Abstract
Plastic pollution has become a critical environmental issue, with vast amounts of plastic waste accumulating in aquatic and terrestrial ecosystems. Plastic pollution poses significant risks to biodiversity by introducing toxic chemicals and disrupting biological functions. The drone fly, Eristalis tenax, is perhaps the most globally widespread hoverfly. This success is aided by its development as a rat-tailed maggot in a wide array of aquatic environments where it feeds on decaying organic matter. As an adult, E. tenax is a vital pollinator, visiting a wide range of crops and wild plants, and has been shown to vector pollen over hundreds of kilometres during seasonal migrations. Exposure to microplastics during larval stages has the potential to alter the provision of these ecosystem services and to provide a route for the long-distance vectoring of microplastics. To investigate this, we rear E. tenax in water contaminated with different concentrations of microplastic particles. We show that these plastics are retained in the gut from larval through to pupal to adult developmental stages. This contamination resulted in reductions of 33% and 60% in pupal and adult weight when exposed to the highest concentrations of microplastic particles but resulted in no detectable effects on mortality or developmental length. Our results demonstrate the potential for the vectoring of microplastics by this highly mobile species. However, the associated reductions in body size likely have profound consequences for movement capability in terms of foraging and migration and should be further investigated for their impact on ecosystem service provision.
1. Introduction
The ubiquity of plastic waste has rapidly risen, and this trend is predicted to worsen—increasing from the 460 million tonnes of total solid plastic waste per annum currently generated to 1231 metric tonnes by 2060 [1]. As of 2015, it has been estimated that around 79% of waste plastic is fated to enter landfills and eventually natural systems [2]. The durability of most commercial plastics enables them to accumulate across these ecosystems, and when they eventually degrade, they are known to fragment and splinter into smaller, equally problematic entities known as microplastics [3]. By 2060, microplastics will constitute 13.2% of the total weight of plastic pollutants worldwide [4]. Due to their small size and low-weight properties, microplastics are easily transported across terrestrial, aquatic, and atmospheric pathways [5,6]. Once in contact with biota, their size facilitates somatic incorporation through multiple pathways [7], be it through (un)intentional ingestion [8,9,10], inhalation [10,11] or to a lesser extent, via external contact [12].
As the most prominent pathway in which microplastics enter the body, the ingestion of these foreign substances can cause destruction to the gastrointestinal system by damaging the gut epithelium, reducing the mucus layer, inducing and creating gastrointestinal blockages, altering the gut microbiome, increasing disease susceptibility and causing tissue inflammation [13]. The presence of bodily microplastics may also have severe implications for reproduction and development [13]: microplastic presence has been linked with reduced growth rates and stunted development across a plethora of species [14,15,16,17,18]. Microplastics can also transfer between different life stages, but this ontogenic pathway has only been demonstrated in a limited range of species such as Culex pipiens, Aedes aegypti and Anopheles spp. [19,20,21]. Moreover, ontogenic transfer and ulterior transfer to mammals by mosquito bites has been experimentally demonstrated using Culex quinquefasciatus [22]. The role of insects in spreading microplastics by ontogenic transfer is potentially huge, as they can be very abundant and exhibit distinct life stages known to develop in habitats high in plastic contamination.
The drone fly Eristalis tenax (Diptera: Syrphidae) is a highly abundant and efficient pollinator, widely distributed across all continents except Antarctica [23]. This species plays a role in the pollination of a wide variety of plant species in both agroecosystems [24,25,26] and natural ecosystems [27,28]. Moreover, E. tenax lay their eggs in aquatic habitats rich in decaying matter, and subsequently their rat-tailed maggot larvae live and feed on microorganisms growing on organic detritus [29] in these aquatic environments until pupation, when they move to dry substrates. As adults, they feed on nectar and pollen and in western Europe and north America are known to undergo seasonal migratory movements [24,30,31,32]. As a migratory pollinator, E. tenax has been shown to vector pollen over hundreds of kilometres of open ocean and may be particularly important in connecting distant or geographically isolated plant populations [28,33,34,35]. This combination of larval development in potentially polluted aquatic environments [36] along with a highly mobile and economically and ecologically important adult stage led us to ask if E. tenax had the potential to vector microplastics and if exposure could affect ecoservice provision.
Here, we exposed E. tenax to varying concentrations of microplastic fragments, to investigate the potential for ontogenic transfer from the larval stage to the adult, and to determine the effects on mortality, developmental time and body size. Ultimately it is hoped that the results of this study may shed some light into some of the challenges that this species will face as the prevalence of plastic pollution increases across terrestrial and aquatic environments.
2. Materials and Methods
2.1. Eristalis tenax Husbandry
Specific E. tenax rearing conditions were established as in [37]. Grass silage was produced to provide ideal conditions for oviposition and larval growth. This silage was produced with a mixture of grass clippings (collected from the University of Exeter campus, Penryn) and deionised water, left to ferment for at least 2 weeks, covered with a muslin cloth to prevent insect infestation. Once fermented, the silage and silage water were used as a food source for E. tenax larvae [37]. To rear a captive colony of E. tenax, males and females were collected at the University of Exeter campus, Penryn, and placed in rearing cages (30 cm × 30 cm × 30 cm) for 7 days with a male-to-female ratio of 10:3. The hoverflies were provided pollen and 40% honey in water solution ad libitum. After 7 days, the gravid females were transferred to a separate cage (45 cm × 45 cm × 45 cm) with the same settings plus an oviposition glass tray filled with a 2:3 grass silage and silage water mixture. This tray was covered with sticks and dry leaves to provide a substrate for oviposition. The captive population was kept at 20 °C (Day), 15 °C (Night), humidity 80%, and 10.5:13.5 h light/dark cycle. The oviposition trays were checked daily for the presence of egg clutches.
2.2. Microplastic Exposure
A stock solution of 3.7 × 105 particles mL−1 was made up of fluorescent green-yellow polyamide fragments, ranging from 9 to 30 µm, in 15 mL of deionised water (UV-responsive RADGLO® EA30 dye, Radiant Colour NV, Houthalen-Helchteren, Belgium). Plastics were kindly provided by the laboratory of Dr Tamara Galloway (University of Exeter). The suspension was placed on a Fixed-Angle Platform Rocker for 12 hrs and vortexed before use to ensure complete homogenisation. Microplastics were pipetted to the glass treatment lagoons to obtain the desired treatment concentrations (0, 50, 500 and 5000 microparticles mL−1). After hatching, the first larval instars were moved to a treatment lagoon, composed of a glass container filled with a substrate made from a 4:1 mixture of decomposed sawdust (made by the same methods as the grass silage) and grass silage solids. A total of 60 g of substrate was added to 200 mL of silage water to create an environment suitable for larval development. These lagoons were placed in a larger container filled with enough sawdust to reach the lip of the lagoon to provide a dry environment in which larvae could exit their lagoons and pupate [37]. Each treatment was composed of one lagoon which contained 10 larvae.
2.3. Pupal and Adult Weight
To obtain pupal weight, individuals were lightly brushed down to remove any foreign material and weighed using a Ohaus Pioneer microscale (accuracy ±0.001 g). Newly emerged adults were placed at −20 °C for 12 h before recording adult weight (accuracy of ±0.001 g).
2.4. Quantifying Bodily Microplastics
The abdomen of the adult was dissected to remove the abdominal section of the gastrointestinal tract (mid and hindgut) and Malpighian tubules. Direct observation under an epifluorescent microscope (Leica DM IRE2 Inverted Fluorescence Microscope) was used to confirm the presence of fluorescent microplastics. These organs were then digested to isolate the plastic particles as described in [38]. In brief, 0.5 mL of Corolase (AB enzymes) diluted in a 1:2 ratio with water was added to the organs and left for 1–2 days, or until the entirety of the gastrointestinal tract and Malpighian tubules were digested [39]. After complete digestion had occurred, the remaining homogenate was pipetted onto a microfiber filter in a Petri dish and dried in an incubator for 24 h at 35 °C. An epifluorescent microscope (Leica DM IRE2 Inverted Fluorescence Microscope) was used to quantify the number of microplastic fragments. Images were taken using the DFC3000 G CCD greyscale Microscope Camera and given false colour using Leica Application Suite X (Leica Application Suite X (RRID:SCR_013673)).
2.5. Statistical Analysis
Data was analysed using R-Studio (v4.1.2; R Core Team 2024). After confirmation of normal distribution of the data using Shapiro–Wilk tests, a one-way Analysis of Variance (ANOVA) was performed to determine the presence of a significant relationship between concentration treatment, body weight and development time. The effect on mortality for both pupa and imago was analysed separately using a binomial generalised linear model. Post hoc analysis was conducted using Tukey’s comparisons where terms were considered to have a significant effect on the response variables at the 95% confidence level.
3. Results
3.1. Microplastic Contamination in E. tenax
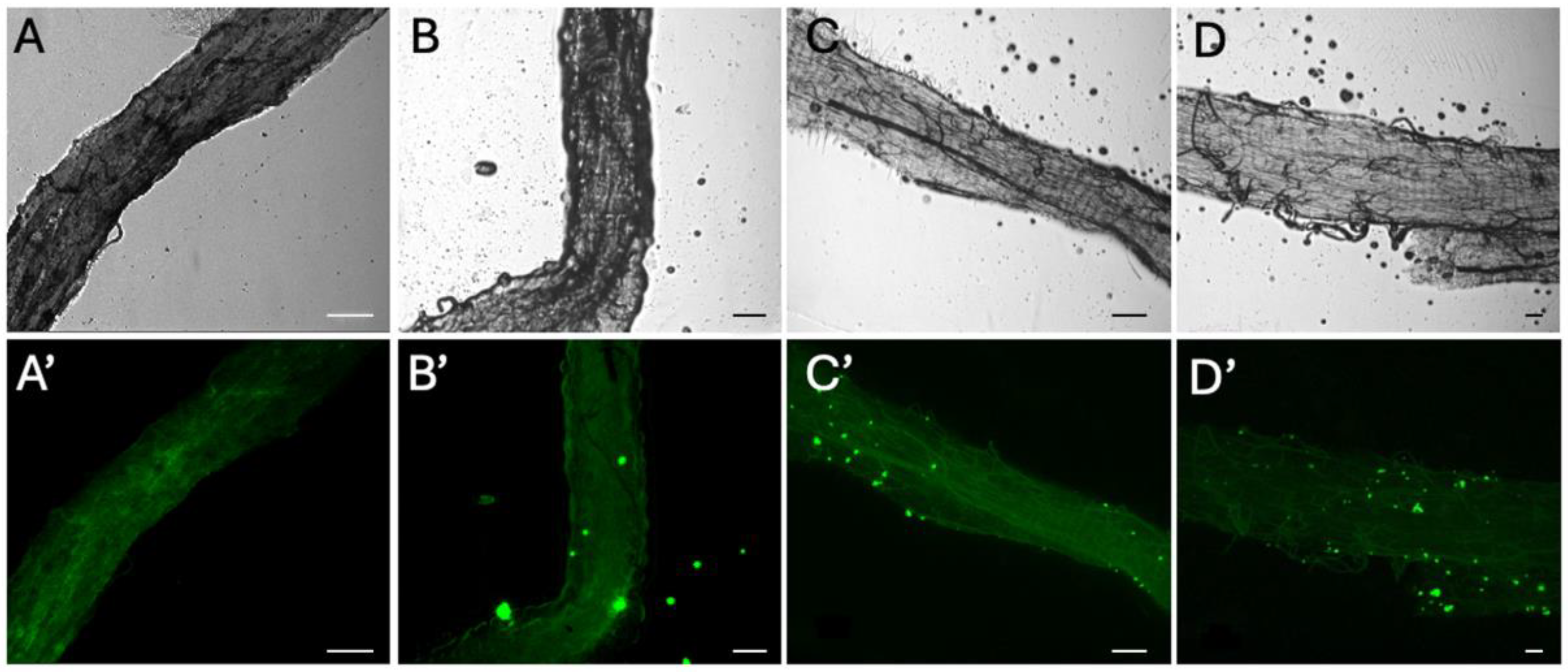
Dissection and digestion of adult E. tenax confirmed no microplastics were found in the abdomens of untreated control groups; further dissection of larvae at different stages which did not survive to the pupal life stage confirmed this. Visual confirmation, via epifluorescent microscopy, determined that fluorescent microplastics were present in the abdomen of individuals across all treatment populations, with microplastics found to accumulate in the hind and midgut (Figure 1). No microplastics were detected in the Malpighian tubule system of any individuals.
Figure 1.
Brightfield (A–D) and epifluorescent (A’–D’) microscope images of adult E. tenax equivalent gut sections from individuals reared in lagoons containing 0 (A,A’), 50 (B,B’), 500 (C,C’) and 5000 (D,D’) microplastic particles mL−1. Scale bars represent 100 μm.
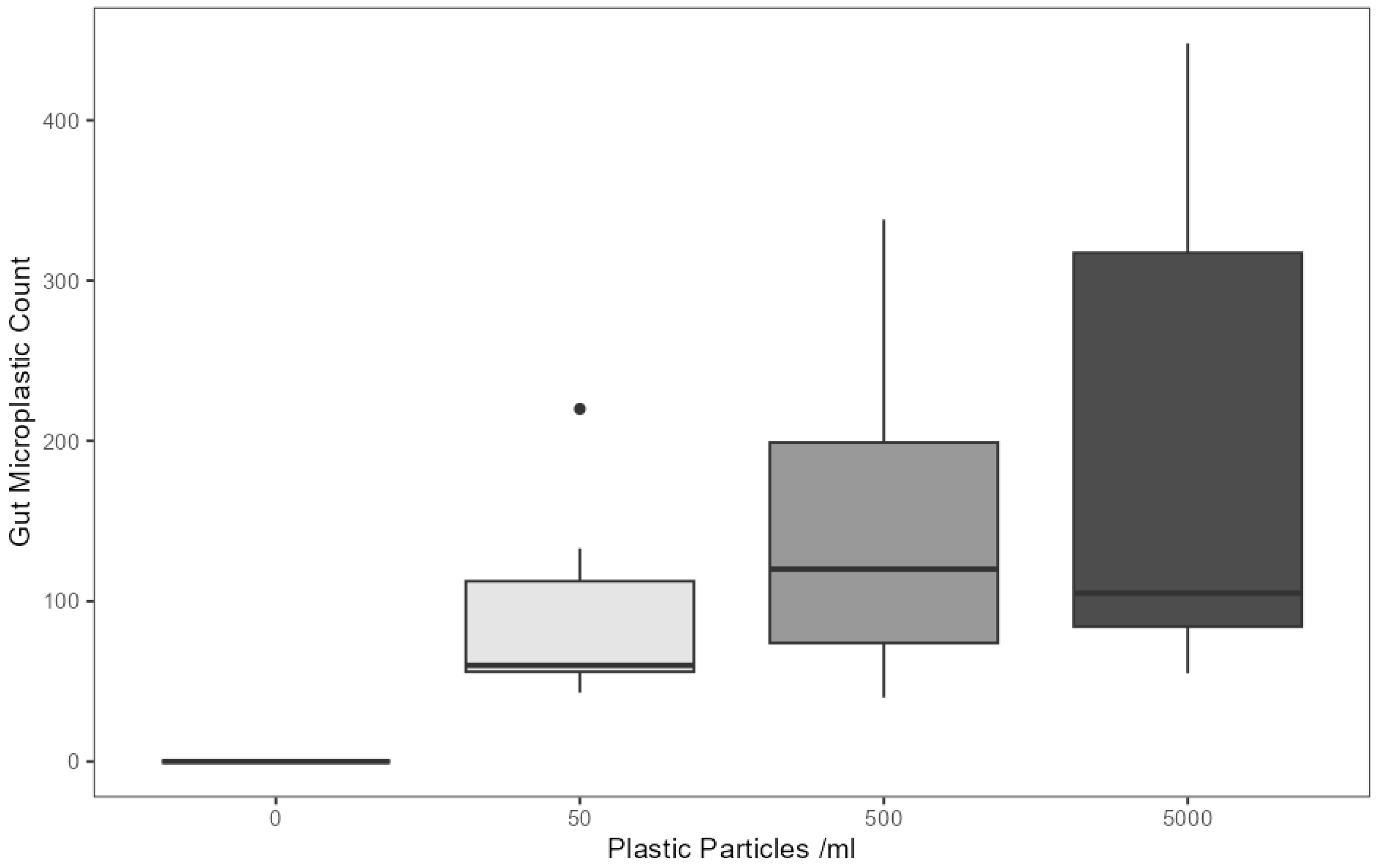
The enzymatic digestion of abdominal organs and subsequent quantification of remaining bodily microplastics revealed the presence of a significant relationship between the concentration of microplastics in the treatment lagoon and the number of microplastics in the gastrointestinal tract, where individuals reared in higher microplastic concentration lagoons retained a greater amount of these pollutants in their adult morphs (F(3,33) = 4.85, p < 0.05) (Figure 2; statistical indicators are presented in a synthetic tabular format in Table S2). Microplastic counts were highest in the 5000 particles mL−1 treatment at an average of 196.92 fragments per individual (SD = 162.1), followed by 500 particles mL−1 at 149.36 fragments (SD = 106.2) and 50 particles mL−1 at 94.59 fragments (SD = 63.33).
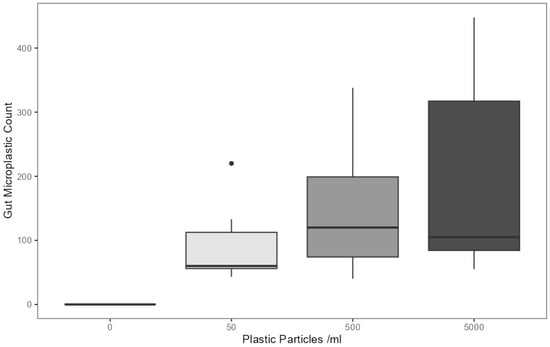
Figure 2.
Microplastic particles detected in the digested gastrointestinal tract of adult E. tenax reared in lagoons treated with different microplastic concentrations (0, 50, 500 and 5000 microplastic particles mL−1). Boxplots show the median value (bold line in box), interquartile range (box), range (whiskers) and outliers (dot).
3.2. Effects of Microplastic Exposure on E. tenax Development
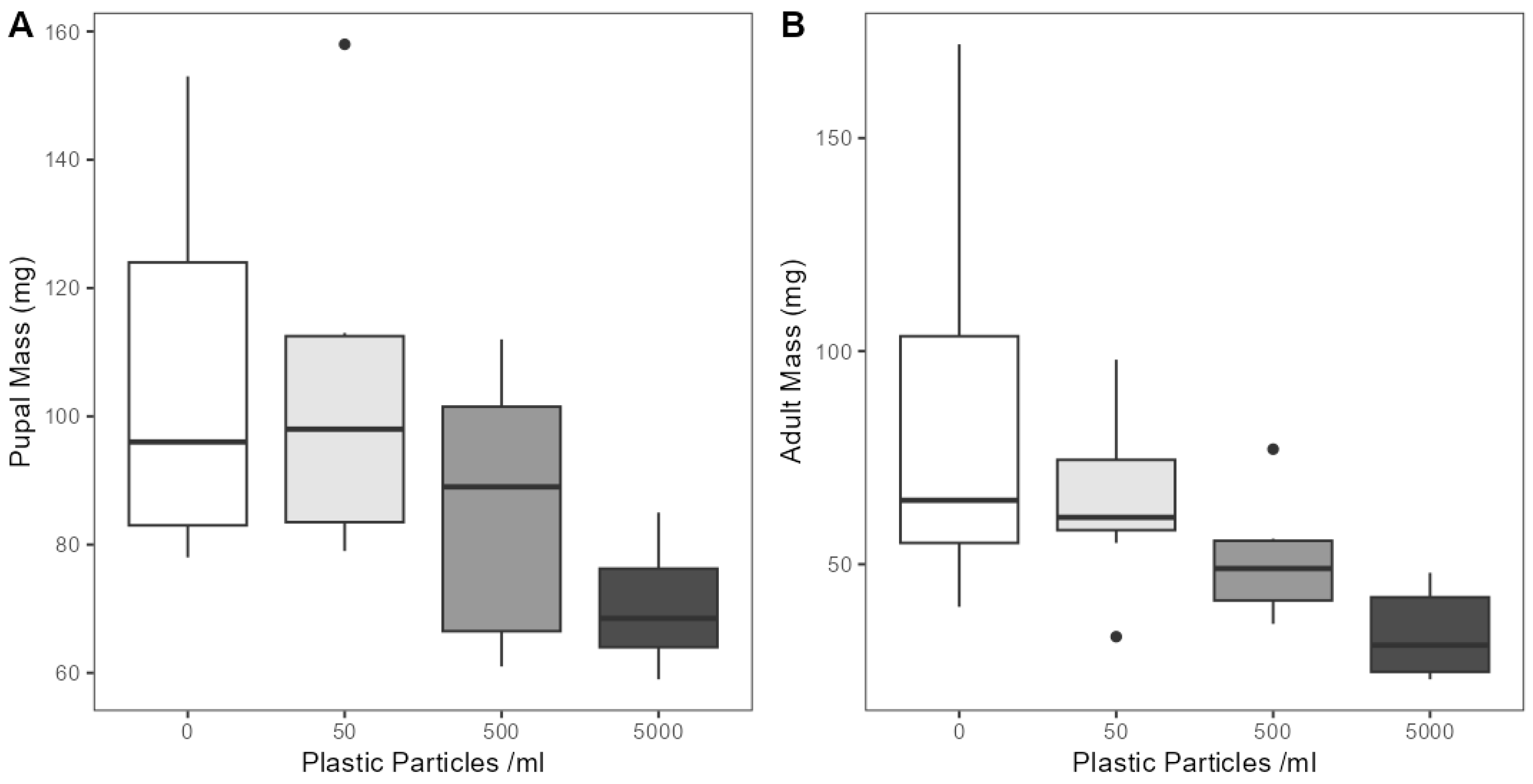
To investigate if microplastic exposure had effects on the development of E. tenax, we measured mortality rates, developmental times and body size. Larvae had similar mortality rates in all treatments including controls (Z(1,39) = 1.228, p = 0.220), with an average mortality rate of 27.5% across all treatments. Moreover, no mortality was detected in pupa, as all pupae developed into adults. We also detected no effect on the period between hatching and pupating (lasting on average 20.45 days across all groups) (F(3,25) = 1.026, p= 0.398) and between pupation and eclosion (lasting on average 14.93 days across all groups) (F(3,25) = 0.264, p= 0.851). However, microplastic contamination was found to have significant negative effects on body weight of pupae (F(3,25) = 4.086, p < 0.05) and adults (F(3,25) = 4.983, p < 0.05) (Figure 3). Post hoc analysis detected significant differences in the pupal mass of individuals from between 5000 particles mL−1 and control treatments (p < 0.05), but not between the other groups. Pupae reared in the 5000 particles mL−1 lagoon had the lowest average body mass at 70.5 mg (SD = 9.5), followed by 500 particles mL−1 at 85 mg (SD = 21.4), 50 particles ml−1 at 104 mg (SD = 27.7) and 0 particles mL−1 at 106 mg (SD = 28.3) (Figure 3A). Similarly, post hoc analysis revealed significant differences between the body weights of E. tenax adults across microplastic concentration treatments, between 5000 particles mL−1 and control treatments (p < 0.05) and 5000 particles mL−1 and 50 particles mL−1 treatments (p < 0.05). No significant difference was detected between the other groups. Adults reared in the 5000 particles mL−1 lagoon were found to have the lowest average body mass at 33 mg (SD = 9.7), followed by 500 particles mL−1 at 51 mg (SD = 14), 50 particles mL−1 at 65 mg (SD = 21) and 0 particles mL−1 at 85 mg (SD = 47) (Figure 3B).
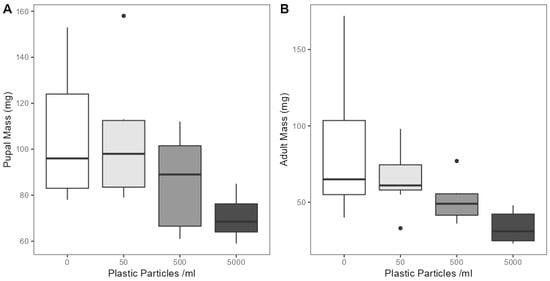
Figure 3.
The weight of (A) pupa and (B) adult E. tenax reared in lagoons treated with different microplastic concentrations (0, 50, 500 and 5000 microplastic particles mL−1). Boxplots show the median value (bold line in box), interquartile range (box), range (whiskers) and outliers (dots).
4. Discussion
Rearing of E. tenax in water contaminated with microplastic particles reveals that they retain these plastics in their body from the larval through pupal to adult developmental stages. Despite this, microplastic exposure had no detectable effects on mortality or developmental length but did reduce pupal and adult weight in animals exposed to the highest concentrations of microplastic particles. The plastic used in this work was fragmented polyamide (9–30 µm), and other plastic polymers or sizes could provide different outcomes. However, this serves as a proof of principle for the possible effects of plastic particles in migratory insects.
4.1. Microplastic Retention
Consistent with prior studies on ontogenic microplastic transfer in the Culex, Aedes and Anopheles mosquitoes [19,20,21,40], the presence of abdominal microplastics in the adult individuals of E. tenax confirms that these contaminants can be passed down from the feeding larval stage to the sedentary, non-feeding pupa and finally to the mobile adult. The localisation of microplastics in the mid and hindgut demonstrates that ingestion is the primary pathway in which these pollutants contaminate E. tenax during the larval stage, whereby microplastic fragments suspended in the water enter the digestive system of rat-tailed maggots during the filter-feeding process and are retained in the pupal and adult stages. The gut microplastic content was greatest in E. tenax reared in lagoons with high microplastic concentrations, where the microplastic gut content was 52.12% greater than in the lowest. This was previously observed in similar studies on ontogenic transfer of plastic particles in Culex mosquitoes [40]. In many holometabolous insects, components of the digestive system are not wholly histolysed during metamorphosis [41,42]. In dipteran species, for instance, the larval gastrointestinal tract is not completely lysed, instead entering a transitory state where, during pupation, the emerging adult gut is formed around the transient larval gut [41]. As such, there is potential for the gut to retain its contents between the life stages of holometabolous insects. Although this pathway has not been explicitly confirmed for microplastic retention, similar mechanics have been identified in the transference of gut microbiomes in Spodoptera littoralis, where Enterococcus spp. are retained across the digestive systems of all life stages [43].
4.2. Effects on Survival
Even at the highest concentration tested (5000 particles mL−1), microplastic contamination had no significant effect on the mortality of E. tenax larvae and pupa. The average mortality for larvae in this study was 27.5% across all treatments and no pupal mortality was detected throughout the study. This observation is very similar to the one from Simakova and collaborators when using Aedes aegipti [20]. No effect on mortality was also observed in Anopheles mosquitoes [19]. Across the literature, there is conflicting evidence that microplastic contamination affects the survival rate of early life stages. In the barnacle Amphibalanus amphitrite larvae, for instance, polystyrene spheres were found to have no significant effect on larval mortality when exposed to microplastic concentrations similar to the ones utilised in this study [44]. Similarly, polyethene microbeads had limited effects on the mortality and overall fitness of sea bass larvae, due to their ability to efficiently eliminate their gut content [45]. The lethality of microplastic contamination, however, has been demonstrated in Japanese rice fish, where the ingestion of microplastics had significant effects on mortality, which were attributed to intestinal lesions and obstruction associated with microplastic ingestion [46]. This variation in fitness response throughout the literature suggests that the toxicity of microplastics may have a species-dependent element, where some species may be robust enough to mitigate against the lethal effects of microplastic contamination. It has also been suggested that the effects of microplastic contamination are highly dose-dependent, such that they do not exhibit any substantial toxic properties at low concentrations and may even have positive fitness effects [47]. The contamination of Drosophila sp. with low doses of polyethylene terephthalate was shown to increase the lifespan of males, possibly because of the hormesis pathway [48]. It is thought that through the hormesis effect, these pollutants stimulate the nervous system, thus increasing overall bodily condition [48,49]. There is, however, limited information regarding this phenomenon. As a final consideration, it is also worth noting that filter-feeding invertebrates are known to possess a level of tolerance against the ingestion of inedible materials (i.e., sediment and inorganic matter) suspended in all aquatic environments. These aquatic organisms are generally thought to be able to resist the lethal effects of such foreign material [50,51].
4.3. Effects on Development Time
Across all treatments, the duration between hatching and pupation was, on average, around 20.5 days, and 15 days for the period between pupation and eclosion. The absence of a significant relationship between larval (the period between hatching and pupation) and pupal development times (the period between pupation and eclosion) and the presence of gut microplastics suggests that the energetic costs accrued during the larval stage of E. tenax were not severe enough to affect the development time of the subsequent life stages. This is consistent with the results found in studies of other holometabolous insects, where the development time (between hatching and adult emergence) of wild-captured populations of both Culex pipiens and Culex tarsalis was unaffected by exposure to microplastics [52]. Similarly, in laboratory-reared populations, direct exposure to microplastics was found to have no significant effect on the development time of mealworms (Tenebrio molitor) or Drosophila melanogaster [53,54]. This phenomenon extends to other invertebrate species. In acorn barnacle (A. amphitrite) larvae, polystyrene ingestion of similar microplastic concentrations as used in the present study was shown to have no significant effect on development time, metamorphosis and settling rates [44]. Contrary to the findings of this study, contamination during the larval stage in other holometabolous insects has been shown to prolong the period between life stages. In the harlequin fly (Chironomus riparius), high concentrations of microplastic contamination were found to increase the development period between the 1st larval instar and adult stage by approximately 15 days [55]. This delayed development is thought to be once again attributed to the energetic costs associated with the long-term presence of gut microplastics. The absence of this effect in E. tenax and other holometabolous species suggests that there is a species-specific element to this phenomenon, where some taxa can better process these pollutants through the digestive system. Characteristics such as body size, for example, may be important for the capacity of excretion of microplastics. E. tenax is about 50 times bigger than C. riparius [56,57], and the particles used in the C. riparius experiments were larger than the ones used in our study [55].
4.4. Effects on Biometric Parameters
Despite no measurable effects on survival rates, the ingestion of microplastics was shown to cause sub-lethal effects in E. tenax. The body weight of pupae and adults in this study was significantly reduced by microplastic exposure. In the pupae, the average weight of individuals reared in the highest microplastic concentration was 33.4% lower than the control, whereas adults were 60% lighter. The disparity between these two values may be due to the cumulative effects that microplastic ingestion has on E. tenax. As these pollutants persist within an organism, their cumulative negative effects increasingly impair somatic growth the longer they remain in the gut [58]. The influence of microplastic ingestion on the growth rates of invertebrates has been identified in several species. In Anopheles mosquitos, low weights of both pupa and adult have also been reported after experimental exposure to very high concentration (8 × 106 particles/mL) of polystyrene particles [19], and studies on the langoustine Nephrops norvegicus revealed that the ingestion of polyethene corresponded to a significant reduction in body weight, where contaminated individuals lost a total of 0.02% body mass per day [59]. These effects are thought to be ubiquitous across invertebrates of different clades, body sizes and habitat requirements [60], with a reduction in growth rates and body mass having been detected in dinoflagellate species [61], Manilla clams (Ruditapes philippinarum) [62] or tropical house crickets (Gryllodes sigillatus) [63]. There are several reasons that may explain this phenomenon. A reduction in body mass and growth is primarily thought to be caused by the energetic costs associated with processing and egesting these non-organic pollutants, which would otherwise be dedicated to somatic growth [60,64]. It is also thought that microplastics may influence nutrient absorption by reducing the bioavailability of key organic compounds. Lipid droplets, for example, have been shown to aggregate on the corona of microplastics (due to their hydrophobic properties), forming large amalgamations of microplastics and lipids that are less available for enzymatic breakdown and absorption [65]. Furthermore, due to their environmental ubiquity and resistance to enzymatic activity, these pollutants persist within the guts of organisms, potentially causing blockages in the gastrointestinal tract, this could be particularly significant for E. tenax larvae whose small size, relative to the microplastic contaminants, may amplify the effects of intestinal blockages, limiting the potential for nutrient absorption [55,66], thus stunting the growth of larvae and subsequent life stages. As the energetic costs that are incurred in the larval stage of holometabolous insects are thought to be reflected in the growth rates and body mass of adults [67,68], intestinal blockages from microplastics that occur in the feeding larvae may explain the reduced body mass of the adult E. tenax. The possible costs associated with damage to the gastrointestinal tract in the feeding larvae of E. tenax may also explain the reduced mass in treatment populations, where lesions and lacerations caused by microplastic processing may incur energetic costs that once again deplete energy reserves needed for growth [69]. These possibilities require further investigation in this species.
Furthermore, the dose-dependent response observed in this study has significant implications for the future fitness of global E. tenax populations (and potentially other holometabolous species). As the abundance of microplastic pollutants is expected to rise in most habitats [70,71], the severity of sub-lethal effects may intensify, potentially resulting in significant consequences for species fitness and, consequently, broader ecosystem functioning [72]. It remains unclear whether these pollutants can translocate during metamorphosis, where the partial lysis of the digestive system might allow microplastics to disperse throughout the body in the adult stage. Studies attempting to determine the effects of somatic microplastics in holometabolous insects have largely been limited to functioning within the digestive system. Understanding how these pollutants could migrate into other organs or tissues may provide insight into how processes like reproduction, dispersal ability and pollination efficiency may be affected by the rising abundance of environmental microplastics.
4.5. Effects on Ecosystem Services
E. tenax is important as a pollinator and decomposer of organic material, so detrimental effects from microplastic contamination may alter the provision of these ecosystem services. E. tenax is known to transport pollen from a wide variety of floral species [27] and its ubiquity and pollination effort rival the efficiency of honeybee colonies, making this species key for several plant taxa [73]. In addition, E. tenax has been shown to vector pollen hundreds of kilometres across oceans [74,75], marking them as potentially important in shaping plant population structure over vast distances. As such, reduced body condition or increased mortality in E. tenax due to microplastic exposure could impair these services, negatively impacting ecosystem functioning and agricultural productivity. In pollinator species of the Apis genus, microplastic ingestion has been evidenced to affect foraging behaviour, memory and potential pollination efficacy [76,77], however, any effects on E. tenax foraging remain to be investigated.
The potential for ontogenic transfer of microplastics in E. tenax has further implications for microplastic vectoring between discrete ecosystems and habitat types, either through direct excretion [78] or via bodily decomposition, where microplastics retained within tissues are released upon death. Moreover, transfer in the food web may lead to bioaccumulation, since E tenax is part of the diet of birds, spiders and wasps. This may be particularly significant due to the highly mobile nature of E. tenax both while foraging and while undertaking long-distance seasonal migration [28,74,79]. Migration is likely a global occurrence in this species with pathways spanning significant landmasses, waterbodies and even entire continents [32,74,79,80]. Although not conclusively confirmed in migratory insects, the ability for microplastics to be vectored by migrants is a phenomenon that has been demonstrated in several taxa, with birds having been identified as major vectors of this pollutant [81,82]. The impact of microplastic pollution on migration ability in E. tenax is unexplored; however, larger body sizes and better body conditions are associated with migratory ability in a range of species, including migratory hoverflies [75,83]. Therefore, the negative effect of microplastic exposure on body size seen here would be predicted to reduce migratory capacity, potentially disrupting a major global flow of biomass and genetic material.
5. Conclusions
The results from this study demonstrate the capacity for ontogenic microplastic transfer in E. tenax from the feeding larval stage to the non-feeding pupa and finally the mobile adult stage. Microplastic contamination displayed a dose-dependent effect; larvae raised in lagoons with higher microplastic concentrations exhibited greater gut microplastic content and had reduced body weights in both pupae and imago. The presence of this pollutant, however, was shown to have no significant lethal effects, where the mortality of individuals across all instars was not affected by the concentration of supplemented microplastics. Similarly, microplastic contamination had no impact on development time, as exposure to this pollutant did not affect the duration of larval or pupal periods in E. tenax. Future studies should investigate the sub-lethal effects of microplastic exposure and the consequences for foraging, migration and reproduction, as disruption of these has the potential to alter abundance, population distribution, migration and the provision of important ecosystem services.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microplastics4020022/s1, Table S1: Raw data of measurements described. Table S2. Summarised results of the effects of microplastic treatment on various response variables.
Author Contributions
Conceptualization, K.R.W. and E.J.-G.; methodology, K.R.W., E.J.-G., M.A. and J.C.B.; validation, M.A. and E.J.-G.; formal analysis, M.A., E.J.-G. and J.C.B.; investigation, M.A., J.C.B. and O.M.P.; resources, K.R.W. and E.J.-G.; data curation, M.A.; writing—original draft preparation, M.A. and E.J.-G.; writing—review and editing, K.R.W., O.M.P., J.C.B., M.A. and E.J-G.; visualization, M.A., E.J.-G. and J.C.B.; supervision, K.R.W. and E.J.-G.; project administration, K.R.W.; funding acquisition, K.R.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Masters programme at the University of Exeter.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of The University of Exeter (protocol code 527784 approved April 2024).
Data Availability Statement
Data supporting this manuscript can be found in the Supplementary Materials.
Acknowledgments
Authors would like to thank the lab of Tamara Galloway for the donation of plastic particles, and Toby Doyle for technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- OECD. Global Plastics Outlook; OECD: Paris, France, 2022. [Google Scholar] [CrossRef]
- Geyer, R. Production, use, and fate of synthetic polymers. In Plastic Waste and Recycling; Elsevier: Amsterdam, The Netherlands, 2020; pp. 13–32. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, Z.; Lei, Y.; Tang, Y.; Wu, L.; Zhang, X.; Naidu, R.; Megharaj, M.; Fang, C. Microplastics generated when opening plastic packaging. Sci. Rep. 2020, 10, 4841. [Google Scholar] [CrossRef]
- Evangeliou, N.; Grythe, H.; Klimont, Z.; Heyes, C.; Eckhardt, S.; Lopez-Aparicio, S.; Stohl, A. Atmospheric transport is a major pathway of microplastics to remote regions. Nat. Commun. 2020, 11, 3381. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Xiong, X.; Zhang, Y.; Wu, C.; Xu, X.; Sun, C.; Shi, H. Global transportation of plastics and microplastics: A critical review of pathways and influences. Sci. Total Environ. 2022, 831, 154884. [Google Scholar] [CrossRef] [PubMed]
- Anbumani, S.; Kakkar, P. Ecotoxicological effects of microplastics on biota: A review. Environ. Sci. Pollut. Res. 2018, 25, 14373–14396. [Google Scholar] [CrossRef]
- Jovanović, B. Ingestion of microplastics by fish and its potential consequences from a physical perspective. Integr. Environ. Assess. Manag. 2017, 13, 510–515. [Google Scholar] [CrossRef]
- Trestrail, C.; Nugegoda, D.; Shimeta, J. Invertebrate responses to microplastic ingestion: Reviewing the role of the antioxidant system. Sci. Total Environ. 2020, 734, 138559. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Environment: Intake through the Food Web, Human Exposure and Toxicological Effects. Toxics 2021, 9, 224. [Google Scholar] [CrossRef]
- Khan, A.; Jia, Z. Recent insights into uptake, toxicity, and molecular targets of microplastics and nanoplastics relevant to human health impacts. Iscience 2023, 26, 106061. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, X.; Mei, T.; Xu, M.; Lu, Z.; Dai, H.; Pi, F.; Wang, J. Estimation of contamination level in microplastic-exposed crayfish by laser confocal micro-Raman imaging. Food Chem. 2022, 397, 133844. [Google Scholar] [CrossRef]
- Zolotova, N.; Kosyreva, A.; Dzhalilova, D.; Fokichev, N.; Makarova, O. Harmful effects of the microplastic pollution on animal health: A literature review. PeerJ 2022, 10, e13503. [Google Scholar] [CrossRef]
- Lo, H.K.A.; Chan, K.Y.K. Negative effects of microplastic exposure on growth and development of Crepidula onyx. Environ. Pollut. 2018, 233, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Gandara e Silva, P.P.; Nobre, C.R.; Resaffe, P.; Pereira, C.D.S.; Gusmão, F. Leachate from microplastics impairs larval development in brown mussels. Water Res. 2016, 106, 364–370. [Google Scholar] [CrossRef]
- Rendell-Bhatti, F.; Paganos, P.; Pouch, A.; Mitchell, C.; D’aniello, S.; Godley, B.J.; Pazdro, K.; Arnone, M.I.; Jimenez-Guri, E. Developmental toxicity of plastic leachates on the sea urchin Paracentrotus lividus. Environ. Pollut. 2021, 269, 115744. [Google Scholar] [CrossRef] [PubMed]
- Paganos, P.; Ullmann, C.V.; Gaglio, D.; Bonanomi, M.; Salmistraro, N.; Arnone, M.I.; Jimenez-Guri, E. Plastic leachate-induced toxicity during sea urchin embryonic development: Insights into the molecular pathways affected by PVC. Sci. Total Environ. 2023, 864, 160901. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Guri, E.; Paganos, P.; La Vecchia, C.; Annona, G.; Caccavale, F.; Molina, M.D.; Ferrández-Roldán, A.; Donnellan, R.D.; Salatiello, F.; Johnstone, A.; et al. Developmental toxicity of pre-production plastic pellets affects a large swathe of invertebrate taxa. Chemosphere 2024, 356, 141887. [Google Scholar] [CrossRef]
- Simakova, A.V.; Varenitsina, A.A.; Babkina, I.B.; Andreeva, Y.V.; Frank, Y.A. Ontogenetic transfer of microplastics in natural populations of malaria mosquitoes in Western Siberia. Entomol. Exp. Appl. 2024, 172, 1046–1053. [Google Scholar] [CrossRef]
- Simakova, A.; Varenitsina, A.; Babkina, I.; Andreeva, Y.; Bagirov, R.; Yartsev, V.; Frank, Y. Ontogenetic Transfer of Microplastics in Bloodsucking Mosquitoes Aedes aegypti L. (Diptera: Culicidae) Is a Potential Pathway for Particle Distribution in the Environment. Water 2022, 14, 1852. [Google Scholar] [CrossRef]
- Al-Jaibachi, R.; Cuthbert, R.N.; Callaghan, A. Up and away: Ontogenic transference as a pathway for aerial dispersal of microplastics. Biol. Lett. 2018, 14, 20180479. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Liang, G.; Gao, H.; Guo, S.; Zhou, X.; Xing, D.; Zhao, T.; Li, C. Microplastics affect mosquito from aquatic to terrestrial lifestyles and are transferred to mammals through mosquito bites. Sci. Total Environ. 2024, 917, 170547. [Google Scholar] [CrossRef]
- Francuski, L.; Djurakic, M.; Ludoški, J.; Milankov, V. Landscape genetics and spatial pattern of phenotypic variation of Eristalis tenax across Europe. J. Zool. Syst. Evol. Res. 2013, 51, 227–238. [Google Scholar] [CrossRef]
- Jarlan, A.; De Oliveira, D.; Gingras, J. Effects of Eristalis tenax (Diptera: Syrphidae) Pollination on Characteristics of Greenhouse Sweet Pepper Fruits. J. Econ. Entomol. 1997, 90, 1650–1654. [Google Scholar] [CrossRef]
- Howlett, B.G.; Gee, M. The potential management of the drone fly (Eristalis tenax) as a crop pollinator in New Zealand. N. Z. Plant Prot. 2019, 72, 221–230. [Google Scholar] [CrossRef]
- Fijen, T.P.M.; Read, S.F.J.; Walker, M.K.; Gee, M.; Nelson, W.R.; Howlett, B.G. Different landscape features within a simplified agroecosystem support diverse pollinators and their service to crop plants. Landsc. Ecol. 2022, 37, 1787–1799. [Google Scholar] [CrossRef]
- Lucas, A.; Bodger, O.; Brosi, B.J.; Ford, C.R.; Forman, D.W.; Greig, C.; Hegarty, M.; Jones, L.; Neyland, P.J.; de Vere, N. Floral resource partitioning by individuals within generalised hoverfly pollination networks revealed by DNA metabarcoding. Sci. Rep. 2018, 8, 5133. [Google Scholar] [CrossRef]
- Doyle, T.; Hawkes, W.L.S.; Massy, R.; Powney, G.D.; Menz, M.H.M.; Wotton, K.R. Pollination by hoverflies in the Anthropocene: Pollination by Hoverflies. Proc. R. Soc. B 2020, 287, 20200508. [Google Scholar] [CrossRef]
- Kamdem, M.M.; Otomo, P.V. Developmental performance of Eristalis tenax larvae (Diptera: Syrphidae): Influence of growth media and yeast addition during captive rearing. J. Exp. Zool. A Ecol. Integr. Physiol. 2023, 339, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Aubert, J.; Aubert, J.-J.; Goeldlin, P. Douze ans de captures systématiques de Syrphides (Diptères) au col de Bretolet (Alpes valaisannes). Mitt. Schweiz. Entomol. Ges. 1976, 49, 115–142. [Google Scholar]
- Owen, D.F. A migration of insects at Spurn Point, Yorkshire. Entomol. Mon. Mag. 1956, 92, 43–44. [Google Scholar]
- Shannon, H.J. A Preliminary Report on the Seasonal Migrations of Insects. J. N. Y. Entomol. Soc. 1926, 34, 199–205. [Google Scholar]
- Hawkes, W.L.; Weston, S.T.; Cook, H.; Doyle, T.; Massy, R.; Guri, E.J.; Jimenez, R.E.W.; Wotton, K.R. Migratory hoverflies orientate north during spring migration. Biol. Lett. 2022, 18, 20220318. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bañón, C.; Petanidou, T.; Marcos-García, M.Á. Pollination in small islands by occasional visitors: The case of Daucus carota subsp. commutatus (Apiaceae) in the Columbretes archipelago, Spain. Plant Ecol. 2007, 192, 133–151. [Google Scholar] [CrossRef]
- Rader, R.; Cunningham, S.A.; Howlett, B.G.; Inouye, D.W. Non-Bee Insects as Visitors and Pollinators of Crops: Biology, Ecology, and Management. Annu. Rev. Entomol. 2020, 65, 391–407. [Google Scholar] [CrossRef]
- Hoellein, T.J.; Schwenk, B.A.; Kazmierczak, E.M.; Petersen, F. Plastic litter is a part of the carbon cycle in an urban river: Microplastic and macroplastic accumulate with organic matter in floating debris rafts. Water Environ. Res. 2024, 96, e11116. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, S.; Thyselius, M.; Holden, M.; Nordström, K. Rearing and Long-Term Maintenance of Eristalis tenax Hoverflies for Research Studies. J. Vis. Exp. 2018, 135, 57711. [Google Scholar] [CrossRef]
- Catarino, A.I.; Thompson, R.; Sanderson, W.; Henry, T.B. Development and optimization of a standard method for extraction of microplastics in mussels by enzyme digestion of soft tissues. Environ. Toxicol. Chem. 2016, 36, 947–951. [Google Scholar] [CrossRef]
- Schlegel, K.; Sontheimer, K.; Eisner, P.; Schweiggert-Weisz, U. Effect of enzyme-assisted hydrolysis on protein pattern, technofunctional, and sensory properties of lupin protein isolates using enzyme combinations. Food Sci. Nutr. 2020, 8, 3041–3051. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaibachi, R.; Cuthbert, R.N.; Callaghan, A. Examining effects of ontogenic microplastic transference on Culex mosquito mortality and adult weight. Sci. Total Environ. 2019, 651, 871–876. [Google Scholar] [CrossRef]
- Mason, C.J.; Auth, J.; Geib, S.M. Gut bacterial population and community dynamics following adult emergence in pest tephritid fruit flies. Sci. Rep. 2023, 13, 13723. [Google Scholar] [CrossRef]
- Takashima, S.; Younossi-Hartenstein, A.; Ortiz, P.A.; Hartenstein, V. A novel tissue in an established model system: The Drosophila pupal midgut. Dev. Genes. Evol. 2011, 221, 69–81. [Google Scholar] [CrossRef]
- Chen, B.; Teh, B.-S.; Sun, C.; Hu, S.; Lu, X.; Boland, W.; Shao, Y. Biodiversity and Activity of the Gut Microbiota across the Life History of the Insect Herbivore Spodoptera littoralis. Sci. Rep. 2016, 6, 29505. [Google Scholar] [CrossRef]
- Yu, S.-P.; Chan, B.K.K. Intergenerational microplastics impact the intertidal barnacle Amphibalanus amphitrite during the planktonic larval and benthic adult stages. Environ. Pollut. 2020, 267, 115560. [Google Scholar] [CrossRef]
- Mazurais, D.; Ernande, B.; Quazuguel, P.; Severe, A.; Huelvan, C.; Madec, L.; Mouchel, O.; Soudant, P.; Robbens, J.; Huvet, A.; et al. Evaluation of the impact of polyethylene microbeads ingestion in European sea bass (Dicentrarchus labrax) larvae. Mar. Environ. Res. 2015, 112, 78–85. [Google Scholar] [CrossRef]
- Pannetier, P.; Morin, B.; Le Bihanic, F.; Dubreil, L.; Clérandeau, C.; Chouvellon, F.; Van Arkel, K.; Danion, M.; Cachot, J. Environmental samples of microplastics induce significant toxic effects in fish larvae. Environ. Int. 2020, 134, 105047. [Google Scholar] [CrossRef]
- Wang, J.; Coffin, S.; Sun, C.; Schlenk, D.; Gan, J. Negligible effects of microplastics on animal fitness and HOC bioaccumulation in earthworm Eisenia fetida in soil. Environ. Pollut. 2019, 249, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhang, D.; Liu, X.; Xu, Y.; Tang, H.; Li, Y.; Shen, J. Sex-specific effects of PET-MPs on Drosophila lifespan. Arch. Insect Biochem. Physiol. 2022, 110, e21909. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liang, B.; Jin, H. The impact of microplastics on insect physiology and the indication of hormesis. TrAC Trends Anal. Chem. 2023, 165, 117130. [Google Scholar] [CrossRef]
- Ogonowski, M.; Schür, C.; Jarsén, Å.; Gorokhova, E. The Effects of Natural and Anthropogenic Microparticles on Individual Fitness in Daphnia magna. PLoS ONE 2016, 11, e0155063. [Google Scholar] [CrossRef]
- Gonçalves, C.; Martins, M.; Sobral, P.; Costa, P.M.; Costa, M.H. An assessment of the ability to ingest and excrete microplastics by filter-feeders: A case study with the Mediterranean mussel. Environ. Pollut. 2019, 245, 600–606. [Google Scholar] [CrossRef]
- Thormeyer, M.; Tseng, M. No Effect of Realistic Microplastic Exposure on Growth and Development of Wild-caught Culex (Diptera: Culicidae) Mosquitoes. J. Med. Entomol. 2023, 60, 604–607. [Google Scholar] [CrossRef]
- Kokalj, A.J.; Nagode, A.; Drobne, D.; Dolar, A. Effects of agricultural microplastics in multigenerational tests with insects; mealworms Tenebrio molitor. Sci. Total Environ. 2024, 946, 174490. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Guri, E.; Roberts, K.E.; García, F.C.; Tourmente, M.; Longdon, B.; Godley, B.J. Transgenerational effects on development following microplastic exposure in Drosophila melanogaster. PeerJ 2021, 9, e11369. [Google Scholar] [CrossRef]
- Stanković, J.; Milošević, D.; Savić-Zdraković, D.; Yalçın, G.; Yildiz, D.; Beklioğlu, M.; Jovanović, B. Exposure to a microplastic mixture is altering the life traits and is causing deformities in the non-biting midge Chironomus riparius Meigen (1804). Environ. Pollut. 2020, 262, 114248. [Google Scholar] [CrossRef] [PubMed]
- Folkö, A. The Relationship Between Body Size and Dry Weight in Hoverflies (Syrphidae), and Their Movements Along an Urban Linear Landscape Element. Bachelor’s Thesis, Uppsala Universitet, Uppsala, Sweden, 2014. [Google Scholar]
- Day, K.E.; Kirby, R.S.; Reynoldson, T.B. Sexual dimorphism in Chironomus riparius (meigen): Impact on interpretation of growth in whole-sediment toxicity tests. Environ. Toxicol. Chem. 1994, 13, 35–39. [Google Scholar] [CrossRef]
- Urbina, M.A.; da Silva Montes, C.; Schäfer, A.; Castillo, N.; Urzúa, Á.; Lagos, M.E. Slow and steady hurts the crab: Effects of chronic and acute microplastic exposures on a filter feeder crab. Sci. Total Environ. 2023, 857, 159135. [Google Scholar] [CrossRef]
- Welden, N.A.C.; Cowie, P.R. Long-term microplastic retention causes reduced body condition in the langoustine. Nephrops norvegicus. Environ. Pollut. 2016, 218, 895–900. [Google Scholar] [CrossRef]
- Arciga, S.M.B.; Soliman, V.S. Microplastics Reduce the Growth of Exposed Marine Invertebrates: A Meta-Analysis. J. Fish. Environ. 2020, 44, 53–60. [Google Scholar]
- Fulfer, V.M.; Menden-Deuer, S. Heterotrophic Dinoflagellate Growth and Grazing Rates Reduced by Microplastic Ingestion. Front. Mar. Sci. 2021, 8, 716349. [Google Scholar] [CrossRef]
- Jiang, W.; Fang, J.; Du, M.; Gao, Y.; Fang, J.; Jiang, Z. Microplastics influence physiological processes, growth and reproduction in the Manila clam, Ruditapes philippinarum. Environ. Pollut. 2022, 293, 118502. [Google Scholar] [CrossRef]
- Fudlosid, S.; Ritchie, M.W.; Muzzatti, M.J.; Allison, J.E.; Provencher, J.; MacMillan, H.A. Ingestion of Microplastic Fibres, But Not Microplastic Beads, Impacts Growth Rates in the Tropical House Cricket Gryllodes Sigillatus. Front. Physiol. 2022, 13, 871149. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Lee, W.T.; Chan, A.K.Y.; Lo, H.S.; Shin, P.K.S.; Cheung, S.G. Microplastic ingestion reduces energy intake in the clam Atactodea striata. Mar. Pollut. Bull. 2017, 124, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Kaseke, T.; Lujic, T.; Velickovic, T.C. Nano- and Microplastics Migration from Plastic Food Packaging into Dairy Products: Impact on Nutrient Digestion, Absorption, and Metabolism. Foods 2023, 12, 3043. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic Ingestion by Zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef]
- Poças, G.M.; Crosbie, A.E.; Mirth, C.K. When does diet matter? The roles of larval and adult nutrition in regulating adult size traits in Drosophila melanogaster. J. Insect Physiol. 2022, 139, 104051. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, E.; Rossi, M.; Niven, J.E. Larval nutrition impacts survival to adulthood, body size and the allometric scaling of metabolic rate in adult honeybees. J. Exp. Biol. 2021, 224, jeb242393. [Google Scholar] [CrossRef]
- Prata, J.C.; Silva, C.J.M.; Serpa, D.; Soares, A.M.V.M.; Gravato, C.; Silva, A.L.P. Mechanisms influencing the impact of microplastics on freshwater benthic invertebrates: Uptake dynamics and adverse effects on Chironomus riparius. Sci. Total Environ. 2023, 859, 160426. [Google Scholar] [CrossRef] [PubMed]
- Ivar do Sul, J.A.; Costa, M.F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352–364. [Google Scholar] [CrossRef]
- Akanyange, S.N.; Zhang, Y.; Zhao, X.; Adom-Asamoah, G.; Ature, A.-R.A.; Anning, C.; Tianpeng, C.; Zhao, H.; Lyu, X.; Crittenden, J.C. A holistic assessment of microplastic ubiquitousness: Pathway for source identification in the environment. Sustain. Prod. Consum. 2022, 33, 113–145. [Google Scholar] [CrossRef]
- Guzzetti, E.; Sureda, A.; Tejada, S.; Faggio, C. Microplastic in marine organism: Environmental and toxicological effects. Environ. Toxicol. Pharmacol. 2018, 64, 164–171. [Google Scholar] [CrossRef]
- Jauker, F.; Bondarenko, B.; Becker, H.C.; Steffan-Dewenter, I. Pollination efficiency of wild bees and hoverflies provided to oilseed rape. Agric. Entomol. 2012, 14, 81–87. [Google Scholar] [CrossRef]
- Hawkes, W.L.S.; Walliker, E.; Gao, B.; Forster, O.; Lacey, K.; Doyle, T.; Massy, R.; Roberts, N.W.; Reynolds, D.R.; Özden, Ö.; et al. Huge spring migrations of insects from the Middle East to Europe: Quantifying the migratory assemblage and ecosystem services. Ecography 2022, 2022, e06288. [Google Scholar] [CrossRef]
- Doyle, T.D.; Poole, O.M.; Barnes, J.C.; Hawkes, W.L.S.; Guri, E.J.; Wotton, K.R. Multiple factors contribute to female dominance in migratory bioflows. Open Biol. 2025, 15, 240235. [Google Scholar] [CrossRef]
- Buteler, M.; Alma, A.M.; Stadler, T.; Gingold, A.C.; Manattini, M.C.; Lozada, M. Acute toxicity of microplastic fibers to honeybees and effects on foraging behavior. Sci. Total Environ. 2022, 822, 153320. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Ilyas, M.; Li, R.; Yang, J.; Yang, F.-L. Microplastics and Nanoplastics Effects on Plant–Pollinator Interaction and Pollination Biology. Environ. Sci. Technol. 2023, 57, 6415–6424. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Guevara, F.; Roy, P.D.; Kutralam-Muniasamy, G.; Shruti, V.C. A central role for fecal matter in the transport of microplastics: An updated analysis of new findings and persisting questions. J. Hazard. Mater. Adv. 2021, 4, 100021. [Google Scholar] [CrossRef]
- Hawkes, W.L.; Doyle, T.; Massy, R.; Weston, S.T.; Davies, K.; Cornelius, E.; Collier, C.; Chapman, J.W.; Reynolds, D.R.; Wotton, K.R. The most remarkable migrants—Systematic analysis of the Western European insect flyway at a Pyrenean mountain pass. Proc. R. Soc. B Biol. Sci. 2024, 291, 20232831. [Google Scholar] [CrossRef]
- Menz, M.H.M.; Brown, B.V.; Wotton, K.R. Quantification of migrant hoverfly movements (Diptera: Syrphidae) on the West Coast of North America. R. Soc. Open Sci. 2019, 6, 190153. [Google Scholar] [CrossRef]
- Bourdages, M.P.T.; Provencher, J.F.; Baak, J.E.; Mallory, M.L.; Vermaire, J.C. Breeding seabirds as vectors of microplastics from sea to land: Evidence from colonies in Arctic Canada. Sci. Total Environ. 2021, 764, 142808. [Google Scholar] [CrossRef]
- Cano-Povedano, J.; López-Calderón, C.; Sánchez, M.I.; Hortas, F.; Cañuelo-Jurado, B.; Martín-Vélez, V.; Ros, M.; Cózar, A.; Green, A.J. Biovectoring of plastic by white storks from a landfill to a complex of salt ponds and marshes. Mar. Pollut. Bull. 2023, 197, 115773. [Google Scholar] [CrossRef]
- Massy, R.; Hawkes, W.; Weston, S.; Doyle, T.; Wotton, K.R. Enhanced flight performance in hoverfly migrants. iScience 2024, 27, 111345. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).