Abstract
Frontotemporal lobar degeneration (FTLD) represents a heterogeneous group of neurodegenerative disorders with overlapping clinical, pathological, and genetic characteristics. Increasing evidence indicates that disease mechanisms begin decades before the appearance of clinical symptoms, highlighting the importance of identifying preclinical and prodromal stages. This review provides a comprehensive synthesis of current knowledge on the complexity of FTLD, emphasizing early detection and intervention strategies. It integrates findings from neuropathological, neuroimaging, fluid biomarker, genetic, and clinical studies in both familial and sporadic forms, with particular attention to gene-specific trajectories, biomarker evolution, and emerging therapeutic approaches targeting presymptomatic and prodromal phases. Recent advances in biomarker discovery and neuroimaging are enabling earlier diagnosis and intervention, offering the potential to delay phenoconversion and preserve brain function.
1. Introduction
The integrity of large-scale brain network dynamics is essential for the normal functioning of the human brain and is markedly compromised in neurodegenerative dementias. Recent research emphasizes the importance of detecting and targeting disease processes at their earliest stages, prior to overt network disruption, as a means of optimizing therapeutic efficacy and prolonging the asymptomatic phase of disease progression [].
Traditionally, clinical focus has centred on manifest dementia syndromes rather than on their preclinical and prodromal stages, while disease classification has primarily relied on clinical phenotypes rather than molecular aetiology. However, there is a growing scientific and ethical impetus to shift both clinical and research paradigms toward early, informed, molecular diagnosis. This approach aims to accurately identify neuropathological processes during the presymptomatic stages, before neural networks are significantly affected and while only minimal neuronal loss or synaptic dysfunction is present, thereby opening potential therapeutic strategies targeted toward disease biology and potentially preventing the phenotypic manifestation of the underlying pathology [].
This shift is particularly relevant in frontotemporal dementia (FTD), the most prevalent clinical form of early-onset neurodegenerative dementia, which now surpasses Alzheimer’s disease in incidence among individuals under 60 years of age [,]. This demographic overlap with peak working and caregiving years exacerbates the social and economic burden of FTD, particularly given the complexity of its clinical management and the high likelihood of genetic inheritance [,,].
The term FTD defines a clinically, pathologically, and genetically heterogeneous group of neurodegenerative disorders characterized by predominant degeneration of the frontal and/or temporal lobes, with neuropathological hallmarks including Tau, TAR DNA-binding protein 43 (TDP-43), and fused in sarcoma (FUS) proteinopathies, followed in incidence by dipeptide repeat proteins (DPR) and proteins altered in frontotemporal lobar degeneration–ubiquitin proteasome system (UPS).
The clinical spectrum of FTD is multifaceted, encompassing progressive changes in behaviour, social conduct, and emotional regulation, and/or language, often accompanied by motor impairments involving either the pyramidal or extrapyramidal systems []. The traditional classification into clinical phenotypes includes: behavioural variant FTD (bvFTD), characterized by progressive deterioration in social conduct and personality changes (apathy or disinhibition, loss of empathy, poor judgment and compulsive or repetitive behaviours) []; semantic variant of primary progressive aphasia (svPPA), characterized by progressive loss of semantic knowledge and naming, non-fluent/agrammatic variant of PPA (nfvPPA), marked by impaired grammar and motor speech deficits and word output []; right temporal variant FTD (rtvFTD), associated with loss of empathy, behavioural changes and prosopagnosia [,,,,,]. A significant proportion of patients that have associated extrapyramidal symptoms form part of either a progressive supranuclear palsy or Richardson syndrome (PSP), characterized by supranuclear gaze palsy, postural instability, axial rigidity, bradykinesia, dysarthria and dysphagia [], and corticobasal syndrome (CBS), characterized by limb apraxia, cortical sensory loss, alien limb, asymmetric rigidity, apraxia, dystonia, myoclonus, and cognitive/behavioural signs. []. Clinical overlap involving the pyramidal system is notably observed with amyotrophic lateral sclerosis (ALS) [], forming the so-called motor neuron disease–FTD continuum, where ALS and FTD represent the phenotypic extremes. Besides the multiform variability within the FTD-spectrum, clinical diagnosis is further complicated by the overlap of features in bvFTD or svPPA with those seen in Alzheimer’s disease or primary psychiatric disorders [,,,].
The clinical heterogeneity of FTD parallels its underpinning neuropathological complexity, characterized by neuronal and glial inclusions leading to neurodegeneration, then damage to frontal and/or temporal lobes, and finally to a more global network dysfunction. Microscopically, this neuropathological substrate is collectively referred to as the umbrella term frontotemporal lobar degeneration (FTLD) [], defined by the abnormal accumulation of pathogenic protein aggregates within the cells of the frontal and/or temporal lobes, including: (i) hyperphosphorylated tau with three- or four-repeat microtubule-binding domains (3R or 4R), underlying FTLD-tau pathologies such as Pick’s disease (predominantly 3R), corticobasal degeneration (4R), PSP (4R), globular glial tauopathy (4R), and FTLD-tau/MAPT (3R or 4R); (ii) TAR DNA-binding protein 43 (TDP-43) inclusions, characterizing FTLD-TDP, further subclassified into subtypes A–E; (iii) Fused in sarcoma–Ewing sarcoma–TATA-binding protein associated factor 2N [FUS-EWS-TAF15] (FET) family proteinopathies, underlying FTLD-FET pathologies including basophilic inclusion body disease (BIBD), atypical FTLD-U (aFTLD-U), neuronal intermediate filament inclusion disease (NIFID), and FUS not otherwise specified (FUS-NOS); (iv) dipeptide repeat proteins (DPR), differentiated in poly-GA (glycine-alanine), poly-GP (glycine-proline), poly-GR (glycine-arginine), poly-PR (proline-arginine), poly-PA (proline-alanine); and (v) altered proteins within the ubiquitin proteasome system (UPS), characterizing FTLD-UPS pathology.
The complexity of FTD is further compounded by the variable relationship between clinical phenotype and underlying pathology, wherein a single pathological subtype tends to occur in isolation in early onset dementia and may manifest across multiple clinical syndromes, but a given clinical syndrome may result from diverse pathological mechanisms in different individuals []. While certain clinicopathological correlations have been established, such as FTLD-TDP C in svPPA [], and FTLD-TDP B in bvFTD-ALS patients [,,], the accurate prediction of underlying FTLD pathology in clinically diagnosed FTD remains a significant diagnostic challenge.
FTLD complexity is further implemented by genetic risk []. In fact, familial aggregation, due to monogenic mutations, is reported in up to 30–40% of FTLD cases, with nearly complete penetrance by age 80. Major causative mutations include (i) MAPT, encoding the microtubule-associated protein tau and affecting tau protein [], (II) GRN gene, encoding progranulin (PGRN) [], (III) C9orf72, encoding chromosome 9 open reading frame 72 [,]. There is significant variability in age at onset, both across and within mutation classes, particularly with GRN and C9orf72 mutations [], with emerging evidence of genetic and environmental modifiers []. Additionally, even in sporadic FTD (50–70% of cases), genetic factors contribute to disease liability to the pathology [].
The above complexity across clinical presentation, neuropathology, and genetic predisposition appears to be consistent across all stages of disease. Theoretically, if the degeneration of specific brain regions, whether due to local accumulation of pathological proteins or long-distance network dysfunction associated with such proteinopathies, results in defined clinical symptoms, it follows that the extent of neuropathological burden correlates with the severity and heterogeneity of the clinical phenotype. This perspective calls for a paradigm shift in the classification and understanding of the syndrome, emphasizing pathology from its earliest stages, including the presymptomatic phases. Conceptually, pathology initiates with a transition from a susceptibility phase (characterized by the absence of symptoms, signs, or biomarker changes) to a presymptomatic phase, defined as the period between the cellular or molecular onset of disease, which is currently undetectable, and phenoconversion to clinically manifest disease. This presymptomatic phase can be subdivided by phenotransition into a “preclinical stage,” characterized by biochemical abnormalities such as proteinopathy, neuronal dysfunction and neurodegeneration without clinical symptoms, and a “prodromal stage,” marked by the emergence of clinical symptoms that do not yet meet criteria for a formal FTD diagnosis. The gradual accumulation of biological (preclinical) and then clinical (prodromal) changes precedes phenoconversion, defined as the onset of detectable and predictable dementia []. During these phases, targeted neuropsychological evaluation, multimodal neuroimaging assessment (including magnetic resonance imaging (MRI) [] and positron emission tomography (PET) []), and established and emerging fluid biomarkers [] in multiple matrices can provide valuable information to (i) define the sequence of pathophysiological changes occurring in FTD, dissecting disease heterogeneity and (ii) predict phenoconversion.
In this narrative review, we will first analyse the complexity of the FTD spectrum and disease terminology. We will then review current evidence and controversies regarding the genetics, imaging, and fluid biomarkers associated with the presymptomatic stages of FTLD, with a focus on proposed diagnostic criteria [,] and future perspectives. Our aim is to provide a framework for clinical and research applications and to encourage therapeutic trials targeting the prodromal and preclinical stages of FTD within large-scale, multicentre collaborative studies.
2. Complexity of the FTD Spectrum
2.1. Complexity of the FTD Spectrum: A Multiform Phenotype
The clinical spectrum with which FTD can present is extraordinarily broad, ranging from behavioural disturbances to motor symptoms (Figure 1B). The symptom marking the disease onset, or the principal symptom dominating the clinical presentation, typically determines the categorization within the phenotypic classification. However, multiple symptoms frequently coexist, with many emerging simultaneously in the more advanced stages of the disease. Indeed, the clinical phenotypes include (Figure 1A superior part):
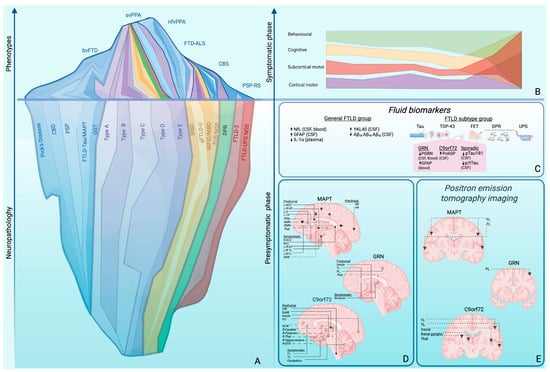
Figure 1.
(A) Phenotypic and neuropathological complexity of FTLD. From bottom to top, the progression from neuropathology to clinical phenotypic manifestation is illustrated. The lower part of the iceberg represents neuropathology, with colour code and band thickness proportional to the frequency of each proteinopathy: blue indicates tauopathies, further subdivided into Pick’s disease, CBD, PSP, FTLD-tau/MAPT, and GGT; purple indicates TDP-43 proteinopathy, further classified into Types A–E; yellow indicates FTLD-FET pathology, including BIBD, aFTLD-U, NIFID/NIBD, and FUS-NOS; green indicates DPR pathology; red indicates UPS pathology, including FTLD-UPS and FTLD-UPS NOS. The upper part represents clinical FTD syndromes, outlined by a blue line and color-coded according to the proportion associated with a specific pathology and its subtypes. (B) Proposed framework for addressing clinical complexity: symptoms are divided into phenotypic axes (behavioural, cognitive, cortical motor [pyramidal], and subcortical motor [extrapyramidal]) which, as the disease progresses, converge into a single spectrum of pathology. (C–E) Strategies to lower the detection threshold. (C) Fluid biomarkers. Biofluid abnormalities are dysregulated either across broad FTD groups (right panel) or within a specific FTLD subtype (left panel), in either blood or CSF. The DPR image illustrates the five dipeptide repeat (DPR) protein subtypes — poly(GP), poly(PA), poly(GA), poly(PR), and poly(GR) — composed respectively of glycine–proline, proline–alanine, glycine–alanine, proline–arginine, and glycine–arginine repeats. Glycine is shown in yellow, alanine in violet, proline in light blue, and arginine in red. (D) MRI progression patterns in familial FTLD. Solid-dot arrows indicate white matter involvement; open-dot arrows indicate gray matter involvement; dashed arrows represent networks with altered functional connectivity; dashed open-dot arrows indicate regions of hypoperfusion identified using ASL. (E) PET-FDG progression patterns in familial FTLD. Abbreviations: ACC, anterior corpus callosum; aFTLD-U, atypical frontotemporal lobar degeneration with ubiquitin-positive inclusions; Aβ, amyloid beta; BIBD, basophilic inclusion body disease; bvFTD, behavioural variant frontotemporal dementia; C, cingulate; CBD, corticobasal degeneration; CSF, cerebrospinal fluid; DAN, dorsal attention network; DFM, default mode network; DPR, dipeptide repeat proteins; FET, family of RNA-binding proteins including FUS, Ewing sarcoma, and TAF15; FL, frontal lobes; FPN, frontoparietal network; FTD-FUS, frontotemporal dementia fused in sarcoma variant; FTD-ALS, frontotemporal dementia with concomitant amyotrophic lateral sclerosis; FTLD, frontotemporal lobar degeneration; GFAP, glial fibrillary acidic protein; GGT, globular glial tauopathy; IL-1α, interleukin-1 alpha; ILF, inferior longitudinal fasciculus; L, left; NIFID, neuronal intermediate filament inclusion disease; NfL, neuro-filament light chain; nfv, non-fluent variant; NOS, not otherwise specified; PiD, Pick’s disease; PL, parietal lobe; PPA, primary progressive aphasia; PSP-RS, progressive supranuclear palsy–Richardson’s syndrome; p-tau, phosphorylated tau; SCC, splenium of the corpus callosum; R, right; SMN, sensorimotor network; SN, salience network; STG, superior temporal gyrus; sv, semantic variant; TDP-43, TAR DNA-binding protein 43; Thal, thalamus; TL, temporal lobes; TP, temporal pole; UF, uncinate fasciculus; UPS, ubiquitin–proteasome system; VN, visual network.
- (I)
- Behavioural variant FTD (bvFTD), characterized by progressive deterioration in social conduct and personality, including apathy or disinhibition, loss of empathy, poor judgment and compulsive or repetitive behaviours []. This variant primarily involves the bilateral frontal cortex (dorsolateral prefrontal cortex which mediates executive functions; orbitofrontal cortex, regulating social behaviour, inhibition, and emotional responses; and medial prefrontal cortex, important for motivation and social cognition) and anterior temporal regions (including the amygdala and temporal pole, involved in emotional processing, social understanding, and semantic memory).
- (II)
- Primary progressive aphasia (PPA), comprising [,]:
- a.
- Semantic variant (svPPA), characterized by progressive loss of semantic knowledge and naming, with involvement of the anterior temporal region.
- b.
- Non fluent/agrammatic variant (nfvPPA), characterized by impaired grammar and motor speech deficits, involving left posterior frontal and insular regions.
- (III)
- Right temporal variant FTD (rtvFTD), characterized by loss of empathy, behavioural changes (disinhibition or apathy, compulsive or ritualistic behaviour), mood changes (irritability, anxiety, or suspiciousness), and prosopagnosia, associated with degeneration in the right anterior temporal lobe, potentially extending into the right orbitofrontal cortex [,].
- (IV)
- Extrapyramidal motor syndromes, including:
- a.
- Progressive supranuclear palsy–Richardson syndrome (PSP-RS) characterized by supranuclear gaze palsy, postural instability, axial rigidity, bradykinesia, dysarthria and dysphagia, cognitive impairment (executive dysfunction, apathy, and reduced verbal fluency), facial expression changes with a “staring” facial appearance due to reduced blinking and facial rigidity. PSP-RS primarily involves the tectum and tegmentum of the midbrain (causing vertical gaze palsy and postural instability), the subthalamic nucleus, globus pallidus, dentate nucleus of the cerebellum, and substantia nigra (motor symptoms), as well as the frontal cortex (executive dysfunction and apathy) [,].
- b.
- Corticobasal syndrome (CBS), characterized by cortical signs (limb apraxia, cortical sensory loss, alien limb), motor signs (asymmetric rigidity, apraxia, dystonia, myoclonus) and cognitive/behavioural signs (executive dysfunction; aphasia; visuospatial dysfunction; behavioural changes). CBS typically involves the frontoparietal lobes asymmetrically [].
- (V)
- Pyramidal motor disorders, most notably amyotrophic lateral sclerosis (ALS) []. Traditionally, ALS and FTD are wider considered the two phenotypic extremes on the so called motor neuron disease–FTD continuum, where (i) about half of all patients exhibit pure motor involvement with preserved cognition during the disease course (classical ALS), (ii) up to half of ALS patients display some degree of cognitive impairment or behavioural changes, without fulfilling the diagnostic criteria for FTD (ALS with executive dysfunction [ALS-eci], ALS with no executive dysfunction but impairment in other cognitive domains [ALS-neci], e.g., memory, or ALS with behavioural changes [ALS-bi]), and (iii) about 5–10% of patients firstly presenting FTD (most frequently bvFTD, occasionally PPA) while developing motor neuron involvement without full ALS manifestation []. Pathologically, ALS-FTD involves the primary motor cortex, corticospinal tracts, and brainstem motor nuclei (motor dysfunction), as well as the frontal lobes (orbitofrontal, medial prefrontal, anterior cingulate), anterior temporal lobes, insular cortex (cognitive/behavioural impairment), and occasionally the striatum and hippocampus [,].
Reasonably, changes in clinical manifestations correspond closely to the anatomical distribution of misfolded pathological proteins within the brain.
2.2. Complexity of the FTD Spectrum: Underpinning Neuropathology
The neuropathological complexity of FTLD is underpinned by the abnormal accumulation of tau, TDP-43, FET family proteins, dipeptide repeat proteins (DPRs), and alterations in the ubiquitin-proteasome system (UPS). Figure 1A inferior part.
2.2.1. Tau Pathology
Tau is a neuronal proteins that can physiologically localize in dendrites, where it plays a role in dendrite and spine maturation, synaptic activity, and microtubule dynamics; or in the nucleus, where it protects DNA/RNA, regulates rDNA transcription, and stabilizes heterochromatin; or in synapses, where it assists in synapse formation, axonal outgrowth, growth cone dynamics, and synaptic transmission; or in the cytoplasm, where it protects RNA and supports translation; or in axons, where it regulates microtubule dynamics and axonal transport. Tau has six brain-specific isoforms based on alternative splicing of exons 2, 3, and 10 of the MAPT gene and is structured into four different domains: the N-terminal projection domain, which projects away from microtubules (MTs), acts as a spacer, and interacts with plasma membrane components; the proline-rich region, which is involved in cellular signalling; the MT-binding domain, which mediates MT polymerization and stability; and the C-terminal region, which also contributes to MT polymerization and tau’s interaction with the plasma membrane. In disease, MAPT mutations, often centred around exons 9–12 or found within intronic regions, frequently lead to impaired splicing of exon 10. Tau with 3R MT-binding domains lacks exon 10, while tau with 4R MT-binding domains includes exon 10. This disruption results in an imbalance between 3R and 4R Tau isoforms. Other missense mutations or deletions diminish tau’s MT-binding affinity, reduce its ability to promote MT assembly, enhance its aggregation propensity, disrupt its interactions with other proteins, or alter its phosphorylation pattern [,,]. Moreover, the dysfunction of other proteins has been associated with the misregulation of tau splicing in sporadic tauopathies, leading to elevated inclusion of exon 10 and resulting in higher expression of 4R tau isoforms. Therefore, hyperphosphorylated tau (3R or 4R) gives rise to the so-called FTLD-tau pathologies (about 45% of FTLD patients), which encompass:
- a.
- Pick’s disease (PiD), with 3R >> 4R tau, forming Pick bodies (intracytoplasmic, eosinophilic, round or oval, strongly argyrophilic neuronal inclusions) in association with ubiquitin. They mainly affect layers II–III of the frontal and temporal cortices and the hippocampus, with a phenotype mostly corresponding to bvFTD and PPA [,].
- b.
- Corticobasal degeneration (CBD) with 4R tau aggregating in neurons and glia (astrocytes and oligodendrocytes). 4R tau forms astrocytic plaques, coiled bodies (cytoplasmic inclusions within oligodendrocytes), tau-positive threads (thin, thread-like structures in neurites and neuropil) in grey matter (GM) and white matter (WM), and neuronal cytoplasmic tau inclusions. They mainly aggregate in layers V–VI of the frontal and parietal cortices, particularly in motor and premotor cortices, but also in the basal ganglia (especially the subthalamic nucleus and globus pallidus) and subcortical WM [,]. Typical phenotype corresponds to CBS, PSP-RS, nfvPPA, frontobehavioural spatial syndrome (FBS) [].
- c.
- Progressive supranuclear palsy (PSP), with 4R tau forming tufted astrocytes (star-like inclusions in proximal astrocytic processes, pathognomonic for PSP), coiled bodies, globose neurofibrillary tangles (NFTs) (round/oval, eosinophilic, silver-positive, intraneuronal inclusions), and tau-positive threads [,]. Cortical involvement mainly affects layers V–VI of the frontal and parietal cortices. Typical phenotype corresponds to PSP-RS, PSP-Parkinson’s (PSP-P), Pure akinesia with gait freezing (PAGF), CBS, nfvPPA, bvFTD [].
- d.
- Globular glial tauopathy (GGT), with 4R tau forming globular oligodendroglial inclusions (GOIs), globular astrocytic inclusions (GAIs), and tau-positive threads in WM, primarily in the frontotemporal cortex, basal ganglia, and brainstem. The clinical presentation often includes bvFTD, parkinsonism, or pyramidal motor disorders [,].
- e.
- FTLD-tau/MAPT, with 3R or 4R tau preferentially localizing in von Economo neurons and fork cells, aggregating in globose NFTs. FTLD-tau/MAPT is linked to genetic causes (familial cases) and presents features resembling PSP, CBD, or Pick’s disease [].
Characteristic phenotypes include bvFTD, PPA, CBS, and PSP. Almost half of bvFTD cases have underlying FTLD-tau pathology, including PiD and, to a lesser extent, CBD and PSP. FTLD-tau has also been reported in patients with PPA and CBS phenotypes. The clinical syndrome of PSP highly correlates with PSP tau pathology. Additionally, parkinsonism associated with bvFTD or PPA has been demonstrated as a predictor of tau pathology [].
2.2.2. TDP-43 Pathology
TDP-43, i.e., transactive response (TAR) DNA-binding protein 43 (TDP-43), is a nuclear major protein component within ubiquitin-positive inclusions, involved in transcription and splicing by RNA processing and transport, comprising two RNA-recognition motifs (RRM1-RRM2) and a glycine-rich C-terminal domain [,]. In disease, it mislocalizes from the nucleus to the cytoplasm in neurons and glia and undergoes aggregation regulated by both N-terminal and C-terminal regions, as well as modifications such as truncation (mainly in RRM2), ubiquitination, phosphorylation (primarily at serine residues near the C-terminal) [,], simulation [], cysteine oxidation [,], finally forming pathological inclusions. This mislocalization, leading to apparent nuclear loss of function and toxic cytoplasmic accumulation, underpins pathology. TDP-43 forms various pathological inclusions: (i) neuronal cytoplasmic inclusions (NCIs), dense, round, or crescent-shaped; (ii) neuronal intranuclear inclusions (NIIs), round or lentiform (less common), (iii) dystrophic neurites (DNs), which are abnormal TDP-43-positive thin, tortuous, or swollen neurites; and (iv) glial cytoplasmic inclusions (GCIs), round or granular TDP-43 inclusions in astrocytes and oligodendrocytes. TDP-43 proteinopathy leads to FTLD-TDP pathologies (approximately 50% of FTLD patients), further classified into five subtypes based on their cortical distribution [,,]:
- a.
- FTLD-TDP subtype A, characterized by moderate to numerous NCIs, numerous short DNs, and occasional NIIs, mainly in upper cortical layers II/III. It also has thread pathology in the subcortical WM, delicate wispy threads in hippocampal CA1, and a predominance of DN and occasional NII in striatum and other subcortical GM regions. Subtype A is often associated with bvFTD (apathy and social withdrawal) and nfvPPA. Executive dysfunction, some degree of memory impairment, and neuropsychiatric manifestations (delusions, hallucinations or obsessive behaviours) are not uncommon.
- b.
- FTLD-TDP subtype B, with moderate to numerous NCIs, sparse DNs, and rare NIIs across all cortical layers. It is also characterized by the presence of glial cytoplasmic inclusions in the subcortical WM, a predominance of diffuse NCIs in subcortical GM, and NCI in lower motor neurons of the medulla and spinal cord. Subtype B is typically linked to the FTD-MND spectrum and to a lesser extent to bvFTD, nfvPPA. Neuropsychiatric manifestation (psychosis) is particularly frequent.
- c.
- FTLD-TDP subtype C, with long, tortuous DNs and infrequent NCIs, primarily in upper cortices. It also has compact “Pick body-like” NCI in dentate granule cells of the hippocampus and striatum. Subtype C is frequently seen in svPPA and sometimes in rtvFTD.
- d.
- FTLD-TDP subtype D, with numerous NIIs, fewer NCIs and DNs concentrated in superficial laminae. Modest numbers of DN and NII are also present in the amygdala, basal ganglia, nucleus basalis, thalamus and midbrain. It is exclusively seen in inclusion body myopathy with Paget disease of bone and frontotemporal dementia (IBMPFD) and FTD-MND spectrum linked to valosin-containing protein (VCP) gene mutations [].
- e.
- FTLD-TDP subtype E, with weakly staining granulofilamentous neuronal cytoplasmic inclusions (GFNI) set in a background of very fine grain-like deposits throughout the neocortex sparing only of the occipital neocortex and cerebellum []. Motor neuron involvement was a typical feature, although only sometimes associated with clinical features of ALS. FTLD-TDP type E is consistently associated with a rapid clinical course of one to three years duration.
FTLD-TDP is commonly associated with genetic causes such as GRN mutations, C9orf72 expansions, and VCP mutations. Characteristic phenotypes include bvFTD, svPPA, CBS, and ALS [,]. Recent studies suggest that psychiatric symptoms in FTD patients are associated with underlying TDP pathology [].
2.2.3. FET Family Pathology
FET proteins are nuclear multifunctional RNA-binding proteins containing RNA recognition motifs (RRMs), glycine-rich domains, nuclear localization signals (NLS), and prion-like domains. Physiologically, FUS is involved in DNA repair, transcription regulation, RNA splicing, and RNA transport; EWS in transcription regulation, RNA splicing, and DNA repair; TAF15, as part of the TFIID complex, in transcription initiation, RNA splicing, and RNA transport. In pathology, FET proteins mislocalize from the nucleus to the cytoplasm and aggregate as hyperphosphorylated, ubiquitinated, and insoluble NCIs, NIIs, and DNs. FET family proteins characterize FTLD-FET pathology (less than 5% of FTLD patients), which includes:
- a.
- Basophilic inclusion body disease (BIBD), characterized by basophilic NCIs, dense, round-to-oval accumulations of FET proteins (mainly FUS) and ubiquitinated proteins within neuronal cytoplasm.
- b.
- Atypical FTLD (aFTLD-U), characterized by FUS positive NCIs, DNs, and rarely NIIs.
- c.
- Neuronal intermediate filament inclusion disease (NIFID/NIBD), characterized by neuronal intermediate filament aggregates, forming NCIs, NIIs, and DNs inclusions.
- d.
- FUS not otherwise specified (FUS NOS), with variably shaped FUS-positive NCIs, NIIs, and DNs.
Characteristic phenotypes associated with FET pathology include ALS, bvFTD, and PPA. Patients with FTLD-FUS pathology often fulfil diagnostic criteria for bvFTD [] and are typically characterized by disease onset before age 40, negative family history, and primarily caudate affection by pathology.
2.2.4. DPR Pathology
DPR are neurotoxic dipeptide aggregates in neurons, generated via RAN translation (repeat-associated non-AUG translation, i.e., without an AUG start codon), of expanded GGGGCC repeats in the C9orf72 gene, in both sense (G4C2) and antisense (C2G4) directions []. Physiologically, a hexanucleotide repeat expansion, consisting of GGGGCC (G4C2) repeats, can be found in the first intron in the reading frame 72 of chromosome 9 (C9orf72) in the non-coding region between exons 1 and 1b [,]. Healthy individuals harbour less than 30 of these G4C2 repeats, while FTLD patients with C9orf72 mutations carry 400 to several thousand G4C2 repeats []. The types of DPRs produced are poly-GA (glycine-alanine), poly-GP (glycine-proline), poly-GR (glycine-arginine), poly-PR (proline-arginine), poly-PA (proline-alanine). Among the different types of DPRs, GA and GR are generated from the sense strand of the expanded repeat, PA and PR from the antisense strand, while GP can be produced from both the sense and antisense strands []. DPRs vary in aggregation patterns, structures, solubility (e.g., soluble like GP and insoluble like GA), and toxicity []. Although the exact contribution of each DPR to disease remains unclear, proposed pathogenic mechanisms include nucleolar dysfunction, altered splicing, impaired nucleocytoplasmic transport, altered RNA granule dynamics, disrupted intracellular transport, and impaired proteasome function. DPRs accumulate as NCIs, NIIs, and DNs, mainly in the neocortex, hippocampus, cerebellum, and spinal cord motor neurons, often coexisting with TDP-43 pathology [,,]. Characteristic phenotypes include bvFTD, FTD-MND, and occasionally nfvPPA [,,].
2.2.5. UPS Pathology
The UPS is the major intracellular protein degradation system, responsible for tagging misfolded, damaged, or short-lived proteins with ubiquitin, regulating protein turnover for cellular homeostasis, and removing abnormal proteins to prevent toxic aggregation. In pathology, the UPS may become overwhelmed or impaired, leading to the accumulation of polyubiquitinated proteins and ubiquitin-positive inclusions. FTLD-UPS has subtypes:
- a.
- FTLD-UPS (FTLD-3 or CHMP2B), characterized by ubiquitin inclusions, mainly localized in the hippocampal dentate gyrus and frontal cortex.
- b.
- FTLD-UPS not otherwise specified (FTLD-UPS NOS), with ubiquitin-immunoreactive NCIs and DNs localized in the frontal and temporal cortices.
Characteristic phenotypes include atypical bvFTD (slowly progressive) with rare motor involvement [,].
Beyond the broad clinical and neuropathological variability, the complexity of FTLD is further highlighted by the, albeit not frequent, yet possible dissociation between clinical phenotype and underlying pathology. Clinicopathological studies have consistently demonstrated that identical clinical presentations can arise from distinct molecular substrates, and conversely, that a single pathological entity may manifest through multiple clinical syndromes. For instance, while most cases of bvFTD are associated with FTLD-TDP or FTLD-tau pathology, up to 10–15% of patients exhibit non-FTLD pathology, including Alzheimer’s disease or Lewy body disease []. Similarly, PPA variants display substantial overlap, with the nfvPPA and svPPA subtypes often associated with TDP-43 or tau inclusions, and the lvPPA variant typically linked to Alzheimer-type pathology []. Moreover, within the FTLD spectrum itself, phenotypic heterogeneity among TDP-43, tau, and FUS subtypes underscores the need for a biologically informed classification system integrating genetic, neuroimaging, and biomarker evidence to refine diagnosis and strengthen clinicopathological correlations [].
2.3. Complexity of the FTD Spectrum: Genetic Underpinnings
The reason why different genes, each associated with distinct molecular pathways, converge to cause a neurodegenerative process predominantly affecting the frontotemporal lobes remains under debate, and the shared mechanisms underlying selective frontal and temporal degeneration are yet to be fully elucidated. The major recognised monogenic mutations, inherited as discrete Mendelian traits, include those in the MAPT [] and GRN genes [,], as well as pathogenic expansions in the C9orf72 gene [,]. A less frequent but noteworthy mutation also occurs in the valosin-containing protein (VCP) gene []. Mutations in MAPT lead to abnormal tau accumulation, whereas pathogenic variations in GRN and C9orf72 are associated with TDP-43 deposition. Although these autosomal dominant mutations generally exhibit poor genotype–phenotype correlations, they are highly predictive of the underlying neuropathology [,]. Specifically:
- (I)
- MAPT is essential for neuronal integrity and axonal transport. Mutations in MAPT, including both missense and splicing mutations, alter tau function, resulting in either a loss of function, via reduced microtubule-binding affinity or impaired microtubule assembly, or a toxic gain of function, characterized by increased aggregation propensity. These mechanisms ultimately lead to aberrant tau aggregation and seeding [,]. Nearly 100 MAPT variants have been linked to FTLD-spectrum disorders, accounting for up to 20% of familial cases (Alzforum database, 08/2025) [,,]. Common genetic variants within the major MAPT haplotypes (H1 and H2) are also associated with an increased risk of sporadic tauopathies []. These mutations are frequently associated with PSP and CBD pathology [,,], and genotype–phenotype correlations indicate that MAPT mutation carriers predominantly present with bvFTD, PPA, or FTD with parkinsonism []. The median age at onset is approximately 50 years, indicating an early onset and rapid progression [].
- (II)
- Over 70 pathogenic mutations in GRN have been identified, most of which result in loss of function due to defective transcription or translational inhibition, leading to GRN haploinsufficiency and a reduction in progranulin levels in serum, blood and CSF to less than 50% of normal []. Loss of progranulin, particularly in lysosomes, results in lysosomal storage disorders characterized by neuronal ceroid lipofuscinosis []. Autosomal dominant loss-of-function mutations in GRN account for 10–15% of FTLD-TDP cases and are specifically associated with FTLD-TDP type A pathology [,,]. Clinically, GRN mutation carriers often exhibit a bvFTD phenotype, although PPA has also been observed []. The median age at onset is about 61 years, reflecting an intermediate onset, incomplete penetrance, and rapid clinical deterioration once symptoms emerge [].
- (III)
- The C9orf72 gene product plays several critical roles: (i) it is involved in RNA metabolism; (ii) it facilitates the accumulation of sense and antisense RNA transcripts of expanded repeats, which serve as templates for the synthesis of dipeptide repeat proteins (DPRs) through repeat-associated non-ATG translation; and (iii) it participates in the autophagy–lysosome pathway. The hexanucleotide repeat expansion (GGGGCC) in the non-coding region of C9orf72 causes disease via multiple mechanisms: (i) reduced gene expression, which disrupts endosomal trafficking and autophagy []; (ii) (ii) toxicity from RNA foci and DPRs generated through non-canonical translation []; (iii) nucleolar stress; (iv) RNA dysregulation; and (v) defects in nucleocytoplasmic transport and protein degradation []. Repeat expansions in C9orf72 account for about 25% of FTLD-TDP cases, primarily involving FTLD-TDP type A and B pathology, and occasionally type C pathology [,,]. Although C9orf72 repeat expansions are typically inherited, they are detected in up to 10% of individuals with apparently sporadic disease. These expansions are twice as common in familial ALS compared to SOD1 mutations, the first gene linked to ALS []. Clinically, C9orf72 expansion carriers with FTD commonly present with bvFTD, and in some cases, with PPA []. The median age at onset is approximately 58 years, and disease expression is marked by wide variability and a prolonged preclinical phase [].
- (IV)
- The VCP gene encodes the valosin-containing protein, an enzyme involved in diverse cellular processes such as intracellular trafficking, proteasomal degradation, and programmed cell death. VCP mutations: (i) alter TDP-43 localization between the nucleus and cytoplasm; (ii) impair proteasome activity; (iii) induce endoplasmic reticulum stress; (iv) increase apoptotic markers; and (v) reduce cell viability. These mutations are associated with FTLD-TDP type D pathology.
In addition to these Mendelian forms, a genetic component also contributes to the risk of developing pathology in sporadic FTD, which accounts for 50–70% of cases [,]. In fact, the determination of this complex disease follows the liability threshold model, where in sporadic FTD inheritance is non-Mendelian but still follows a binary outcome relative to a threshold on a graded liability scale, above which individuals manifest the disease. Here, liability encompasses both innate susceptibility and the combination of external circumstances that modulate the likelihood of disease development, with genetic factors constituting part of this burden. Therefore, while familial FTD (monogenic forms) can be attributed to risk genes with large effects and clear binary outcomes, sporadic FTD forms are influenced by many factors, among which gene variants act as susceptibility alleles, increasing liability and nudging individuals towards disease manifestation. Genome-wide association studies (GWAS) have identified susceptibility gene variants in FTLD-TDP. UNC13A has emerged as the strongest overall risk factor, while TNIP1 represents a novel risk locus. Additional genome-wide significant loci associated with specific FTLD-TDP subtypes include: FARP2, TINAG, MZT1, and GRN in subtype A; TNIP1, RCL1, PDS5B, and UNC13A in subtype B; and C9orf72, TRPC4, TMEM135, LRP1B, and COL22A1 in subtype C. Other genes, such as TBK1, C3AR1, SMG8, VIPR1, RBPJL, L3MBTL1, and ANO9, have also been identified as subtype-specific risk genes, reinforcing the involvement of immune pathways and Notch signalling in FTLD-TDP pathogenesis [,]. A few additional rare genetic causes of FTD (e.g., TARDBP, CHCHD10, OPTN, SQSTM1) have been reported with TDP-43 pathology, but insufficient data exist to characterize their specific patterns []. Genes that confer an increased risk of FTLD-tau include MOBP, STX6, EIF2AK3, and TRIM11, as they play a role in tau misfolding, impaired protein clearance and repair, and dysmyelination [].
Beyond risk and susceptibility genes, additional factors influence disease manifestation, including (i) penetrance, the probability that a genotype results in a phenotype (in C9orf72, penetrance is age-related, i.e., 0% at age 35, ~50% at 58, and almost fully penetrant by age 80 [], in MAPT, it is nearly complete; in GRN, it is estimated around 71%); (ii) pleiotropy, where a gene variant contributes to different pathologies, such as ALS and FTD; (iii) oligogenic inheritance, the combined effect of two or three genes producing a trait, as seen in approximately 6% of ALS cases; (iv) long non-coding RNAs, which are ~200 bp transcripts that modulate gene expression, recruiting chromatin-modifying complex and acting as scaffolds for histone-modifying enzymes; and (v) epistasis, where a variant at one locus interacts with another locus, potentially switching from beneficial to deleterious effects depending on the genetic background, as exemplified by the TMEM106B gene. The TMEM106B rs1990622 polymorphism has been shown to modulate plasma progranulin levels, thereby influencing the age of symptom onset in GRN mutation carriers [,,]. Specifically, individuals with prodromal FTD due to GRN mutations who carry the TMEM106B TT genotype exhibit more extensive functional brain damage compared to those with the CT or CC genotypes []. In contrast, among individuals with prodromal FTD associated with C9orf72 expansions, TMEM106B may influence disease pathology in an opposite manner [,]. Finally, the risk of disease progression and the natural history of FTD may also be influenced by various modifiable factors [], such as cognitive reserve, comprising educational attainment, and bilingualism, and an active lifestyle. In fact, higher educational levels have been associated with increased GM volumes, suggesting that individuals with greater educational attainment may better withstand the effects of pathogenic mutations []. Bilingualism has been linked to a delayed onset of dementia in individuals with bvFTD []. These factors are considered protective against disease progression, as they may enhance both brain reserve and brain maintenance during the prodromal stages of genetic FTD, thereby conferring greater clinical resilience, even in autosomal dominant forms [,]. Among the modifiable factors that may contribute to disease development, particularly in tauopathies, are annonacin, chemical agents, metals, and pesticides, which exacerbate mitochondrial dysfunction, as well as certain medications (such as glucocorticoids and beta-blockers) that ultimately promote tau aggregation, either directly or indirectly.
3. Pathophysiological Mechanisms
To date, the mechanisms that trigger the initial conversion of normally soluble proteins into pathological filamentous polymers remain unresolved. According to the template-directed misfolding hypothesis, a misfolded version of a protein directly interacts with its native counterpart, inducing a conformational change that converts it into a misfolded replica. This process promotes the formation of fibrillar ultrastructures composed of misfolded proteins, which ultimately aggregate into neurotoxic oligomers. The precise mechanisms through which these oligomers propagate between neurons remain to be fully elucidated. Evidence suggests that FTLD-associated pathological proteins which form intracellular aggregates (particularly tau and TDP-43), require release from the originating neuron or glial cell and subsequent uptake by neighbouring neurons or glial cells to propagate. This intercellular transfer necessitates crossing cell membranes, which can occur through extracellular release and uptake as fibrils or via trans-synaptic mechanisms, such as exosomes, tunnelling nanotubes, or synaptic vesicles, in non-fibrillar forms.
This postulated neurobiological mechanism possibly underlying the progression of the disease therefore comprises the spreading of disease proteins from cell to cell, mimicking the prions diffusion. However, the chained events that begin with misfolding and continue with cell–cell propagation, culminate with spreading of disease proteins within targeted brain regions []. In fact, disease progression is not random but follows the architecture of structural and functional brain networks [,]. Highly connected hubs (regions with elevated connectivity and metabolic activity) are particularly vulnerable and serve as critical nodes for the propagation of pathology []. These hubs are especially susceptible due to: (i) high baseline activity and metabolic demands, increasing vulnerability to oxidative stress; (ii) extensive connectivity, heightening the probability of acquiring and transmitting pathological proteins; and (iii) their central role in cognitive and motor processing, such that damage results in widespread network dysfunction. Distinct pathological proteins exhibit stereotypical patterns of progression across connected brain regions, with lesions forming sequentially at specific CNS sites. As disease advances, there is an increase both in the size of protein aggregates and in the number of affected cells displaying pathological inclusions.
Tau pathology follows a predictable progression pattern, with a consistent predilection for neurons of the pallido–nigro–luysian axis, although progression varies substantially across major subtypes (Pick’s disease, PSP, CBD, and GGT) correlating with clinical manifestations (Figure 2A). In Pick’s disease, pathology begins in the frontal cortex, anterior temporal lobes, and hippocampus (particularly CA1 and subiculum), before extending to the posterior temporal and parietal cortices, and ultimately involving the basal ganglia and brainstem nuclei [] (Figure 2(A.1)). In PSP, early pathological involvement includes the globus pallidus, subthalamic nucleus, substantia nigra, dentate nucleus of the cerebellum, and midbrain tectum, with subsequent spread to the prefrontal, premotor, and parietal cortices, as well as deeper structures such as the superior colliculus, pontine tegmentum, and spinal cord at later stages [] (Figure 2(A.2)). In CBD, early pathology involves the motor and premotor cortices, supplementary motor area, superior frontal gyrus, and basal ganglia (putamen and globus pallidus), progressing later to the parietal cortex, brainstem nuclei, and WM tracts (e.g., corona radiata) [] (Figure 2(A.3)). GGT typically involves the frontal and temporal cortex, pyramidal tracts, subcortical WM, and brainstem [] (Figure 2(A.4)). However, although prion-like spreading across connected neurons is well established, explanations of tau propagation in tauopathies must also account for tau accumulation in astroglial and oligodendroglial cells.
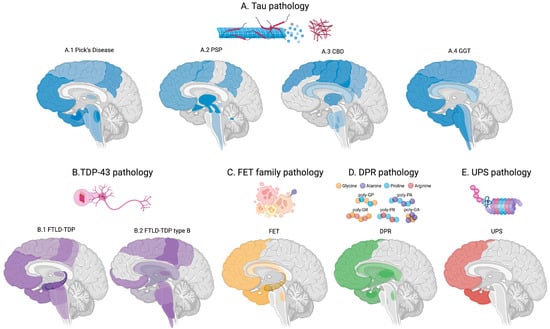
Figure 2.
Proteinopathy neuropathology and progression, colour-code. Darker colours indicate the anatomical regions affected first, with progressively lighter shades representing later involvement. (A) blue indicates tauopathies. further subdivided into Pick’s disease (A.1), PSP (A.2), CBD (A.3), and GGT (A.4). (B) Purple indicates TDP-43 proteinopathy further classified into FTLD-TDP (B.1)—and FTLD-subtype B/ALS (B.2). (C) Yellow indicates FTLD-FET pathology. (D) Green indicates DPR pathology. (E) red indicates UPS pathology.
TDP-43 pathology also follows a stereotyped progression, spreading via corticofugal pathways with regional involvement that parallels the development of cognitive and motor symptoms (Figure 2B). It predominantly affects cortical layers in the frontal, temporal, and motor cortices, as well as the spinal cord, hippocampus (especially the dentate gyrus), and subcortical WM. In FTLD-TDP, pathology typically follows the Mackenzie staging system, beginning in the amygdala and hippocampus, then extending to the frontal and temporal neocortex, and ultimately reaching the motor cortex, basal ganglia, and brainstem in advanced stages (Figure 2(B.1)). In ALS (FTLD-TDP type B), the Brettschneider staging system applies: Stage 1 involves the agranular motor cortex, brainstem motor nuclei, and anterior horn cells of the spinal cord; stage 2 includes the prefrontal neocortex and reticular formation; stage 3 adds the postcentral gyrus and striatum; stage 4 involves temporal lobe structures, including the hippocampus [,] (Figure 2(B.2)). Therefore, the pattern of progression may vary according to the clinical phenotype.
Although the stereotypical patterns of pathology progression for FUS, DPRs, and FTLD-UPS are less well established compared to tau and TDP-43, certain patterns have emerged from neuropathological studies.
FET pathology is primarily localized to frontotemporal regions rather than motor or pyramidal tracts. The initial sites of pathology include the frontal and temporal neocortex, hippocampus, amygdala, and in some cases, the caudate and putamen [], especially in basophilic inclusion body disease (BIBD) []. Pathology can later spread to cortico-striatal circuits and, in certain cases such as neuronal intermediate filament inclusion disease (NIFID), to brainstem nuclei like the substantia nigra (Figure 2C).
DPR pathology, associated with C9orf72 expansions, follows a stereotyped distribution pattern, initially affecting the neocortex (particularly frontal and temporal lobes), hippocampus, cerebellum (notably the dentate gyrus), thalamus, striatum, and brainstem nucle (Figure 2D) []. Notably, DPR inclusions have been found in clinically unaffected individuals with C9orf72 expansions, indicating early, possibly pre-symptomatic, accumulation []. Importantly, the extent of DPR pathology does not correlate strongly with the severity of neurodegeneration, suggesting that DPRs may act more as disease triggers rather than direct markers of symptom progression. Among DPR species, poly-GA is the most abundant and widely distributed, while poly-GR and poly-PR are considered more toxic due to their effects on nucleolar function and RNA metabolism [].
FTLD-UPS pathology, although rare, most commonly initiates in the frontal and temporal cortices, with variable patterns of subsequent spread [,] (Figure 2E).
Because FTLD pathology typically begins focally and progresses through anatomically connected cortical and subcortical networks, defining cell-specific stages of protein aggregation is crucial for identifying preclinical or early-stage disease. This knowledge enhances our understanding of the earliest pathogenic events, provides insights into the subtype-specific dynamics of disease propagation, and informs protein-neuroimaging research aimed at characterizing spatial distribution patterns [].
But when does the pathology truly begin? Although FTD is traditionally considered a mid- to late-onset disorder, with most patients presenting symptoms or receiving diagnoses in their 50s or 60s (and occasionally in their 30s or 40s), recent findings suggest that disease pathology may emerge much earlier. Some studies have detected evidence of pathological changes as early as the mid-30s, or even mid-20s. These observations raise the question of whether the pathogenesis of FTLD, particularly in genetic forms, might actually begin during neurodevelopment. The concept that a neurodegenerative disorder manifesting in mid-life may have developmental origins is supported by evidence that genetic mutations exert neurodevelopmental effects on brain structure and function []. While these effects may initially confer compensatory advantages, they may later lead to selective vulnerability in the neural networks targeted by FTLD pathology. In vitro studies have demonstrated that C9orf72 expansion carriers exhibit early reductions in thalamic volume and cortical thickness []; GRN mutation carriers display altered dendritic spine morphology and impaired synaptic plasticity []; MAPT mutation carriers show delays in neuronal migration during prenatal development. These findings support the hypothesis that FTD-related mutations initiate subtle yet measurable changes during childhood and adolescence, potentially establishing the substrate for later neurodegeneration [].
4. Defining Preclinical and Prodromal FTD
The theoretical course of the disease begins with a “no disease-stage”, switches to a “preclinical stage”, advances through a “prodromal stage”, and ultimately phenoconverts to “clinically manifest stage”. This timeline of the natural history of FTLD conceptually includes an initial healthy phase, characterized by the absence of clinical symptoms and neuropathological abnormalities. However, as abovementioned, in monogenic subtypes, this conceptualization may be challenged by the observation that certain biomarkers are already altered from birth and remain abnormal throughout young adulthood in individuals carrying pathogenic mutations. Indeed, it may be more appropriate to define the period preceding disease onset as a “susceptibility phase,” reflecting a state of biological vulnerability to environmental or intrinsic disease-triggering factors.
From a biological standpoint, disease onset is conceptually marked by the occurrence of first signs of protein misfolding, which defines the beginning of the preclinical stage. This phase involves an active, ongoing neuropathological process but ever without clinical abnormalities. The gradual accumulation of misfolded proteins leads to neuronal dysfunction via multiple cellular mechanisms, including impaired mitochondrial function, stress granule dysregulation, defective autophagy, and altered transcriptional processes. This progressive dysfunction ultimately culminates in neuronal loss, i.e., neurodegeneration. Therefore, the sequence of protein misfolding, cellular dysfunction, and neuronal death constitutes the successive phases of the preclinical stage, preceding the emergence of clinical symptoms. The subsequent prodromal stage is defined by the onset and gradual progression of subtle clinical changes, resulting from the ongoing accumulation of toxic protein aggregates, neuronal dysfunction, and neurodegeneration, particularly in disease-specific brain regions. These symptoms, although observable by patients, caregivers, or clinicians as significant deviations from prior functioning, do not yet fulfil the diagnostic criteria for a formal FTLD diagnosis []. Finally, phenoconversion denotes the transition to the clinically manifest stage, characterized by the onset of mild cognitive, behavioral, or motor symptoms (MCBMI) that meet established diagnostic thresholds.
Regarding the presymptomatic stage, which encompasses the preclinical and prodromal stages, current research efforts aim to identify the earliest biological hallmarks of disease. Although protein misfolding and the formation of neurotoxic oligomers are conceptually considered the first pathophysiological events, the most detectable feature remains the intracellular accumulation of pathogenic protein aggregates. However, reliable In vivo biomarkers capable of quantifying FTLD-related proteinopathies at this early stage are not yet available. Consequently, another major research goal is to detect and quantify the earliest signs of neuronal dysfunction and to track the subsequent progression of neurodegeneration. Moreover, given the complex clinical presentation of FTD, which can be heralded by different phenotypes, defining the prodromal stage of FTD is fraught with difficulties. Recent efforts have focused on characterizing early cognitive and behavioural symptoms under the frameworks of mild cognitive impairment (MCI) [,,,,] and mild behavioural impairment (MBI) []. However, the more inclusive term “mild cognitive and/or behavioural and/or motor impairment” (MCBMI) has been proposed to encompass the full spectrum of early manifestations under a single, unified classification []. For bvFTD specifically, research criteria have been proposed to delineate the prodromal stage and harmonize early-case identification [].
The unification of FTLD’s pleiotropic clinical manifestations into parallel phenotypic axes, including frontotemporal (encompassing behavioural, and cognitive features), and motor (extrapyramidal and pyramidal), would offer a more precise framework than the traditional unitary approach of combining all symptoms into a single diagnostic category. This phenotypic axis model, like the “Miami Framework” proposed for ALS [], would facilitate more subtle understanding of disease progression and symptomatology. Such a shift in classification also demands standardization of the terminology used to describe these domains, both in terms of the domains themselves and the degree of deviation from normal functioning. Throughout the disease course, individuals may present a variety of signs and symptoms to varying extents, meaning that a patient may fall at different points along each of the three axes. For instance, individuals with clinically manifest motor impairment often also exhibit mild cognitive and behavioural symptoms. Identifying group-level differences can guide the prediction of domain-specific impairments in individual patients, but it is equally important to capture sub-threshold abnormalities. These may represent the earliest clinical indicators during the prodromal stage or reflect subtle longitudinal variations throughout FTLD progression.
From a pathological perspective, this shift toward early detection underscores the need for robust biomarkers that reflect distinct aspects of the underlying pathology and biology of neurodegeneration. With this shift toward earlier detection and intervention, the evolving clinical and diagnostic framework must centre on biomarkers. These will be essential for identifying disease in its presymptomatic and prodromal stages, and for reflecting the underlying biology and pathology of neurodegeneration with increasing precision.
5. Clinical Characterization and Early Symptomatology
According to the proposed unifying framework of frontotemporal lobar degeneration, which conceptualizes the disease along parallel phenotypic axes, namely, the frontotemporal axis (encompassing cognitive and behavioural features) and the motor axis (including extrapyramidal and pyramidal signs), specific signs and symptoms should be analysed in relation to clinical characterization and early presentation (Figure 1B). In this context, genetic forms of FTD provide a unique opportunity to investigate the pathophysiological mechanisms underlying the disease.
Analysis of the frontotemporal axis highlights cognition and behaviour as key domains, with particular emphasis on neuropsychiatric manifestations. The cognitive prodromal phase of FTD typically begins with gradual and progressive executive dysfunction, which may occur in isolation or alongside other subtle cognitive changes, such as impairments in social cognition or language []. However, comprehensive neuropsychological assessments have demonstrated that a more global cognitive decline may already be present during the asymptomatic and prodromal phases of genetic FTD. Specifically, standardized tests assessing attention, executive function, language, and memory have revealed clear baseline differences between mutation carriers and non-carriers, although the rate of cognitive decline varies depending on the specific genetic mutation and stage of disease. Cross-sectional analyses of individuals carrying FTD-related mutations at baseline have revealed intergroup differences, suggesting that during the prodromal stage C9orf72 expansion carriers may be more severely affected. These individuals exhibit notable deficits in cognitive and behavioural functions compared to non-carriers, particularly in the domains of attention, executive function, and verbal fluency []. During the prodromal phase, also episodic memory deficits are relatively common, with gene-specific patterns: MAPT mutation carriers demonstrate poorer performance in list recall, recognition memory, and both nonverbal and semantic memory tasks; C9orf72 carriers show reduced psychomotor speed; while GRN carriers display more variable cognitive profiles [,]. Language impairment is present across all three major genetic forms of FTD, with overlapping but distinct characteristics. Reduced letter fluency often emerges first, followed by difficulties in planning, judgment, cognitive flexibility, and increasing perseveration. Word retrieval deficits are observed in all three genetic groups, whereas significant impairments in grammar and syntax are more prominent in C9orf72 and GRN carriers. Articulatory deficits are specific to C9orf72. Category fluency impairment is seen in both C9orf72 and MAPT carriers, though only the latter group exhibits significantly reduced performance on naming tasks [,]. In the subsequent stage, corresponding to mild cognitive impairment, C9orf72 and GRN carriers often show deficits in social cognition. GRN carriers also experience executive dysfunction, while MAPT carriers predominantly exhibit memory deficits [,]. Longitudinal studies have identified gene-specific cognitive trajectories: during MCI, GRN carriers show declines in verbal fluency and visuoconstruction, while MAPT carriers experience progressive language deterioration. At the dementia stage, attention and language impairments are common across all mutation groups. Verbal fluency remains affected in C9orf72 and GRN carriers, social cognition in GRN carriers, and executive function in MAPT carriers []. Executive dysfunction emerges very early in individuals with C9orf72 mutations and worsens with disease progression, whereas similar impairments typically appear only in the later stages in GRN and MAPT mutation carriers [].
The behavioural prodromal phase may include symptoms such as inertia and apathy, disinhibition and asocial behaviour, loss of empathy, compulsiveness with perseveration and stereotypies, and changes in eating habits (e.g., hyperorality, hyperphagia, stereotyped eating patterns) [,,,,,,]. Additional commonly observed features include irritability, loss of insight, and, in some cases, psychosis. Interestingly, early conceptualizations of FTD, such as those proposed by the Lund–Manchester group, included so-called “early and evanescent” symptoms like depression, anxiety, suicidality, sentimentality, delusions, hypochondriasis, and somatic preoccupation, which were observed to evolve into emotional blunting []. Although not formal diagnostic criteria, these early symptoms may reflect the initial phase of a broader neuropsychiatric evolution []. FTD can present subtly and may initially resemble primary psychiatric disorders. Symptoms such as anhedonia, psychomotor slowing, low motivation, and fatigue are commonly seen in depression; logorrhoea, distractibility, and impulsivity may mimic mania; compulsions can resemble obsessive–compulsive disorder; and disorganized speech, affective flattening, logopenia, and lack of motivation are reminiscent of schizophrenia-like syndromes []. Genotype–phenotype correlations have elucidated specific patterns of neuropsychiatric symptoms across the genetic subtypes. Apathy and depression are prevalent across C9orf72, GRN, and MAPT mutations. Disinhibition and compulsive behaviours are more prominent in MAPT carriers, psychosis is characteristic of C9orf72, and irritability is particularly frequent in GRN carriers. These findings support a tentative chronology of neuropsychiatric symptomatology for each genotype: C9orf72 carriers may progress through psychosis, apathy, anxiety, and depression; GRN carriers through apathy, depression, and irritability; and MAPT carriers through anxiety, compulsions, and disinhibition [,,,,,]. Prospective cohorts of prodromal FTD identify baseline clinical features that predict progression and conversion to dementia []. The behavioral domain represents a predominant component of the clinical spectrum of FTLD, to the extent that the bvFTD is the most frequent clinical phenotype in both sporadic and genetic forms of the disease. This has led to recent efforts to develop research diagnostic criteria for prodromal bvFTD in a cohort of early symptomatic (“prodromal”) individuals carrying a pathogenic MAPT, GRN, or C9orf72 mutation who subsequently progressed to overt bvFTD, compared to healthy control subjects, defined here as non-mutation carrier family members. A diagnosis of possible MBCI-FTD requires the presence of either three core features, or two core features plus one supportive feature; a diagnosis of probable MBCI-FTD additionally requires imaging or biomarker evidence, or the presence of a pathogenic genetic mutation. Seven core features have been identified: apathy without moderate-to-severe dysphoria, behavioral disinhibition, irritability/agitation, reduced empathy/sympathy, repetitive behaviours (simple and/or complex), joviality/gregariousness, and appetite changes/hyperorality. Supportive features include a neuropsychological profile showing impaired executive function or naming ability with preserved orientation and visuospatial skills, reduced awareness of cognitive or behavioral changes, and poor social cognition. The proposed MBCI-FTD criteria correctly classified 95% of the prodromal bvFTD Development Group and 74% of the prodromal bvFTD Validation Group, with a false positive rate of <10% among healthy controls [].
Regarding motor axes, pyramidal and extrapyramidal motor symptoms are essential components to be looked at. Motor symptoms are common in genetic FTD, affecting C9orf72 (31.7%) more than MAPT (19.3%) or GRN (18.8%) mutation carriers. Within the genetic mutation groups, C9orf72 expansion carriers present features mostly suggestive of ALS, including both limb and bulbar features, whereas GRN and MAPT mutation carriers are more likely to exhibit parkinsonian features, with minimal bulbar involvement.
During the prodromal stage, the most frequently reported motor impairments include weakness, gait disturbances, and functional difficulties using the hands. However, slowness and gait abnormalities are the most consistent motor features, both across and within genetic groups, and their frequency increases with disease progression, rising from 6% in asymptomatic individuals to 21% during the prodromal phase, and up to 63% at the symptomatic stage. These motor deficits may reflect extrapyramidal pathology (e.g., bradykinesia) or pyramidal involvement (e.g., weakness, rigidity), as well as overlap with motor neuron disease. Other observed symptoms include dysarthria, dysphagia, tremor, and falls [].
Beyond the genetic forms, where the clinical spectrum, although complex, is more amenable to systematic investigation and standardization of onset and phenotypic progression, FTD remains a type of dementia characterized by a markedly heterogeneous clinical presentation [,]. This extensive variability makes the identification and definition of prodromal symptoms particularly challenging and highly dependent on the specific FTD subtype.
While the availability of large genetic cohorts such as GENFI and ALLFTD has substantially advanced our understanding of early disease trajectories, current prodromal FTD definitions remain largely derived from these familial populations, potentially introducing diagnostic and selection bias. This reliance may limit generalizability to sporadic cases, which often present with more heterogeneous onset profiles, variable comorbidities, and environmental influences []. Comparative studies directly contrasting sporadic and genetic FTD remain scarce, though recent evidence suggests overlapping neuropsychological signatures but divergent biomarker trajectories between the two groups []. Addressing this imbalance will be critical to refining prodromal staging frameworks and ensuring applicability across the full FTLD spectrum.
Accurate and early diagnosis of FTD requires not only a refined clinical framework but also the systematic implementation of validated and standardized assessment tools.
Given the clinical and genetic heterogeneity of FTD phenotypes, it is essential to employ cognitive, behavioural, and motor assessments that are sensitive to the subtle prodromal manifestations of both familial and sporadic variants.
From a methodological standpoint, many existing cognitive and functional rating instruments were originally developed for Alzheimer’s disease and thus fail to capture the distinct behavioral, language, and social cognition changes characteristic of FTD.
Harmonization of testing protocols and interpretive criteria is critical to enable comparability across studies and clinical settings. This includes aligning severity descriptors with the actual degree of functional and pathological involvement, as determined by normative reference data. Standardization of test batteries and scoring methods will support early detection, longitudinal tracking, and phenotypic stratification across the FTLD spectrum [,,].
To date, the CDR scale remains the most widely used tool to assess dementia severity. However, it is not well-suited for capturing the clinical subtleties of FTD. As such, modified versions like the CDR plus NACC FTLD [,,] and the FTD rating scale have been introduced to improve sensitivity to FTD-specific domains. The Clinical Dementia Rating (CDR) and its FTLD-modified version offer useful staging metrics, yet their sensitivity in the prodromal phase remains suboptimal, particularly for non-amnestic and behavioral presentations []. Recent psychometric analyses have highlighted a potential ceiling effect in early disease and a limited capacity to differentiate mild bvFTD from psychiatric phenocopies. Novel multidomain tools, such as the Amsterdam IADL Questionnaire (A-IADL-Q) [], the MiND-SET battery for social and emotional cognition, the Global Assessment of Functioning (GAF) [] and digital behavioral monitoring platforms, show promise in capturing subtle, ecologically valid manifestations []. A comprehensive cognitive assessment should encompass evaluation of multiple domains. Language testing should include object naming, conversational abilities, comprehension, repetition, fluency, reading, and writing. Executive functions should be assessed through tasks evaluating planning, organization, working memory, verbal fluency, and symbol-digit substitution. Social cognition measures should address theory of mind, empathy, perspective-taking, mental flexibility, apathy, self-awareness, and emotional recognition, with additional neuropsychological testing when needed. Visuoperceptual and spatial skills should be examined through figure copying and angle discrimination. Episodic memory evaluation should include both verbal and visual learning tasks. Attention should be assessed using sustained attention paradigms, such as responding selectively to specific stimuli within a time-limited sequence []. The Wechsler Memory Scale-Revised (WMS-R) Digit Span Backwards (DSB) can be employed to measure auditory working memory and attention [], the Wechsler Adult Intelligence Scale-Revised Digit Symbol task to assess processing speed and visual attention, the Trail Making Test (Parts A & B) to evaluate visual attention, processing speed, and cognitive flexibility [], and the Delis-Kaplan Executive Function System Color Word Interference Test to examine cognitive flexibility and inhibitory control []. The A-IADL-Q, available in both 70- and 30-item versions, benefits from strong psychometric properties and accounts for the functional impact of motor symptoms, though it is not specific to FTD. The GAF, currently used in psychiatric settings, could be adapted to incorporate anchors for motor, cognitive/behavioural, and functional dimensions. Cognitive assessment should rely on standardized tools that minimize the confounding influence of motor impairments [,]. Instruments such as the Edinburgh Cognitive and Behavioural ALS Screen (ECAS) and the ALS Cognitive Behavioural Screen (ALS-CBS) have proven effective in ALS and may serve as useful models for FTD, particularly during the prodromal phase. Nevertheless, overlapping cognitive domains complicate interpretation. For instance, impaired performance on memory tasks may stem from executive dysfunction rather than primary memory deficits. This underscores the need for consensus-driven, domain-specific definitions and validated outcome measures applicable across both clinical and research settings. Clarifying the temporal dynamics and hierarchical involvement of cognitive domains is critical for improving diagnostic specificity and establishing reliable clinical trial endpoints. Although memory impairments have been reported in FTLD, these findings must be interpreted cautiously, as many memory tests also engage executive processes. A refined cognitive taxonomy is therefore needed to distinguish primary deficits from secondary effects and to better characterize disease progression.
The scales used to assess the behavioural domain include behavioural and neuropsychiatric questionnaires. The Neuropsychiatric Inventory Questionnaire (NPI-Q) [] comprises items such as apathy/indifference, depression/dysphoria, delusions, hallucinations, disinhibition, irritability/lability, agitation/aggression, anxiety, night-time behaviours, elation/euphoria, motor disturbances, and appetite/eating changes. Each feature was rated as present (mild, moderate, or severe) or absent. The 15-item version of the Geriatric Depression Scale (GDS) [] was employed as a measure of depressive symptoms, particularly dysphoria. Self-awareness represents a key aspect for patient management and caregiver support. This dimension was investigated using the Interpersonal Reactivity Index (IRI), specifically the Empathic Concern and Perspective Taking subscales, as a measure of informant-reported empathy [].
Assessment of motor function would also benefit from integration of digital biomarkers and wearable sensor technologies capable of detecting subtle, early motor changes in real-world settings. These tools could provide ecologically valid, continuous data to support longitudinal tracking of disease evolution from the prodromal phase onward.
Despite recent progress, defining the prodromal stage of FTD remains challenging. Current diagnostic frameworks, particularly the Barker [] and Benussi [] criteria, represent an important step toward operationalizing early disease stages but also have notable limitations. Their reliance on the FTLD-CDR plus NACC FTLD score of 0.5 may restrict sensitivity and specificity for detecting the earliest subclinical manifestations and introduces a potential cohort bias, as most validation studies were conducted in familial or research-based populations.
Recent research efforts have therefore focused on integrating multimodal and longitudinal indicators, including subtle changes in social cognition, apathy, executive function, and digital or ecological behavioral metrics, to refine the delineation of the prodromal phase. Incorporating biomarker-supported staging models and neuroimaging markers of network dysfunction (e.g., salience and default-mode connectivity) could further enhance early diagnostic precision and allow for the identification of preclinical conversion markers.
In this context, the tri-axial model, encompassing the frontotemporal (cognitive–behavioral), motor (pyramidal and extrapyramidal), and neuropsychiatric axes, offers a comprehensive perspective to capture the heterogeneity of prodromal manifestations and to align clinical characterization with underlying pathophysiological mechanisms. However, harmonized methodologies that integrate both familial and sporadic cases are still lacking. Future validation efforts should systematically compare genetic and sporadic cohorts using standardized behavioral, cognitive, and biomarker endpoints, to delineate shared versus divergent prodromal trajectories. This approach would not only mitigate cohort bias but also facilitate the cross-validation of staging instruments. Developing FTD-specific composite indices that integrate cognitive, behavioural, and functional measures, analogous to AD composite scores, may ultimately enhance diagnostic granularity and trial readiness.
In conclusion, the development and validation of harmonized, domain-specific, and disease-sensitive assessment tools, along with standardized terminology and scoring systems, are imperative for advancing diagnostic precision, enhancing longitudinal phenotyping, and guiding targeted therapeutic strategies across the FTLD disease spectrum.
6. Biomarkers for Preclinical and Prodromal FTD
Diagnosing FTLD pathology and differentiating it from related diseases that often co-exist in co-pathology, such as sporadic AD [] or the non-neurodegenerative primary psychiatric disorders [], as well as identifying prodromal cases within the FTLD spectrum and monitoring disease progression, represent key goals for improving diagnostic precision. Specifically, Neuroimaging and fluid biomarkers represent pivotal tools for both research and clinical practice FTLD. Beyond their role in early and differential diagnosis, these modalities provide unique In vivo insights into the pathophysiological processes underlying disease progression. Structural and functional imaging techniques enable the detection of subtle regional brain changes long before clinical onset, while advanced fluid biomarkers, including neurofilament light chain (NfL) and emerging markers of synaptic and glial dysfunction, offer sensitive indicators of neuronal injury and disease activity.
Importantly, the integration of multimodal biomarker data is transforming the clinical management of FTLD, from improving diagnostic accuracy and staging to monitoring disease progression and evaluating therapeutic efficacy in clinical trials. As the field moves toward precision medicine, neuroimaging and fluid biomarkers are becoming indispensable for defining individualized disease trajectories and identifying windows of therapeutic opportunity during the preclinical and prodromal phases.
6.1. Fluid Biomarkers (Figure 1C)
6.1.1. Tauopathy
FTLD has been included as a contrast group in many AD studies of tau and amyloid beta (Aβ) fluid biomarkers providing relevant information. CSF biomarkers have shown potentiality both differentiating FTLD from other dementias and non-degenerative disorders and utility in distinguish among FTLD spectrum.
Tau-associated FTLD subtypes do not typically show elevated cerebrospinal fluid (CSF) levels of total tau (t-tau) or phosphorylated tau at residues 181 or 217 (p-tau181 and p-tau217, respectively), unlike AD, where these markers are significantly increased and disease-specific [,,,,]. In FTLD, CSF t-tau and p-tau181 levels have been inconsistently reported as either elevated or reduced [,]. Accordingly, an increased CSF tau/Aβ42 ratio is a useful tool to distinguish AD from FTLD, showing high diagnostic accuracy (specificity of 70% in AD vs. bvFTD, and 86% in AD vs. svPPA) [,,]. In FTLD, CSF levels of Aβ species (Aβ38, Aβ40, Aβ42) and soluble amyloid precursor protein-β (sAPPβ) are generally reduced compared to controls [,], but the CSF Aβ42/Aβ40 ratio remains within normal range in FTLD, differentiating it from AD, which typically shows a selective reduction in Aβ42 [].
Among FTLD spectrum, lower CSF p-tau levels may help discriminate FTLD-TDP from FTLD-tau in sporadic, pure FTLD cases after excluding AD []. The CSF p-tau/t-tau ratio is decreased in FTLD-TDP, distinguishing it from FTLD-tau with sensitivity and specificity around 82% and 62%, respectively, particularly in cases without co-pathology [,,,,,]. Specific tau isoforms such as 4R tau species (MTBR-tau275 and MTBR-tau282) have shown potential in differentiating CBD from PSP [].
As regards plasma biomarkers some help arrives for the two previous goals. Plasma biomarkers such as p-tau181, p-tau217, and the p-tau181/NfL ratio are decreased in CBS, PSP, bvFTD, nfvPPA, and svPPA [,,]. The discrepancy about plasma p-tau181, p-tau217 and p-tau231 rate that is high in AD, relative to controls, but not in FTD allow to differentiate the two diseases with close to 100% accuracy using a simple blood test [,]. Interestingly, plasma p-tau181 and p-tau217 may be elevated in ALS [,,].
Emerging evidence supports the use of 4R tau seed amplification assays (SAA) from skin biopsy specimens for PSP diagnosis. The accuracy of this method depends on anatomical site and biopsy count, with a reported specificity of 93% and sensitivity of 87.5%) [,].
6.1.2. TDP-43 Proteinopathy
Despite efforts to develop fluid biomarkers for TDP-43, their specificity for FTLD pathology remains limited []. Most antibodies target phosphorylated C-terminal TDP-43, a hallmark of pathological aggregates. Phosphorylated TDP-43 detection and CSF-based RT-QuIC assays may improve specificity, though further validation is needed [,]. It is necessary to keep in mind that since TDP-43 aggregates may be found in copathology (i.e., in AD patients, in Limbic-predominant age-related TDP-43 encephalopathy [LATE], in other neurodegenerative disorders, or in some aged people) lack of specificity for TDP-43 biomarkers persists yet. However, these markers have shown promise in distinguishing FTLD and ALS from controls with >80% sensitivity and 80% specificity [,,]. In familial FTLD-TDP due to GRN mutations, both CSF and plasma PGRN levels are markedly reduced, with nearly 100% diagnostic accuracy [,,]. Glial fibrillary acidic protein (GFAP) levels are increased in blood. In C9orf72 expansion carriers, CSF poly-GP levels are elevated []. It is worth of mention that TDP-43 regulates UNC13A cryptic exon (CE) splicing; its dysfunction results in stable CE protein accumulation detectable in FTLD/ALS and AD postmortem tissue and CSF []. Moreover, A novel candidate biomarker, hepatoma-derived growth factor-like protein 2 (HDGFL2-CE) that is a protein encoded by some CE, accumulates in the amygdala of FTLD-TDP patients and correlates with earlier disease onset.
6.1.3. FET Proteinopathy
There are currently no reliable fluid biomarkers for FET pathologies due to their rarity and lack of consistent CSF/plasma detectability. CSF FUS levels have been preliminarily investigated in ALS but not in FTLD, with inconclusive outcomes [].
No markers exist for ubiquitinated protein aggregates [,]. Ideally, a combined panel measuring markers of tau, TDP-43, and FUS would allow for accurate pathologic diagnosis of FTD, but these have so far posed important challenges.
6.1.4. DPR Proteinopathy
In C9orf72 expansion carriers, CSF poly-GP is detectable in both symptomatic and pre-symptomatic stages. Levels are stable and correlate with mutation status, though not with disease progression [,].
CSF poly-GA, poly-GR, and poly-PR lack reliability due to insolubility and poor detectability []. No approved plasma biomarkers for DPRs currently exist [,].
Although cerebrospinal fluid poly-GP has emerged as a promising biomarker of target engagement in therapeutic trials, the analytical sensitivity and translational feasibility of assays for DPR remain limited. Quantification is challenged by the insolubility and conformational heterogeneity of DPR aggregates, leading to variability across assay platforms. Standardization of detection methods and cross-validation among laboratories are therefore essential steps toward the clinical implementation of DPR-based biomarkers in C9orf72-related FTLD [].
Regardless of FTLD subtype group, both CSF and blood NfL, a general biomarker for neurodegeneration, levels are elevated in CBS, PSP, bvFTD, nfvPPA and svPPA, and are extremely high in ALS. In fact, NfL are emerging as markers in the differential diagnosis of ALS (extremely high), FTD (very high), and atypical Parkinson’s syndrome (discretely high), AD (high). Thus, high levels of NfL in combination with negative AD biomarkers strongly suggest a non-AD neurodegenerative disease [,,]. Moreover, neurofilament light chain (NfL) shows promise as a blood-based biomarker for symptomatic genetic FTD carriers. It demonstrates moderate accuracy in distinguishing primary psychiatric disorders (PPD) from mild or early forms of FTD, including C9orf72 mutation carriers. Blood NfL levels are elevated in all symptomatic genetic mutations (MAPT, C9orf72, and GRN). An optimal NfL cutoff of 22.1 pg/mL (J = 0.647) distinguishes symptomatic genetic FTD from PPD with 78.5% sensitivity and 86.2% specificity, whereas a lower cutoff of 16.2 pg/mL for mildly symptomatic individuals provides 86.7% sensitivity and 73.5% specificity. These findings highlight NfL as a valuable tool for early detection and differential diagnosis within the FTLD spectrum [].
Other biomarkers include increased CSF levels of YKL-40 (CHI3L1) and GFAP, and reduced levels of CSF Aβ38, Aβ40, Aβ42, and sAPPβ [,,].
Therefore, these multiple findings suggest that the assessment of CSF and blood t-Tau, pTau (281/217/231), Aβ42, Aβ40, Aβ42/Aβ40 ratio, and the assessment of serum neurofilament (NfL) in co-evaluation of other biomarkers improves FTLD vs. AD differential diagnosis, although FTLD-specific biomarkers are still lacking.
Recent studies suggest that combining CSF NfL, YKL-40, and sAPPβ may discriminate FTLD syndromes from controls [,], since YKL40 is elevated also in other neurodegenerative disorders including AD, while sAPPβ concentrations were specifically lower in FTLD syndromes [,], with similarity of results in an autopsy-confirmed FTLD cohort [,]. Moreover, plasma pTau181 and pTau2017 are close to clinical application and CSF poly-GP may reflect target engagement in therapeutic trials. Recent findings suggest that candidates next to NfL, phosphorylated neurofilament heavy chain (pNfH), GFAP and PGRN are chitinase 1 (CHIT-1), aquaporin-4 (AQP4), pentraxins, synaptic markers (e.g., neurogranin, SNAP-25, beta-synuclein) []. CSF samples from 238 participants of the GENFI cohort, including 107 presymptomatic (44 C9orf72, 38 GRN, and 25 MAPT) and 55 symptomatic (27 C9orf72, 17 GRN, and 11 MAPT) mutation carriers, as well as 76 mutation-negative controls, were analysed using tandem mass tag proteomics, an untargeted mass spectrometric approach, to identify proteomic signatures in genetic FTLD. Among the proteins consistently altered across all three genetic forms, neuronal pentraxin 2 and fatty acid binding protein 3 were prominently affected. Notably, lysosomal proteins exhibited a marked decrease exclusively in MAPT mutation carriers, whereas other genetic groups did not show such changes. Importantly, proteomic alterations were detectable even in presymptomatic mutation carriers, underscoring the potential of CSF proteomic profiling for the early identification of biomarkers in genetic FTLD.
In addition to these pathology-biomarkers, plasma profiling of inflammatory factors, including cytokines and chemokines, has highlighted the central role of neuroinflammation in FTLD pathogenesis, particularly in genetic forms []. GRN haploinsufficiency leads—among others—to PGRN deficits, resulting in complement activation, neuroinflammation, astrogliosis, and microglial alterations, which exacerbate lysosomal dysfunction and synaptic pruning [,]. MAPT mutations, by disrupting the balance between protein phosphatase 2A (PP2A) and glycogen synthase kinase-3β (GSK-3β), similarly drive neuroinflammation, oxidative stress, and cognitive decline, while evidence suggests C9orf72 mutations contribute to inflammatory mechanisms []. Circulating microRNAs and long non-coding RNAs in GRN and C9orf72 carriers further modulate cytokine and chemokine expression []. A profiling study on a well-characterized genetic GENFI cohort revealed widespread upregulation of TNF-α, IL-7, IL-15, IL-16, and IL-17A in MAPT and GRN carriers, with additional inflammatory mediators, including IL-1α, IL-6, IL-10, IL-12/IL-23p40, eotaxin, eotaxin-3, IP-10, and MCP-4, specifically elevated in GRN carriers. Notably, IL-1α was downregulated even in presymptomatic carriers, suggesting its early reduction may serve as a longitudinal biomarker preceding clinical symptom onset [].
6.2. Neuroimaging Biomarkers
6.2.1. Magnetic Resonance Imaging (MRI)
The diagnostic value of anatomical MRI in frontotemporal lobar degeneration (FTLD) is well established and is incorporated into current diagnostic criteria. The location of maximal atrophy within behavioral and/or language-related neural networks appears to determine the clinical phenotypes of FTLD variants []. Structural MRI and diffusion tensor imaging (DTI) provide detailed insights into brain anatomy, allowing measurement of GM thickness and WM tract integrity. Importantly, MRI can detect neuroanatomical changes up to 10 years before symptom onset. Atrophy typically begins in the insular and temporal cortices (approximately 10 years before expected symptom onset), followed by the frontal cortex and subcortical areas (about 5 years before onset), the parietal and cingulate cortices (around onset), and, finally, the occipital cortex (5 years after onset) and the cerebellum (10 years after onset) []. MRI thus plays a central role in the diagnostic evaluation of FTLD, enabling both syndrome-level differentiation and inference of underlying pathology.
Across FTLD syndromes, group-level analyses of brain volume loss have revealed characteristic and relatively syndrome-specific atrophy patterns when compared with healthy controls. In bvFTD, atrophy involves the prefrontal cortex, insula, and anterior temporal lobes bilaterally. Indeed, frontal, insular, or anterior temporal atrophy (or hypometabolism on FDG-PET) is a mandatory criterion for a “probable” bvFTD diagnosis []. Within bvFTD, the subgroup of patients presenting socially maladaptive behaviours, including criminal conduct, and who tend to exhibit higher rates of disinhibition, lower levels of apathy, and better performance in verbal fluency, show reduced GM density and cortical thickness in the temporal lobe, predominantly in the left hemisphere. Structural brain damage associated with disinhibition appears to localize to the superior temporal gyrus and is accompanied by functional disconnection, as demonstrated by resting-state fMRI, between the left anterior superior temporal gyrus and widely distributed cortical regions, including the precentral sulcus and inferior frontal junction, associated with executive control []. From a network perspective, brain organization is thought to follow a developmental trajectory of increasing differentiation, while aging and disease promote “de-differentiation.” Supporting this framework, a DTI study on 90 bvFTD patients and 71 cognitively normal controls generated probabilistic tractography maps and calculated system segregation, a metric quantifying the balance of within- versus between-network connectivity. Patients with bvFTD who exhibited greater executive dysfunction showed significantly lower segregation in both the salience network and the global brain network. These findings suggest that salience network de-differentiation contributes to executive impairment in bvFTD, and more broadly, that reduced neural specialization may underlie executive dysfunction in this disorder []. In nfvPPA, atrophy predominantly involves the left inferior frontal gyrus (Broca’s area), with potential extension into the left middle and superior premotor cortices and their right-hemisphere homologues as the disease progresses. Predominant left posterior fronto-insular atrophy is considered supportive evidence for the diagnosis of nfvPPA []. In svPPA, degeneration initially affects the left anteromedial temporal lobe, subsequently extending to the right anteromedial temporal lobe and left orbitofrontal cortex. Predominant anterior temporal lobe atrophy provides strong imaging support for the diagnosis of semantic dementia []. The chronological anatomical progression of the more common sporadic FTD variants (bvFTD, nfvPPA, svPPA) from the preclinical stage to death has been investigated using large-scale neuroimaging data sharing combined with lifespan modeling of brain volume changes [] (brain charts) []. This analysis included 8022 quality-controlled MRIs from multiple databases, encompassing 107 bvFTD, 44 svPPA, and 38 nfvPPA cases []. Examination of the sequential divergence of volumetric trajectories between normal aging and FTD variants revealed that subcortical atrophy antedates focal cortical atrophy within specific behavioral and language networks, with a “radiological” prodromal phase lasting 8–10 years, defined as the interval between the first detectable structural alteration and canonical cortical atrophy. Five major stages of atrophy progression were identified for each variant. In bvFTD, these stages progressively involve: (1) bilateral amygdala; (2) bilateral striatum; (3) bilateral anterior insula; (4) bilateral hippocampus and thalamus; and (5) right temporal pole and middle temporal gyrus, bilateral prefrontal cortex (gyrus rectus, inferior frontal gyrus), orbital gyri, and frontal poles. In svPPA: (1) bilateral amygdala; (2) left temporal pole, left hippocampus, and left anterior insula; (3) left middle temporal gyrus and striatum; (4) other left temporal structures; and (5) right-lateralized temporal atrophy. In nfvPPA: (1) left striatum; (2) bilateral anterior insula; (3) left thalamus; (4) left (and subsequently right) operculum; and (5) left precuneus and cuneus. The prodromal phase lasted approximately 8 years in bvFTD (from right amygdala to right insular atrophy), 10 years in svPPA (from left amygdala to left temporal pole atrophy), and 8 years in nfvPPA (from left putamen to left insular atrophy). Across the disease course, the most severely affected structures were subcortical regions, underscoring their central role in FTLD pathophysiology. In bvFTD, the right caudate, right nucleus accumbens, and left nucleus accumbens were the most affected (divergence from healthy aging trajectories at 90 years: 9.2, 8.8, and 8.4, respectively). In svPPA, the most affected regions were the left inferior temporal gyrus, left accumbens, and left hippocampus (8.1, 7.1, and 6.5). In nfvPPA, the left accumbens, right accumbens, and left anterior insula were the most affected []. Other FTLD-related syndromes also demonstrate characteristic atrophy patterns. PSP is associated with atrophy along the dentatorubrothalamic WM pathway, extending from the dentate nucleus of the cerebellum through the superior cerebellar peduncle to the midbrain and thalamus, with occasional mild frontal involvement. CBS typically presents with asymmetric degeneration of the frontoparietal lobes, while FTD-ALS most often presents with mild frontal lobe atrophy [].
Distinct patterns of atrophy have also been identified across genetic FTLD groups, reflecting the selective vulnerability of large-scale neural networks and the progressive spread of pathological proteins through these networks as the disease advances [] (Figure 1D).
MAPT mutation carriers exhibit greater medial temporal lobe (MTL) atrophy than controls in the preclinical stage [], with early involvement of the hippocampus and amygdala, followed by the temporal lobe and, later, the insula in the prodromal phase [,,,,,,,]. A recent study in a large GENFI cohort of MAPT mutation carriers, stratified into asymptomatic, prodromal, and symptomatic groups, applied linear mixed models to measures of cortical thickness, WM integrity, and functional connectivity (FC) []. This study demonstrated that disease propagation follows a progressive, staged trajectory of structural and functional alterations, with distinct patterns at each stage. In asymptomatic carriers, early FC changes were detected in the salience network (reduced eigenvector centrality, degree, and strength) and in the visual network (increased connectivity), associated with only subtle cortical thinning in the left anterior cingulate gyrus, while WM integrity remained preserved. In the prodromal stage, FC alterations were accompanied by structural damage and WM tract disruptions in regions directly connected to early-affected networks. Cortical atrophy was observed in the left cingulate and left temporal pole with medium effect sizes. WM alterations included reduced fractional anisotropy in the rostrum of the corpus callosum, bilateral uncinate fasciculus, and left cingulum, as well as increased radial diffusivity in the left uncinate fasciculus. FC changes largely mirrored the asymptomatic-stage pattern, with additional eigenvector reductions in the frontoparietal network, increased connectivity in the sensorimotor network, and higher degree centrality in the default mode network. In symptomatic carriers, cortical thinning extended to the bilateral temporal lobes (both lateral and mesial), left cingulate, and bilateral frontal lobes, with large effect sizes. WM integrity was reduced in the anterior corpus callosum (rostrum, genu, rostral body, anterior midbody) and splenium, as well as in the bilateral uncinate and inferior longitudinal fasciculi and left cingulum. FC alterations at this stage included reduced eigenvector centrality in the dorsal attention network. The early occurrence of FC changes in the absence of structural abnormalities, as observed in the preclinical stage, may reflect both pathological processes and compensatory mechanisms. The former aligns with the concept of connectomal diaschisis, where a focal “lesion” induces distal network changes, either reductions due to pathological disruption or increases resulting from network overexcitation secondary to diminished inhibitory control. Conversely, compensatory mechanisms may involve pathology-driven disruptions in some networks that promote increased connectivity in others, highlighting the dynamic balance between resilience and vulnerability in early MAPT-related neurodegeneration. These findings align with the close pathology–phenotype correspondence: the first structural changes in the prodromal stage occur in regions and tracts associated with the earliest cognitive symptoms in MAPT carriers, including anomia and semantic or episodic memory impairment. WM integrity becomes compromised in the prodromal phase, when subtle clinical symptoms may emerge but remain insufficient for a definitive diagnosis. This pattern supports the hypothesis that tau pathology propagates along WM tracts in a prion-like manner, spreading through connected networks. Thus, the sequential progression from FC alterations to structural degeneration, consistent with the hypothesis of tau propagation along axonal pathways, provides a potential theoretical model for the development of early biomarkers. Functional networks may therefore sustain long-term compensation for a genetically determined disease, with clinical symptoms emerging only when structural damage exceeds a critical threshold [].
In GRN mutation carriers, the preclinical stage is not marked by the characteristic asymmetry observed in later phases []. During the prodromal stage, the insula is the first region affected, followed by the temporal and parietal lobes, and subsequently the striatum, typically with asymmetric involvement [,,,,,,,]. The asymmetric involvement of cerebral hemispheres gives rise to two distinct anatomical asymmetry patterns depending on the side of disease onset, each associated with differences in clinical presentation, baseline severity, and disease progression. Specifically, right-sided onset is associated with more severe baseline disease but slower progression, while left-sided onset predicts faster decline. This anatomical asymmetry is detectable in GRN carriers up to 10.4 years before symptom onset, highlighting its potential utility as a prodromal biomarker for predicting conversion in these individuals [].
C9orf72 expansion carriers show early involvement of subcortical, insular, and occipital regions, followed by progressive degeneration of the frontal and temporal lobes and, ultimately, the cerebellum [,,,,,,,]. As C9orf72 gene expansion represents the principal genetic cause of the FTD-ALS phenotype, making the assessment of corticospinal tract WM volumes a relevant neuroimaging biomarker for disease monitoring. A consistent reduction in the volumetric proportion of WM across all brainstem subregions has been observed in symptomatic carriers. Among these, patients with ALS phenotype exhibited lower WM ratios in the pons and medulla compared to patients with FTD phenotype. Clinical severity was negatively associated with WM ratios. Furthermore, C9orf72 carriers demonstrated a more pronounced age-related loss of WM compared to non-carriers, with MND patients showing significantly greater atrophy in the pons and medulla.
Patterns of atrophy in MAPT mutation carriers resemble those of FTLD-tauopathy, whereas those in GRN and C9orf72 carriers parallel FTLD-TDP-43 proteinopathy. No single visual rating scale can optimally distinguish FTLD subtypes, but frontal scales (orbitofrontal, anterior cingulate, frontoinsular) are most effective for differentiating FTLD-tau, while temporal scales (anterior and medial temporal) best identify FTLD-TDP [,,,]. WM changes tend to be more severe and widespread in FTLD-TDP-43 proteinopathies (particularly in GRN mutations), whereas FTLD-tau typically produces more localized WM involvement [,].
The recent development of lifespan brain charts has enabled the modelling of anatomical progression in genetic FTLD, combining 37,532 MRIs from healthy controls spanning the entire lifespan with 1341 MRIs from carriers of pathogenic FTLD mutations aged 18–86 years []. In C9orf72 and MAPT mutation carriers, the first significant regional brain volume differences were detected on average at 27 years of age, whereas in GRN carriers they appeared at 42 years. The presymptomatic phase, defined as the interval between the onset of anatomical changes and the average age of symptom onset, was estimated at 13 years for MAPT, 17 years for GRN, and 34 years for C9orf72 carriers. Cumulative atrophy across the lifespan was approximately twice as severe in affected regions in MAPT compared with GRN or C9orf72 carriers. However, the spatial extent of neurodegeneration was greatest in C9orf72 carriers (35 of 61 brain regions affected), compared to GRN (25 regions) and MAPT (18 regions). Chronological staging revealed that in C9orf72 carriers, atrophy begins in the thalamus and progresses to frontotemporo-insular regions, striatum, and amygdala. In GRN carriers, atrophy first involves fronto-insular areas before extending to subcortical structures. In MAPT carriers, degeneration originates in the anterior temporal pole, amygdala, and hippocampus, and subsequently progresses to fronto-insular regions and the striatum. Overall, these findings highlight that C9orf72-related FTLD is the most spatially diffuse yet slowest to emerge, MAPT-related FTLD is more focal but aggressive, and GRN-related FTLD is also rapidly progressive but with a later onset of the presymptomatic phase.
Given the need for longitudinal biomarkers to monitor disease progression, cerebral perfusion has been evaluated in presymptomatic genetic FTD. Longitudinal arterial spin labelling (ASL) MRI in 47 C9orf72, 70 GRN, and 31 MAPT carriers, compared with 158 non-carriers, revealed distinct patterns of decline. All genetic subgroups exhibited greater global GM perfusion reduction, detectable from year 1 in GRN and MAPT carriers and from year 2 in C9orf72 carriers. Regional analyses showed subgroup-specific profiles: C9orf72 carriers showed predominant right frontal hypoperfusion (pars opercularis, orbitalis, triangularis, orbitofrontal cortex) with additional involvement of right superior temporal gyrus, caudate, putamen, hippocampus, and thalamus; GRN carriers displayed more widespread, left-lateralized decline; and MAPT carriers exhibited selective hypoperfusion in the left thalamus, a region also affected in GRN and C9orf72, suggesting a shared vulnerability. These findings implicate early perfusion decline in C9orf72 and GRN carriers involved core salience network nodes (insula, anterior cingulate, posterior orbitofrontal cortex), crucial for behavioural regulation and attention, consistent with the behavioral variant FTD phenotype most frequently associated with these mutations. This vulnerability may reflect selective susceptibility of von Economo neurons, where TDP-43 pathology (the hallmark inclusion of C9orf72 and GRN FTD) has been identified in the right frontoinsular region and linked to salience network atrophy. Importantly, converters to symptomatic FTD showed greater perfusion decline than non-converters, particularly in right frontal regions, reinforcing cerebral perfusion as a potential prodromal biomarker of disease progression.
6.2.2. Positron Emission Tomography (PET) Imaging
Despite significant advances in PET imaging, proteinopathy-specific tracers for FTLD remain lacking. Tau PET ligands face several limitations, including non-specific binding and poor affinity for 3R and 4R tau isoforms. While existing tracers reliably detect AD-type tau deposits, only a few exhibit reactivity with non-AD tau pathologies. Cryo-electron microscopy has further revealed at least two distinct binding modes of these compounds to tau protofibrils, explaining their selective affinity for AD versus non-AD tau []. Several radioligands have been investigated, including ^18F-flortaucipir (^18F-AV1451), ^18F-MK6240, ^18F-THK5351, and ^18F-THK5317. These ligands can capture neuronal somatodendritic tau inclusions composed of all six isoforms observed in AD, but in vivo detection of 3R tau isoforms remains challenging due to low signal, off-target binding, and the lack of robust postmortem validation for non-AD tau aggregates. For instance, ^18F-AV1451 shows limited utility in FTLD because of its low sensitivity to 3R and 4R tau [,]; in PiD, despite AT8 immunohistochemistry–confirmed high tau burden in the temporal and parietal lobes, uptake remained relatively uniform across brain regions []. By contrast, ^18F-PI-2620 demonstrates higher affinity for 4R tau in the putamen and greater binding in PSP-RS and CBS compared with ^18F-AV1451, though its ability to detect 3R tauopathies has yet to be established []. A promising advance is ^18F-florzolotau (^18F-APN-1607, ^18F-PM-PBB3), a next-generation tau radioligand enabling high-contrast imaging of tau fibrils in both AD and non-AD tauopathies, including 3R and 4R isoforms. It has shown capacity to identify a broad spectrum of tau aggregates in vitro and in vivo and may stratify patients with clinical FTD into neuropathology-based subgroups on visual and quantitative PET assessments: 3R-like (predominant frontotemporal and frontolimbic accumulation), 4R-like (posterior cortical and subcortical accumulation), AD-like (both amyloid and tau), and tau-negative (absence of overt tau/amyloid pathology) []. However, neuropathological correlation remains limited, with only one study demonstrating significant concordance between ^18F-florzolotau PET signals and Pick body density (AT8 immunochemistry) in PiD, particularly in frontotemporal regions (most prominent the orbital and inferior frontal, superior frontal and inferior temporal cortices). []. To date, no PET tracers targeting TDP-43, FET, or UPS proteinopathies are available, though ongoing research is attempting to overcome these challenges [].
Despite remarkable progress, current in vivo tools remain limited in their ability to detect early protein misfolding events, particularly during preclinical and prodromal stages. The specificity of tau PET ligands for non-AD tau isoforms (3R and 4R) remains suboptimal due to low binding affinity, off-target signals, and limited postmortem validation. Similarly, the lack of reliable in vivo markers for TDP-43 and FET proteinopathies continues to represent a major barrier to accurate molecular classification and early therapeutic targeting [].
In contrast, FDG-PET is a well-established marker of neuronal dysfunction, detecting glucose hypometabolism early in the disease course. The correspondence between neural dysfunction and hypometabolism on PET FDG has allowed to correlate specifical pattern to the phenotype. In bvFTD, FDG-PET typically demonstrates bilateral hypometabolism in the prefrontal and anterior temporal lobes, accompanied by reduced structural and functional connectivity within and between frontotemporal regions. Several regions are almost universally involved, including the anterior cingulate cortex, anterior insula, orbital and medial frontal cortices, and the temporal poles, consistent with the concept of disease epicentres. Degeneration of basal and limbic networks is a hallmark feature of bvFTD [,]. Early phase of bvFTD typically shows frontoinsular, anterior cingulate, and orbitofrontal hypometabolism, which can be present when MRI is normal or equivocal, useful for ruling in neurodegeneration and excluding “phenocopy” cases.
In svPPA, hypometabolism predominantly affects the left anteromedial temporal lobe in early phase, with posterior spread within the same hemisphere and eventual extension to the right anteromedial temporal lobe, insula, and orbitofrontal cortex []. The nfvPPA exhibits firstly marked hypometabolism in the left posterior–inferior frontal regions, including Broca’s area (linked to agrammatism) and the superior premotor cortex (associated with apraxia of speech), with then progression into the prefrontal cortex, basal ganglia, and posterior motor cortex [].
CBS is associated with asymmetric hypometabolism involving the posterior frontal and anterior parietal lobes, in addition to basal ganglia involvement [].
In PSP-RS, mild frontal hypometabolism may be present, but the dominant features include metabolic and connectivity disruption along the dentatorubrothalamic tract, encompassing the midbrain and superior cerebellar peduncle []. Converging evidence suggests that these regional patterns of hypometabolism reflect the brain’s intrinsic functional connectivity, with disease spread occurring from epicentres through highly interconnected cortical and subcortical networks.
In regard to underlying proteinopathy (Figure 1E), FTLD-Tauopathy is typically characterized by glucose hypometabolism predominantly in frontal and temporal lobes, often symmetric, whereas FTLD-TDP proteinopathy shows more asymmetric hypometabolism, sometimes more posterior (e.g., parietal), especially in GRN mutations. Presymptomatic C9orf72 carriers show hypometabolism in frontotemporal, insular, basal ganglia, and thalamic regions [,].
Additional tools include dopaminergic imaging with ^123I-FP-CIT SPECT (DaTScan) or ^11C-DOPA PET, useful in FTLD-tau syndromes with parkinsonism (PSP, CBD), and [^11C]UCB-J PET, which quantifies synaptic density and reveals early synaptic loss. In C9orf72 carriers, [^11C]UCB-J detects thalamic synaptic loss in presymptomatic stages, with widespread frontotemporal involvement in symptomatic phases [].
Neurotransmitter pathway alterations represent also an emerging imaging biomarker in prodromal FTD. While post-mortem studies have shown dopaminergic, serotonergic, GABAergic, and glutamatergic deficits [], a recent study employing JuSpace toolbox has allowed in vivo mapping of grey matter atrophy patterns against neurotransmitter system distribution []. In prodromal stages, atrophy co-localizes with specific pathways, dopaminergic and cholinergic in C9orf72 carriers, dopaminergic and serotonergic in MAPT carriers, with no significant changes in GRN carriers, whereas symptomatic stages show widespread involvement of dopaminergic, serotonergic, and cholinergic systems across all genetic subtypes. These imaging findings align with clinical phenotypes: dopaminergic and serotonergic dysfunction contribute to impaired social cognition and empathy [], dopamine additionally to reward and emotion processing [], and serotonin to emotion regulation and social behaviour [,].
It is increasingly evident that a relationship exists between the regional burden of proteinopathy and the clinical manifestations of disease, as well as a substantial correlation between the spatial progression of proteinopathy and the functional connections originating from salient pathological epicentres. The study of these relationships provides a crucial opportunity to investigate the universality of connectivity as a scaffold for the propagation of proteinopathy.
Nevertheless, despite these advances, capturing the earliest molecular events underlying protein misfolding and aggregation in vivo remains a major challenge. The transition from physiological to pathological protein states often occurs below the detection threshold of current imaging and biochemical tools, underscoring the need for next-generation assays capable of tracking these processes at preclinical stages [].
A comprehensive evaluation of multiple biomarkers currently represents the most effective approach for improving the overall understanding of FTLD []. This strategy aids in differential diagnosis, staging disease severity, and estimating survival probability [,]. In the largest observational cohort study of genetic forms of FTLD, incorporating families from the GENFI and ALLFTD consortia, MRI and NfL have emerged as sensitive indicators in the presymptomatic stages [,]. In particular, temporal patterns and the sequence of biomarker alterations vary significantly across genetic subgroups. In carriers of the C9orf72 repeat expansion, MRI appears to be the earliest detectable biomarker, with brain volumes diverging from control values up to 40 years before the expected onset of symptoms []. Between −40 and −10 years before phenoconversion, widespread reductions in brain volume were observed across several regions of interest (ROIs), with the thalamus demonstrating the most pronounced effect []. Between −10 and 0 years from expected onset, the temporal lobe showed the greatest volumetric decline, followed by the parietal and frontal lobes. Notably, the rate of volume loss in C9orf72 carriers appears to remain relatively stable over time compared with other genetic subtypes. Plasma NfL levels begin to diverge from those of healthy controls approximately 30 years before symptom onset. In GRN mutation carriers, the earliest detectable biomarker is an elevation in plasma NfL levels, which occurs around 15 years before the expected onset of symptoms. These levels remain significantly elevated across all time intervals and show a steep increase during the symptomatic phase. MRI-detected morphometric changes follow, becoming evident approximately 5 to 10 years prior to symptom onset. These changes affect nearly all brain regions except the striatum. The earliest significant volumetric deviations are observed in the frontal and temporal lobes, approximately one year before clinical onset. During the symptomatic stage, the greatest volume loss is found in the frontal, temporal (including medial regions), insular, and striatal areas. In MAPT mutation carriers, atrophy of the medial temporal lobe (MTL) is the earliest observable biomarker, detectable around 10 years before symptom onset. The remaining temporal regions and the insula demonstrate later and partially overlapping atrophy. In the symptomatic phase, the most affected areas include the temporal, frontal, medial, insular, and striatal regions. As the disease progresses, the MTL, the rest of the temporal lobe, the striatum, and the insula exhibit accelerated volume loss. In contrast to the other groups, plasma NfL levels in MAPT carriers show a significant increase only after the onset of clinical symptoms [].
The importance of an integrated, multimodal evaluation of available biomarkers in the diagnosis of FTLD has been confirmed by a recent study which, leveraging deep learning, demonstrated that the use of a cascaded multimodal system enhances both diagnostic and prognostic accuracy, even in the early stages of disease, when multimodal data integration is applied as opposed to unimodal modelling. Notably, cerebrospinal fluid (CSF) biomarkers were shown to play a key role in driving accurate model decisions [].
A multimodal assessment remains the most effective strategy to detect the earliest changes along the disease time course and to investigate the associated pathological alterations, which may themselves represent potential biomarkers of conversion. For instance, neuroinflammation and iron accumulation have been demonstrated in FTLD; however, the timing of these processes, their clinical relevance, and whether they represent a cause or a consequence of the disease remain uncertain. This uncertainty is further compounded by the emerging role of cerebral glymphatic clearance as a potential contributor to disease pathophysiology. Recently, approval was obtained for a multimodal biomarker study employing a combination of ultra–high field (7T) MRI, blood-based biomarkers, and cerebrospinal fluid measures to investigate the role of neuroinflammation, iron accumulation, and brain clearance in FTLD, as well as to identify biomarkers capable of distinguishing FTLD-TDP from FTLD-tau [].
Despite all these advances, predicting the underlying FTLD pathology in clinically diagnosed FTD cases remains challenging due to partial discordance among clinical presentation, neuroimaging patterns, and biomarker profiles. For example, cases of bvFTD with predominant medial prefrontal or anterior cingulate atrophy on MRI may still harbor Alzheimer-type pathology, while conversely, TDP-43 pathology can manifest with relatively preserved imaging findings in early stages. Fluid biomarkers such as plasma NfL, although highly sensitive to neurodegeneration, lack disease specificity and can overlap with other dementias, further complicating the prediction of molecular pathology. To improve diagnostic accuracy in these overlapping syndromes—particularly distinguishing FTLD from Alzheimer’s disease and primary psychiatric disorders—current strategies combine neuropsychological profiling (focusing on social cognition, emotion recognition, and language features) with advanced neuroimaging approaches. Structural MRI and FDG-PET contribute to detecting frontotemporal hypometabolism or atrophy patterns, while amyloid- and tau-PET imaging assist in excluding Alzheimer’s disease in ambiguous cases. Recent machine-learning studies integrating clinical, cognitive, and multimodal biomarker data have demonstrated improved differentiation of FTLD from psychiatric phenocopies, underscoring the importance of comprehensive, multimodal diagnostic frameworks in clinical practice.
7. Innovative Interventional Approaches in Early Disease Stages
To date, the treatment of FTLD has been primarily symptomatic, with limited clinical benefits and, in some cases, potential exacerbation of symptoms. The main pharmacological classes which have been tested include acetylcholinesterase inhibitors, NMDA receptor antagonists, selective serotonin reuptake inhibitors, and antipsychotics []. The overarching aim of current research is to leverage the progressively expanding understanding of disease pathogenesis to develop disease-modifying therapies [,].
For FTLD-TDP, therapeutic strategies include inhibition of TDP-43 aggregation, enhancement of TDP-43 aggregate clearance, upregulation of PGRN levels, activation of the autophagy–lysosome system, and modulation of the ubiquitin–proteasome system. In familial FTLD-TDP, the principal genetic causes are GRN mutations and C9orf72 repeat expansions [].
In GRN mutation carriers, the therapeutic goal is to restore PGRN to physiological levels in order to normalize lysosomal function and modulate inflammatory responses.
Latozinemab, a human monoclonal antibody currently under investigation in the pivotal Phase 3 INFRONT study, targets heterozygous loss-of-function GRN mutations. It acts by blocking and downregulating the sortilin receptor, a critical regulator of plasma and CNS PGRN concentrations. Previous studies demonstrated that latozinemab effectively restored PGRN levels to the physiological range and slowed disease progression compared with matched controls. The ongoing INFRONT-3 trial is evaluating its efficacy and safety across a spectrum of disease severity, from at-risk individuals (asymptomatic but with elevated serum NfL) to symptomatic participants (mild dementia with at least one of six features required for possible bvFTD or PPA diagnosis) [].
TAK-594/DNL593 is a novel replacement therapy under evaluation in a Phase 1/2 Denali Therapeutics–Takeda study. It consists of PGRN fused to an antibody fragment targeting the transferrin receptor, engineered to cross the blood–brain barrier and deliver PGRN to lysosomes. Dose-dependent increases in CSF PGRN have been observed, consistent with CNS delivery [].
Recent human trials of gene replacement therapy in FTD-GRN (e.g., PR006) have demonstrated feasibility and favorable safety profiles, with evidence of restored progranulin levels in CSF and plasma. Gene therapy approaches include PR006 and PBFT02, single-dose adeno-associated virus–based vectors (AAV9 and AAV1, respectively) designed to deliver a functional GRN gene. These are administered via cisterna magna injection in Phase 1/2 studies (PROCLAIM and UpliFT-D). PBFT02 has shown consistent CSF PGRN elevation in all participants, plateauing by six months and remaining durable through 18 months []. AVB-101 is another single-dose gene therapy under Phase 1/2 evaluation (ASPIRE-FTD), delivering a functional GRN copy directly to the brain via bilateral MRI-guided intrathalamic infusion, aiming to restore progranulin levels in the central nervous system (CNS) [].
In C9orf72 expansion carriers, antisense oligonucleotides (ASOs) designed to reduce repeat-associated toxic products are in development. Afinersen selectively depletes V1 and V3 transcripts, suppressing poly(GP) levels in C9orf72-ALS patients in a single pilot study []. WVE-004, based on a similar mechanism, was discontinued after 24 weeks due to lack of improvement in exploratory clinical outcomes []. BIIB078, targeting toxic RNA and dipeptide repeat (DPR) proteins, failed to show significant slowing of clinical decline [].
Small-molecule approaches for C9orf72 pathology include repeat-targeting agents and autophagy modulators: TPN101, a nucleoside analog LINE-1 reverse transcriptase inhibitor, suppresses retrotransposon activity implicated in innate immune activation that contributes to the diseases. A Phase 2 (ART-AD) trial showed reductions in NfL []. Apilimod dimesylate, a phosphatidylinositol phosphate kinase inhibitor, enhances trafficking and clearance of aggregation-prone proteins []. Biological approach directed to FTDL-C9orf72 includes metabolic or autophagy modulators, i.e., metformin (safety/biomarker study in C9orf72-ALS) and trimetazidine (Phase 2a trial in ALS); iron chelation/oxidative stress modulators, i.e., deferiprone (FAIR-ALS II, Phase II/III), anti-inflammatory JAK–STAT pathway modulators, i.e., baricitinib (NADALS, Phase 1/2 basket trial in ALS and asymptomatic C9orf72 carriers); and mitochondrial modulators, such as ropinirole (ALS). Neuroimaging and neuropathological studies have shown that C9orf72 promoter hypermethylation protects the hippocampus, frontal cortex, and thalamus from neuronal loss and correlates with reduced longitudinal decline in preserved GM regions. This suggests C9orf72 promoter hypermethylation as a potential neuroprotective therapeutic target [].
Other biological approach directed to FTLD-TDP are loss-of-function repair ASOs, that include QRL-201 and BIIB105/ION541 []; neuroprotective and aggregate-clearing small molecules, that include PrimeC (ciprofloxacin + celecoxib), buntanetap (a translation inhibitor lowering TDP-43), and verdiperstat (a myeloperoxidase inhibitor) [,,]. CTx1000 is a promising gene therapy that has been shown in preclinical models to selectively target pathological TDP-43 aggregation, potentially allowing reversal of existing pathology while preserving normal TDP-43 function.
For FTLD-tau, strategies include antisense oligonucleotide suppression of MAPT, inhibition of tau phosphorylation and acetylation, stabilization of microtubules, prevention of tau aggregation, and promotion of aggregate clearance []. Tau immunotherapies: Tilavonemab and gosuranemab (anti-tau monoclonal antibodies targeting the N-terminus) were tested in PSP but discontinued after Phase 2 trials (ARISE and PASSPORT, respectively) due to lack of efficacy [,]. Bepranemab remains in clinical testing for PSP []. AADvac1, an active peptide vaccine against pathological tau, was safe, immunogenic, and slowed blood NfL increase in AD; the ADAMANT Phase 2 trial in nfvPPA is ongoing [].
The second anti FTLD-tau strategy uses tau-lowering ASOs, such as NIO752 that is being evaluated in PSP []. The third strategy is aggregation inhibitors/clearance boosters, including sodium selenate sodium that is under investigation in bvFTD [].
For FTLD-FUS, ulefnersen, an ASO targeting the neurotoxic form of FUS, is in Phase 3 (FUSION trial) in ALS-FUS patients [].
These approaches highlight the shift from symptomatic to disease-modifying interventions, underscoring the urgency of translating mechanistic insights into therapeutic innovation in FTLD.
Transcranial stimulation in FTLD, both transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS), has also highlighted therapeutic promise and biomarker potentiality []. A randomized, double-blind, sham-controlled trial showed that 2 weeks of anodal left-prefrontal tDCS in 70 participants (55 symptomatic FTD; 15 presymptomatic carriers) restored intracortical inhibitory/excitatory balance and produced measurable cognitive–behavioural gains, supporting both target-engagement and clinical effects in symptomatic and presymptomatic disease []. In PPA, multiple controlled studies indicate that tDCS augments language therapy across variants, most consistently in nonfluent/agrammatic and logopenic PPA, with improvements in trained (and sometimes untrained) items and concomitant network-level changes in connectivity [,,,,]. For TMS, prefrontal rTMS has been associated with short-term improvements in global cognition and frontal behaviours in bvFTD, and DLPFC rTMS can acutely enhance action naming in PNFA, with deep-TMS case evidence in logopenic PPA [,,]. TMS measures also proven to predict severity and 12-month decline in FTD and to highlight distinct pattern of FTD from AD [].
On the biomarker side, paired-pulse TMS metrics (e.g., SICI/ICF, LICI, SICF) correlate with clinical severity and predict 12-month functional decline better than clinical indices alone in large FTD cohorts The same neurophysiological signatures reliably distinguish FTLD from AD and, in multigroup analyses, from DLB, improving diagnostic confidence and combining additively with amyloid/tau or plasma markers [,,,,,,,,,,,,,,,,]. Finally, mechanistic neuromodulation of disease-relevant rhythms is under active investigation: a multicentre, double-blind trial of 40 Hz tACS in bvFTD (GIFTeD) is assessing safety, cognition, EEG gamma activity and multimodal imaging/biomarker readouts, underscoring the field’s shift toward objective target engagement [].
In addition to the aforementioned pharmacological strategies, non-pharmacological interventions hold substantial importance, particularly when implemented during the presymptomatic phases (preclinical and prodromal stages), to maximize their potential benefit. Early cognitive rehabilitation, psychoeducational and behavioural strategies, as well as motor rehabilitation and structured physical exercise, are essential components of a comprehensive management plan [,,,]. Initiating these interventions at the earliest stages may not only support cognitive and behavioural functioning but also help to counteract the onset or progression of even mild motor symptoms, thereby contributing to the preservation of autonomy and quality of life.
Overall, these rapidly evolving therapeutic strategies reflect a paradigm shift from purely symptomatic management toward disease-modifying interventions, emphasizing the importance of early identification, biomarker-driven patient stratification, and the integration of multimodal approaches to optimize outcomes in FTLD.
8. Discussion
Frontotemporal dementia (FTD) is now recognized as a molecular disease that begins decades before the onset of clinically manifest dementia. The definition and characterization of the presymptomatic phase, subdivided into susceptibility, preclinical, and prodromal stages, are crucial for enabling timely interventions that may modify the otherwise inevitable course of neurodegeneration. From a clinical perspective, we believe that detecting pathology in its early prodromal stage requires moving beyond the rigid phenotypic categorizations typical of the fully symptomatic phases. Instead, FTD should be conceptualized along a spectrum defined by three major clinical axes, behavioural, cognitive, and motor, that may emerge at different times, with variable intensity, and potentially at distinct points along the severity spectrum simultaneously. Consequently, the clinical definition of the prodromal phase should remain flexible and inclusive. Cognitive symptoms are generally easier to define objectively, whereas behavioral changes are more difficult to capture, as they are influenced by cultural background, sex, personality traits, and behavioral reserve. With respect to motor symptoms, the terminology also requires refinement. The commonly used label of “extrapyramidal” may be inaccurate, since many features of PSP and CBS have cortical rather than subcortical origins. A more precise classification would distinguish Parkinsonian (subcortical) from corticobasal (cortical) motor features. Similarly, relying solely on dementia-based definitions (i.e., impairment of activities of daily living) does not adequately capture subtle yet clinically meaningful behavioral alterations. In this regard, the psychiatric definition of impairment may be more appropriate, describing dysfunctions not readily controlled by the individual that cause clinically significant distress or interfere with social, occupational, or interpersonal functioning. To improve the characterization of the prodromal stage, the framework of “transitional decline” proposed by Jack et al. (2024) for Alzheimer’s disease may be effectively adapted to FTD []. In this model, transitional decline is defined as a mild but detectable change with minimal impact on daily function, characterized by: (i) performance within the normal range on objective cognitive tests; (ii) evidence of decline from the individual’s prior baseline within the past 1–3 years, persisting for at least 6 months; (iii) documentation through subtle decline on longitudinal cognitive testing, which may involve memory or other domains but still remain within normal limits; (iv) subjective reports of decline from the patient or informant; and (v) recent-onset changes in mood, anxiety, or motivation not explained by life events. Importantly, individuals remain fully independent, with no or minimal impact on activities of daily living (ADLs). The subsequent stage, cognitive impairment with early functional impact, is instead characterized by: (i) performance in the abnormal range on cognitive tests; (ii) evidence of decline from baseline, documented by self-report, observer report, or longitudinal testing; and (iii) independence in ADLs, though with detectable inefficiency or slowing in complex tasks. We argue that this transitional decline framework, applicable to both cognitive and behavioral symptoms, is more suitable for FTD than definitions based solely on subjective decline. It captures subtle yet clinically meaningful changes, identified by patients, informants, or clinicians, that still fall within normal performance ranges. This is particularly relevant given that even the most sensitive neuropsychological tests may fail to detect early changes in individuals with high cognitive or behavioral reserve.
From a neuropathological perspective, we strongly advocate for an integrated approach that lowers the temporal threshold for disease detection by combining neuroimaging, fluid biomarkers, and the expansion of international cohort studies to include both sporadic and genetic FTLD. Biomarkers are essential to the definition of the prodromal syndrome. Clinical experience and published evidence demonstrate that patients with initial cognitive or behavioural symptoms often already exhibit elevated NfL levels or abnormal FDG-PET findings. Neuroimaging is particularly valuable when combining PET, which detects early neurodegeneration with high sensitivity and wide availability, with MRI, which provides more pathology-specific signatures of atrophy and subcortical degeneration. Identifying disease epicentres may offer the neuropathological correlate of the proposed multidimensional, axis-based clinical framework: the clinical manifestations of FTD can be seen as expressions of neurodegeneration, whether through compensatory mechanisms in the earliest stages or functional failure in later ones, arising from the progressive disruption of distributed brain networks.
Alongside imaging, fluid biomarkers are critical not only for early detection of neurodegeneration in general but also for identifying proteinopathy-specific signatures. The combined use of general and pathology-specific biomarkers could substantially enhance the timeliness of diagnosis and the feasibility of applying targeted interventions. The limited specificity of early clinical features, and their overlap with psychiatric conditions, underscores the central role of biomarkers in the prodromal definition. While current biomarkers are imperfect, they remain the most effective tools available, and their application will continue to evolve, much as the ATN framework has advanced from 2018 to 2024, adapting to scientific progress. With respect to pathology-specific biomarkers, it remains premature to draw firm conclusions. Although some markers such as DPR (for C9orf72) and PGRN (for GRN) are altered very early, it is unclear whether these changes reflect the underlying pathology or simply the presence of a genetic variant. RT-QuIC assays for TDP-43 (including from olfactory mucosa) show promise but require further validation, while biomarkers for SOD1, tau, and FUS are even more preliminary.
Genetic characterization of FTD has thus far focused primarily on familial forms. Accordingly, current criteria for prodromal FTD have largely been developed in genetic cohorts, where clinicians knew whether a patient carried a pathogenic variant, potentially biasing diagnostic judgment. Given that only a few studies have directly compared sporadic and genetic FTD, it is important to acknowledge both their similarities and their differences. Therefore, exclusive reliance on genetic cases should be avoided.
The Barker [] and Benussi [] criteria, currently available for prodromal FTD, also exhibit notable limitations. These definitions rely primarily on an FTLD-CDR plus NACC FTLD global score of 0.5, which broadly but not specifically reflects a mild or prodromal stage. The Benussi criteria [] expanded this by also incorporating motor features. A modified version of the CDR plus NACC FTLD scale, which explicitly assesses neuropsychiatric, language, and motor domains, may better capture the subtle and multidimensional manifestations of prodromal FTD, though it should likely be expanded to more fully account for motor neuron disease (MND) features. Current approaches still reveal biases towards particular clinical traditions. For example, the Benussi criteria [] grouped parkinsonism and MND features under a broad “motor” axis while separating cognition and behaviour, whereas the Miami framework [] grouped cognition and behaviour together as “frontotemporal” while separating MND from extrapyramidal symptoms. Ideally, future frameworks should adopt a balanced, multidimensional approach that acknowledges all relevant clinical domains without disproportionately emphasizing any particular axis.
9. Conclusions
Frontotemporal lobar degeneration is no longer a clinical riddle to be solved only at the stage of manifest dementia. Advances in genetics, biomarker discovery, and neuroimaging have reframed it as a disease process that often begins decades before symptoms emerge, with identifiable preclinical and prodromal stages shaped by a complex interplay of molecular pathology, network vulnerability, and genetic risk. This paradigm shift brings both opportunity and responsibility: the opportunity to intervene before irreversible network breakdown, and the responsibility to develop precise, biologically anchored diagnostic frameworks that reflect disease heterogeneity.
The challenge ahead is twofold. First, to refine and validate biomarker panels (fluid, imaging, and neurophysiological) that can detect the earliest pathogenic events, distinguish between pathological subtypes, and track progression across parallel phenotypic axes. Second, to translate these insights into targeted interventions, from gene therapies and proteinopathy-directed agents to network-level neuromodulation, that are deployed at a stage when they can meaningfully alter the disease course.
Equally crucial is the harmonization of clinical assessments and the integration of digital and ecological measures, enabling sensitive, real-world tracking of cognitive, behavioural, and motor changes. For genetic forms, longitudinal multinational cohorts already have clarified the temporal sequence of biomarker change; extending such frameworks to sporadic cases will be essential.
The future of FTLD research will require coordinated, large-scale, multidisciplinary efforts that dissolve the boundaries between laboratory science, clinical neurology, and trial methodology. This means embedding biomarker endpoints in all therapeutic studies, designing prevention trials in at-risk populations, and exploring synergistic effects of pharmacological and non-pharmacological interventions.
If the last two decades were about recognising FTLD’s complexity, the next will be about mastering it, transforming early molecular insights into precision prevention and intervention strategies. The ultimate goal is not only to delay phenoconversion but to preserve brain network integrity, cognitive autonomy, and quality of life for those at risk. The tools are within reach; the task is to deploy them decisively.
Author Contributions
Conceptualization, A.B.; methodology, F.P. and A.B.; data curation, F.P. and A.B.; writing—original draft preparation, F.P. and A.B.; writing—review and editing, F.P., P.M. and A.B.; supervision, P.M. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Acknowledgments
We sincerely thank all patients and their families for their invaluable participation in the clinical trials referenced in this work. Their dedication, trust, and generosity have been essential to advancing our understanding of non-invasive brain stimulation in ALS. Without their commitment, these research efforts would not have been possible. The authors acknowledge the use of AI for language proofreading and rephrasing to improve clarity and readability. The AI tool was not involved in data analysis, interpretation, or the drawing of scientific conclusions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| A-IADL-Q | Amsterdam instrumental activities of daily living questionnaire |
| AD | Alzheimer’s disease |
| aFTLD-U | atypical frontotemporal lobar degeneration with ubiquitin-positive inclusions |
| ALS | amyotrophic lateral sclerosis |
| ALS-CBS | ALS cognitive behavioural screen |
| AQP4 | aquaporin-4 |
| ASO | antisense oligonucleotide |
| BIBD | basophilic inclusion body disease |
| bvFTD | behavioural variant frontotemporal dementia |
| C9orf72 | chromosome 9 open reading frame 72 |
| CBD | corticobasal degeneration |
| CBS | corticobasal syndrome |
| CDR | clinical dementia rating |
| CHIT-1 | chitinase 1 |
| CSF | cerebrospinal fluid |
| DaTScan | dopamine transporter scan |
| DLPFC | dorsolateral prefrontal cortex |
| DNs | dystrophic neurites |
| DPR | dipeptide repeat proteins |
| DTI | diffusion tensor imaging |
| ECAS | Edinburgh cognitive and behavioural ALS screen |
| FBS | frontobehavioural spatial syndrome |
| FDG-PET | fluorodeoxyglucose positron emission tomography |
| FTD | frontotemporal dementia |
| FTLD | frontotemporal lobar degeneration |
| FUS | fused in sarcoma |
| GAF | global assessment of functioning |
| GAIs | globular astrocytic inclusions |
| GCIs | glial cytoplasmic inclusions |
| GENFI | GENetic Frontotemporal dementia Initiative |
| GFAP | glial fibrillary acidic protein |
| GGT | globular glial tauopathy |
| GM | grey matter |
| GOIs | globular oligodendroglial inclusions |
| GRN | progranulin |
| ICF | intracortical facilitation |
| LICI | long-interval intracortical inhibition |
| lvPPA | logopenic variant primary progressive aphasia |
| MAPT | microtubule-associated protein tau |
| MBI | mild behavioural impairment |
| MCBMI | mild cognitive and/or behavioural and/or motor impairment |
| MRI | magnetic resonance imaging |
| MTL | medial temporal lobe |
| NACC | national Alzheimer’s coordinating centre |
| NCIs | neuronal cytoplasmic inclusions |
| NfL | neurofilament light chain |
| NFTs | neurofibrillary tangles |
| nfvPPA | non-fluent/agrammatic variant primary progressive aphasia |
| NIFID | neuronal intermediate filament inclusion disease |
| NOS | FUS not otherwise specified |
| NIIs | neuronal intranuclear inclusions |
| PET | positron emission tomography |
| PiD | Pick’s disease |
| PGRN | progranulin |
| PSP | progressive supranuclear palsy |
| rtvFTD | right temporal variant frontotemporal dementia |
| SAA | seed amplification assay |
| sAPPβ | soluble amyloid precursor protein beta |
| SICF | short-interval intracortical facilitation |
| SICI | short-interval intracortical inhibition |
| SPECT | single-photon emission computed tomography |
| svPPA | semantic variant primary progressive aphasia |
| tACS | transcranial alternating current stimulation |
| tDCS | transcranial direct current stimulation |
| TDP-43 | TAR DNA-binding protein 43 |
| TMS | transcranial magnetic stimulation |
| UPS | ubiquitin proteasome system |
| VCP | valosin-containing protein |
| WM | White matter |
References
- Pievani, M.; de Haan, W.; Wu, T.; Seeley, W.W.; Frisoni, G.B. Functional network disruption in the degenerative dementias. Lancet Neurol. 2011, 10, 829–843. [Google Scholar] [CrossRef]
- Russell, L.L.; Rohrer, J.D. Defining the presymptomatic phase of frontotemporal dementia. Curr. Opin. Neurol. 2023, 36, 276–282. [Google Scholar] [CrossRef]
- Onyike, C.U.; Diehl-Schmid, J. The epidemiology of frontotemporal dementia. Int. Rev. Psychiatry 2013, 25, 130–137. [Google Scholar] [CrossRef]
- Logroscino, G.; Piccininni, M.; Binetti, G.; Zecca, C.; Turrone, R.; Capozzo, R.; Tortelli, R.; Battista, P.; Bagoj, E.; Barone, R.; et al. Incidence of frontotemporal lobar degeneration in Italy: The Salento-Brescia Registry study. Neurology 2019, 92, e2355–e2363, Erratum in Neurology 2019, 93, 608. [Google Scholar] [CrossRef] [PubMed]
- Boeve, B.F.; Boxer, A.L.; Kumfor, F.; Pijnenburg, Y.; Rohrer, J.D. Advances and controversies in frontotemporal dementia: Diagnosis, biomarkers, and therapeutic considerations. Lancet Neurol. 2022, 21, 258–272. [Google Scholar] [CrossRef]
- Benussi, A.; Padovani, A.; Borroni, B. Phenotypic Heterogeneity of Monogenic Frontotemporal Dementia. Front. Aging Neurosci. 2015, 7, 171. [Google Scholar] [CrossRef]
- Borroni, B.; Benussi, A. Recent advances in understanding frontotemporal degeneration. F1000Research 2019, 8, 2098. [Google Scholar] [CrossRef]
- Josephs, K.A.; Hodges, J.R.; Snowden, J.S.; Mackenzie, I.R.; Neumann, M.; Mann, D.M.; Dickson, D.W. Neuropathological background of phenotypical variability in frontotemporal dementia Transactive response DNA binding protein of 43 kD FUS Fused in sarcoma. Acta Neuropathol. 2011, 122, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; Van Swieten, J.C.; Seelaar, H.; Dopper, E.G.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef] [PubMed]
- Gorno-Tempini, M.L.; Hillis, A.E.; Weintraub, S.; Kertesz, A.; Mendez, M.; Cappa, S.F.; Ogar, J.M.; Rohrer, J.D.; Black, S.; Boeve, B.F.; et al. Classification of primary progressive aphasia and its variants. Neurology 2011, 76, 1006–1014. [Google Scholar] [CrossRef]
- Chan, D.; Anderson, V.; Pijnenburg, Y.; Whitwell, J.; Barnes, J.; Scahill, R.; Stevens, J.M.; Barkhof, F.; Scheltens, P.; Rossor, M.N.; et al. The clinical profile of right temporal lobe atrophy. Brain 2009, 132, 1287–1298. [Google Scholar] [CrossRef]
- Edwards-Lee, T.; Miller, B.L.; Benson, D.F.; Cummings, J.L.; Russell, G.L.; Boone, K.; Mena, I. The temporal variant of frontotemporal dementia. Brain A J. Neurol. 1997, 120, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.A.; Patterson, K.; Hodges, J.R. Left/right asymmetry of atrophy in semantic dementia: Behavioral-cognitive implications. Neurology 2003, 61, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Josephs, K.A.; Whitwell, J.L.; Knopman, D.S.; Boeve, B.F.; Vemuri, P.; Senjem, M.L.; Parisi, J.E.; Ivnik, R.J.; Dickson, D.W.; Petersen, R.C.; et al. Two distinct subtypes of right temporal variant frontotemporal dementia. Neurology 2009, 73, 1443–1450. [Google Scholar] [CrossRef]
- Seeley, W.W.; Bauer, A.M.; Miller, B.L.; Gorno-Tempini, M.L.; Kramer, J.H.; Weiner, M.; Rosen, H.J. The natural history of temporal variant frontotemporal dementia. Neurology 2005, 64, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.L.; Chang, L.; Mena, I.; Boone, K.; Lesser, I.M. Progressive right frontotemporal degeneration: Clinical, neuropsychological and SPECT characteristics. Dementia 1993, 4, 204–213. [Google Scholar] [CrossRef]
- Höglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Müller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Litvan, I.; Lang, A.E.; Bak, T.H.; Bhatia, K.P.; Borroni, B.; Boxer, A.L.; Dickson, D.W.; Grossman, M.; Hallett, M.; et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013, 80, 496–503. [Google Scholar] [CrossRef]
- Strong, M.J.; Abrahams, S.; Goldstein, L.H.; Woolley, S.; Mclaughlin, P.; Snowden, J.; Mioshi, E.; Roberts-South, A.; Benatar, M.; HortobáGyi, T.; et al. Amyotrophic lateral sclerosis—frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph. Lateral Scler. Front. Degener. 2017, 18, 153–174. [Google Scholar] [CrossRef]
- Balasa, M.; Gelpi, E.; Martín, I.; Antonell, A.; Rey, M.J.; Grau-Rivera, O.; Molinuevo, J.L.; Sánchez-Valle, R.; Lladó, A.; Catalan collaborative Study Group for FTLD. Diagnostic accuracy of behavioral variant frontotemporal dementia consortium criteria (FTDC) in a clinicopathological cohort. Neuropathol. Appl. Neurobiol. 2015, 41, 882–892. [Google Scholar] [CrossRef]
- Lanata, S.C.; Miller, B.L. The behavioural variant frontotemporal dementia (bvFTD) syndrome in psychiatry. J. Neurol. Neurosurg. Psychiatry 2016, 87, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Woolley, J.D.; Khan, B.K.; Murthy, N.K.; Miller, B.L.; Rankin, K.P. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: Rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J. Clin. Psychiatry 2011, 72, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Misirocchi, F.; Zilioli, A.; Benussi, A.; Cappellari, S.; Mutti, C.; Florindo, I.; Spallazzi, M.; Parrino, L. A Novel CSF1R Mutation Mimicking Frontotemporal Dementia: A Glimpse into a Microgliopathy. Can. J. Neurol. Sci. 2023, 50, 642–644. [Google Scholar] [CrossRef]
- Riedl, L.; Mackenzie, I.R.; Förstl, H.; Kurz, A.; Diehl-Schmid, J. Frontotemporal lobar degeneration: Current perspectives. Neuropsychiatr. Dis. Treat. 2014, 10, 297–310. [Google Scholar] [CrossRef]
- Robinson, J.L.; Lee, E.B.; Xie, S.X.; Rennert, L.; Suh, E.; Bredenberg, C.; Caswell, C.; Van Deerlin, V.M.; Yan, N.; Yousef, A.; et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 2018, 141, 2181–2193. [Google Scholar] [CrossRef]
- Mackenzie, I.R.A.; Baborie, A.; Pickering-Brown, S.; Plessis DDu Jaros, E.; Perry, R.H.; Neary, D.; Snowden, J.S.; Mann, D.M. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: Classification and relation to clinical phenotype. Acta Neuropathol. 2006, 112, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, I.R.; Neumann, M.; Baborie, A.; Sampathu, D.M.; Du Plessis, D.; Jaros, E.; Perry, R.H.; Trojanowski, J.Q.; Mann, D.M.; Lee, V.M.; et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011, 122, 111–113. [Google Scholar] [CrossRef]
- Mackenzie, I.R.; Neumann, M. Reappraisal of TDP-43 pathology in FTLD-U subtypes. Acta Neuropathol. 2017, 134, 79–96. [Google Scholar] [CrossRef]
- Borroni, B.; Padovani, A. Dementia: A new algorithm for molecular diagnostics in FTLD. Nat. Rev. Neurol. 2013, 9, 241–242. [Google Scholar] [CrossRef]
- Grover, A.; Houlden, H.; Baker, M.; Adamson, J.; Lewis, J.; Prihar, G.; Pickering-Brown, S.; Duff, K.; Hutton, M. 5’ splice site mutations in tau associated with the inherited dementia FTDP-17 affect a stem-loop structure that regulates alternative splicing of exon 10. J. Biol. Chem. 1999, 274, 15134–15143. [Google Scholar] [CrossRef]
- Baker, M.; Mackenzie, I.R.; Pickering-Brown, S.M.; Gass, J.; Rademakers, R.; Lindholm, C.; Snowden, J.; Adamson, J.; Sadovnick, A.D.; Rollinson, S.; et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006, 442, 916–919. [Google Scholar] [CrossRef]
- Renton, A.E.; Majounie, E.; Waite, A.; Simón-Sánchez, J.; Rollinson, S.; Gibbs, J.R.; Schymick, J.C.; Laaksovirta, H.; van Swieten, J.C.; Myllykangas, L.; et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 2011, 72, 257–268. [Google Scholar] [CrossRef]
- DeJesus-Hernandez, M.; Mackenzie, I.R.; Boeve, B.F.; Boxer, A.L.; Baker, M.; Rutherford, N.J.; Nicholson, A.M.; Finch, N.A.; Flynn, H.; Adamson, J.; et al. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron 2011, 72, 245–256. [Google Scholar] [CrossRef]
- Pengo, M.; Alberici, A.; Libri, I.; Benussi, A.; Gadola, Y.; Ashton, N.J.; Zetterberg, H.; Blennow, K.; Borroni, B. Sex influences clinical phenotype in frontotemporal dementia. Neurol. Sci. 2022, 43, 5281–5287. [Google Scholar] [CrossRef]
- Falconer, D.S. The inheritance of liability to certain diseases, estimated from the incidence among relatives. Ann. Hum. Genet. 1965, 29, 51. [Google Scholar] [CrossRef]
- Benussi, A.; Alberici, A.; Samra, K.; Russell, L.L.; Greaves, C.V.; Bocchetta, M.; Ducharme, S.; Finger, E.; Fumagalli, G.; Galimberti, D.; et al. Conceptual framework for the definition of preclinical and prodromal frontotemporal dementia. Alzheimers Dement. 2022, 18, 1408–1423. [Google Scholar] [CrossRef]
- Whiteside, D.J.; Malpetti, M.; Jones, P.S.; Ghosh, B.C.P.; Coyle-Gilchrist, I.; van Swieten, J.C.; Seelaar, H.; Jiskoot, L.; Borroni, B.; Sanchez-Valle, R.; et al. Temporal dynamics predict symptom onset and cognitive decline in familial frontotemporal dementia. Alzheimers Dement. 2023, 19, 1947–1962. [Google Scholar] [CrossRef] [PubMed]
- De Vocht, J.; Blommaert, J.; Devrome, M.; Radwan, A.; Van Weehaeghe, D.; De Schaepdryver, M.; Ceccarini, J.; Rezaei, A.; Schramm, G.; van Aalst, J.; et al. Use of multimodal imaging and clinical biomarkers in presymptomatic carriers of C9orf72 repeat expansion. JAMA Neurol. 2020, 77, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Van Der Ende, E.L.; Bron, E.E.; Poos, J.M.; Jiskoot, L.C.; Panman, J.L.; Papma, J.M.; Meeter, L.H.; Dopper, E.G.P.; Wilke, C.; Synofzik, M.; et al. A data-driven disease progression model of fluid biomarkers in genetic frontotemporal dementia. Brain 2022, 145, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Premi, E.; Grassi, M.; Alberici, A.; Cantoni, V.; Gazzina, S.; Archetti, S.; Gasparotti, R.; Fumagalli, G.G.; Bouzigues, A.; et al. Diagnostic accuracy of research criteria for prodromal frontotemporal dementia. Alzheimers Res. Ther. 2024, 16, 10. [Google Scholar] [CrossRef]
- Barker, M.S.; Gottesman, R.T.; Manoochehri, M.; Chapman, S.; Appleby, B.S.; Brushaber, D.; Devick, K.L.; Dickerson, B.C.; Domoto-Reilly, K.; Fields, J.A.; et al. Proposed research criteria for prodromal behavioural variant frontotemporal dementia. Brain 2022, 145, 1079–1097. [Google Scholar] [CrossRef]
- Wilson, S.M.; Ogar, J.M.; Laluz, V.; Growdon, M.; Jang, J.; Glenn, S.; Miller, B.L.; Weiner, M.W.; Gorno-Tempini, M.L. Automated MRI-based classification of primary progressive aphasia variants. Neuroimage 2009, 47, 1558–1567. [Google Scholar] [CrossRef]
- Younes, K.; Borghesani, V.; Montembeault, M.; Spina, S.; Mandelli, M.L.; Welch, A.E.; Weis, E.; Callahan, P.; Elahi, F.M.; Hua, A.Y.; et al. Right temporal degeneration and socioemotional semantics: Semantic behavioural variant frontotemporal dementia. Brain 2022, 145, 4080–4096. [Google Scholar] [CrossRef]
- Ulugut Erkoyun, H.; Groot, C.; Heilbron, R.; Nelissen, A.; van Rossum, J.; Jutten, R.; Koene, T.; Van der Flier, W.M.; Wattjes, M.P.; Scheltens, P.; et al. A clinical-radiological framework of the right temporal variant of frontotemporal dementia. Brain 2020, 143, 2831–2843. [Google Scholar] [CrossRef]
- Litvan, I.; Agid, Y.; Calne, D.; Campbell, G.; Dubois, B.; Duvoisin, R.C.; Goetz, C.G.; Golbe, L.I.; Grafman, J.; Growdon, J.H.; et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome). Neurology 1996, 47, 1–9. [Google Scholar] [CrossRef] [PubMed]
- van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; Van den Berg, L.H. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef] [PubMed]
- Mioshi, E.; Lillo, P.; Yew, B.; Hsieh, S.; Savage, S.; Hodges, J.R.; Kiernan, M.C.; Hornberger, M. Cortical atrophy in ALS is critically associated with neuropsychiatric and cognitive changes. Neurology 2013, 80, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Hu, N.; Xiao, Y.; Zhang, W.; Gong, Q.; Lui, S. Comparison of Gray Matter Atrophy in Behavioral Variant Frontal Temporal Dementia and Amyotrophic Lateral Sclerosis: A Coordinate-Based Meta-Analysis. Front. Aging Neurosci. 2020, 12, 500359. [Google Scholar] [CrossRef]
- Lee Virginia, M.Y.; Zhukareva, V.; Vogelsberg-Ragaglia, V.; Wszolek, Z.; Reed, L.; Miller, B.I.; Geschwind, D.H.; Bird, T.D.; McKeel, D.; Goate, A.; et al. Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science 1998, 282, 1914–1917. [Google Scholar] [CrossRef]
- D’Souza, I.; Poorkaj, P.; Hong, M.; Nochlin, D.; Lee, V.M.Y.; Bird, T.D.; Schellenberg, G.D. Missense and silent tau gene mutations cause frontotemporal dementia with parkinsonism-chromosome 17 type, by affecting multiple alternative RNA splicing regulatory elements. Proc. Natl. Acad. Sci. USA 1999, 96, 5598. [Google Scholar] [CrossRef] [PubMed]
- Von Bergen, M.; Barghorn, S.; Li, L.; Marx, A.; Biernat, J.; Mandelkow, E.M.; Mandelkow, E. Mutations of Tau Protein in Frontotemporal Dementia Promote Aggregation of Paired Helical Filaments by Enhancing Local β-Structure. J. Biol. Chem. 2001, 276, 48165–48174. [Google Scholar] [CrossRef] [PubMed]
- Takeda, N.; Kishimoto, Y.; Yokota, O. Pick’s disease. Adv. Exp. Med. Biol. 2012, 724, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W. Neuropathology of Pick’s disease. Neurology 2001, 56 (Suppl. S4), S16–S20. [Google Scholar] [CrossRef]
- Ling, H.; O’Sullivan, S.S.; Holton, J.L.; Revesz, T.; Massey, L.A.; Williams, D.R.; Paviour, D.C.; Lees, A.J. Does corticobasal degeneration exist? A clinicopathological re-evaluation. Brain 2010, 133, 2045–2057. [Google Scholar] [CrossRef]
- Kovacs, G.G.; Lukic, M.J.; Irwin, D.J.; Arzberger, T.; Respondek, G.; Lee, E.B.; Coughlin, D.; Giese, A.; Grossman, M.; Kurz, C.; et al. Distribution patterns of tau pathology in progressive supranuclear palsy. Acta Neuropathol. 2020, 140, 99–119. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R.; Lees, A.J. Progressive supranuclear palsy: Clinicopathological concepts and diagnostic challenges. Lancet Neurol. 2009, 8, 270–279. [Google Scholar] [CrossRef]
- Ahmed, Z.; Bigio, E.H.; Budka, H.; Dickson, D.W.; Ferrer, I.; Ghetti, B.; Giaccone, G.; Hatanpaa, K.J.; Holton, J.L.; Josephs, K.A.; et al. Globular glial tauopathies (GGT): Consensus recommendations. Acta Neuropathol. 2013, 126, 537–544. [Google Scholar] [CrossRef]
- Chung, D.E.C.; Carlomagno, Y.; Cook, C.N.; Jansen-West, K.; Daughrity, L.; Lewis-Tuffin, L.J.; Castanedes-Casey, M.; DeTure, M.; Dickson, D.W.; Petrucelli, L.; et al. Tau exhibits unique seeding properties in globular glial tauopathy. Acta Neuropathol. Commun. 2019, 7, 36. [Google Scholar] [CrossRef]
- Lin, L.C.; Nana, A.L.; Hepker, M.; Hwang, J.H.L.; Gaus, S.E.; Spina, S.; Cosme, C.G.; Gan, L.; Grinberg, L.T.; Geschwind, D.H.; et al. Preferential tau aggregation in von Economo neurons and fork cells in frontotemporal lobar degeneration with specific MAPT variants. Acta Neuropathol. Commun. 2019, 7, 159. [Google Scholar] [CrossRef]
- Boeve, B.F. Links between frontotemporal lobar degeneration, corticobasal degeneration, progressive supranuclear palsy, and amyotrophic lateral sclerosis. Alzheimer Dis. Assoc. Disord. 2007, 21, S31–S38. [Google Scholar] [CrossRef]
- Janssens, J.; Van Broeckhoven, C. Pathological mechanisms underlying TDP-43 driven neurodegeneration in FTLD–ALS spectrum disorders. Hum. Mol. Genet. 2013, 22, R77–R87. [Google Scholar] [CrossRef]
- Buratti, E.; Baralle, F.E. Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Front. Biosci. 2008, 13, 867–878. [Google Scholar] [CrossRef]
- Hasegawa, M.; Arai, T.; Nonaka, T.; Kametani, F.; Yoshida, M.; Hashizume, Y.; Beach, T.G.; Buratti, E.; Baralle, F.; Morita, M.; et al. Phosphorylated TDP-43 in frontotemporal lobar degeneration and ALS. Ann. Neurol. 2008, 64, 60. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M.; et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006, 314, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, N.T.; Gozal, Y.M.; Dammer, E.B.; Xia, Q.; Duong, D.M.; Cheng, D.; Lah, J.J.; Levey, A.I.; Peng, J. Multiplex SILAC analysis of a cellular TDP-43 proteinopathy model reveals protein inclusions associated with SUMOylation and diverse polyubiquitin chains. Mol. Cell. Proteom. 2010, 9, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.J.; Hwang, A.W.; Unger, T.; Trojanowski, J.Q.; Lee, V.M.Y. Redox signalling directly regulates TDP-43 via cysteine oxidation and disulphide cross-linking. EMBO J. 2012, 31, 1241–1252. [Google Scholar] [CrossRef]
- Buratti, E. TDP-43 post-translational modifications in health and disease. Expert. Opin. Ther. Targets 2018, 22, 279–293. [Google Scholar] [CrossRef]
- Lee, E.B.; Porta, S.; Michael Baer, G.; Xu, Y.; Suh, E.R.; Kwong, L.K.; Elman, L.; Grossman, M.; Lee, V.M.; Irwin, D.J.; et al. Expansion of the classification of FTLD-TDP: Distinct pathology associated with rapidly progressive frontotemporal degeneration. Acta Neuropathol. 2017, 134, 65–78. [Google Scholar] [CrossRef]
- Neumann, M.; Lee, E.B.; Mackenzie, I.R. FTLD-TDP pathological subtypes: Clinical and mechanistic significance. Adv. Exp. Med. Biol. 2021, 1281, 201. [Google Scholar] [CrossRef]
- Gitcho, M.A.; Strider, J.; Carter, D.; Taylor-Reinwald, L.; Forman, M.S.; Goate, A.M.; Cairns, N.J. VCP Mutations Causing Frontotemporal Lobar Degeneration Disrupt Localization of TDP-43 and Induce Cell Death. J. Biol. Chem. 2009, 284, 12384–12398. [Google Scholar] [CrossRef]
- Forman, M.S.; Farmer, J.; Johnson, J.K.; Clark, C.M.; Arnold, S.E.; Coslett, H.B.; Chatterjee, A.; Hurtig, H.I.; Karlawish, J.H.; Rosen, H.J.; et al. Frontotemporal dementia: Clinicopathological correlations. Ann. Neurol. 2006, 59, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Scarioni, M.; Gami-Patel, P.; Timar, Y.; Seelaar, H.; van Swieten, J.C.; Rozemuller, A.J.M.; Dols, A.; Scarpini, E.; Galimberti, D.; Netherlands Brain Bank; et al. Frontotemporal Dementia: Correlations Between Psychiatric Symptoms and Pathology. Ann. Neurol. 2020, 87, 950. [Google Scholar] [CrossRef]
- Chornenka, K.; Hirsch-Reinshagen, V.; Perez-Rosendahl, M.; Feldman, H.; Segal-Gidan, F.; Vinters, H.V.; Mackenzie, I.R. Expanding the Phenotype of Frontotemporal Lobar Degeneration with FUS-Positive Pathology (FTLD-FUS). J. Neuropathol. Exp. Neurol. 2020, 79, 809–812. [Google Scholar] [CrossRef]
- Schmitz, A.; Pinheiro Marques, J.; Oertig, I.; Maharjan, N.; Saxena, S. Emerging Perspectives on Dipeptide Repeat Proteins in C9ORF72 ALS/FTD. Front. Cell. Neurosci. 2021, 15, 637548. [Google Scholar] [CrossRef]
- Taylor, J.P.; Brown, R.H.; Cleveland, D.W. Decoding ALS: From genes to mechanism. Nature 2016, 539, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, N.; Puangmalai, N.; Sengupta, U.; Jerez, C.; Kidd, M.; Gandhi, S.; Kayed, R. C9orf72-associated dipeptide protein repeats form A11-positive oligomers in amyotrophic lateral sclerosis and frontotemporal dementia. J. Biol. Chem. 2024, 300, 105628. [Google Scholar] [CrossRef]
- Liu, F.; Morderer, D.; Wren, M.C.; Vettleson-Trutza, S.A.; Wang, Y.; Rabichow, B.E.; Salemi, M.R.; Phinney, B.S.; Oskarsson, B.; Dickson, D.W.; et al. Proximity proteomics of C9orf72 dipeptide repeat proteins identifies molecular chaperones as modifiers of poly-GA aggregation. Acta Neuropathol. Commun. 2022, 10, 22. [Google Scholar] [CrossRef]
- Krishnan, G.; Raitcheva, D.; Bartlett, D.; Prudencio, M.; McKenna-Yasek, D.M.; Douthwright, C.; Oskarsson, B.E.; Ladha, S.; King, O.D.; Barmada, S.J.; et al. Poly(GR) and poly(GA) in cerebrospinal fluid as potential biomarkers for C9ORF72-ALS/FTD. Nat. Commun. 2022, 13, 2799. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, J.; Vercruysse, T.; Boeynaems, S.; Sicart, A.; Van Damme, P.; Daelemans, D.; Van Den Bosch, L. C9orf72-generated poly-GR and poly-PR do not directly interfere with nucleocytoplasmic transport. Sci. Rep. 2019, 9, 15728. [Google Scholar] [CrossRef]
- Mackenzie, I.R.A. The role of dipeptide-repeat protein pathology in C9orf72 mutation cases. Neuropathol. Appl. Neurobiol. 2016, 42, 217–219. [Google Scholar] [CrossRef]
- Gendron, T.F.; Chew, J.; Stankowski, J.N.; Hayes, L.R.; Zhang, Y.J.; Prudencio, M.; Carlomagno, Y.; Daughrity, L.M.; Jansen-West, K.; Perkerson, E.A.; et al. Poly(GP) proteins are a useful pharmacodynamic marker for C9ORF72-associated amyotrophic lateral sclerosis. Sci. Transl. Med. 2017, 9, eaai7866. [Google Scholar] [CrossRef]
- Rubio-Guerra, S.; Bernal, S.; Almenta, D.; Pérez-Blanco, J.; Camacho, V.; Sala, I.; Sánchez-Saudinós, M.B.; García Castro, J.; Selma-González, J.; Santos-Santos, M.Á.; et al. A Novel CHMP2B Splicing Variant in Atypical Presentation of Familial Frontotemporal Lobar Degeneration. Ann. Clin. Transl. Neurol. 2025, 12, 1894–1900. [Google Scholar] [CrossRef]
- Rizzu, P.; Van Mil, S.E.; Anar, B.; Rosso, S.M.; Kaat, L.D.; Heutink, P.; Van Swieten, J.C. CHMP2B mutations are not a cause of dementia in Dutch patients with familial and sporadic frontotemporal dementia. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2006, 141, 944–946. [Google Scholar] [CrossRef] [PubMed]
- Carbayo, Á.; Borrego-Écija, S.; Turon-Sans, J.; Cortés-Vicente, E.; Molina-Porcel, L.; Gascón-Bayarri, J.; Rubio, M.Á.; Povedano, M.; Gámez, J.; Sotoca, J.; et al. Clinicopathological correlates in the frontotemporal lobar degeneration-motor neuron disease spectrum. Brain 2024, 147, 2357–2367. [Google Scholar] [CrossRef]
- Belder, C.R.S.; Marshall, C.R.; Jiang, J.; Mazzeo, S.; Chokesuwattanaskul, A.; Rohrer, J.D.; Volkmer, A.; Hardy, C.J.D.; Warren, J.D. Primary progressive aphasia: Six questions in search of an answer. J. Neurol. 2024, 271, 1028–1046. [Google Scholar] [CrossRef] [PubMed]
- Cruts, M.; Gijselinck, I.; Van Der Zee, J.; Engelborghs, S.; Wils, H.; Pirici, D.; Rademakers, R.; Vandenberghe, R.; Dermaut, B.; Martin, J.J.; et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 2006, 442, 920–924. [Google Scholar] [CrossRef]
- Rohrer, J.D.; Guerreiro, R.; Vandrovcova, J.; Uphill, J.; Reiman, D.; Beck, J.; Isaacs, A.M.; Authier, A.; Ferrari, R.; Fox, N.C.; et al. The heritability and genetics of frontotemporal lobar degeneration. Neurology 2009, 73, 1451–1456. [Google Scholar] [CrossRef]
- Greaves, C.V.; Rohrer, J.D. An update on genetic frontotemporal dementia. J. Neurol. 2019, 266, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Strang, K.H.; Golde, T.E.; Giasson, B.I. MAPT mutations, tauopathy, and mechanisms of neurodegeneration. Lab. Investig. 2019, 99, 912–928. [Google Scholar] [CrossRef]
- Strang, K.H.; Croft, C.L.; Sorrentino, Z.A.; Chakrabarty, P.; Golde, T.E.; Giasson, B.I. Distinct differences in prion-like seeding and aggregation between Tau protein variants provide mechanistic insights into tauopathies. J. Biol. Chem. 2018, 293, 2408–2421, Erratum in J. Biol. Chem. 2018, 293, 4579. [Google Scholar] [CrossRef]
- Bang, J.; Spina, S.; Miller, B.L. Non-Alzheimer’s dementia 1: Frontotemporal dementia. Lancet 2015, 386, 1672. [Google Scholar] [CrossRef]
- Arendt, T.; Stieler, J.T.; Holzer, M. Tau and tauopathies. Brain Res. Bull. 2016, 126, 238–292. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G.; Ghetti, B.; Goedert, M. Classification of Diseases with Accumulation of Tau Protein. Neuropathol. Appl. Neurobiol. 2022, 48, e12792, Erratum in Neuropathol. Appl. Neurobiol. 2022, 48, e12821. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, J.S.; Karch, C.M.; Fan, C.C.; Bonham, L.W.; Kouri, N.; Ross, O.A.; Rademakers, R.; Kim, J.; Wang, Y.; Höglinger, G.U.; et al. Shared genetic risk between corticobasal degeneration, progressive supranuclear palsy, and frontotemporal dementia. Acta Neuropathol. 2017, 133, 825–837. [Google Scholar] [CrossRef]
- Irwin, D.J.; Cairns, N.J.; Grossman, M.; McMillan, C.T.; Lee, E.B.; Van Deerlin, V.M.; Lee, V.M.; Trojanowski, J.Q. Frontotemporal Lobar Degeneration: Defining Phenotypic Diversity Through Personalized Medicine. Acta Neuropathol. 2014, 129, 469. [Google Scholar] [CrossRef] [PubMed]
- Kouri, N.; Ross, O.A.; Dombroski, B.; Younkin, C.S.; Serie, D.J.; Soto-Ortolaza, A.; Baker, M.; Finch, N.C.A.; Yoon, H.; Kim, J.; et al. Genome-wide association study of corticobasal degeneration identifies risk variants shared with progressive supranuclear palsy. Nat. Commun. 2015, 6, 7247. [Google Scholar] [CrossRef]
- Höglinger, G.U.; Melhem, N.M.; Dickson, D.W.; Sleiman, P.M.A.; Wang, L.S.; Klei, L.; Rademakers, R.; de Silva, R.; Litvan, I.; Riley, D.E.; et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat. Genet. 2011, 43, 699–705. [Google Scholar] [CrossRef]
- Gossye, H.; Van Broeckhoven, C.; Engelborghs, S. The Use of Biomarkers and Genetic Screening to Diagnose Frontotemporal Dementia: Evidence and Clinical Implications. Front. Neurosci. 2019, 13, 757. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.M.; Nicholas, J.; Grossman, M.; McMillan, C.T.; Irwin, D.J.; Massimo, L.; Van Deerlin, V.M.; Warren, J.D.; Fox, N.C.; Rossor, M.N.; et al. Age at symptom onset and death and disease duration in genetic frontotemporal dementia: An international retrospective cohort study. Lancet Neurol. 2020, 19, 145–156, Erratum in Lancet Neurol. 2020, 19, 12. [Google Scholar] [CrossRef]
- Finch, N.; Baker, M.; Crook, R.; Swanson, K.; Kuntz, K.; Surtees, R.; Bisceglio, G.; Rovelet-Lecrux, A.; Boeve, B.; Petersen, R.C.; et al. Plasma progranulin levels predict progranulin mutation status in frontotemporal dementia patients and asymptomatic family members. Brain 2009, 132, 583–591. [Google Scholar] [CrossRef]
- Smith, K.R.; Damiano, J.; Franceschetti, S.; Carpenter, S.; Canafoglia, L.; Morbin, M.; Rossi, G.; Pareyson, D.; Mole, S.E.; Staropoli, J.F.; et al. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am. J. Hum. Genet. 2012, 90, 1102–1107. [Google Scholar] [CrossRef]
- Mackenzie, I.R.A.; Baker, M.; Pickering-Brown, S.; Hsiung, G.Y.R.; Lindholm, C.; Dwosh, E.; Gass, J.; Cannon, A.; Rademakers, R.; Hutton, M.; et al. The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain 2006, 129, 3081–3090. [Google Scholar] [CrossRef]
- Farg, M.A.; Sundaramoorthy, V.; Sultana, J.M.; Yang, S.; Atkinson, R.A.K.; Levina, V.; Halloran, M.A.; Gleeson, P.A.; Blair, I.P.; Soo, K.Y.; et al. C9ORF72, implicated in amytrophic lateral sclerosis and frontotemporal dementia, regulates endosomal trafficking. Hum. Mol. Genet. 2014, 23, 3579–3595. [Google Scholar] [CrossRef]
- Van Blitterswijk, M.; Dejesus-Hernandez, M.; Rademakers, R. How do C9ORF72 repeat expansions cause amyotrophic lateral sclerosis and frontotemporal dementia: Can we learn from other noncoding repeat expansion disorders? Curr. Opin. Neurol. 2012, 25, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Gendron, T.F.; Petrucelli, L. Disease mechanisms of c9orf72 repeat expansions. Cold Spring Harb. Perspect. Med. 2017, 8, a024224. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Libri, I.; Premi, E.; Alberici, A.; Cantoni, V.; Gadola, Y.; Rivolta, G.; Pengo, M.; Gazzina, S.; Calhoun, V.D.; et al. Differences and similarities between familial and sporadic frontotemporal dementia: An Italian single-center cohort study. Alzheimers Dement. Transl. Res. Clin. Interv. 2022, 8, e12326. [Google Scholar] [CrossRef] [PubMed]
- Pottier, C.; Küçükali, F.; Baker, M.; Batzler, A.; Jenkins, G.D.; van Blitterswijk, M.; Vicente, C.T.; De Coster, W.; Wynants, S.; Van de Walle, P.; et al. Deciphering distinct genetic risk factors for FTLD-TDP pathological subtypes via whole-genome sequencing. Nat. Commun. 2025, 16, 3914. [Google Scholar] [CrossRef]
- van der Ende, E.L.; Heller, C.; Sogorb-Esteve, A.; Swift, I.J.; McFall, D.; Peakman, G.; Bouzigues, A.; Poos, J.M.; Jiskoot, L.C.; Panman, J.L.; et al. Elevated CSF and plasma complement proteins in genetic frontotemporal dementia: Results from the GENFI study. J. Neuroinflamm. 2022, 19, 217. [Google Scholar] [CrossRef]
- Pottier, C.; Ravenscroft, T.A.; Sanchez-Contreras, M.; Rademakers, R. Genetics of FTLD: Overview and what else we can expect from genetic studies. J. Neurochem. 2016, 138 (Suppl. S1), 32–53. [Google Scholar] [CrossRef]
- Müller, U.; Höglinger, G.; Dickson, D.W. Multifactorial etiology of progressive supranuclear palsy (PSP): The genetic component. Acta Neuropathol. 2025, 149, 58. [Google Scholar] [CrossRef]
- Van Langenhove, T.; Van Der Zee, J.; Gijselinck, I.; Engelborghs, S.; Vandenberghe, R.; Vandenbulcke, M.; De Bleecker, J.; Sieben, A.; Versijpt, J.; Ivanoiu, A.; et al. Distinct Clinical Characteristics of C9orf72 Expansion Carriers Compared with GRN, MAPT, and Nonmutation Carriers in a Flanders-Belgian FTLD Cohort. JAMA Neurol. 2013, 70, 365–373. [Google Scholar] [CrossRef]
- Pottier, C.; Ren, Y.; Perkerson, R.B.; Baker, M.; Jenkins, G.D.; van Blitterswijk, M.; DeJesus-Hernandez, M.; van Rooij, J.G.J.; Murray, M.E.; Christopher, E.; et al. Genome-wide analyses as part of the international FTLD-TDP whole-genome sequencing consortium reveals novel disease risk factors and increases support for immune dysfunction in FTLD. Acta Neuropathol. 2019, 137, 879–899. [Google Scholar] [CrossRef] [PubMed]
- Van Deerlin, V.M.; Sleiman, P.M.A.; Martinez-Lage, M.; Chen-Plotkin, A.; Wang, L.S.; Graff-Radford, N.R.; Dickson, D.W.; Rademakers, R.; Boeve, B.F.; Grossman, M.; et al. Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat. Genet. 2010, 42, 234–239. [Google Scholar] [CrossRef]
- Premi, E.; Grassi, M.; Van Swieten, J.; Galimberti, D.; Graff, C.; Masellis, M.; Tartaglia, C.; Tagliavini, F.; Rowe, J.B.; Laforce, R., Jr.; et al. Cognitive reserve and TMEM106B genotype modulate brain damage in presymptomatic frontotemporal dementia: A GENFI study. Brain 2017, 140, 1784–1791. [Google Scholar] [CrossRef] [PubMed]
- Premi, E.; Formenti, A.; Gazzina, S.; Archetti, S.; Gasparotti, R.; Padovani, A.; Borroni, B. Effect of TMEM106B polymorphism on functional network connectivity in asymptomatic GRN mutation carriers. JAMA Neurol. 2014, 71, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Busch, J.I.; Unger, T.L.; Jain, N.; Skrinak, R.T.; Charan, R.A.; Chen-Plotkin, A.S. Increased expression of the frontotemporal dementia risk factor TMEM106B causes C9orf72-dependent alterations in lysosomes. Hum. Mol. Genet. 2016, 25, 2681–2697. [Google Scholar] [CrossRef]
- Van Blitterswijk, M.; Mullen, B.; Nicholson, A.M.; Bieniek, K.F.; Heckman, M.G.; Baker, M.C.; DeJesus-Hernandez, M.; Finch, N.A.; Brown, P.H.; Murray, M.E.; et al. TMEM106B protects C9ORF72 expansion carriers against frontotemporal dementia. Acta Neuropathol. 2014, 127, 397–406. [Google Scholar] [CrossRef]
- Soppela, H.; Katisko, K.; Gadola, Y.; Krüger, J.; Hartikainen, P.; Alberici, A.; Benussi, A.; Koivisto, A.; Haapasalo, A.; Remes, A.M.; et al. Modifiable potential risk factors in familial and sporadic frontotemporal dementia. Ann. Clin. Transl. Neurol. 2022, 9, 1195–1205. [Google Scholar] [CrossRef]
- Gazzina, S.; Grassi, M.; Premi, E.; Cosseddu, M.; Alberici, A.; Archetti, S.; Gasparotti, R.; Van Swieten, J.; Galimberti, D.; Sanchez-Valle, R.; et al. Education modulates brain maintenance in presymptomatic frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1124–1130. [Google Scholar] [CrossRef]
- Alladi, S.; Bak, T.H.; Shailaja, M.; Gollahalli, D.; Rajan, A.; Surampudi, B.; Hornberger, M.; Duggirala, V.; Chaudhuri, J.R.; Kaul, S. Bilingualism delays the onset of behavioral but not aphasic forms of frontotemporal dementia. Neuropsychologia 2017, 99, 207–212. [Google Scholar] [CrossRef]
- Casaletto, K.B.; Staffaroni, A.M.; Wolf, A.; Appleby, B.; Brushaber, D.; Coppola, G.; Dickerson, B.; Domoto-Reilly, K.; Elahi, F.M.; Fields, J.; et al. Active lifestyles moderate clinical outcomes in autosomal dominant frontotemporal degeneration. Alzheimers Dement. 2020, 16, 91–105. [Google Scholar] [CrossRef]
- Jucker, M.; Walker, L.C. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 2013, 501, 45–51. [Google Scholar] [CrossRef]
- Shafiei, G.; Bazinet, V.; Dadar, M.; Manera, A.L.; Collins, D.L.; Dagher, A.; Borroni, B.; Sanchez-Valle, R.; Moreno, F.; Laforce, R.; et al. Network structure and transcriptomic vulnerability shape atrophy in frontotemporal dementia. Brain 2023, 146, 321–336. [Google Scholar] [CrossRef]
- Franciotti, R.; Moretti, D.V.; Benussi, A.; Ferri, L.; Russo, M.; Carrarini, C.; Barbone, F.; Arnaldi, D.; Falasca, N.W.; Koch, G. Cortical network modularity changes along the course of frontotemporal and Alzheimer’s dementing diseases. J. Neurol. Sci. 2021, 429, 118988. [Google Scholar] [CrossRef]
- Brettschneider, J.; Del Tredici, K.; Toledo, J.B.; Robinson, J.L.; Irwin, D.J.; Grossman, M.; Suh, E.; Van Deerlin, V.M.; Wood, E.M.; Baek, Y.; et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann. Neurol. 2013, 74, 20–38. [Google Scholar] [CrossRef]
- Fatima, M.; Tan, R.; Halliday, G.M.; Kril, J.J. Spread of pathology in amyotrophic lateral sclerosis: Assessment of phosphorylated TDP-43 along axonal pathways. Acta Neuropathol. Commun. 2015, 3, 47. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Rademakers, R.; Roeber, S.; Baker, M.; Kretzschmar, H.A.; MacKenzie, I.R.A. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain 2009, 132, 2922–2931. [Google Scholar] [CrossRef] [PubMed]
- Munoz, D.G.; Neumann, M.; Kusaka, H.; Yokota, O.; Ishihara, K.; Terada, S.; Kuroda, S.; Mackenzie, I.R. FUS pathology in basophilic inclusion body disease. Acta Neuropathol. 2009, 118, 617–627. [Google Scholar] [CrossRef]
- Schludi, M.H.; May, S.; Grässer, F.A.; Rentzsch, K.; Kremmer, E.; Küpper, C.; Klopstock, T.; German Consortium for Frontotemporal Lobar Degeneration; Bavarian Brain Banking Alliance; Arzberger, T.; et al. Distribution of dipeptide repeat proteins in cellular models and C9orf72 mutation cases suggests link to transcriptional silencing. Acta Neuropathol. 2015, 130, 537–555, Erratum in Acta Neuropathol. 2015, 130, 557–558. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, I.R.; Arzberger, T.; Kremmer, E.; Troost, D.; Lorenzl, S.; Mori, K.; Weng, S.M.; Haass, C.; Kretzschmar, H.A.; Edbauer, D.; et al. Dipeptide repeat protein pathology in C9ORF72 mutation cases: Clinico-pathological correlations. Acta Neuropathol. 2013, 126, 859–879. [Google Scholar] [CrossRef]
- Quaegebeur, A.; Glaria, I.; Lashley, T.; Isaacs, A.M. Soluble and insoluble dipeptide repeat protein measurements in C9orf72-frontotemporal dementia brains show regional differential solubility and correlation of poly-GR with clinical severity. Acta Neuropathol. Commun. 2020, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, I.R.A.; Neumann, M.; Bigio, E.H.; Cairns, N.J.; Alafuzoff, I.; Kril, J.; Kovacs, G.G.; Ghetti, B.; Halliday, G.; Holm, I.E.; et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: An update. Acta Neuropathol. 2010, 119, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Götzl, J.K.; Lang, C.M.; Haass, C.; Capell, A. Impaired protein degradation in FTLD and related disorders. Ageing Res. Rev. 2016, 32, 122–139. [Google Scholar] [CrossRef]
- Finger, E.; Malik, R.; Bocchetta, M.; Coleman, K.; Graff, C.; Borroni, B.; Masellis, M.; Laforce, R.; Greaves, C.V.; Russell, L.L.; et al. Neurodevelopmental effects of genetic frontotemporal dementia in young adult mutation carriers. Brain 2022, 146, 2120–2131. [Google Scholar] [CrossRef]
- Hendricks, E.; Quihuis, A.M.; Hung, S.T.; Chang, J.; Dorjsuren, N.; Der, B.; Staats, K.A.; Shi, Y.; Sta Maria, N.S.; Jacobs, R.E. The C9ORF72 repeat expansion alters neurodevelopment. Cell Rep. 2023, 42, 112983. [Google Scholar] [CrossRef]
- Longhena, F.; Zaltieri, M.; Grigoletto, J.; Faustini, G.; La Via, L.; Ghidoni, R.; Benussi, L.; Missale, C.; Spano, P.; Bellucci, A. Depletion of Progranulin Reduces GluN2B-Containing NMDA Receptor Density, Tau Phosphorylation, and Dendritic Arborization in Mouse Primary Cortical Neurons. J. Pharmacol. Exp. Ther. 2017, 363, 164–175. [Google Scholar] [CrossRef]
- Hefti, M.M.; Farrell, K.; Kim, S.H.; Bowles, K.R.; Fowkes, M.E.; Raj, T.; Crary, J.F. High-resolution temporal and regional mapping of MAPT expression and splicing in human brain development. PLoS ONE 2018, 13, e0195771. [Google Scholar] [CrossRef]
- Petersen, R.C. Mild Cognitive Impairment. Continuum 2016, 22, 404–418. [Google Scholar] [CrossRef]
- Petersen, R.C. Clinical practice. Mild cognitive impairment. N. Engl. J. Med. 2011, 364, 2227–2234. [Google Scholar] [CrossRef]
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Negash, S. Mild cognitive impairment: An overview. CNS Spectr. 2008, 13, 45–53. [Google Scholar] [CrossRef]
- Petersen, R.C.; Roberts, R.O.; Knopman, D.S.; Boeve, B.F.; Geda, Y.E.; Ivnik, R.J.; Smith, G.E.; Jack, C.R., Jr. Mild cognitive impairment: Ten years later. Arch. Neurol. 2009, 66, 1447. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Z.; Smith, E.E.; Geda, Y.; Sultzer, D.; Brodaty, H.; Smith, G.; Agüera-Ortiz, L.; Sweet, R.; Miller, D.; Lyketsos, C.G.; et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: Provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016, 12, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Benatar, M.; Wuu, J.; Huey, E.D.; McMillan, C.T.; Petersen, R.C.; Postuma, R.; McHutchison, C.; Dratch, L.; Arias, J.J.; Crawley, A.; et al. The Miami Framework for ALS and related neurodegenerative disorders: An integrated view of phenotype and biology. Nat. Rev. Neurol. 2024, 20, 364–376, Erratum in Nat. Rev. Neurol. 2024, 20, 377. [Google Scholar] [CrossRef]
- Franklin, H.D.; Russell, L.L.; Peakman, G.; Greaves, C.V.; Bocchetta, M.; Nicholas, J.; Poos, J.; Convery, R.S.; Cash, D.M.; van Swieten, J.; et al. The Revised Self-Monitoring Scale detects early impairment of social cognition in genetic frontotemporal dementia within the GENFI cohort. Alzheimers Res. Ther. 2021, 13, 127. [Google Scholar] [CrossRef]
- Lule, D.E.; Müller, H.P.; Finsel, J.; Weydt, P.; Knehr, A.; Winroth, I.; Andersen, P.; Weishaupt, J.; Uttner, I.; Kassubek, J.; et al. Deficits in verbal fluency in presymptomatic C9orf72 mutation gene carriers—A developmental disorder. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Barker, M.S.; Manoochehri, M.; Rizer, S.J.; Appleby, B.S.; Brushaber, D.; Dev, S.I.; Devick, K.L.; Dickerson, B.C.; Fields, J.A.; Foroud, T.M.; et al. Recognition memory and divergent cognitive profiles in prodromal genetic frontotemporal dementia. Cortex 2021, 139, 99–115. [Google Scholar] [CrossRef]
- Poos, J.M.; Russell, L.L.; Peakman, G.; Bocchetta, M.; Greaves, C.V.; Jiskoot, L.C.; van der Ende, E.L.; Seelaar, H.; Papma, J.M.; van den Berg, E.; et al. Impairment of episodic memory in genetic frontotemporal dementia: A genfi study. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2021, 13, e12185. [Google Scholar] [CrossRef]
- Samra, K.; MacDougall, A.M.; Bouzigues, A.; Bocchetta, M.; Cash, D.M.; Greaves, C.V.; Convery, R.S.; van Swieten, J.C.; Jiskoot, L.; Seelaar, H.; et al. Prodromal language impairment in genetic frontotemporal dementia within the GENFI cohort. J. Neurol. Sci. 2023, 451, 120711. [Google Scholar] [CrossRef]
- Bouzigues, A.; Russell, L.L.; Peakman, G.; Bocchetta, M.; Greaves, C.V.; Convery, R.S.; Todd, E.; Rowe, J.B.; Borroni, B.; Galimberti, D.; et al. Anomia is present pre-symptomatically in frontotemporal dementia due to MAPT mutations. J. Neurol. 2022, 269, 4322–4332. [Google Scholar] [CrossRef]
- Russell, L.L.; Bouzigues, A.; Convery, R.S.; Foster, P.H.; Ferry-Bolder, E.; Cash, D.M.; Van Swieten, J.C.; Jiskoot, L.C.; Seelaar, H.; Moreno, F.; et al. Executive Function Deficits in Genetic Frontotemporal Dementia. Neurol. Genet. 2025, 11, e200248. [Google Scholar] [CrossRef]
- Rohrer, J.D.; Nicholas, J.M.; Cash, D.M.; van Swieten, J.; Dopper, E.; Jiskoot, L.; van Minkelen, R.; Rombouts, S.A.; Cardoso, M.J.; Clegg, S.; et al. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: A cross-sectional analysis. Lancet Neurol. 2015, 14, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Malpetti, M.; Jones, P.S.; Tsvetanov, K.A.; Rittman, T.; van Swieten, J.C.; Borroni, B.; Sanchez-Valle, R.; Moreno, F.; Laforce, R.; Graff, C.; et al. Apathy in presymptomatic genetic frontotemporal dementia predicts cognitive decline and is driven by structural brain changes. Alzheimers Dement. 2021, 17, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Tavares, T.P.; Mitchell, D.G.V.; Coleman, K.K.L.; Coleman, B.L.; Shoesmith, C.L.; Butler, C.R.; Santana, I.; Danek, A.; Gerhard, A.; de Mendonca, A.; et al. Early symptoms in symptomatic and preclinical genetic frontotemporal lobar degeneration. J. Neurol. Neurosurg. Psychiatry 2020, 91, 975–984, Correction in J. Neurol. Neurosurg. Psychiatry 2020, 91, e3. [Google Scholar] [CrossRef]
- Nelson, A.; Russell, L.L.; Peakman, G.; Convery, R.S.; Bouzigues, A.; Greaves, C.V.; Bocchetta, M.; Cash, D.M.; van Swieten, J.C.; Jiskoot, L.; et al. The CBI-R detects early behavioural impairment in genetic frontotemporal dementia. Ann. Clin. Transl. Neurol. 2022, 9, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Foster, P.H.; Russell, L.L.; Peakman, G.; Convery, R.S.; Bouzigues, A.; Greaves, C.V.; Bocchetta, M.; Cash, D.M.; van Swieten, J.C.; Jiskoot, L.C.; et al. Examining empathy deficits across familial forms of frontotemporal dementia within the GENFI cohort. Cortex 2022, 150, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Neary, D.; Brun, A.; Englund, B.; Gustafson, L.; Passant, U.; Mann, D.M.A.; Snowden, J.S. Clinical and neuropathological criteria for frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 1994, 57, 416–418. [Google Scholar] [CrossRef]
- Cosseddu, M.; Benussi, A.; Gazzina, S.; Alberici, A.; Dell’Era, V.; Manes, M.; Cristillo, V.; Borroni, B.; Padovani, A. Progression of behavioural disturbances in frontotemporal dementia: A longitudinal observational study. Eur. J. Neurol. 2020, 27, 265–272. [Google Scholar] [CrossRef]
- Wylie, M.A.; Shnall, A.; Onyike, C.U.; Huey, E.D. Management of frontotemporal dementia in mental health and multidisciplinary settings. Int. Rev. Psychiatry 2013, 25, 230–236. [Google Scholar] [CrossRef]
- Sha, S.J.; Takada, L.T.; Rankin, K.P.; Yokoyama, J.S.; Rutherford, N.J.; Fong, J.C.; Khan, B.; Karydas, A.; Baker, M.C.; DeJesus-Hernandez, M.; et al. Frontotemporal dementia due To C9ORF72 mutations clinical and imaging features. Neurology 2012, 79, 1002–1011. [Google Scholar] [CrossRef]
- Benussi, A.; Borroni, B. Advances in the treatment and management of frontotemporal dementia. Expert. Rev. Neurother. 2023, 23, 621–639. [Google Scholar] [CrossRef]
- Devenney, E.M.; Ahmed, R.M.; Halliday, G.; Piguet, O.; Kiernan, M.C.; Hodges, J.R. Psychiatric disorders in C9orf72 kindreds study of 1,414 family members. Neurology 2018, 91, e1498–e1507, Correction in Neurology 2019, 93, 1022. [Google Scholar] [CrossRef]
- Benussi, A.; Premi, E.; Gazzina, S.; Brattini, C.; Bonomi, E.; Alberici, A.; Jiskoot, L.; van Swieten, J.C.; Sanchez-Valle, R.; Moreno, F.; et al. Progression of Behavioral Disturbances and Neuropsychiatric Symptoms in Patients with Genetic Frontotemporal Dementia. JAMA Netw. Open 2021, 4, e2030194. [Google Scholar] [CrossRef]
- Silvestri, C.; Almici, V.; Libri, I.; Mattioli, I.; Cosseddu, M.; Turrone, R.; Rivolta, J.; Grassini, C.; Caratozzolo, S.; Alberici, A.; et al. Sex Differences in the Severity and Progression of Neuropsychiatric Symptoms Across Different Dementia Types. Neurol. Clin. Pract. 2024, 14, e200299. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Ashton, N.J.; Karikari, T.K.; Alberici, A.; Saraceno, C.; Ghidoni, R.; Benussi, L.; Zetterberg, H.; Blennow, K.; Borroni, B.; et al. Prodromal frontotemporal dementia: Clinical features and predictors of progression. Alzheimers Res. Ther. 2021, 13, 188. [Google Scholar] [CrossRef]
- Samra, K.; MacDougall, A.M.; Peakman, G.; Bouzigues, A.; Bocchetta, M.; Cash, D.M.; Greaves, C.V.; Convery, R.S.; van Swieten, J.C.; Jiskoot, L.; et al. Motor symptoms in genetic frontotemporal dementia: Developing a new module for clinical rating scales. J. Neurol. 2022, 270, 1466. [Google Scholar] [CrossRef]
- Peakman, G.; Russell, L.L.; Convery, R.S.; Nicholas, J.M.; van Swieten, J.C.; Jiskoot, L.C.; Moreno, F.; Sanchez-Valle, R.; Laforce, R.; Graff, C.; et al. Comparison of clinical rating scales in genetic frontotemporal dementia within the GENFI cohort. J. Neurol. Neurosurg. Psychiatry 2021, 93, 158–168. [Google Scholar] [CrossRef]
- Öijerstedt, L.; Andersson, C.; Jelic, V.; van Swieten, J.C.; Jiskoot, L.C.; Seelaar, H.; Borroni, B.; Sanchez-Valle, R.; Moreno, F.; Laforce, R., Jr.; et al. Practice effects in genetic frontotemporal dementia and at-risk individuals: A GENFI study. J. Neurol. Neurosurg. Psychiatry 2022, 93, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Poos, J.M.; Moore, K.M.; Nicholas, J.; Russell, L.L.; Peakman, G.; Convery, R.S.; Jiskoot, L.C.; van der Ende, E.; van den Berg, E.; Papma, J.M.; et al. Cognitive composites for genetic frontotemporal dementia: GENFI-Cog. Alzheimers Res. Ther. 2022, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, T.; Brushaber, D.; Syrjanen, J.; Kremers, W.; Fields, J.; Forsberg, L.K.; Heuer, H.W.; Knopman, D.; Kornak, J.; Boxer, A.; et al. Use of the CDR® plus NACC FTLD in mild FTLD: Data from the ARTFL/LEFFTDS consortium. Alzheimers Dement. 2020, 16, 79–90. [Google Scholar] [CrossRef]
- Knopman, D.S.; Kramer, J.H.; Boeve, B.F.; Caselli, R.J.; Graff-Radford, N.R.; Mendez, M.F.; Miller, B.L.; Mercaldo, N. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain 2008, 131, 2957–2968. [Google Scholar] [CrossRef] [PubMed]
- Samra, K.; Peakman, G.; MacDougall, A.M.; Bouzigues, A.; Greaves, C.V.; Convery, R.S.; Van Swieten, J.C.; Jiskoot, L.; Seelaar, H.; Moreno, F.; et al. Extending the phenotypic spectrum assessed by the CDR plus NACC FTLD in genetic frontotemporal dementia. Alzheimers Dement. Diagn. Assess. Dis. Monit. 2024, 16, e12571. [Google Scholar] [CrossRef]
- Sikkes, S.A.M.; Knol, D.L.; Pijnenburg, Y.A.L.; De Lange-De Klerk, E.S.M.; Uitdehaag, B.M.J.; Scheltens, P. Validation of the amsterdam IADL questionnaire©, a new tool to measure instrumental activities of daily living in dementia. Neuroepidemiology 2013, 41, 35–41. [Google Scholar] [CrossRef]
- Aas, I.M. Global Assessment of Functioning (GAF): Properties and frontier of current knowledge. Ann. Gen. Psychiatry 2010, 9, 20. [Google Scholar] [CrossRef]
- Tanguy, D.; Batrancourt, B.; Estudillo-Romero, A.; Baxter, J.S.H.; Le Ber, I.; Bouzigues, A.; Godefroy, V.; Funkiewiez, A.; Chamayou, C.; Volle, E.; et al. An ecological approach to identify distinct neural correlates of disinhibition in frontotemporal dementia. Neuroimage Clin. 2022, 35, 103079. [Google Scholar] [CrossRef]
- Grossman, M.; Seeley, W.W.; Boxer, A.L.; Hillis, A.E.; Knopman, D.S.; Ljubenov, P.A.; Miller, B.; Piguet, O.; Rademakers, R.; Whitwell, J.L.; et al. Frontotemporal lobar degeneration. Nat. Rev. Dis. Primers 2023, 9, 40. [Google Scholar] [CrossRef]
- Crawford, J.R.; Allan, K.M.; Stephen, D.W.; Parker, D.M.; Besson, J.A.O. The Wechsler Adult Intelligence Scale-Revised (WAIS-R): Factor structure in a UK sample. Personal. Individ. Differ. 1989, 10, 1209–1212. [Google Scholar] [CrossRef]
- Ryan, J.J.; Lopez, S.J. Wechsler Adult Intelligence Scale-III. In Understanding Psychological Assessment; Springer: Boston, MA, USA, 2001; pp. 19–42. [Google Scholar] [CrossRef]
- Delis, D.C.; Kaplan, E.; Kramer, J.H. Delis-Kaplan Executive Function System; PsycTESTS Dataset; The Psychological Corporation: San Antonio, TX, USA, 2001. [Google Scholar] [CrossRef]
- Kaufer, D.I.; Cummings, J.L.; Ketchel, P.; Smith, V.; MacMillan, A.; Shelley, T.; Lopez, O.L.; DeKosky, S.T. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 233–239. [Google Scholar] [CrossRef]
- Sheikh, J.I.; Yesavage, J.A. 9/geriatric depression scale (Gds) recent evidence and development of a shorter version. Clin. Gerontol. 1986, 5, 165–173. [Google Scholar] [CrossRef]
- Davis, M.H. Measuring individual differences in empathy: Evidence for a multidimensional approach. J. Pers. Soc. Psychol. 1983, 44, 113–126. [Google Scholar] [CrossRef]
- Vijverberg, E.G.B.; Dols, A.; Krudop, W.A.; Del Campo Milan, M.; Kerssens, C.J.; Gossink, F.; Prins, N.D.; Stek, M.L.; Scheltens, P.; Teunissen, C.E.; et al. Cerebrospinal fluid biomarker examination as a tool to discriminate behavioral variant frontotemporal dementia from primary psychiatric disorders. Alzheimers Dement. Diagn. Assess. Dis. Monit. 2017, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.J.; Lleó, A.; Xie, S.X.; McMillan, C.T.; Wolk, D.A.; Lee, E.B.; Van Deerlin, V.M.; Shaw, L.M.; Trojanowski, J.Q.; Grossman, M. Ante mortem CSF tau levels correlate with post mortem tau pathology in FTLD. Ann. Neurol. 2017, 82, 247. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Ashton, N.J.; Karikari, T.K.; Gazzina, S.; Premi, E.; Benussi, L.; Ghidoni, R.; Rodriguez, J.L.; Emeršič, A.; Binetti, G.; et al. Serum Glial Fibrillary Acidic Protein (GFAP) Is a Marker of Disease Severity in Frontotemporal Lobar Degeneration. J. Alzheimers Dis. 2020, 77, 1129–1141. [Google Scholar] [CrossRef]
- Benussi, A.; Karikari, T.K.; Ashton, N.; Gazzina, S.; Premi, E.; Benussi, L.; Ghidoni, R.; Rodriguez, J.L.; Emeršič, A.; Simrén, J.; et al. Diagnostic and prognostic value of serum NfL and p-Tau 181 in frontotemporal lobar degeneration. J. Neurol. Neurosurg. Psychiatry 2020, 91, 960–967. [Google Scholar] [CrossRef]
- Benussi, A.; Huber, H.; Tan, K.; Cantoni, V.; Rivolta, J.; Cotelli, M.S.; Benedet, A.L.; Blennow, K.; Zetterberg, H.; Ashton, N.J.; et al. Plasma p-tau217 and neurofilament/p-tau217 ratio in differentiating Alzheimer’s disease from syndromes associated with frontotemporal lobar degeneration. Alzheimers Dement. 2025, 21, e14482. [Google Scholar] [CrossRef]
- Benussi, A.; Cantoni, V.; Rivolta, J.; Archetti, S.; Micheli, A.; Ashton, N.; Zetterberg, H.; Blennow, K.; Borroni, B. Classification accuracy of blood-based and neurophysiological markers in the differential diagnosis of Alzheimer’s disease and frontotemporal lobar degeneration. Alzheimers Res. Ther. 2022, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Schöll, M.; Maass, A.; Mattsson, N.; Ashton, N.J.; Blennow, K.; Zetterberg, H.; Jagust, W. Biomarkers for tau pathology. Mol. Cell Neurosci. 2019, 97, 18. [Google Scholar] [CrossRef]
- Borroni, B.; Benussi, A.; Cosseddu, M.; Archetti, S.; Padovani, A. Cerebrospinal fluid tau levels predict prognosis in non-inherited frontotemporal dementia. Neurodegener. Dis. 2014, 13, 224–229. [Google Scholar] [CrossRef]
- Norise, C.; Ungrady, M.; Halpin, A.; Jester, C.; McMillan, C.T.; Irwin, D.J.; Cousins, K.A.; Grossman, M. Clinical Correlates of Alzheimer’s Disease Cerebrospinal Fluid Analytes in Primary Progressive Aphasia. Front. Neurol. 2019, 10, 485. [Google Scholar] [CrossRef]
- Townley, R.A.; Graff-Radford, J.; Mantyh, W.G.; Botha, H.; Polsinelli, A.J.; Przybelski, S.A.; Machulda, M.M.; Makhlouf, A.T.; Senjem, M.L.; Murray, M.E.; et al. Progressive dysexecutive syndrome due to Alzheimer’s disease: A description of 55 cases and comparison to other phenotypes. Brain Commun. 2020, 2, fcaa068. [Google Scholar] [CrossRef]
- Paterson, R.W.; Slattery, C.F.; Poole, T.; Nicholas, J.M.; Magdalinou, N.K.; Toombs, J.; Chapman, M.D.; Lunn, M.P.; Heslegrave, A.J.; Foiani, M.S.; et al. Cerebrospinal fluid in the differential diagnosis of Alzheimer’s disease: Clinical utility of an extended panel of biomarkers in a specialist cognitive clinic. Alzheimers Res. Ther. 2018, 10, 32. [Google Scholar] [CrossRef]
- Illán-Gala, I.; Pegueroles, J.; Montal, V.; Alcolea, D.; Vilaplana, E.; Bejanin, A.; Borrego-Écija, S.; Sampedro, F.; Subirana, A.; Sánchez-Saudinós, M.B.; et al. APP-derived peptides reflect neurodegeneration in frontotemporal dementia. Ann. Clin. Transl. Neurol. 2019, 6, 2518–2530. [Google Scholar] [CrossRef]
- Gabelle, A.; Roche, S.; Gény, C.; Bennys, K.; Labauge, P.; Tholance, Y.; Quadrio, I.; Tiers, L.; Gor, B.; Boulanghien, J.; et al. Decreased sAβPPβ, Aβ38, and Aβ40 cerebrospinal fluid levels in frontotemporal dementia. J. Alzheimers Dis. 2011, 26, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Lleó, A.; Irwin, D.J.; Illán-Gala, I.; McMillan, C.T.; Wolk, D.A.; Lee, E.B.; Van Deerlin, V.M.; Shaw, L.M.; Trojanowski, J.Q.; Grossman, M. A 2-Step Cerebrospinal Algorithm for the Selection of Frontotemporal Lobar Degeneration Subtypes. JAMA Neurol. 2018, 75, 738. [Google Scholar] [CrossRef] [PubMed]
- del Campo, M.; Galimberti, D.; Elias, N.; Boonkamp, L.; Pijnenburg, Y.A.; van Swieten, J.C.; Watts, K.; Paciotti, S.; Beccari, T.; Hu, W.; et al. Novel CSF biomarkers to discriminate FTLD and its pathological subtypes. Ann. Clin. Transl. Neurol. 2018, 5, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Borroni, B.; Benussi, A.; Archetti, S.; Galimberti, D.; Parnetti, L.; Nacmias, B.; Sorbi, S.; Scarpini, E.; Padovani, A. Csf p-tau181/tau ratio as biomarker for TDP pathology in frontotemporal dementia. Amyotroph. Lateral Scler. Front. Degener. 2015, 16, 86–91. [Google Scholar] [CrossRef]
- Hu, W.T.; Watts, K.; Grossman, M.; Glass, J.; Lah, J.J.; Hales, C.; Shelnutt, M.; Van Deerlin, V.; Trojanowski, J.Q.; Levey, A.I. Reduced CSF p-Tau181 to Tau ratio is a biomarker for FTLD-TDP. Neurology 2013, 81, 1945. [Google Scholar] [CrossRef]
- Meeter, L.H.H.; Vijverberg, E.G.; Del Campo, M.; Rozemuller, A.J.M.; Donker Kaat, L.; de Jong, F.J.; van der Flier, W.M.; Teunissen, C.E.; van Swieten, J.C.; Pijnenburg, Y.A.L. Clinical value of neurofilament and phospho-tau/tau ratio in the frontotemporal dementia spectrum. Neurology 2018, 90, e1231–e1239. [Google Scholar] [CrossRef]
- Pijnenburg, Y.A.L.; Verwey, N.A.; van der Flier, W.M.; Scheltens, P.; Teunissen, C.E. Discriminative and prognostic potential of cerebrospinal fluid phosphoTau/tau ratio and neurofilaments for frontotemporal dementia subtypes. Alzheimers Dement. Diagn. Assess. Dis. Monit. 2015, 1, 505. [Google Scholar] [CrossRef]
- Horie, K.; Barthélemy, N.R.; Spina, S.; VandeVrede, L.; He, Y.; Paterson, R.W.; Wright, B.A.; Day, G.S.; Davis, A.A.; Karch, C.M.; et al. CSF tau microtubule-binding region identifies pathological changes in primary tauopathies. Nat. Med. 2022, 28, 2547–2554. [Google Scholar] [CrossRef]
- Thijssen, E.H.; La Joie, R.; Wolf, A.; Strom, A.; Wang, P.; Iaccarino, L.; Bourakova, V.; Cobigo, Y.; Heuer, H.; Spina, S.; et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat. Med. 2020, 26, 387. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, E.H.; La Joie, R.; Strom, A.; Fonseca, C.; Iaccarino, L.; Wolf, A.; Spina, S.; Allen, I.E.; Cobigo, Y.; Heuer, H.; et al. Association of Plasma P-tau217 and P-tau181 with clinical phenotype, neuropathology, and imaging markers in Alzheimer’s disease and frontotemporal lobar degeneration: A retrospective diagnostic performance study. Lancet Neurol. 2021, 20, 739, Erratum in Lancet Neurol. 2021, 20, e6. [Google Scholar] [CrossRef]
- Karikari, T.K.; Pascoal, T.A.; Ashton, N.J.; Janelidze, S.; Benedet, A.L.; Rodriguez, J.L.; Chamoun, M.; Savard, M.; Kang, M.S.; Therriault, J.; et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: A diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020, 19, 422–433. [Google Scholar] [CrossRef]
- Ashton, N.J.; Pascoal, T.A.; Karikari, T.K.; Benedet, A.L.; Lantero-Rodriguez, J.; Brinkmalm, G.; Snellman, A.; Schöll, M.; Troakes, C.; Hye, A.; et al. Plasma p-tau231: A new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol. 2021, 141, 709–724. [Google Scholar] [CrossRef]
- Cousins, K.A.Q.; Shaw, L.M.; Shellikeri, S.; Dratch, L.; Rosario, L.; Elman, L.B.; Quinn, C.; Amado, D.A.; Wolk, D.A.; Tropea, T.F.; et al. Elevated Plasma Phosphorylated Tau 181 in Amyotrophic Lateral Sclerosis. Ann. Neurol. 2022, 92, 807–818. [Google Scholar] [CrossRef]
- Vacchiano, V.; Mastrangelo, A.; Zenesini, C.; Baiardi, S.; Avoni, P.; Polischi, B.; Capellari, S.; Salvi, F.; Liguori, R.; Parchi, P.; et al. Elevated plasma p-tau181 levels unrelated to Alzheimer’s disease pathology in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2023, 94, 428–435. [Google Scholar] [CrossRef]
- Abu-Rumeileh, S.; Scholle, L.; Mensch, A.; Großkopf, H.; Ratti, A.; Kölsch, A.; Stoltenburg-Didinger, G.; Conrad, J.; De Gobbi, A.; Barba, L.; et al. Phosphorylated tau 181 and 217 are elevated in serum and muscle of patients with amyotrophic lateral sclerosis. Nat. Commun. 2025, 16, 2019. [Google Scholar] [CrossRef]
- Martinez-Valbuena, I.; Tartaglia, M.C.; Fox, S.H.; Lang, A.E.; Kovacs, G.G. Four-Repeat Tau Seeding in the Skin of Patients with Progressive Supranuclear Palsy. JAMA Neurol. 2024, 81, 1228–1230. [Google Scholar] [CrossRef]
- Dellarole, I.L.; Vacchi, E.; Ruiz-Barrio, I.; Pinton, S.; Raimondi, A.; Rossi, S.; Morandi, S.; Bianco, G.; Begum Bacinoglu, M.; Lombardo, A.; et al. Tau seeding activity in skin biopsy differentiates tauopathies from synucleinopathies. NPJ Park. Dis. 2024, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Goossens, J.; Vanmechelen, E.; Trojanowski, J.Q.; Lee, V.M.Y.; Van Broeckhoven, C.; van der Zee, J.; Engelborghs, S. TDP-43 as a possible biomarker for frontotemporal lobar degeneration: A systematic review of existing antibodies. Acta Neuropathol. Commun. 2015, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Scialò, C.; Tran, T.H.; Salzano, G.; Novi, G.; Caponnetto, C.; Chiò, A.; Calvo, A.; Canosa, A.; Moda, F.; Caroppo, P.; et al. TDP-43 real-Time quaking induced conversion reaction optimization and detection of seeding activity in CSF of amyotrophic lateral sclerosis and frontotemporal dementia patients. Brain Commun. 2020, 2, fcaa142. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Calvet, M.; Dols-Icardo, O.; Lladó, A.; Sánchez-Valle, R.; Hernández, I.; Amer, G.; Antón-Aguirre, S.; Alcolea, D.; Fortea, J.; Ferrer, I.; et al. Plasma phosphorylated TDP-43 levels are elevated in patients with frontotemporal dementia carrying a C9orf72 repeat expansion or a GRN mutation. J. Neurol. Neurosurg. Psychiatry 2014, 85, 684–691. [Google Scholar] [CrossRef]
- Fontana, E.; Bongianni, M.; Benussi, A.; Bronzato, E.; Scialo, C.; Sacchetto, L.; Cagnin, A.; Castriciano, S.; Buratti, E.; Gardoni, F.; et al. Detection of TDP-43 seeding activity in the olfactory mucosa from patients with frontotemporal dementia. Alzheimers Dement. 2023, 20, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Vizziello, M.; Dellarole, I.L.; Ciullini, A.; Pascuzzo, R.; Lombardo, A.; Bellandi, F.; Celauro, L.; Battipaglia, C.; Ciusani, E.; Rizzo, A.; et al. TDP-43 seeding activity in the olfactory mucosa of patients with amyotrophic lateral sclerosis. Mol. Neurodegener. 2025, 20, 49. [Google Scholar] [CrossRef]
- Goossens, J.; Bjerke, M.; Van Mossevelde, S.; Van Den Bossche, T.; Goeman, J.; De Vil, B.; Sieben, A.; Martin, J.J.; Cras, P.; De Deyn, P.P.; et al. Diagnostic value of cerebrospinal fluid tau, neurofilament, and progranulin in definite frontotemporal lobar degeneration. Alzheimers Res. Ther. 2018, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Huin, V.; Barbier, M.; Bottani, A.; Lobrinus, J.A.; Clot, F.; Lamari, F.; Chat, L.; Rucheton, B.; Fluchère, F.; Auvin, S.; et al. Homozygous GRN mutations: New phenotypes and new insights into pathological and molecular mechanisms. Brain 2020, 143, 303–319. [Google Scholar] [CrossRef]
- Meeter, L.H.H.; Gendron, T.F.; Sias, A.C.; Jiskoot, L.C.; Russo, S.P.; Donker Kaat, L.; Papma, J.M.; Panman, J.L.; van der Ende, E.L.; Dopper, E.G.; et al. Poly(GP), neurofilament and grey matter deficits in C9orf72 expansion carriers. Ann. Clin. Transl. Neurol. 2018, 5, 583–597. [Google Scholar] [CrossRef]
- del Campo, M.; Zetterberg, H.; Gandy, S.; Onyike, C.U.; Oliveira, F.; Udeh-Momoh, C.; Lleó, A.; Teunissen, C.E.; Pijnenburg, Y. New developments of biofluid-based biomarkers for routine diagnosis and disease trajectories in frontotemporal dementia. Alzheimers Dement. 2022, 18, 2292–2307. [Google Scholar] [CrossRef]
- Seddighi, S.; Qi, Y.A.; Brown, A.L.; Wilkins, O.G.; Bereda, C.; Belair, C.; Zhang, Y.J.; Prudencio, M.; Keuss, M.J.; Khandeshi, A.; et al. Mis-spliced transcripts generate de novo proteins in TDP-43–related ALS/FTD. Sci. Transl. Med. 2024, 16, eadg7162. [Google Scholar] [CrossRef]
- Deng, H.; Gao, K.; Jankovic, J. The role of FUS gene variants in neurodegenerative diseases. Nat. Rev. Neurol. 2014, 10, 337–348. [Google Scholar] [CrossRef]
- Olney, N.T.; Spina, S.; Miller, B.L. Frontotemporal Dementia. Neurol. Clin. 2017, 35, 339–374. [Google Scholar] [CrossRef]
- Lehmer, C.; Oeckl, P.; Weishaupt, J.H.; Volk, A.E.; Diehl-Schmid, J.; Schroeter, M.L.; Lauer, M.; Kornhuber, J.; Levin, J.; Fassbender, K.; et al. Poly-GP in cerebrospinal fluid links C9orf72-associated dipeptide repeat expression to the asymptomatic phase of ALS/FTD. EMBO Mol. Med. 2017, 9, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Salomonsson, S.E.; Maltos, A.M.; Gill, K.; Aladesuyi Arogundade, O.; Brown, K.A.; Sachdev, A.; Sckaff, M.; Lam, K.J.K.; Fisher, I.J.; Chouhan, R.S.; et al. Validated assays for the quantification of C9orf72 human pathology. Sci. Rep. 2024, 14, 828. [Google Scholar] [CrossRef]
- Bridel, C.; Van Wieringen, W.N.; Zetterberg, H.; Tijms, B.M.; Teunissen, C.E.; Alvarez-Cermeño, J.C.; Andreasson, U.; Axelsson, M.; Bäckström, D.C.; Bartos, A.; et al. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurol. 2019, 76, 1035. [Google Scholar] [CrossRef]
- Wilke, C.; Reich, S.; van Swieten, J.C.; Borroni, B.; Sanchez-Valle, R.; Moreno, F.; Laforce, R.; Graff, C.; Galimberti, D.; Rowe, J.B.; et al. Stratifying the Presymptomatic Phase of Genetic Frontotemporal Dementia by Serum NfL and pNfH: A Longitudinal Multicentre Study. Ann. Neurol. 2022, 91, 33–47. [Google Scholar] [CrossRef]
- Liu, E.; Jones, S.L.; Light, V.; Teunissen, C.; Bouzigues, A.; Russell, L.L.; Foster, P.; Ferry-Bolder, E.; van Swieten, J.; Jiskoot, L.; et al. Accuracy of blood-based neurofilament light to different genetic frontotemporal dementia from primary psychiatric disorders. J. Alzheimers Dis. 2025, 106, 1337–1354. [Google Scholar] [CrossRef] [PubMed]
- Woollacott, I.O.C.; Swift, I.J.; Sogorb-Esteve, A.; Heller, C.; Knowles, K.; Bouzigues, A.; Russell, L.L.; Peakman, G.; Greaves, C.V.; Convery, R.; et al. CSF glial markers are elevated in a subset of patients with genetic frontotemporal dementia. Ann. Clin. Transl. Neurol. 2022, 9, 1764–1777. [Google Scholar] [CrossRef] [PubMed]
- Alcolea, D.; Vilaplana, E.; Suárez-Calvet, M.; Illán-Gala, I.; Blesa, R.; Clarimón, J.; Lladó, A.; Sánchez-Valle, R.; Molinuevo, J.L.; García-Ribas, G.; et al. CSF sAPPβ, YKL-40, and neurofilament light in frontotemporal lobar degeneration. Neurology 2017, 89, 178–188. [Google Scholar] [CrossRef]
- Bergström, S.; Öijerstedt, L.; Remnestål, J.; Olofsson, J.; Ullgren, A.; Seelaar, H.; van Swieten, J.C.; Synofzik, M.; Sanchez-Valle, R.; Moreno, F.; et al. A panel of CSF proteins separates genetic frontotemporal dementia from presymptomatic mutation carriers: A GENFI study. Mol. Neurodegener. 2021, 16, 79. [Google Scholar] [CrossRef]
- Craig-Schapiro, R.; Perrin, R.J.; Roe, C.M.; Xiong, C.; Carter, D.; Cairns, N.J.; Mintun, M.A.; Peskind, E.R.; Li, G.; Galasko, D.R.; et al. YKL-40: A novel prognostic fluid biomarker for preclinical Alzheimer’s disease. Biol. Psychiatry 2010, 68, 903–912. [Google Scholar] [CrossRef]
- Alcolea, D.; Irwin, D.J.; Illán-Gala, I.; Muñoz, L.; Clarimón, J.; McMillan, C.T.; Fortea, J.; Blesa, R.; Lee, E.B.; Trojanowski, J.Q.; et al. Elevated YKL-40 and low sAPPβ:YKL-40 ratio in antemortem cerebrospinal fluid of patients with pathologically confirmed FTLD. J. Neurol. Neurosurg. Psychiatry 2018, 90, 180. [Google Scholar] [CrossRef]
- Sogorb-Esteve, A.; Weiner, S.; Simrén, J.; Swift, I.J.; Bocchetta, M.; Todd, E.G.; Cash, D.M.; Bouzigues, A.; Russell, L.L.; Foster, P.H.; et al. Proteomic analysis reveals distinct cerebrospinal fluid signatures across genetic frontotemporal dementia subtypes. Sci. Transl. Med. 2025, 17, eadm9654. [Google Scholar] [CrossRef]
- Sogorb-Esteve, A.; Nilsson, J.; Swift, I.J.; Heller, C.; Bocchetta, M.; Russell, L.L.; Peakman, G.; Convery, R.S.; van Swieten, J.C.; Seelaar, H.; et al. Differential impairment of cerebrospinal fluid synaptic biomarkers in the genetic forms of frontotemporal dementia. Alzheimers Res. Ther. 2022, 14, 118. [Google Scholar] [CrossRef]
- Galimberti, D.; Schoonenboom, N.; Scheltens, P.; Fenoglio, C.; Venturelli, E.; Pijnenburg, Y.A.L.; Bresolin, N.; Scarpini, E. Intrathecal chemokine levels in Alzheimer disease and frontotemporal lobar degeneration. Neurology 2006, 66, 146–147. [Google Scholar] [CrossRef]
- Huang, G.; Jian, J.; Liu, C.J. Progranulinopathy: A diverse realm of disorders linked to progranulin imbalances. Cytokine Growth Factor. Rev. 2024, 76, 142–159. [Google Scholar] [CrossRef]
- Boylan, M.A.; Pincetic, A.; Romano, G.; Tatton, N.; Kenkare-Mitra, S.; Rosenthal, A. Targeting Progranulin as an Immuno-Neurology Therapeutic Approach. Int. J. Mol. Sci. 2023, 24, 15946. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.D.; Abdul Hamid, H.; Md Hashim, N.F.; Cheema, M.S.; Chiroma, S.M.; Mustapha, M.; Mehat, M.Z. Could protein phosphatase 2A and glycogen synthase kinase-3 beta be targeted by natural compounds to ameliorate Alzheimer’s pathologies? Brain Res. 2024, 1829, 148793. [Google Scholar] [CrossRef]
- Fenoglio, C.; Serpente, M.; Visconte, C.; Arcaro, M.; Sorrentino, F.; D’Anca, M.; Arighi, A.; Rotondo, E.; Vimercati, R.; Rossi, G.; et al. Circulating Non-Coding RNA Levels Are Altered in Autosomal Dominant Frontotemporal Dementia. Int. J. Mol. Sci. 2022, 23, 14723. [Google Scholar] [CrossRef] [PubMed]
- Fenoglio, C.; Serpente, M.; Arcaro, M.; Carandini, T.; Sacchi, L.; Pintus, M.; Rotondo, E.; Borracci, V.; Ghezzi, L.; Bouzigues, A.; et al. Inflammatory plasma profile in genetic symptomatic and presymptomatic Frontotemporal Dementia—A GENFI study. Brain Behav. Immun. 2024, 122, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.; Barkhof, F.; Scheltens, P.; Schott, J.M.; Fox, N.C. An algorithmic approach to structural imaging in dementia. J. Neurol. Neurosurg. Psychiatry 2014, 85, 692–698. [Google Scholar] [CrossRef]
- Rascovsky, K.; Hodges, J.R.; Kipps, C.M.; Johnson, J.K.; Seeley, W.W.; Mendez, M.F.; Knopman, D.; Kertesz, A.; Mesulam, M.; Salmon, D.P.; et al. Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): Current limitations and future directions. Alzheimer Dis. Assoc. Disord. 2007, 21, S14–S18. [Google Scholar] [CrossRef]
- Mueller, K.; Scherf, N.; Grimmer, T.; Diehl-Schmid, J.; Danek, A.; Levin, J.; Wiltfang, J.; Anderl-Straub, S.; Otto, M.; Schroeter, M.L. Criminal Behavior in Frontotemporal Dementia: A Multimodal MRI Study. Hum. Brain Mapp. 2025, 46, e70308. [Google Scholar] [CrossRef]
- Matyi, M.A.; Radhakrishnan, H.; Olm, C.A.; Phillips, J.S.; Cook, P.A.; Rhodes, E.; Gee, J.C.; Irwin, D.J.; McMillan, C.T.; Massimo, L. Executive dysfunction relates to salience network desegregation in behavioural variant frontotemporal dementia. Neuroimage Clin. 2025, 48, 103853. [Google Scholar] [CrossRef]
- Coupé, P.; Catheline, G.; Lanuza, E.; Manjón, J.V. Towards a unified analysis of brain maturation and aging across the entire lifespan: A MRI analysis. Hum. Brain Mapp. 2017, 38, 5501–5518. [Google Scholar] [CrossRef]
- Bethlehem, R.A.I.; Seidlitz, J.; White, S.R.; Vogel, J.W.; Anderson, K.M.; Adamson, C.; Adler, S.; Alexopoulos, G.S.; Anagnostou, E.; Areces-Gonzalez, A.; et al. Brain charts for the human lifespan. Nature 2022, 604, 525–533, Erratum in Nature 2022, 610, E6. [Google Scholar] [CrossRef] [PubMed]
- Planche, V.; Mansencal, B.; Manjon, J.V.; Tourdias, T.; Catheline, G.; Coupé, P. Anatomical MRI staging of frontotemporal dementia variants. Alzheimers Dement. 2023, 19, 3283–3294. [Google Scholar] [CrossRef]
- Fumagalli, G.G.; Basilico, P.; Arighi, A.; Bocchetta, M.; Dick, K.M.; Cash, D.M.; Harding, S.; Mercurio, M.; Fenoglio, C.; Pietroboni, A.M.; et al. Distinct patterns of brain atrophy in Genetic Frontotemporal Dementia Initiative (GENFI) cohort revealed by visual rating scales. Alzheimers Res. Ther. 2018, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Bocchetta, M.; Todd, E.G.; Peakman, G.; Cash, D.M.; Convery, R.S.; Russell, L.L.; Thomas, D.L.; Eugenio Iglesias, J.; van Swieten, J.C.; Jiskoot, L.C.; et al. Differential early subcortical involvement in genetic FTD within the GENFI cohort. Neuroimage Clin. 2021, 30, 102646. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Millan, A.; Borrego-Écija, S.; van Swieten, J.C.; Jiskoot, L.; Moreno, F.; Laforce, R.; Graff, C.; Masellis, M.; Tartaglia, M.C.; Rowe, J.B.; et al. Loss of brainstem white matter predicts onset and motor neuron symptoms in C9orf72 expansion carriers: A GENFI study. J. Neurol. 2023, 270, 1573–1586. [Google Scholar] [CrossRef]
- Gazzina, S.; Grassi, M.; Premi, E.; Alberici, A.; Benussi, A.; Archetti, S.; Gasparotti, R.; Bocchetta, M.; Cash, D.M.; Todd, E.G.; et al. Structural brain splitting is a hallmark of Granulin-related frontotemporal dementia. Neurobiol. Aging 2022, 114, 94–104. [Google Scholar] [CrossRef]
- Bruffaerts, R.; Gors, D.; Bárcenas Gallardo, A.; Vandenbulcke, M.; Van Damme, P.; Suetens, P.; van Swieten, J.C.; Borroni, B.; Sanchez-Valle, R.; Moreno, F.; et al. Hierarchical spectral clustering reveals brain size and shape changes in asymptomatic carriers of C9orf72. Brain Commun. 2022, 4, fcac182. [Google Scholar] [CrossRef] [PubMed]
- Premi, E.; Costa, T.; Gazzina, S.; Benussi, A.; Cauda, F.; Gasparotti, R.; Archetti, S.; Alberici, A.; van Swieten, J.C.; Sanchez-Valle, R.; et al. An Automated Toolbox to Predict Single Subject Atrophy in Presymptomatic Granulin Mutation Carriers. J. Alzheimers Dis. 2022, 86, 205–218. [Google Scholar] [CrossRef]
- Pasternak, M.; Mirza, S.S.; Luciw, N.; Mutsaerts, H.J.M.M.; Petr, J.; Thomas, D.; Cash, D.; Bocchetta, M.; Tartaglia, M.C.; Mitchell, S.B.; et al. Longitudinal cerebral perfusion in presymptomatic genetic frontotemporal dementia: GENFI results. Alzheimers Dement. 2024, 20, 3525–3542. [Google Scholar] [CrossRef]
- Giunta, M.; Libri, I.; Premi, E.; Brattini, C.; Paghera, B.; Archetti, S.; Gasparotti, R.; Padovani, A.; Borroni, B.; Benussi, A. Clinical and radiological features of Posterior Cortical Atrophy (PCA) in a GRN mutation carrier: A case report. Eur. J. Neurol. 2021, 28, 344–348. [Google Scholar] [CrossRef]
- Bouzigues, A.; Du VLe Joulot, M.; Peysson, N.; Houot, M.; Béranger, B.; Russell, L.L.; Foster, P.H.; Ferry-Bolder, E.; van Swieten, J.C.; Jiskoot, L.; et al. Structural and functional connectivity in tau mutation carriers: From presymptomatic to symptomatic frontotemporal dementia. Alzheimers Dement. 2025, 21, e70367. [Google Scholar] [CrossRef]
- Borrego-Ecija, S.; Juncà-Parella, J.; Vandebergh, M.; Pérez Millan, A.; Balasa, M.; Llado, A.; Bouzigues, A.; Russell, L.L.; Foster, P.H.; Ferry-Bolder, E.; et al. Association of Initial Side of Brain Atrophy with Clinical Features and Disease Progression in Patients with GRN Frontotemporal Dementia. Neurology 2024, 103, e209944. [Google Scholar] [CrossRef]
- Harper, L.; Fumagalli, G.G.; Barkhof, F.; Scheltens, P.; O’Brien, J.T.; Bouwman, F.; Burton, E.J.; Rohrer, J.D.; Fox, N.C.; Ridgway, G.R.; et al. MRI visual rating scales in the diagnosis of dementia: Evaluation in 184 post-mortem confirmed cases. Brain 2016, 139, 1211. [Google Scholar] [CrossRef]
- Assogna, M.; Premi, E.; Gazzina, S.; Benussi, A.; Ashton, N.J.; Zetterberg, H.; Blennow, K.; Gasparotti, R.; Padovani, A.; Tadayon, E.; et al. Association of Choroid Plexus Volume with Serum Biomarkers, Clinical Features, and Disease Severity in Patients with Frontotemporal Lobar Degeneration Spectrum. Neurology 2023, 101, e1218–e1230. [Google Scholar] [CrossRef] [PubMed]
- Manera, A.L.; Dadar, M.; Van Swieten, J.C.; Borroni, B.; Sanchez-Valle, R.; Moreno, F.; Laforce, R., Jr.; Graff, C.; Synofzik, M.; Galimberti, D.; et al. MRI data-driven algorithm for the diagnosis of behavioural variant frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 2021, 92, 608–616. [Google Scholar] [CrossRef]
- Premi, E.; Giunta, M.; Iraji, A.; Rachakonda, S.; Calhoun, V.D.; Gazzina, S.; Benussi, A.; Gasparotti, R.; Archetti, S.; Bocchetta, M.; et al. Dissemination in time and space in presymptomatic granulin mutation carriers: A GENFI spatial chronnectome study. Neurobiol. Aging 2021, 108, 155–167. [Google Scholar] [CrossRef] [PubMed]
- McMillan, C.T.; Brun, C.; Siddiqui, S.; Churgin, M.; Libon, D.; Yushkevich, P.; Zhang, H.; Boller, A.; Gee, J.; Grossman, M. White matter imaging contributes to the multimodal diagnosis of frontotemporal lobar degeneration. Neurology 2012, 78, 1761–1768. [Google Scholar] [CrossRef]
- McMillan, C.T.; Irwin, D.J.; Avants, B.B.; Powers, J.; Cook, P.A.; Toledo, J.B.; McCarty Wood, E.; Van Deerlin, V.M.; Lee, V.M.; Trojanowski, J.Q.; et al. White Matter Imaging Helps Dissociate Tau from TDP-43 in Frontotemporal Lobar Degeneration. J. Neurol. Neurosurg. Psychiatry 2013, 84, 949. [Google Scholar] [CrossRef] [PubMed]
- Planche, V.; Mansencal, B.; Fonov, V.; Manjon, J.V.; Tourdias, T.; Bouzigues, A.; Russell, L.L.; Foster, P.H.; Ferry-Bolder, E.; van Swieten, J.C.; et al. Anatomical progression of genetic frontotemporal lobar degeneration across the lifespan. Brain 2025, 148, 3880–3892. [Google Scholar] [CrossRef]
- Higuchi, M.; Tagai, K.; Takahata, K.; Endo, H. Advances in PET imaging of protein aggregates associated with neurodegenerative disease. Nat. Rev. Neurol. 2025, 21, 506–522. [Google Scholar] [CrossRef] [PubMed]
- Bevan-Jones, R.W.; Cope, T.E.; Jones, S.P.; Passamonti, L.; Hong, Y.T.; Fryer, T.; Arnold, R.; Coles, J.P.; Aigbirhio, F.A.; Patterson, K.; et al. [18F]AV-1451 binding is increased in frontotemporal dementia due to C9orf72 expansion. Ann. Clin. Transl. Neurol. 2018, 5, 1292–1296. [Google Scholar] [CrossRef]
- Jones, D.T.; Knopman, D.S.; Graff-Radford, J.; Syrjanen, J.A.; Senjem, M.L.; Schwarz, C.G.; Dheel, C.; Wszolek, Z.; Rademakers, R.; Kantarci, K.; et al. In vivo 18 F-AV-1451 tau PET signal in MAPT mutation carriers varies by expected tau isoforms. Neurology 2018, 90, e947–e954. [Google Scholar] [CrossRef] [PubMed]
- Gatto, R.G.; Carlos, A.F.; Reichard, R.R.; Lowe, V.J.; Whitwell, J.L.; Josephs, K.A. Comparative assessment of regional tau distribution by Tau-PET and Post-mortem neuropathology in a representative set of Alzheimer’s & frontotemporal lobar degeneration patients. PLoS ONE 2023, 18, e0284182. [Google Scholar] [CrossRef]
- Malarte, M.L.; Gillberg, P.G.; Kumar, A.; Bogdanovic, N.; Lemoine, L.; Nordberg, A. Discriminative binding of tau PET tracers PI2620, MK6240 and RO948 in Alzheimer’s disease, corticobasal degeneration and progressive supranuclear palsy brains. Mol. Psychiatry 2022, 28, 1272–1283, Erratum in Mol. Psychiatry 2023, 29, 38. [Google Scholar] [CrossRef]
- Kubota, M.; Endo, H.; Takahata, K.; Tagai, K.; Suzuki, H.; Onaya, M.; Sano, Y.; Yamamoto, Y.; Kurose, S.; Matsuoka, K.; et al. In vivo PET classification of tau pathologies in patients with frontotemporal dementia. Brain Commun. 2024, 6, fcae075. [Google Scholar] [CrossRef]
- Suzuki, H.; Kubota, M.; Kurose, S.; Tagai, K.; Endo, H.; Onaya, M.; Yamamoto, Y.; Sahara, N.; Ohgidani, M.; Haga, C.; et al. Neuropathological correlations of 18F-florzolotau PET in a case with pick’s disease. EJNMMI Res. 2025, 15, 96. [Google Scholar] [CrossRef]
- Vanderlinden, G.; Vandenberghe, R.; Vandenbulcke, M.; Van Laere, K. The Current Role of Tau PET Imaging in Neurodegeneration. Semin. Nucl. Med. 2025, 55, 548–564. [Google Scholar] [CrossRef]
- Nigro, S.; Tafuri, B.; Urso, D.; De Blasi, R.; Cedola, A.; Gigli, G.; Logroscino, G. Altered structural brain networks in linguistic variants of frontotemporal dementia. Brain Imaging Behav. 2022, 16, 1113–1122. [Google Scholar] [CrossRef]
- Neumann, M.; Kwong, L.K.; Sampathu, D.M.; Trojanowski, J.Q.; Lee, V.M.Y. TDP-43 Proteinopathy in Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis: Protein Misfolding Diseases Without Amyloidosis. Arch. Neurol. 2007, 64, 1388–1394. [Google Scholar] [CrossRef]
- Tetzloff, K.A.; Duffy, J.R.; Clark, H.M.; Utianski, R.L.; Strand, E.A.; Machulda, M.M.; Botha, H.; Martin, P.R.; Schwarz, C.G.; Senjem, M.L.; et al. Progressive agrammatic aphasia without apraxia of speech as a distinct syndrome. Brain 2019, 142, 2466–2482. [Google Scholar] [CrossRef]
- Boxer, A.L.; Geschwind, M.D.; Belfor, N.; Gorno-Tempini, M.L.; Schauer, G.F.; Miller, B.L.; Weiner, M.W.; Rosen, H.J. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch. Neurol. 2006, 63, 81–86. [Google Scholar] [CrossRef]
- Whitwell, J.L.; Höglinger, G.U.; Antonini, A.; Bordelon, Y.; Boxer, A.L.; Colosimo, C.; Van Eimeren, T.; Golbe, L.I.; Kassubek, J.; Kurz, C.; et al. Radiological biomarkers for diagnosis in PSP: Where are we and where do we need to be? Mov. Disord. 2017, 32, 955–971. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Gao, H.; Xie, H.; Jia, Z.; Chen, Q. Molecular imaging biomarkers in familial frontotemporal lobar degeneration: Progress and prospects. Front. Neurol. 2022, 13, 933217. [Google Scholar] [CrossRef]
- Popuri, K.; Beg, M.F.; Lee, H.; Balachandar, R.; Wang, L.; Sossi, V.; Jacova, C.; Baker, M.; Shahinfard, E.; Rademakers, R.; et al. FDG-PET in presymptomatic C9orf72 mutation carriers. Neuroimage Clin. 2021, 31, 102687. [Google Scholar] [CrossRef]
- Malpetti, M.; Holland, N.; Jones, P.S.; Ye, R.; Cope, T.E.; Fryer, T.D.; Hong, Y.T.; Savulich, G.; Rittman, T.; Passamonti, L.; et al. Synaptic density in carriers of C9orf72 mutations: A [11C]UCB-J PET study. Ann. Clin. Transl. Neurol. 2021, 8, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Murley, A.G.; Rowe, J.B. Neurotransmitter deficits from fronto temporal lobar degeneration. Brain 2018, 141, 1263–1285. [Google Scholar] [CrossRef] [PubMed]
- Premi, E.; Pengo, M.; Mattioli, I.; Cantoni, V.; Dukart, J.; Gasparotti, R.; Buratti, E.; Padovani, A.; Bocchetta, M.; Todd, E.G.; et al. Early neurotransmitters changes in prodromal frontotemporal dementia: A GENFI study. Neurobiol. Dis. 2023, 179, 106068. [Google Scholar] [CrossRef] [PubMed]
- Toller, G.; Cobigo, Y.; Callahan, P.; Appleby, B.S.; Brushaber, D.; Domoto-Reilly, K.; Forsberg, L.K.; Ghoshal, N.; Graff-Radford, J.; Graff-Radford, N.R. Multisite ALLFTD study modeling progressive empathy loss from the earliest stages of behavioral variant frontotemporal dementia. Alzheimers Dement. 2023, 19, 2842–2852. [Google Scholar] [CrossRef]
- Schuster, B.A.; Sowden, S.; Rybicki, A.J.; Fraser, D.S.; Press, C.; Holland, P.; Cook, J.L. Dopaminergic Modulation of Dynamic Emotion Perception. J. Neurosci. 2022, 42, 4394–4400. [Google Scholar] [CrossRef]
- Duerler, P.; Vollenweider, F.X.; Preller, K.H. A neurobiological perspective on social influence: Serotonin and social adaptation. J. Neurochem. 2022, 162, 60–79. [Google Scholar] [CrossRef]
- Kanen, J.W.; Arntz, F.E.; Yellowlees, R.; Cardinal, R.N.; Price, A.; Christmas, D.M.; Apergis-Schoute, A.M.; Sahakian, B.J.; Robbins, T.W. Serotonin depletion amplifies distinct human social emotions as a function of individual differences in personality. Transl. Psychiatry 2021, 11, 81. [Google Scholar] [CrossRef]
- Gérard, T.; Colmant, L.; Malotaux, V.; Salman, Y.; Huyghe, L.; Quenon, L.; Boyer, E.; Dricot, L.; Ivanoiu, A.; Lhommel, R.; et al. Tau PET Imaging with [18F]MK-6240: Limited Affinity for Primary Tauopathies and High Specificity for Alzheimer’s Disease. Eur. J. Neurol. 2025, 32, e70068. [Google Scholar] [CrossRef] [PubMed]
- Borroni, B.; Benussi, A.; Premi, E.; Alberici, A.; Marcello, E.; Gardoni, F.; Di Luca, M.; Padovani, A. Biological, Neuroimaging, and Neurophysiological Markers in Frontotemporal Dementia: Three Faces of the Same Coin. J. Alzheimers Dis. 2018, 62, 1113–1123. [Google Scholar] [CrossRef]
- Cosseddu, M.; Benussi, A.; Gazzina, S.; Turrone, R.; Archetti, S.; Bonomi, E.; Biasiotto, G.; Zanella, I.; Ferrari, R.; Cotelli, M.S.; et al. Mendelian forms of disease and age at onset affect survival in frontotemporal dementia. Amyotroph. Lateral Scler. Front. Degener. 2018, 19, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Bocchetta, M.; Todd, E.G.; Bouzigues, A.; Cash, D.M.; Nicholas, J.M.; Convery, R.S.; Russell, L.L.; Thomas, D.L.; Malone, I.B.; Iglesias, J.E.; et al. Structural MRI predicts clinical progression in presymptomatic genetic frontotemporal dementia: Findings from the GENetic Frontotemporal dementia Initiative cohort. Brain Commun. 2023, 5, fcad061. [Google Scholar] [CrossRef]
- McCarthy, J.; Borroni, B.; Sanchez-Valle, R.; Moreno, F.; Laforce, R.; Graff, C.; Synofzik, M.; Galimberti, D.; Rowe, J.B.; Masellis, M.; et al. Data-driven staging of genetic frontotemporal dementia using multi-modal MRI. Hum. Brain Mapp. 2022, 43, 1821–1835. [Google Scholar] [CrossRef]
- Staffaroni, A.M.; Quintana, M.; Wendelberger, B.; Heuer, H.W.; Russell, L.L.; Cobigo, Y.; Wolf, A.; Goh, S.M.; Petrucelli, L.; Gendron, T.F.; et al. Temporal order of clinical and biomarker changes in familial frontotemporal dementia. Nat. Med. 2022, 28, 2194–2206. [Google Scholar] [CrossRef]
- Guarnier, G.; Reinelt, J.; Molloy, E.N.; Mihai, P.G.; Einaliyan, P.; Valk, S.; Modestino, A.; Ugolini, M.; Mueller, K.; Wu, Q.; et al. Cascaded Multimodal Deep Learning in the Differential Diagnosis, Progression Prediction, and Staging of Alzheimer’s and Frontotemporal Dementia. MedRxiv 2025, in press. [Google Scholar] [CrossRef]
- Prinse, F.A.M.; Weerd Lvan der Swieten JCvan Ronen, I.; Seelaar, H.; Hirschler, L.; Najac, C.; Dopper, E.G.P. Investigating the role of neuroinflammation and brain clearance in frontotemporal lobar degeneration using 7T MRI and fluid biomarkers: Protocol for a cross-sectional study in a tertiary care setting. BMJ Open 2025, 15, e102668. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Seripa, D.; Daniele, A.; Watling, M.; Giannelli, G.; Imbimbo, B.P. Development of disease-modifying drugs for frontotemporal dementia spectrum disorders. Nat. Rev. Neurol. 2020, 16, 213–228. [Google Scholar] [CrossRef]
- Giunta, M.; Solje, E.; Gardoni, F.; Borroni, B.; Benussi, A. Experimental Disease-Modifying Agents for Frontotemporal Lobar Degeneration. J. Exp. Pharmacol. 2021, 13, 359–376. [Google Scholar] [CrossRef]
- Carter, L.; Borroni, B.; Mummery, C.; Ber ILe Boeve, B.; Boxer, A.; Smithey, M.; Chow, T.; Huang, J.; Guizzetti, L.; Salvadore, G.; et al. Baseline Characteristics for INFRONT-3: A Phase 3 Double-Blind, Placebo-Controlled 96-Week Study Evaluating Latozinemab in FTD-GRN (P8-3.005). Neurology 2025, 104, 4085. [Google Scholar] [CrossRef]
- Chang Berger, A.; Cohen, I.; Jafarnejad, M.; Chiu, C.-L.; Bhalla, A.; Damo, L.; Shanbhag, N.M.; Simen, A.; Lu, H.; Zicha, S.; et al. Safety and pharmacokinetics of single ascending doses of TAK-594/DNL593, a brain-penetrant progranulin replacement therapy, in healthy volunteers: Interim results from Part A of a Phase 1/2 clinical trial. Alzheimers Dement. 2023, 19, e075068. [Google Scholar] [CrossRef]
- Study Details|A Study of PBFT02 in Participants with FTD and Mutations in the Granulin Precursor (GRN) or C9ORF72 Genes|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT04747431 (accessed on 8 August 2025).
- AviadoBio Announces Completion of Second Cohort in Phase 1/2 ASPIRE-FTD Clinical Trial Studying AVB-101 for FTD-GRN. Available online: https://aviadobio.com/aviadobio-announces-completion-of-second-cohort-in-phase-1-2-aspire-ftd-clinical-trial/ (accessed on 8 August 2025).
- Tran, H.; Moazami, M.P.; Yang, H.; McKenna-Yasek, D.; Douthwright, C.L.; Pinto, C.; Metterville, J.; Shin, M.; Sanil, N.; Dooley, C.; et al. Suppression of mutant C9orf72 expression by a potent mixed backbone antisense oligonucleotide. Nat. Med. 2022, 28, 117–124. [Google Scholar] [CrossRef]
- Liu, Y.; Andreucci, A.; Iwamoto, N.; Yin, Y.; Yang, H.; Liu, F.; Bulychev, A.; Hu, X.S.; Lin, X.; Lamore, S.; et al. Preclinical evaluation of WVE-004, aninvestigational stereopure oligonucleotide forthe treatment of C9orf72-associated ALS or FTD. Mol. Ther. Nucleic Acids 2022, 28, 558–570. [Google Scholar] [CrossRef]
- van den Berg, L.H.; Rothstein, J.D.; Shaw, P.J.; Babu, S.; Benatar, M.; Bucelli, R.C.; Genge, A.; Glass, J.D.; Hardiman, O.; Libri, V.; et al. Safety, tolerability, and pharmacokinetics of antisense oligonucleotide BIIB078 in adults with C9orf72-associated amyotrophic lateral sclerosis: A phase 1, randomised, double blinded, placebo-controlled, multiple ascending dose study. Lancet Neurol. 2024, 23, 901–912. [Google Scholar] [CrossRef]
- Sullivan, A.C.; Zuniga, G.; Ramirez, P.; Fernandez, R.; Wang, C.-P.; Li, J.; Davila, L.; Pelton, K.; Gomez, S.; Sohn, C.; et al. A Phase IIa clinical trial to evaluate the effects of anti-retroviral therapy in Alzheimer’s disease (ART-AD). NPJ Dement. 2025, 1, 2. [Google Scholar] [CrossRef]
- Babu, S.; Nicholson, K.A.; Rothstein, J.D.; Swenson, A.; Sampognaro, P.J.; Pant, P.; Macklin, E.A.; Spruill, S.; Paganoni, S.; Gendron, T.F.; et al. Apilimod dimesylate in C9orf72 amyotrophic lateral sclerosis: A randomized phase 2a clinical trial. Brain 2024, 147, 2998–3008. [Google Scholar] [CrossRef]
- McMillan, C.T.; Russ, J.; Wood, E.M.; Irwin, D.J.; Grossman, M.; McCluskey, L.; Elman, L.; Van Deerlin, V.; Lee, E.B. C9orf72 promoter hypermethylation is neuroprotective: Neuroimaging and neuropathologic evidence. Neurology 2015, 84, 1622. [Google Scholar] [CrossRef]
- Boros, B.D.; Schoch, K.M.; Kreple, C.J.; Miller, T.M. Antisense Oligonucleotides for the Study and Treatment of ALS. Neurotherapeutics 2022, 19, 1145. [Google Scholar] [CrossRef]
- Study Details|Ciprofloxacin/Celecoxib Combination in Patients with ALS|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT04090684 (accessed on 8 August 2025).
- Writing Committee for the HEALEY ALS Platform Trial. Verdiperstat in Amyotrophic Lateral Sclerosis: Results from the Randomized HEALEY ALS Platform Trial. JAMA Neurol. 2025, 82, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Babazadeh, A.; Rayner, S.L.; Lee, A.; Chung, R.S. TDP-43 as a therapeutic target in neurodegenerative diseases: Focusing on motor neuron disease and frontotemporal dementia. Ageing Res. Rev. 2023, 92, 102085. [Google Scholar] [CrossRef] [PubMed]
- Höglinger, G.U.; Litvan, I.; Mendonca, N.; Wang, D.; Zheng, H.; Rendenbach-Mueller, B.; Lon, H.K.; Jin, Z.; Fisseha, N.; Budur, K.; et al. Safety and efficacy of tilavonemab in progressive supranuclear palsy: A phase 2, randomised, placebo-controlled trial. Lancet Neurol. 2021, 20, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Shulman, M.; Kong, J.; O’Gorman, J.; Ratti, E.; Rajagovindan, R.; Viollet, L.; Huang, E.; Sharma, S.; Racine, A.M.; Czerkowicz, J.; et al. TANGO: A placebo-controlled randomized phase 2 study of efficacy and safety of the anti-tau monoclonal antibody gosuranemab in early Alzheimer’s disease. Nat. Aging 2023, 3, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. Anti-tau antibody stumbles in phase II Alzheimer trial. Nat. Rev. Drug Discov. 2024, 23, 883. [Google Scholar] [CrossRef]
- Novak, P.; Kovacech, B.; Katina, S.; Schmidt, R.; Scheltens, P.; Kontsekova, E.; Ropele, S.; Fialova, L.; Kramberger, M.; Paulenka-Ivanovova, N.; et al. ADAMANT: A placebo-controlled randomized phase 2 study of AADvac1, an active immunotherapy against pathological tau in Alzheimer’s disease. Nat. Aging 2021, 1, 521–534. [Google Scholar] [CrossRef]
- Study of Safety, Tolerability, Pharmacodynamics and Pharmacokinetics of NIO752 in Early Alzheimer’s Disease Participants|Novartis. Available online: https://www.novartis.com/clinicaltrials/study/nct05469360 (accessed on 8 August 2025).
- Vivash, L.; Malpas, C.B.; Meletis, C.; Gollant, M.; Eratne, D.; Li, Q.X.; McDonald, S.; O’Brien, W.T.; Brodtmann, A.; Darby, D.; et al. A phase 1b open-label study of sodium selenate as a disease-modifying treatment for possible behavioral variant frontotemporal dementia. Alzheimers Dement. Transl. Res. Clin. Interv. 2022, 8, e12299. [Google Scholar] [CrossRef]
- Codron, P.; Cassereau, J.; Vourc’h, P. InFUSing antisense oligonucleotides for treating ALS. Trends Mol. Med. 2022, 28, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Altomare, D.; Benussi, A.; Bréchet, L.; Casula, E.P.; Dodich, A.; Pievani, M.; Santarnecchi, E.; Frisoni, G.B. The emerging field of non-invasive brain stimulation in Alzheimer’s disease. Brain 2024, 147, 4003–4016. [Google Scholar] [CrossRef]
- Benussi, A.; Dell’era, V.; Cosseddu, M.; Cantoni, V.; Cotelli, M.S.; Cotelli, M.; Manenti, R.; Benussi, L.; Brattini, C.; Alberici, A.; et al. Transcranial stimulation in frontotemporal dementia: A randomized, double-blind, sham-controlled trial. Alzheimers Dement. Transl. Res. Clin. Interv. 2020, 6, e12033. [Google Scholar] [CrossRef]
- Teichmann, M.; Lesoil, C.; Godard, J.; Vernet, M.; Bertrand, A.; Levy, R.; Dubois, B.; Lemoine, L.; Truong, D.Q.; Bikson, M.; et al. Direct current stimulation over the anterior temporal areas boosts semantic processing in primary progressive aphasia. Ann. Neurol. 2016, 80, 693–707. [Google Scholar] [CrossRef]
- Gervits, F.; Ash, S.; Coslett, H.B.; Rascovsky, K.; Grossman, M.; Hamilton, R. Transcranial direct current stimulation for the treatment of primary progressive aphasia: An open-label pilot study. Brain Lang. 2016, 162, 35–41. [Google Scholar] [CrossRef]
- Cotelli, M.; Manenti, R.; Petesi, M.; Brambilla, M.; Cosseddu, M.; Zanetti, O.; Miniussi, C.; Padovani, A.; Borroni, B. Treatment of primary progressive aphasias by transcranial direct current stimulation combined with language training. J. Alzheimers Dis. 2014, 39, 799–808. [Google Scholar] [CrossRef]
- Godoi, D.C.; Pandiá, E.; Rodrigues, M.E.; Araújo, L.; Barbosa, M.; Alhwaishel, K.; Godoi, A.; McGonigle, D.J. Linguistic effects of transcranial Direct Current Stimulation (tDCS) in patients with primary progressive aphasia: A systematic review and meta-analysis of randomised controlled trials. Neurosci. Biobehav. Rev. 2025, 176, 106264. [Google Scholar] [CrossRef] [PubMed]
- Cotelli, M.; Adenzato, M.; Cantoni, V.; Manenti, R.; Alberici, A.; Enrici, I.; Benussi, A.; Dell’Era, V.; Bonetta, E.; Padovani, A.; et al. Enhancing theory of mind in behavioural variant frontotemporal dementia with transcranial direct current stimulation. Cogn. Affect. Behav. Neurosci. 2018, 18, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Antczak, J.; Kowalska, K.; Klimkowicz-Mrowiec, A.; Wach, B.; Kasprzyk, K.; Banach, M.; Rzeźnicka-Brzegowy, K.; Kubica, J.; Słowik, A. Repetitive transcranial magnetic stimulation for the treatment of cognitive impairment in frontotemporal dementia: An open-label pilot study. Neuropsychiatr. Dis. Treat. 2018, 14, 749. [Google Scholar] [CrossRef]
- Cotelli, M.; Manenti, R.; Alberici, A.; Brambilla, M.; Cosseddu, M.; Zanetti, O.; Miozzo, A.; Padovani, A.; Miniussi, C.; Borroni, B. Prefrontal cortex rTMS enhances action naming in progressive non-fluent aphasia. Eur. J. Neurol. 2012, 19, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Trebbastoni, A.; Raccah, R.; de Lena, C.; Zangen, A.; Inghilleri, M. Repetitive Deep Transcranial Magnetic Stimulation Improves Verbal Fluency and Written Language in a Patient with Primary Progressive Aphasia-Logopenic Variant (LPPA). Brain Stimul. 2013, 6, 545–553. [Google Scholar] [CrossRef]
- Benussi, A.; Di Lorenzo, F.; Dell’Era, V.; Cosseddu, M.; Alberici, A.; Caratozzolo, S.; Cotelli, M.S.; Micheli, A.; Rozzini, L.; Depari, A.; et al. Transcranial magnetic stimulation distinguishes Alzheimer disease from frontotemporal dementia. Neurology 2017, 89, 665–672. [Google Scholar] [CrossRef]
- Benussi, A.; Dell’Era, V.; Cantoni, V.; Cotelli, M.S.; Cosseddu, M.; Spallazzi, M.; Micheli, A.; Turrone, R.; Alberici, A.; Borroni, B. TMS for staging and predicting functional decline in frontotemporal dementia. Brain Stimul. 2020, 13, 386–392. [Google Scholar] [CrossRef]
- Vucic, S.; Stanley Chen, K.H.; Kiernan, M.C.; Hallett, M.; Benninger, D.H.; Di Lazzaro, V.; Rossini, P.M.; Benussi, A.; Berardelli, A.; Currà, A.; et al. Clinical diagnostic utility of transcranial magnetic stimulation in neurological disorders. Updated report of an IFCN committee. Clin. Neurophysiol. 2023, 150, 131–175. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Bella, R.; Benussi, A.; Bologna, M.; Borroni, B.; Capone, F.; Chen, K.S.; Chen, R.V.; Chistyakov, A.V.; Classen, J.; et al. Diagnostic contribution and therapeutic perspectives of transcranial magnetic stimulation in dementia. Clin. Neurophysiol. 2021, 132, 2568–2607. [Google Scholar] [CrossRef]
- Benussi, A.; Cantoni, V.; Borroni, B. The role of transcranial magnetic stimulation in the diagnosis of dementia. In Horizons in Neuroscience Research; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2021; Volume 44, pp. 55–104. [Google Scholar]
- Benussi, A.; Premi, E.; Gazzina, S.; Cantoni, V.; Cotelli, M.S.; Giunta, M.; Gasparotti, R.; Calhoun, V.D.; Borroni, B. Neurotransmitter imbalance dysregulates brain dynamic fluidity in frontotemporal degeneration. Neurobiol. Aging 2020, 94, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Oberman, L.M.; Benussi, A. Transcranial Magnetic Stimulation Across the Lifespan: Impact of Developmental and Degenerative Processes. Biol. Psychiatry 2023, 95, 581–591. [Google Scholar] [CrossRef]
- Padovani, A.; Benussi, A.; Cotelli, M.S.; Ferrari, C.; Cantoni, V.; Dell’Era, V.; Turrone, R.; Paghera, B.; Borroni, B. Transcranial magnetic stimulation and amyloid markers in mild cognitive impairment: Impact on diagnostic confidence and diagnostic accuracy. Alzheimers Res. Ther. 2019, 11, 95. [Google Scholar] [CrossRef]
- Benussi, A.; Dell’Era, V.; Cantoni, V.; Cotelli, M.S.; Cosseddu, M.; Spallazzi, M.; Alberici, A.; Padovani, A.; Borroni, B. Neurophysiological Correlates of Positive and Negative Symptoms in Frontotemporal Dementia. J. Alzheimers Dis. 2020, 73, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Gazzina, S.; Benussi, A.; Premi, E.; Paternicò, D.; Cristillo, V.; Dell’Era, V.; Cosseddu, M.V.; Archetti, S.; Alberici, A.; Gasparotti, R.; et al. Neuroanatomical Correlates of Transcranial Magnetic Stimulation in Presymptomatic Granulin Mutation Carriers. Brain Topogr. 2018, 31, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Palese, F.; Bonomi, E.; Nuzzo, T.; Benussi, A.; Mellone, M.; Zianni, E.; Cisani, F.; Casamassa, A.; Alberici, A.; Scheggia, D.; et al. Anti-GluA3 antibodies in frontotemporal dementia: Effects on glutamatergic neurotransmission and synaptic failure. Neurobiol. Aging 2020, 86, 143–155. [Google Scholar] [CrossRef]
- Burrell, J.R.; Kiernan, M.C.; Vucic, S.; Hodges, J.R. Motor Neuron dysfunction in frontotemporal dementia. Brain 2011, 134, 2582–2594. [Google Scholar] [CrossRef]
- Benussi, A.; Grassi, M.; Palluzzi, F.; Cantoni, V.; Cotelli, M.S.; Premi, E.; Di Lorenzo, F.; Pellicciari, M.C.; Ranieri, F.; Musumeci, G.; et al. Classification accuracy of TMS for the diagnosis of mild cognitive impairment. Brain Stimul. 2021, 14, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Gazzina, S.; Premi, E.; Cosseddu, M.; Archetti, S.; Dell’Era, V.; Cantoni, V.; Cotelli, M.S.; Alberici, A.; Micheli, A.; et al. Clinical and biomarker changes in presymptomatic genetic frontotemporal dementia. Neurobiol. Aging 2019, 76, 133–140. [Google Scholar] [CrossRef]
- Benussi, A.; Dell’Era, V.; Cantoni, V.; Ferrari, C.; Caratozzolo, S.; Rozzini, L.; Alberici, A.; Padovani, A.; Borroni, B. Discrimination of atypical parkinsonisms with transcranial magnetic stimulation. Brain Stimul. 2018, 11, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Alberici, A.; Buratti, E.; Ghidoni, R.; Gardoni, F.; Di Luca, M.; Padovani, A.; Borroni, B. Toward a Glutamate Hypothesis of Frontotemporal Dementia. Front. Neurosci. 2019, 13, 304. [Google Scholar] [CrossRef]
- Benussi, A.; Grassi, M.; Palluzzi, F.; Koch, G.; Di Lazzaro, V.; Nardone, R.; Cantoni, V.; Dell’Era, V.; Premi, E.; Martorana, A.; et al. Classification Accuracy of Transcranial Magnetic Stimulation for the Diagnosis of Neurodegenerative Dementias. Ann. Neurol. 2020, 87, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Assogna, M.; Sprugnoli, G.; Press, D.; Dickerson, B.; Macone, J.; Bonnì, S.; Borghi, I.; Connor, A.; Hoffman, M.; Grover, N.; et al. Gamma-induction in frontotemporal dementia (GIFTeD) randomized placebo-controlled trial: Rationale, noninvasive brain stimulation protocol, and study design. Alzheimers Dement. Transl. Res. Clin. Interv. 2021, 7, e12219. [Google Scholar] [CrossRef]
- Pagnoni, I.; Gobbi, E.; Premi, E.; Borroni, B.; Binetti, G.; Cotelli, M.; Manenti, R. Language training for oral and written naming impairment in primary progressive aphasia: A review. Transl. Neurodegener. 2021, 10, 24. [Google Scholar] [CrossRef]
- Massimo, L.; Hirschman, K.B.; Aryal, S.; Quinn, R.; Fisher, L.; Sharkey, M.; Thomas, G.; Bowles, K.H.; Riegel, B. iCare4Me for FTD: A pilot randomized study to improve self-care in caregivers of persons with frontotemporal degeneration. Alzheimers Dement. Transl. Res. Clin. Interv. 2023, 9, e12381. [Google Scholar] [CrossRef] [PubMed]
- Tsai, R.M.; Boxer, A.L. Treatment of frontotemporal dementia. Curr. Treat. Options Neurol. 2014, 16, 319. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).