blount, J.D. Life History Correlates of Oxidative Damage in a Free-Living Mammal Population. Funct. Ecol. 2009, 23, 809–817. [Google Scholar] [CrossRef]
- Zhang, M.; Dunshea, F.R.; Warner, R.D.; DiGiacomo, K.; Joy, A.; Abhijith, A.; Prathap, P.; Ma, T.; Chauhan, S.S. Short Duration Heatwaves Increase Body Temperature and Alter Blood Gas Balance but May Not Cause Oxidative Stress and Intestinal Structure Variations in Lambs. Small Rumin. Res. 2024, 240, 107367. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; Kumar, P.; Choudhary, P.K.; Srivastava, A.; Nirala, R.K. Study of Oxidative Stress Biomarkers and Physio-Haematological Conditions during Different Seasons in Sonadi Corriedale Sheep. Int. J. Adv. Biochem. Res. 2025, 9, 688–693. [Google Scholar] [CrossRef]
- Al-Dawood, A. Towards Heat Stress Management in Small Ruminants—A Review. Ann. Anim. Sci. 2017, 17, 59–88. [Google Scholar] [CrossRef]
- Theusme, C.; Avendaño-Reyes, L.; Macías-Cruz, U.; Correa-Calderón, A.; García-Cueto, R.O.; Mellado, M.; Vargas-Villamil, L.; Vicente-Pérez, A. Climate Change Vulnerability of Confined Livestock Systems Predicted Using Bioclimatic Indexes in an Arid Region of México. Sci. Total Environ. 2021, 751, 141779. [Google Scholar] [CrossRef]
- NOM-062-ZOO-1999; Technical Specifications for the Production, Care, and Use of Laboratory Animals. Ciudad de México, México. 2001. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 10 September 2025).
- FASS—Federation Animal Science Society. Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching, 3rd ed.; Federation Animal Science Society: Champaign, IL, USA, 2010; p. 177. [Google Scholar]
- NRC. Nutrient Requirements of Small Ruminants; National Academies Press: Washington, DC, USA, 2007; Volume 9, ISBN 978-0-309-10213-1. [Google Scholar]
- AOAC. Official Methods of Analysis, 21st ed.; Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2019; Volume I, p. 700. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Sheep; National Academy of Press: Washington, DC, USA, 1985; ISBN 0309035961. [Google Scholar]
- Marai, I.F.M.; El-Darawany, A.A.; Fadiel, A.; Abdel-Hafez, M.A.M. Physiological Traits as Affected by Heat Stress in Sheep-A Review. Small Rumin. Res. 2007, 71, 1–12. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced Oxidation Protein Products as a Novel Marker of Oxidative Stress in Uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Khoa, T.N.; Capeillère-Blandin, C.; Nguyen, A.T.; Canteloup, S.; Dayer, J.-M.; Jungers, P.; Drüeke, T.; Descamps-Latscha, B. Advanced Oxidation Protein Products as Novel Mediators of Inflammation and Monocyte Activation in Chronic Renal Failure. J. Immunol. 1998, 161, 2524–2532. [Google Scholar] [CrossRef]
- Erel, O. A New Automated Colorimetric Method for Measuring Total Oxidant Status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Erel, O. A Novel Automated Direct Measurement Method for Total Antioxidant Capacity Using a New Generation, More Stable ABTS Radical Cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Karaagac, L.; Koruk, S.T.; Koruk, I.; Aksoy, N. Decreasing Oxidative Stress in Response to Treatment in Patients with Brucellosis: Could It Be Used to Monitor Treatment? Int. J. Infect. Dis. 2011, 15, e346–e349. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I.T. Discarding Variables in a Principal Component Analysis. II: Real Data. Appl. Stat. 1973, 22, 160–173. [Google Scholar] [CrossRef]
- Valadez-García, K.M.; Avendaño-Reyes, L.; Meza-Herrera, C.A.; Mellado, M.; Díaz-Molina, R.; González-Ríos, H.; Macías-Cruz, U. Ferulic Acid in Animal Feeding: Mechanisms of Action, Productive Benefits, and Future Perspectives in Meat Production. Food Biosci. 2021, 43, 101247. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, Z.; Guo, J.; Wang, W.; Duan, Y.; Hao, X.; Wang, R.; An, X.; Qi, J. Effect of Wheat Bran Feruloyl Oligosaccharides on the Performance, Blood Metabolites, Antioxidant Status and Rumen Fermentation of Lambs. Small Rumin. Res. 2019, 175, 65–71. [Google Scholar] [CrossRef]
- Macías-Cruz, U.; López-Baca, M.A.; Vicente, R.; Mejía, A.; Álvarez, F.D.; Correa-Calderón, A.; Meza-Herrera, C.A.; Mellado, M.; Guerra-Liera, J.E.; Avendaño-Reyes, L. Effects of Seasonal Ambient Heat Stress (Spring vs. Summer) on Physiological and Metabolic Variables in Hair Sheep Located in an Arid Region. Int. J. Biometeorol. 2016, 60, 1279–1286. [Google Scholar] [CrossRef]
- Shi, L.; Xu, Y.; Mao, C.; Wang, Z.; Guo, S.; Jin, X.; Yan, S.; Shi, B. Effects of Heat Stress on Antioxidant Status and Immune Function and Expression of Related Genes in Lambs. Int. J. Biometeorol. 2000, 64, 2093–2104. [Google Scholar] [CrossRef]
- Christensen, L.L.; Selman, C.; Blount, J.D.; Pilkington, J.G.; Watt, K.A.; Pemberton, J.M.; Reid, J.M.; Nussey, D.H. Marker-Dependent Associations among Oxidative Stress, Growth and Survival during Early Life in a Wild Mammal. Proc. Biol. Sci. 2016, 283, 20161407. [Google Scholar] [CrossRef]
- Kandiel, M.M.M.; El-Khaiat, H.M.; Mahmoud, K.G.M. Changes in Some Hematobiochemical and Hormonal Profile in Barki Sheep with Various Reproductive Statuses. Small Rumin. Res. 2016, 136, 87–95. [Google Scholar] [CrossRef]
- Çelik, H.T.; Aslan, F.A.; Altay, D.U.; Kahveci, M.E.; Konanç, K.; Noyan, T.; Ayhan, S. Effects of Transport and Altitude on Hormones and Oxidative Stress Parameters in Sheep. PLoS ONE 2021, 16, e0244911. [Google Scholar] [CrossRef]
- Celi, P. Biomarkers of Oxidative Stress in Ruminant Medicine. Immunopharmacol. Immunotoxicol. 2011, 33, 233–240. [Google Scholar] [CrossRef]
- Di Trana, A.; Celi, P.; Claps, S.; Fedele, V.; Rubino, R. The Effect of Hot Season and Nutrition on the Oxidative Status and Metabolic Profile in Dairy Goats during Mid Lactation. Anim. Sci. 2006, 82, 717–722. [Google Scholar] [CrossRef]
- Bezerra, H.V.A.; Gallo, S.B.; Rosa, A.F.; Fernandes, A.C.; e Silva, S.d.L.; Leme, P.R. Impact of Purified Lignin on Performance, Rumen Health, Oxidative Stress Control and Meat Quality of Lambs Fed a High-Concentrate Diet. Livest. Sci. 2020, 231, 103882. [Google Scholar] [CrossRef]
- Celi, P.; Gabai, G. Oxidant/Antioxidant Balance in Animal Nutrition and Health: The Role of Protein Oxidation. Front. Vet. Sci. 2015, 2, 48. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Abad-Jiménez, Z.; Martínez de Marañón, A.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.S.; Kaufman, R.J. Endoplasmic Reticulum Stress and Oxidative Stress in Cell Fate Decision and Human Disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef]
- Collier, R.J.; Baumgard, L.H.; Zimbelman, R.B.; Xiao, Y. Heat Stress: Physiology of Acclimation and Adaptation. Anim. Front 2019, 9, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Rebez, E.B.; Sejian, V.; Silpa, M.V.; Kalaignazhal, G.; Devaraj, C.; Nikhil, K.T.; Ninan, J.; Tüfekci, H.; de Franca Carvalho Fonsêca, V.; Chauhan, S.S.; et al. Feed Additives Supplementation: A Potential Strategy to Ameliorate Heat Stress in Sheep—A Review. Ann. Anim. Sci. 2025, 25, 845–864. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).