Abdominal Surgery Performed in Awake Patients Under Neuraxial Anesthesia: A Systematic Review Across Surgical Specialties
Abstract
1. Introduction
2. Materials and Methods
2.1. Objectives
2.2. Protocol and Registration
2.3. Eligibility Criteria
2.4. Information Sources
2.5. Search
- -
- awake AND surgery NOT neurosur*
- -
- neuraxial AND minimally AND invasive
- -
- neuraxial AND surgery NOT neurosur*
- -
- neuraxial AND colect*neuraxial AND abdom*
- -
- (non-intubated) AND surgery NOT neurosurg*
- -
- neuraxial AND laparosc*
- -
- thoracic spinal-epidural anesthesia
- -
- spinal AND abdom* AND surgery
- -
- ((laparosc*) AND (conscious)) AND (sedation)
2.6. Study Selection
2.7. Data Extraction
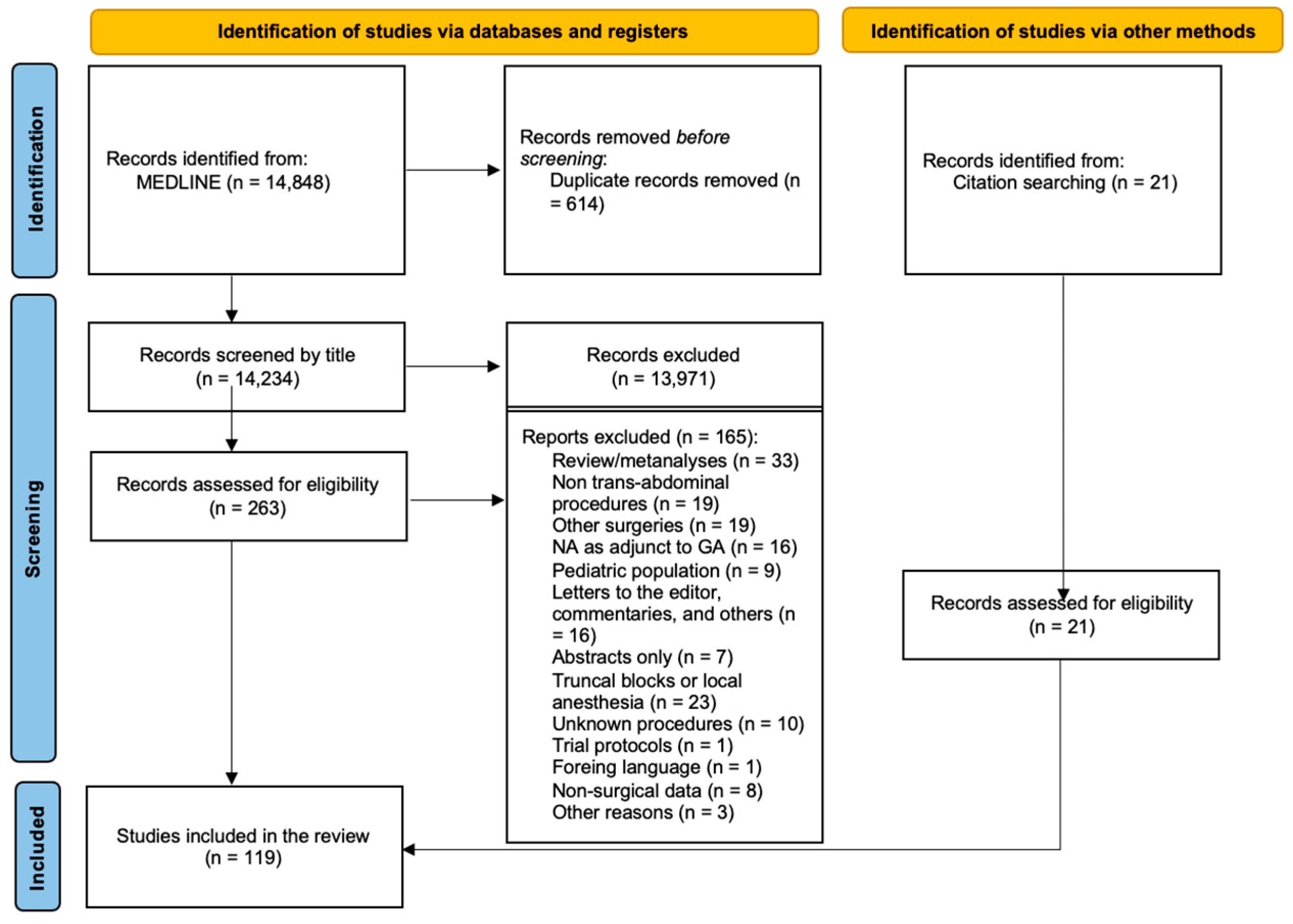
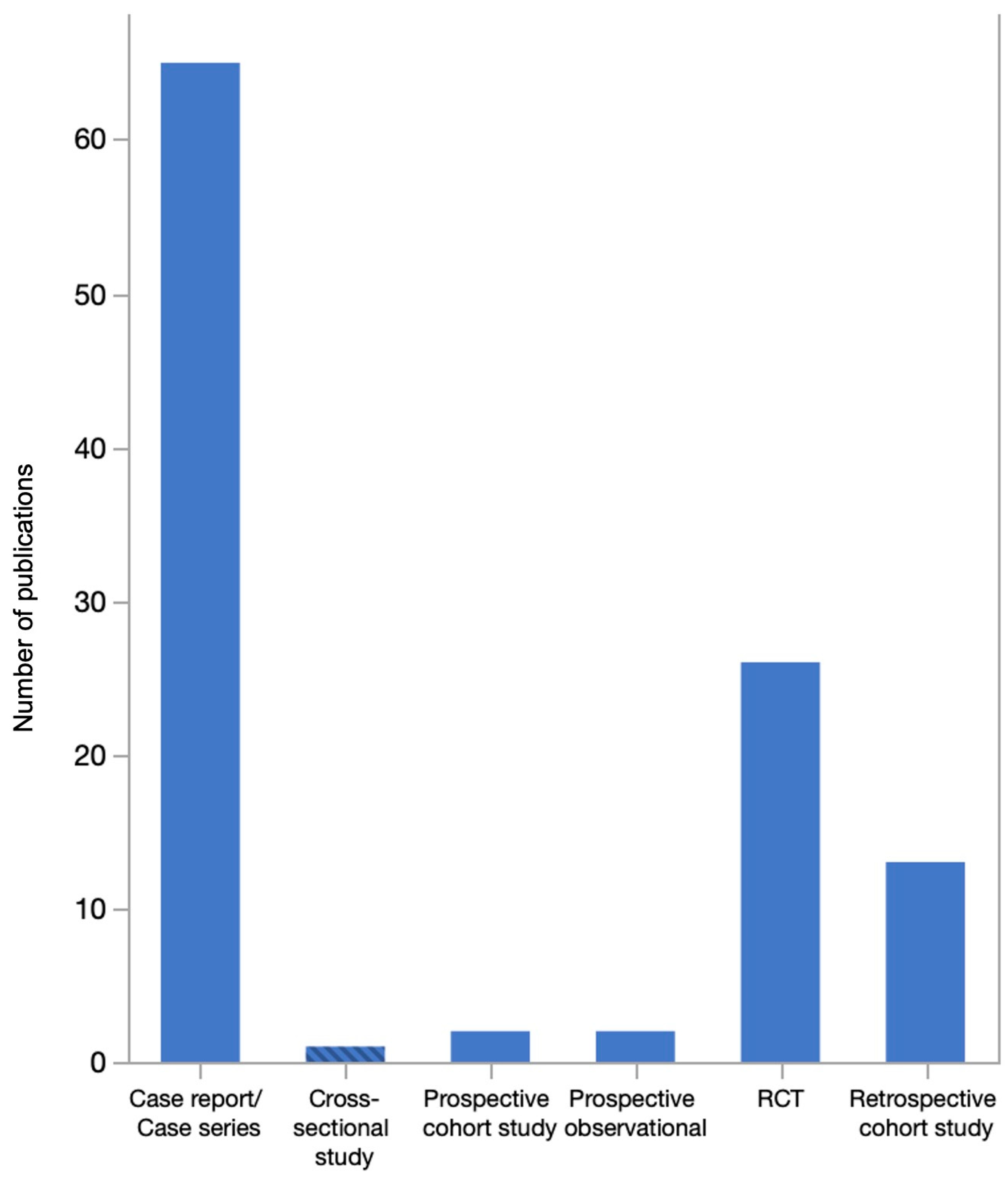
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mandabach, M.G. The early history of spinal anesthesia. Int. Congr. Ser. 2002, 1242, 163–168. [Google Scholar] [CrossRef]
- Corning, J.L. Spinal anaesthesia and local medication of the cord. N. Y. Med. J. 1885, 42, 483–485. [Google Scholar]
- Robinson, D.H.; Toledo, A.H. Historical Development of Modern Anesthesia. J. Investig. Surg. 2012, 25, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Gulur, P.; Nishimori, M.; Ballantyne, J.C. Regional anaesthesia versus general anaesthesia, morbidity and mortality. Best Pract. Res. Clin. Anaesthesiol. 2006, 20, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Tsui, B.C.H.; Wagner, A.; Finucane, B. Regional Anaesthesia in the Elderly: A Clinical Guide. Drugs Aging 2004, 21, 895–910. [Google Scholar] [CrossRef]
- Li, Y.-W.; Li, H.-J.; Li, H.-J.; Zhao, B.-J.; Guo, X.-Y.; Feng, Y.; Zuo, M.-Z.; Yu, Y.-P.; Kong, H.; Zhao, Y.; et al. Delirium in Older Patients after Combined Epidural–General Anesthesia or General Anesthesia for Major Surgery: A Randomized Trial. Anesthesiology 2021, 135, 218–232. [Google Scholar] [CrossRef]
- Hotta, K. Regional anesthesia in the time of COVID-19: A minireview. J. Anesth. 2021, 35, 341–344. [Google Scholar] [CrossRef]
- Uppal, V.; Sondekoppam, R.V.; Landau, R.; El-Boghdadly, K.; Narouze, S.; Kalagara, H.K.P. Neuraxial anaesthesia and peripheral nerve blocks during the COVID-19 pandemic: A literature review and practice recommendations. Anaesthesia 2020, 75, 1350–1363. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Stanley, L.L. Spinal anesthesia in upper abdominal surgery. Calif. State J. Med. 1919, 17, 183–184. [Google Scholar]
- Khan, K.T.; Hemati, K.; Donovan, A.L. Geriatric Physiology and the Frailty Syndrome. Anesthesiol. Clin. 2019, 37, 453–474. [Google Scholar] [CrossRef]
- Hewitt, J.; Long, S.; Carter, B.; Bach, S.; McCarthy, K.; Clegg, A. The prevalence of frailty and its association with clinical outcomes in general surgery: A systematic review and meta-analysis. Age Ageing 2018, 47, 793–800. [Google Scholar] [CrossRef]
- Bilotta, F.; Gelb, A.W.; Stazi, E.; Titi, L.; Paoloni, F.P.; Rosa, G. Pharmacological perioperative brain neuroprotection: A qualitative review of randomized clinical trials. Br. J. Anaesth. 2013, 110, i113–i120. [Google Scholar] [CrossRef]
- Alba, S.; Fimognari, D.; Crocerossa, F.; Ascalone, L.; Pullano, C.; Chiaravalloti, F.; Chiaradia, F.; Carbonara, U.; Ferro, M.; De Cobelli, O.; et al. Neuraxial anesthesia versus general anesthesia in patients undergoing three-dimensional laparoscopic radical prostatectomy: Preliminary results of a prospective comparative study. Asian J. Urol. 2023, 10, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Tseng, K.S.; Kulkarni, S.; Humphreys, E.B.; Ballentine Carter, H.; Mostwin, J.L.; Partin, A.W.; Han, M.; Wu, C.L. Spinal Anesthesia Does Not Impact Prostate Cancer Recurrence in a Cohort of Men Undergoing Radical Prostatectomy: An Observational Study. Reg. Anesth. Pain Med. 2014, 39, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, S.S.; Kaur, J.; Singh, A. A comparative evaluation of epidural and general anaesthetic technique for renal surgeries: A randomised prospective study. Indian J. Anaesth. 2014, 58, 410. [Google Scholar] [CrossRef] [PubMed]
- Friedrich-Freksa, M.; Schulz, E.; Nitzke, T.; Wenzel, O.; Popken, G. Cystectomy and urinary diversion in the treatment of bladder cancer without artificial respiration. Int. Braz. J. Urol. 2012, 38, 645–651. [Google Scholar] [CrossRef][Green Version]
- Piana, A.; Chiaravalloti, F.; Chiaradia, F.; Greco, A.; Lauria, J.; Zappalà, G.; Cappa, M.; Pagliarulo, V.; Pullano, C.; Checcucci, E.; et al. A New Concept in Minimally Invasive Surgical Treatment in Renal Cancer: The Use of Neuroaxial Anesthesia During Laparoscopic Partial Nephrectomy. Eur. Urol. Open Sci. 2023, 57, 16–21. [Google Scholar] [CrossRef]
- Sprung, J.; Scavonetto, F.; Yeoh, T.Y.; Kramer, J.M.; Karnes, R.J.; Eisenach, J.H.; Schroeder, D.R.; Weingarten, T.N. Outcomes After Radical Prostatectomy for Cancer: A Comparison Between General Anesthesia and Epidural Anesthesia with Fentanyl Analgesia A Matched Cohort Study. Anesth. Analg. 2014, 119, 859–866. [Google Scholar] [CrossRef]
- Castellani, D.; Starnari, R.; Faloia, L.; Stronati, M.; Venezia, A.; Gasparri, L.; Claudini, R.; Branchi, A.; Giampieri, M.; Dellabella, M. Radical cystectomy in frail octogenarians in thoracic continuous spinal anesthesia and analgesia: A pilot study. Ther. Adv. Urol. 2018, 10, 343–349. [Google Scholar] [CrossRef]
- Kofler, O.; Prueckner, S.; Weninger, E.; Tomasi, R.; Karl, A.; Niedermayer, S.; Jovanovic, A.; Müller, H.H.; Stief, C.; Zwissler, B.; et al. Anesthesia for Open Radical Retropubic Prostatectomy: A Comparison between Combined Spinal Epidural Anesthesia and Combined General Epidural Anesthesia. Prostate Cancer 2019, 2019, 4921620. [Google Scholar] [CrossRef] [PubMed]
- Ehdaie, B.; Sjoberg, D.D.; Dalecki, P.H.; Scardino, P.T.; Eastham, J.A.; Amar, D. Association of anesthesia technique for radical prostatectomy with biochemical recurrence: A retrospective cohort study. Can. J. Anesth./J. Can. Anesth. 2014, 61, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, A.J.; Tucker, V.; Gibbs, P. Awake Renal Transplantation; A Realistic Alternative to General Anesthesia. Transplant. Proc. 2010, 42, 1677–1678. [Google Scholar] [CrossRef] [PubMed]
- Karl, A.; Schneevoigt, B.; Weninger, E.; Grimm, T.; Stief, C. Feasibility of radical cystectomy in exclusive spinal and/or epidural anaesthesia. World J. Urol. 2013, 31, 1279–1284. [Google Scholar] [CrossRef]
- Salonia, A.; Crescenti, A.; Suardi, N.; Memmo, A.; Naspro, R.; Bocciardi, A.M.; Colombo, R.; Da Pozzo, L.F.; Rigatti, P.; Montorsi, F. General versus spinal anesthesia in patients undergoing radical retropubic prostatectomy: Results of a prospective, randomized study. Urology 2004, 64, 95–100. [Google Scholar] [CrossRef]
- Bhosale, G.; Shah, V. Combined Spinal-Epidural Anesthesia for Renal Transplantation. Transplant. Proc. 2008, 40, 1122–1124. [Google Scholar] [CrossRef]
- Soltan, W.A.; Fathy, E.; Khattab, M.; Mostafa, M.S.; Hasan, H.; Refaat, A.; Eltantawy, M.A.M.; Ziada, H.F.M.; Sarhan, M.D. Combined Thoracic Spinal-Epidural Anesthesia for Laparoscopic Sleeve Gastrectomy; One Hundred Case Experience. Obes. Surg. 2022, 32, 457–462. [Google Scholar] [CrossRef]
- El Fawal, M.H.; Mohammed, D.A.; Abou-Abbass, H.; Abbas, M.; Tamim, H.; Kanawati, S. Laparoscopic Sleeve Gastrectomy under Awake Paravertebral Blockade Versus General Anesthesia: Comparison of Short-Term Outcomes. Obes. Surg. 2021, 31, 1921–1928. [Google Scholar] [CrossRef]
- Scimia, P.; Sciamanna, P.; D’Agostino, M.L.; Venturoni, F.; Sepolvere, G.; Starnari, R. Neuraxial anesthesia for open gastrectomy: When the benefits outweigh the risks. Minerva Anestesiol. 2024, 90, 584–586. [Google Scholar] [CrossRef]
- Chandra, R.; Misra, G.; Datta, G. Thoracic Spinal Anesthesia for Laparoscopic Cholecystectomy: An Observational Feasibility Study. Cureus 2023, 15, e36617. [Google Scholar] [CrossRef]
- Agrawala, M.; Verma, A.; Kang, L. Thoracic epidural anesthesia for laparoscopic cholecystectomy using either bupivacaine or a mixture of bupivacaine and clonidine: A comparative clinical study. Anesth. Essays Res. 2013, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Dar, M.; Sharma, S.; Mehta, K. Thoracic combined spinal epidural anesthesia for laparoscopic cholecystectomy: A feasibility study. J. Anaesthesiol. Clin. Pharmacol. 2016, 32, 224. [Google Scholar] [CrossRef]
- Mehta, N.; Gupta, K.; Sharma, S.; Dar, M. Thoracic combined spinal epidural anesthesia in patient of dilated cardiomyopathy undergoing laparoscopic cholecystectomy. J. Anaesthesiol. Clin. Pharmacol. 2016, 32, 269. [Google Scholar] [CrossRef]
- Reidwan Dar, M.; Mehta, N.; Gupta, S.; Sharma, A. Thoracic combined spinal epidural anesthesia for laparoscopic cholecystectomy in a geriatric patient with ischemic heart disease and renal insufficiency. Local Reg. Anesth. 2015, 8, 101–104. [Google Scholar] [CrossRef]
- Daszkiewicz, A.; Copik, M.; Misiolek, H. Thoracic combined spinal-epidural anesthesia for laparoscopic cholecystectomy in an obese patient with asthma and multiple drug allergies: A case report. Innov. Surg. Sci. 2016, 1, 105–108. [Google Scholar] [CrossRef]
- Gautam, B. Spinal anaesthesia for laparoscopic cholecystectomy: A feasibility and safety study. Kathmandu Univ. Med. J. 2009, 7, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Gautam, B.; Baral, B. Spinal Anaesthesia for Laparoscopic Cholecystectomy in Parkinson’s Disease. JNMA J. Nepal Med. Assoc. 2018, 56, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Kar, M.; Kar, J.; Debnath, B. Experience of laparoscopic cholecystectomy under spinal anesthesia with low-pressure pneumoperitoneum—Prospective study of 300 cases. Saudi J. Gastroenterol. 2011, 17, 203. [Google Scholar] [CrossRef]
- Vincenzi, P.; Stronati, M.; Garelli, P.; Gaudenzi, D.; Boccoli, G.; Starnari, R. Segmental Thoracic Spinal Anesthesia for Laparoscopic Cholecystectomy with the “Hypobaric” Technique: A Case Series. Local Reg. Anesth. 2023, 16, 31–40. [Google Scholar] [CrossRef]
- Mazzone, C.; Sofia, M.; Sarvà, I.; Litrico, G.; Di Stefano, A.M.L.; La Greca, G.; Latteri, S. Awake laparoscopic cholecystectomy: A case report and review of literature. World J. Clin. Cases 2023, 11, 3002–3009. [Google Scholar] [CrossRef]
- Aissaoui, Y.; Bahi, M.; El Khader, A.; El Barni, R.; Belhadj, A. Thoracic spinal anaesthesia for abdominal surgery in a humanitarian military field hospital: A prospective observational study. BMJ Mil. Health 2024, 170, 26–30. [Google Scholar] [CrossRef]
- Aljuba, Y.M.; Amro, A.M.; Alkadi, A.T.; Taamrah, H.; Hamamdh, M.G. Thoracic Segmental Spinal Anesthesia for Emergency Cholecystectomy: A Case Report. Cureus 2022, 14, e30184. [Google Scholar] [CrossRef]
- Saini, H.; Angral, R.; Sharma, S.; Sharma, R.; Kumar, R. Comparision of dexmedetomidine and propofol in patients undergoing laparoscopic cholecystectomy under spinal anesthesia. Anesth. Essays Res. 2020, 14, 194. [Google Scholar] [CrossRef]
- Ross, S.B.; Christodoulou, M.; Ross, N.; Sucandy, I.; Lubrice, K.; Saravanan, S.; Rosemurgy, A. Epidural versus general anesthesia for laparo-endoscopic single-site cholecystectomy: A randomized controlled trial. Surg. Endosc. 2024, 38, 1414–1421. [Google Scholar] [CrossRef]
- Arati, S.; Ashutosh, N. Comparative analisys of spinal vs general anaesthesia for laparoscopic cholecystectomy: A prospective randomized study. Internet J. Anesthesiol. 2009, 24. Available online: https://api.semanticscholar.org/CorpusID:55677029 (accessed on 30 June 2024).
- Bessa, S.S.; Katri, K.M.; Abdel-Salam, W.N.; El-Kayal, E.-S.A.; Tawfik, T.A. Spinal Versus General Anesthesia for Day-Case Laparoscopic Cholecystectomy: A Prospective Randomized Study. J. Laparoendosc. Adv. Surg. Tech. 2012, 22, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Bessa, S.S.; El-Sayes, I.A.; El-Saiedi, M.K.; Abdel-Baki, N.A.; Abdel-Maksoud, M.M. Laparoscopic Cholecystectomy Under Spinal Versus General Anesthesia: A Prospective, Randomized Study. J. Laparoendosc. Adv. Surg. Tech. 2010, 20, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Ellakany, M. Comparative study between general and thoracic spinal anesthesia for laparoscopic cholecystectomy. Egypt. J. Anaesth. 2013, 29, 375–381. [Google Scholar] [CrossRef]
- Imbelloni, L.E.; Fornasari, M.; Fialho, J.C.; Sant’Anna, R.; Cordeiro, J.A. General Anesthesia versus Spinal Anesthesia for Laparoscopic Cholecystectomy. Braz. J. Anesthesiol. 2010, 60, 217–227. [Google Scholar] [CrossRef]
- Kalaivani, V.; Pujari, V.S.; Sreevathsa, M.R.; Hiremath, B.V.; Bevinaguddaiah, Y. Laparoscopic Cholecystectomy Under Spinal Anaesthesia vs. General Anaesthesia: A Prospective Randomised Study. J. Clin. Diagn. Res. 2014, 8, NC01–NC04. [Google Scholar] [CrossRef]
- Mehta, P.; Chavda, H.; Wadhwana, A.; Porecha, M. Comparative analysis of spinal versus general anesthesia for laparoscopic cholecystectomy: A controlled, prospective, randomized trial. Anesth. Essays Res. 2010, 4, 91. [Google Scholar] [CrossRef]
- Ross, S.B.; Mangar, D.; Karlnoski, R.; Camporesi, E.; Downes, K.; Luberice, K.; Haines, K.; Rosemurgy, A.S. Laparo-endoscopic single-site (LESS) cholecystectomy with epidural vs. general anesthesia. Surg. Endosc. 2013, 27, 1810–1819. [Google Scholar] [CrossRef]
- Tiwari, S.; Chauhan, A.; Chaterjee, P.; Alam, M. Laparoscopic cholecystectomy under spinal anaesthesia: A prospective, randomised study. J. Minimal Access Surg. 2013, 9, 65. [Google Scholar] [CrossRef]
- Turkstani, A.; Ibraheim, O.A.; Khairy, G.A.; Alseif, A.; Khalil, N.; Arab; Anesth, B. Spinal versus general anesthesia for laparoscopic cholecystectomy: A comparative study of cost effectiveness and side effects. Anaesth. Pain Intensive Care 2019, 13, 9–14. [Google Scholar]
- Tzovaras, G.; Fafoulakis, F.; Pratsas, K.; Georgopoulou, S.; Stamatiou, G.; Hatzitheofilou, C. Spinal vs general anesthesia for laparoscopic cholecystectomy: Interim analysis of a controlled randomized trial. Arch. Surg. 2008, 143, 497–501. [Google Scholar] [CrossRef]
- Gramatica, L.; Brasesco, O.E.; Mercado Luna, A.; Martinessi, V.; Panebianco, G.; Labaque, F.; Rosin, D.; Rosenthal, R.J.; Gramatica, L. Laparoscopic cholecystectomy performed under regional anesthesia in patients with chronic obstructive pulmonary disease. Surg. Endosc. 2002, 16, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Gurwara, A.K.; Gupta, S.C. Laparoscopic Cholecystectomy Under Spinal Anesthesia: A Study of 3492 Patients. J. Laparoendosc. Adv. Surg. Tech. 2009, 19, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Donmez, T.; Erdem, V.M.; Uzman, S.; Yildirim, D.; Avaroglu, H.; Ferahman, S.; Sunamak, O. Laparoscopic cholecystectomy under spinal-epidural anesthesia vs general anaesthesia: A prospective randomised study. Ann. Surg. Treat. Res. 2017, 92, 136. [Google Scholar] [CrossRef]
- Longo, M.A.; Cavalheiro, B.T.; De Oliveira Filho, G.R. Laparoscopic cholecystectomy under neuraxial anesthesia compared with general anesthesia: Systematic review and meta-analyses. J. Clin. Anesth. 2017, 41, 48–54. [Google Scholar] [CrossRef]
- Papagni, V.; Piacente, C.; Varvara, M.; Vincenti, L. Unexpected duodenopancreatectomy in an “awake” gastrectomized patient: Case report and technical notes. Int. J. Surg. Case Rep. 2021, 81, 105781. [Google Scholar] [CrossRef]
- Elzohry, A.A.M.; Hegab, A.S.; Khalifa, O.Y.A.; Elhossieny, K.M.; Abdel Hameed, F.A.Z.H. Safety and Efficacy of Ultrasound-Guided Combined Segmental Thoracic Spinal Epidural Anesthesia in Abdominal Surgeries and Laparoscopic Procedures: A Prospective Randomized Clinical Study. Anesth. Pain Med. 2024, 13, e138825. [Google Scholar] [CrossRef]
- Graham, R.R.; Brown, W.E. Spinal anesthesia in abdominal surgery. Ann. Surg. 1939, 110, 863–871. [Google Scholar] [CrossRef]
- Rocca, A.; Porfidia, C.; Russo, R.; Tamburrino, A.; Avella, P.; Vaschetti, R.; Bianco, P.; Calise, F. Neuraxial anesthesia in hepato-pancreatic-bilio surgery: A first western pilot study of 46 patients. Updates Surg. 2023, 75, 481–491. [Google Scholar] [CrossRef]
- Delvecchio, A.; Pavone, G.; Conticchio, M.; Piacente, C.; Varvara, M.; Ferraro, V.; Stasi, M.; Casella, A.; Filippo, R.; Tedeschi, M.; et al. Awake robotic liver surgery: A case report. World J. Gastrointest. Surg. 2023, 15, 2954–2961. [Google Scholar] [CrossRef] [PubMed]
- Gontero, P.; Oderda, M.; Calleris, G.; Allasia, M.; Balagna, R.; Gobbi, F. Awake Da Vinci robotic partial nephrectomy: First case report ever in a situation of need. Urol. Case Rep. 2022, 42, 102008. [Google Scholar] [CrossRef] [PubMed]
- Uzman, S.; Donmez, T.; Erdem, V.M.; Hut, A.; Yildirim, D.; Akinci, M. Combined spinal-epidural anesthesia in laparoscopic appendectomy: A prospective feasibility study. Ann. Surg. Treat. Res. 2017, 92, 208. [Google Scholar] [CrossRef] [PubMed]
- El Moheb, M.; Han, K.; Breen, K.; El Hechi, M.; Jia, Z.; Mokhtari, A.; Kongkaewpaisan, N.; Kongwibulwut, M.; Rodriguez, G.; Ortega, C.; et al. General Versus Neuraxial Anesthesia for Appendectomy: A Multicenter International Study. World J. Surg. 2021, 45, 3295–3301. [Google Scholar] [CrossRef]
- Mane, R.; Patil, M.; Kedareshvara, K.; Sanikop, C. Combined spinal epidural anesthesia for laparoscopic appendectomy in adults: A case series. Saudi J. Anaesth. 2012, 6, 27. [Google Scholar] [CrossRef]
- De Cassai, A.; Bertoncello, F.; Correale, C.; Sandei, L. Spinal anesthesia is a viable option for emergent laparoscopic procedure in high-risk patients. Saudi J. Anaesth. 2020, 14, 115. [Google Scholar] [CrossRef]
- Germanò, P.; Siboni, S.; Milito, P.; Mautone, G.; Resta, M.; Bonavina, L. Ventral hernia repair under neuraxial anesthesia. Eur. Surg. 2022, 54, 54–58. [Google Scholar] [CrossRef]
- Thalji, M.; Tarayrah, R.; Ruzaygat, A.; Motawe, D.; Ibedo, F. Classic incisional hernia repair under awake thoracic combined spinal -epidural anesthesia in a geriatric patient with multiple co-morbidities. Int. J. Surg. Case Rep. 2024, 119, 109744. [Google Scholar] [CrossRef]
- Ali, Y.; Elmasry, M.N.; Negmi, H.; Al Ouffi, H.; Fahad, B.; Rahman, S.A. The feasibility of spinal anesthesia with sedation for laparoscopic general abdominal procedures in moderate risk patients. Middle East J. Anesthesiol. 2008, 19, 1027–1039. [Google Scholar]
- Janež, J.; Preskar, J.; Avguštin, M.; Štor, Z. Surgical repair of a large ventral hernia under spinal anaesthesia: A case report. Ann. Med. Surg. 2019, 40, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, H.; Liu, D.; Pan, X.; Wu, W.; Hu, Z.; Zhang, H. Non-intubated laparoscopic repair of giant Morgagni’s hernia for a young man. J. Thorac. Dis. 2016, 8, E698–E701. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harold, K.H.; Webster, M. Spinal Anesthesia for Emergent Abdominal Surgery in a Patient with a Tricuspid Valvectomy: A Case Report. AA Pract. 2018, 10, 185–187. [Google Scholar] [CrossRef]
- Sarakatsianou, C.; Baloyiannis, I.; Perivoliotis, K.; Georgopoulou, S.; Tzovaras, G. Quality of life after laparoscopic trans-abdominal pre-peritoneal inguinal hernia repair: Spinal vs general anesthesia. Hernia 2021, 25, 789–796. [Google Scholar] [CrossRef]
- Sada, F.; Kavaja, F.; Hamza, A.; Ukperaj, B.M. A 74-Year-Old Man with Severe Comorbidities and Successful Abdominal Aortic Aneurysm Repair with Thoracic Segmental Spinal Anesthesia: A Case Report. Am. J. Case Rep. 2024, 25, e943702. [Google Scholar] [CrossRef]
- Berardi, G.; Ferrero, E.; Fadde, M.; Lojacono, N.; Ferri, M.; Viazzo, A.; Gaggiano, A.; Bianchi, A.; Maggio, D.; Ganzaroli, M.; et al. Combined spinal and epidural anesthesia for open abdominal aortic aneurysm surgery in vigil patients with severe chronic obstructive pulmonary disease ineligible for endovascular aneurysm repair. Analysis of results and description of the technique. Int. Angiol. 2010, 29, 278–283. [Google Scholar] [PubMed]
- Flores, J.A.; Nishibe, T.; Koyama, M.; Imai, T.; Kudo, F.; Miyazaki, K.; Yasuda, K. Combined spinal and epidural anesthesia for abdominal aortic aneurysm surgery in patients with severe chronic pulmonary obstructive disease. Int. Angiol. 2002, 21, 218–221. [Google Scholar]
- Meecham, L.; Torrance, A.; Vijay, S.; Burtenshaw, A.; Downing, R. Open Abdominal Aortic Aneurysm Replacement in the Awake Patient. Int. J. Angiol. 2015, 26, 064–067. [Google Scholar] [CrossRef][Green Version]
- Toledano, R.D.; Leffert, L. What’s New in Neuraxial Labor Analgesia. Curr. Anesthesiol. Rep. 2021, 11, 340–347. [Google Scholar] [CrossRef]
- Al-Husinat, L.; Barletta, F.; Gammaldi, V.; Alsabbah, A.; Gammaldi, D. Double neuraxial catheter (Subarachnoid and epidural) in obese patient cancer surgery: A case report. Ann. Med. Surg. 2022, 81, 104446. [Google Scholar] [CrossRef]
- Holyachi, R.; Patil, B.; Karigar, S.L. Anesthetic management of a patient with bicuspid aortic valve and Hashimoto’s thyroiditis posted for abdominal hysterectomy. Indian J. Med. Sci. 2012, 66, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Ghirardini, G.; Baraldi, R.; Bertellini, C.; Bertoli, C.; Bianchini, A.; Castigliani, G.P.; Pellegrino, A.; Capelli, E.; Canova, S. Advantages of spinal anesthesia in abdominal gynecologic surgery. Clin. Exp. Obstet. Gynecol. 1998, 25, 105–106. [Google Scholar] [PubMed]
- de Carli, D.; Meletti, J.F.A.; Camargo, R.P.S.D.; Gratacós, L.S.; Gomes, V.C.R.; Marques, N.D. Effect of anesthetic technique on the quality of anesthesia recovery for abdominal histerectomy: A cross-observational study. Braz. J. Anesthesiol. (Engl. Ed.) 2021, 71, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Gautam, B.; Tabdar, S.; Shrestha, U. Comparison of Fentanyl and Dexmedetomidine as Intrathecal Adjuvants to Spinal Anaesthesia for Abdominal Hysterectomy. J. Nepal Med. Assoc. 2018, 56, 848–855. [Google Scholar] [CrossRef]
- Major, A.L.; Jumaniyazov, K.; Jabbarov, R.; Razzaghi, M.; Mayboroda, I. Gynecological Laparoscopic Surgeries under Spinal Anesthesia: Benefits and Challenges. J. Pers. Med. 2024, 14, 633. [Google Scholar] [CrossRef]
- Massicotte, L.; Chalaoui, K.D.; Beaulieu, D.; Roy, J.-D.; Bissonnette, F. Comparison of spinal anesthesia with general anesthesia on morphine requirement after abdominal hysterectomy. Acta Anaesthesiol. Scand. 2009, 53, 641–647. [Google Scholar] [CrossRef]
- Lal, M.; Singh, S.; Gupta, A.; Rao, B. Combined spinal and epidural anaesthesia for abdominal surgery: A new technique. Med. J. Armed Forces India 1996, 52, 166–168. [Google Scholar] [CrossRef]
- Lin, L.; Liu, C.; Tan, H.; Ouyang, H.; Zhang, Y.; Zeng, W. Anaesthetic technique may affect prognosis for ovarian serous adenocarcinoma: A retrospective analysis. Br. J. Anaesth. 2011, 106, 814–822. [Google Scholar] [CrossRef]
- Giampaolino, P.; Della Corte, L.; Di Spiezio Sardo, A.; Zizolfi, B.; Manzi, A.; De Angelis, C.; Bifulco, G.; Carugno, J. Emergent Laparoscopic Removal of a Perforating Intrauterine Device During Pregnancy Under Regional Anesthesia. J. Minim. Invasive Gynecol. 2019, 26, 1013–1014. [Google Scholar] [CrossRef]
- Chauvet, P.; Storme, B.; Bonnin, M.; Legros, M.; Pinot, A.; Canis, M.; Bourdel, N. Laparoscopic adnexectomy under regional anaesthesia: It is possible! J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101803. [Google Scholar] [CrossRef] [PubMed]
- Asgari, Z.; Rezaeinejad, M.; Hosseini, R.; Nataj, M.; Razavi, M.; Sepidarkish, M. Spinal Anesthesia and Spinal Anesthesia with Subdiaphragmatic Lidocaine in Shoulder Pain Reduction for Gynecological Laparoscopic Surgery: A Randomized Clinical Trial. Pain Res. Manag. 2017, 2017, 1721460. [Google Scholar] [CrossRef]
- Pusapati, R.N.; Sivashanmugam, T.; Ravishankar, M. Respiratory changes during spinal anaesthesia for gynaecological laparoscopic surgery. J. Anaesthesiol. Clin. Pharmacol. 2010, 26, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Kontrimaviciute, E.; Baublys, A.; Ivaskevicius, J. Postoperative nausea and vomiting in patients undergoing total abdominal hysterectomy under spinal anaesthesia: A randomized study of ondansetron prophylaxis. Eur. J. Anaesthesiol. 2005, 22, 504–509. [Google Scholar] [CrossRef]
- Borendal Wodlin, N.; Nilsson, L.; Kjølhede, P.; For the GASPI Study Group. The impact of mode of anaesthesia on postoperative recovery from fast-track abdominal hysterectomy: A randomised clinical trial: Fast-track hysterectomy and mode of anaesthesia. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Kim, B.W. Comparison of General Anesthesia and Combined Spinal and Epidural Anesthesia for Gasless Laparoscopic Surgery in Gynecology. JSLS 2022, 26, e2022.00004. [Google Scholar] [CrossRef]
- Moawad, N.S.; Santamaria Flores, E.; Le-Wendling, L.; Sumner, M.T.; Enneking, F.K. Total Laparoscopic Hysterectomy Under Regional Anesthesia. Obstet. Gynecol. 2018, 131, 1008–1010. [Google Scholar] [CrossRef]
- Vailati, D.; Bonvecchio, E.; Secco, G.; Magistro, C.; Basta, B. Neuraxial Anesthesia for Combined Left Nephrectomy and Left Hemicolectomy in a One-Lung Patient. Cureus 2024, 16, e59854. [Google Scholar] [CrossRef]
- Marrone, F.; Fusco, P.; Lepre, L.; Giulii Capponi, M.; Villani, A.; Paventi, S.; Tomei, M.; Starnari, R.; Pullano, C. Neuraxial Anesthesia for an Open Low Anterior Rectal Resection: Tip the Scales in Patient’s Favor. Cureus 2024, 16, e57094. [Google Scholar] [CrossRef]
- Romanzi, A.; Dragani, T.A.; Adorni, A.; Colombo, M.; Farro, A.; Maspero, M.; Zamburlini, B.; Vannelli, A. Neuraxial anesthesia for abdominal surgery, beyond the pandemic: A feasibility pilot study of 70 patients in a suburban hospital. Updates Surg. 2023, 75, 1691–1697. [Google Scholar] [CrossRef]
- Kumar, C.M.; Corbett, W.A.; Wilson, R.G. Spinal anaesthesia with a micro-catheter in high-risk patients undergoing colorectal cancer and other major abdominal surgery. Surg. Oncol. 2008, 17, 73–79. [Google Scholar] [CrossRef]
- Ellakany, M. Thoracic spinal anesthesia is safe for patients undergoing abdominal cancer surgery. Anesth. Essays Res. 2014, 8, 223. [Google Scholar] [CrossRef]
- Romanzi, A.; Boleso, N.; Di Palma, G.; La Regina, D.; Mongelli, F.; Milanesi, M.; Putortì, A.; Rossi, F.; Scolaro, R.; Zanardo, M.; et al. Awake Major Abdominal Surgeries in the COVID-19 Era. Pain Res. Manag. 2021, 2021, 8763429. [Google Scholar] [CrossRef]
- Oshimizu, M.; Yamaguchi, Y.; Tsuboi, S.; Sugawara, Y.; Hayami, H.; Tobias, J.D.; Inagawa, G. Combined Spinal-Epidural Anesthesia for Subtotal Colectomy in a Patient with Hamman Syndrome and Epidural Pneumatosis: A Case Report. AA Pract. 2021, 15, e01511. [Google Scholar] [CrossRef] [PubMed]
- Nimma, S.; Gans, A.; Wardhan, R.; Allen, W. Remimazolam Sedation and Neuraxial Anesthesia in a Patient with Amyotrophic Lateral Sclerosis Undergoing an Open Colectomy: A Case Report. AA Pract. 2023, 17, e01733. [Google Scholar] [CrossRef] [PubMed]
- Romanzi, A.; Galletti, M.; Macchi, L.; Putortì, A.; Rossi, F.; Scolaro, R.; Vannelli, A. Awake laparotomy: Is locoregional anesthesia a functional option for major abdominal surgeries in the COVID-19 era? Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5162–5166. [Google Scholar] [CrossRef]
- Coe, C.; Shuttleworth, P.W.; Rangappa, D.; Abdel-Halim, M. Locoregional Anaesthesia for Laparotomy: A Literature Review and Subsequent Case Series Highlighting the Potential of an Alternative Anaesthetic Technique. Cureus 2023, 15, e45529. [Google Scholar] [CrossRef]
- Romanzi, A.; Moroni, R.; Rongoni, E.; Scolaro, R.; La Regina, D.; Mongelli, F.; Putortì, A.; Rossi, F.; Zanardo, M.; Vannelli, A. The management of “fragile” and suspected COVID-19 surgical patients during pandemic: An Italian single-center experience. Minerva Chir. 2020, 75, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Koltun, W.A.; McKenna, K.J.; Rung, G. Awake epidural anesthesia is effective and safe in the high-risk colectomy patient. Dis. Colon Rectum 1994, 37, 1236–1241. [Google Scholar] [CrossRef]
- Kopacz, D.J. Continuous spinal anaesthesia for abdominal surgery in a patient receiving amiodarone. Can. J. Anaesth. 1991, 38, 341–344. [Google Scholar] [CrossRef][Green Version]
- Kao, Y.-T.; Chang, C.-C.; Yeh, C.-C.; Hu, C.-J.; Cherng, Y.-G.; Chen, T.-L.; Liao, C.-C. Complications and Mortality after Surgeries in Patients with Prior Stroke Who Received General and Neuraxial Anesthesia: A Propensity-Score Matched Study. J. Clin. Med. 2022, 11, 1490. [Google Scholar] [CrossRef]
- Roth, A.F.; Harris, M.J. Combined Spinal-Epidural for Loop Ileostomy in a Patient with End-Stage Amyotrophic Lateral Sclerosis: A Case Report. AA Pract. 2022, 16, e01588. [Google Scholar] [CrossRef]
- Tsuji, H.; Asoh, T.; Takeuchi, Y.; Shirasaka, C. Attenuation of adrenocortical response to upper abdominal surgery with epidural blockade. J. Br. Surg. 1983, 70, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Corda Teixeira, J.S.; Correia, M.J.D.; Haas, A.; Tralhão, A. Continuous spinal anaesthesia for partial gastrectomy in an adult patient with unrepaired tetralogy of Fallot. Cardiol. Young 2019, 29, 845–846. [Google Scholar] [CrossRef] [PubMed]
- Savas, J.F.; Litwack, R.; Davis, K.; Miller, T.A. Regional anesthesia as an alternative to general anesthesia for abdominal surgery in patients with severe pulmonary impairment. Am. J. Surg. 2004, 188, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Spannella, F.; Giulietti, F.; Damiani, E.; Faloia, L.; Stronati, M.; Venezia, A.; Vincenzi, P.; Castellani, D.; Boccoli, G.; Dellabella, M.; et al. Thoracic continuous spinal anesthesia for high-risk comorbid older patients undergoing major abdominal surgery: One-year experience of an Italian geriatric hospital. Minerva Anestesiol 2020, 86, 261–269. [Google Scholar] [CrossRef]
- Vincenzi, P.; Starnari, R.; Faloia, L.; Grifoni, R.; Bucchianeri, R.; Chiodi, L.; Venezia, A.; Stronati, M.; Giampieri, M.; Montalti, R.; et al. Continuous thoracic spinal anesthesia with local anesthetic plus midazolam and ketamine is superior to local anesthetic plus fentanyl in major abdominal surgery. Surg. Open Sci. 2020, 2, 5–11. [Google Scholar] [CrossRef]
- Samuel, H.; Girma, B.; Negash, M.; Muluneh, E. Comparison of spinal versus general anesthesia on the perioperative blood glucose levels in patients undergoing lower abdominal and pelvic surgery: A prospective cohort study, Ethiopia. Ann. Med. Surg. 2023, 85, 849–855. [Google Scholar] [CrossRef]
- Han, Q.; Wu, S.; Chen, H.; Wang, L.; Zhang, C. The choice of anesthesia for acute abdomen surgery patients and its influence on gastrointestinal function recovery. Am. J. Transl. Res. 2021, 13, 9621–9626. [Google Scholar]
- Brill, J.; Stewart, D.E. Fractional spinal anesthesia for short upper abdominal surgery. Calif. Med. 1947, 66, 238–239. [Google Scholar]
- Tran, Q.H.D.; Kaufman, I.; Schricker, T. Spinal anesthesia for a patient with type I sialidosis undergoing abdominal surgery. Acta Anaesthesiol. Scand. 2001, 45, 919–921. [Google Scholar] [CrossRef]
- Stechishin, O. Nupercaine spinal anesthesia for upper abdominal surgery of long duration. Anesthesiology 1949, 10, 494–504. [Google Scholar] [CrossRef]
- Sakamoto, M.; Kano, T.; Nakamura, M.; Higashi, K.; Sadanaga, M.; Morioka, T. Perioperative management of two patients with respiratory problems undergoing abdominal surgery with high spinal anesthesia. J. Anesth. 1993, 7, 108–112. [Google Scholar] [CrossRef]
- Michaloudis, D.; Petrou, A.; Bakos, P.; Chatzimichali, A.; Kafkalaki, K.; Papaioannou, A.; Zeaki, M.; Flossos, A. Continuous spinal anaesthesia/analgesia for the perioperative management of high-risk patients. Eur. J. Anaesthesiol. 2000, 17, 239–247. [Google Scholar] [CrossRef]
- Abd Elrazek, E.; Thornton, M.; Lannigan, A. Effective awake thoracic epidural anesthetic for major abdominal surgery in two high-risk patients with severe pulmonary disease—A case report. Middle East J. Anesthesiol. 2010, 20, 891–895. [Google Scholar]
- Consani, G.; Amorese, G.; Boggi, U.; Comite, C.; Avagliano, E. Laparotomic sub-total gastrectomy under awake thoracic epidural anaesthesia: A successful experience. Updates Surg. 2013, 65, 255–256. [Google Scholar] [CrossRef]
- Karaca, O. Laparotomic gastrostomy under aweake thoracic epidural anaesthesia: A prospering experience. Agri 2018, 30, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, J.J.; Wakabayashi, K.; Jooma, Z. Emergency Awake Abdominal Surgery Under Thoracic Epidural Anaesthesia in a High-Risk Patient Within a Resource-Limited Setting. Cureus 2023, 15, e34856. [Google Scholar] [CrossRef] [PubMed]
- Emyedu, A.; Kyoheirwe, B.; Atumanya, P. Continuous Spinal Anesthesia following Inadvertent Dural Puncture during Epidural Placement for an Emergency Laparotomy. Case Rep. Anesthesiol. 2021, 2021, 8819864. [Google Scholar] [CrossRef] [PubMed]
- Manasra, M.R.; Heih, O.Q.; Adwan, R.F.; Maraqa, M.A. The Use of Thoracic Segmental Spinal Anaesthesia for Thoracoscopic Diaphragmatic Hernia Repair in an Adult with Cardiac Compromise. Cureus 2024, 16, e56029. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Gurwara, A.K.; Gupta, S.C. Laparoscopic surgery using spinal anesthesia. JSLS 2008, 12, 133–138. [Google Scholar]
- Singh, R.K.; Saini, A.M.; Goel, N.; Bisht, D.; Seth, A. Major laparoscopic surgery under regional anesthesia: A prospective feasibility study. Med. J. Armed Forces India 2015, 71, 126–131. [Google Scholar] [CrossRef][Green Version]
- Gerges, F.J.; Kanazi, G.E.; Jabbour-Khoury, S.I. Anesthesia for laparoscopy: A review. J. Clin. Anesth. 2006, 18, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Hahnenkamp, K.; Herroeder, S.; Hollmann, M.W. Regional anaesthesia, local anaesthetics and the surgical stress response. Best Pract. Res. Clin. Anaesthesiol. 2004, 18, 509–527. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, H.; Wetterslev, J.; Møiniche, S.; Dahl, J.B. Epidural local anaesthetics versus opioid-based analgesic regimens for postoperative gastrointestinal paralysis, PONV and pain after abdominal surgery. In Cochrane Database of Systematic Reviews; The Cochrane Collaboration, Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2001; p. CD001893. [Google Scholar] [CrossRef]
- Jørgensen, H.; Fomsgaard, J.S.; Dirks, J.; Wetterslev, J.; Andreasson, B.; Dahl, J.B. Effect of peri- and postoperative epidural anaesthesia on pain and gastrointestinal function after abdominal hysterectomy. Br. J. Anaesth. 2001, 87, 577–583. [Google Scholar] [CrossRef]
- Neuman, M.D.; Feng, R.; Carson, J.L.; Gaskins, L.J.; Dillane, D.; Sessler, D.I.; Sieber, F.; Magaziner, J.; Marcantonio, E.R.; Mehta, S.; et al. Spinal Anesthesia or General Anesthesia for Hip Surgery in Older Adults. N. Engl. J. Med. 2021, 385, 2025–2035. [Google Scholar] [CrossRef]
- Li, T.; Li, J.; Yuan, L.; Wu, J.; Jiang, C.; Daniels, J.; Mehta, R.L.; Wang, M.; Yeung, J.; Jackson, T.; et al. Effect of Regional vs General Anesthesia on Incidence of Postoperative Delirium in Older Patients Undergoing Hip Fracture Surgery: The RAGA Randomized Trial. JAMA 2022, 327, 50. [Google Scholar] [CrossRef]
- Staender, S.; Smith, A. Enhancing the quality and safety of the perioperative patient. Curr. Opin. Anaesthesiol. 2017, 30, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Strodtbeck, W.M.; Richman, J.M.; Wu, C.L. A Comparison of Regional Versus General Anesthesia for Ambulatory Anesthesia: A Meta-Analysis of Randomized Controlled Trials. Anesth. Analg. 2005, 101, 1634–1642. [Google Scholar] [CrossRef]
- Le Roux, J.J.; Wakabayashi, K.; Jooma, Z. Defining the role of thoracic spinal anaesthesia in the 21st century: A narrative review. Br. J. Anaesth. 2023, 130, e56–e65, Correction in Br. J. Anaesth. 2025, 135, 520–521. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, F.; Bauerle, J. Awareness Under Anesthesia. Mo. Med. 2023, 120, 459–463. [Google Scholar]
- Sandhu, K.; Dash, H. Awareness during anaesthesia. Indian J. Anaesth. 2009, 53, 148–157. [Google Scholar]
- Osterman, J.E.; Hopper, J.; Heran, W.J.; Keane, T.M.; Van Der Kolk, B.A. Awareness under anesthesia and the development of posttraumatic stress disorder. Gen. Hosp. Psychiatry 2001, 23, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Magistro, C.; Ferrari, C.; Vailati, D.; Basta, B.; Colasuonno, M.; Tresoldi, M.; Barbaro, S.; Crippa, J. Awareness surgery: Laparoscopic right colectomy in a heart transplant patient—A case report. J. Vis. Surg. 2024, 10, 26. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, C.; Crippa, J.; Floris, P.; Vailati, D.; Basta, B.; Santalucia, R.; Barbaro, S.; Magistro, C. Abdominal Surgery Performed in Awake Patients Under Neuraxial Anesthesia: A Systematic Review Across Surgical Specialties. Int. J. Transl. Med. 2025, 5, 53. https://doi.org/10.3390/ijtm5040053
Ferrari C, Crippa J, Floris P, Vailati D, Basta B, Santalucia R, Barbaro S, Magistro C. Abdominal Surgery Performed in Awake Patients Under Neuraxial Anesthesia: A Systematic Review Across Surgical Specialties. International Journal of Translational Medicine. 2025; 5(4):53. https://doi.org/10.3390/ijtm5040053
Chicago/Turabian StyleFerrari, Carlo, Jacopo Crippa, Paola Floris, Davide Vailati, Benedetta Basta, Roberto Santalucia, Salvatore Barbaro, and Carmelo Magistro. 2025. "Abdominal Surgery Performed in Awake Patients Under Neuraxial Anesthesia: A Systematic Review Across Surgical Specialties" International Journal of Translational Medicine 5, no. 4: 53. https://doi.org/10.3390/ijtm5040053
APA StyleFerrari, C., Crippa, J., Floris, P., Vailati, D., Basta, B., Santalucia, R., Barbaro, S., & Magistro, C. (2025). Abdominal Surgery Performed in Awake Patients Under Neuraxial Anesthesia: A Systematic Review Across Surgical Specialties. International Journal of Translational Medicine, 5(4), 53. https://doi.org/10.3390/ijtm5040053





