Impact of Microplastics on Human Health: Risks, Diseases, and Affected Body Systems
Abstract
1. Introduction
2. Sources of Microplastics
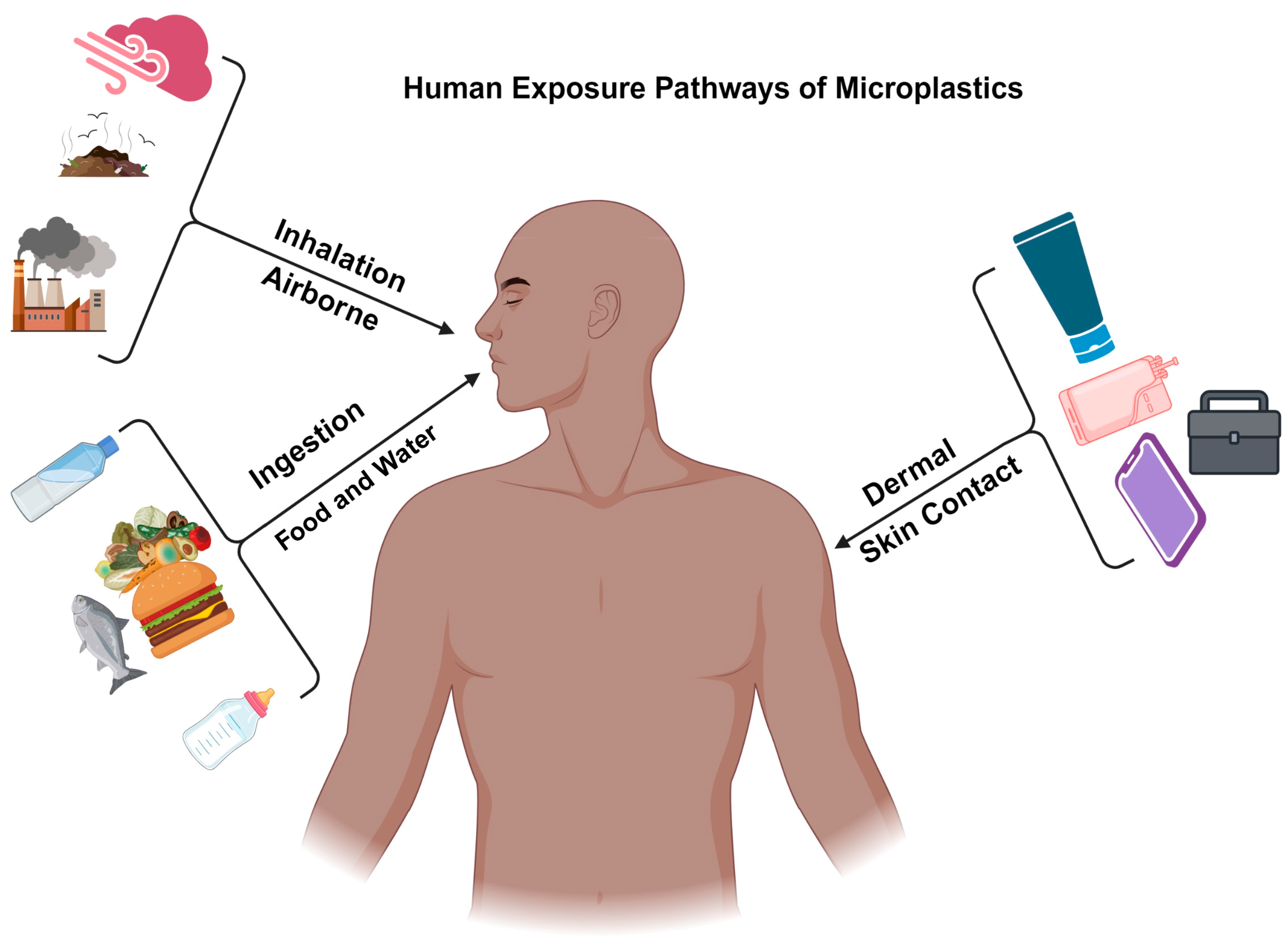
3. Human Exposure Pathways of Microplastics
3.1. Ingestion
Food Consumption
3.2. Inhalation
Airborne Microplastics
3.3. Dermal Contact
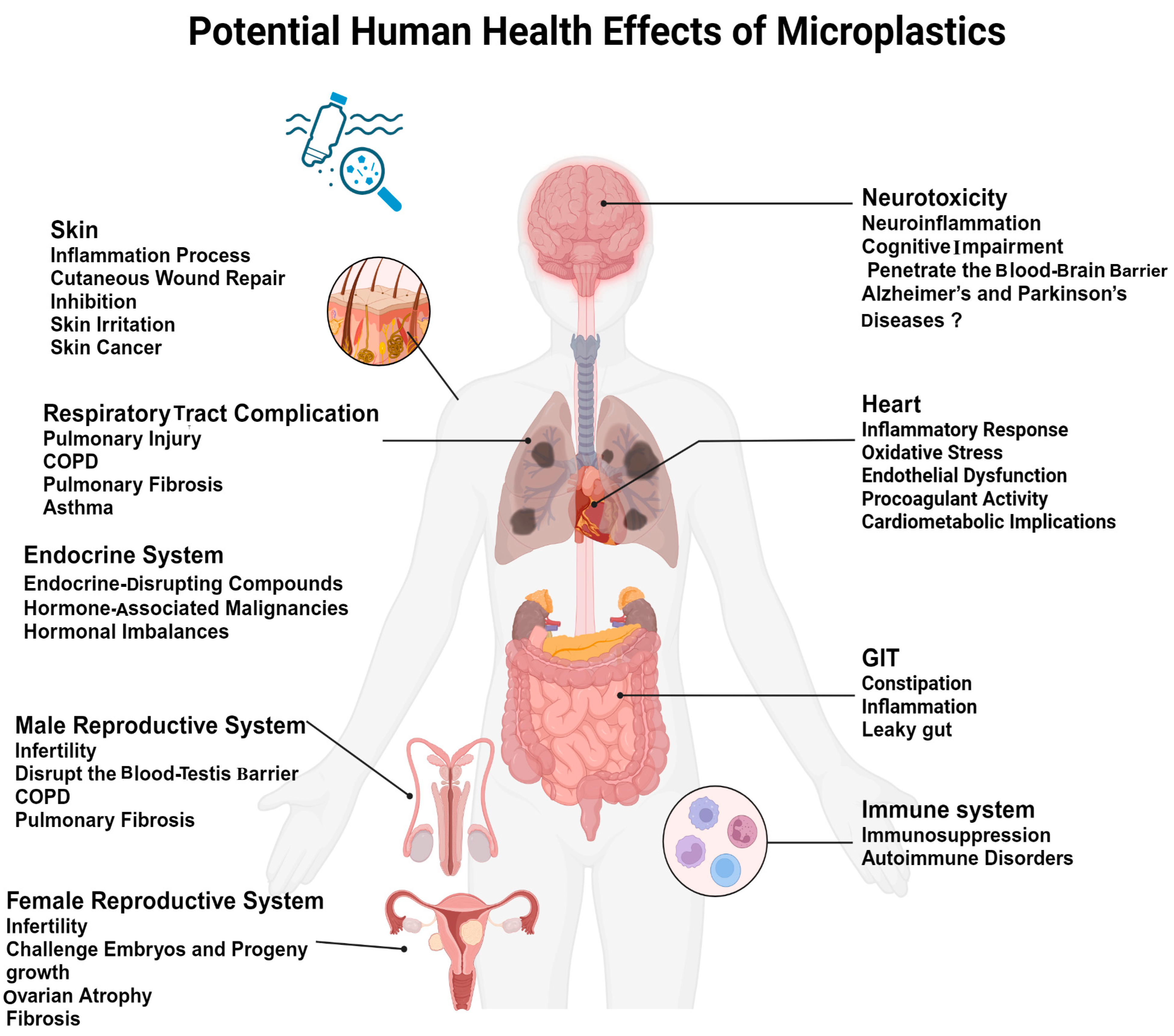
4. Potential Human Health Effects of Microplastics
4.1. MPs’ Impact on the Immune System
4.2. Respiratory Health Issues in Response to MPs
4.3. Gastrointestinal and Metabolic Impacts in Response to MPs
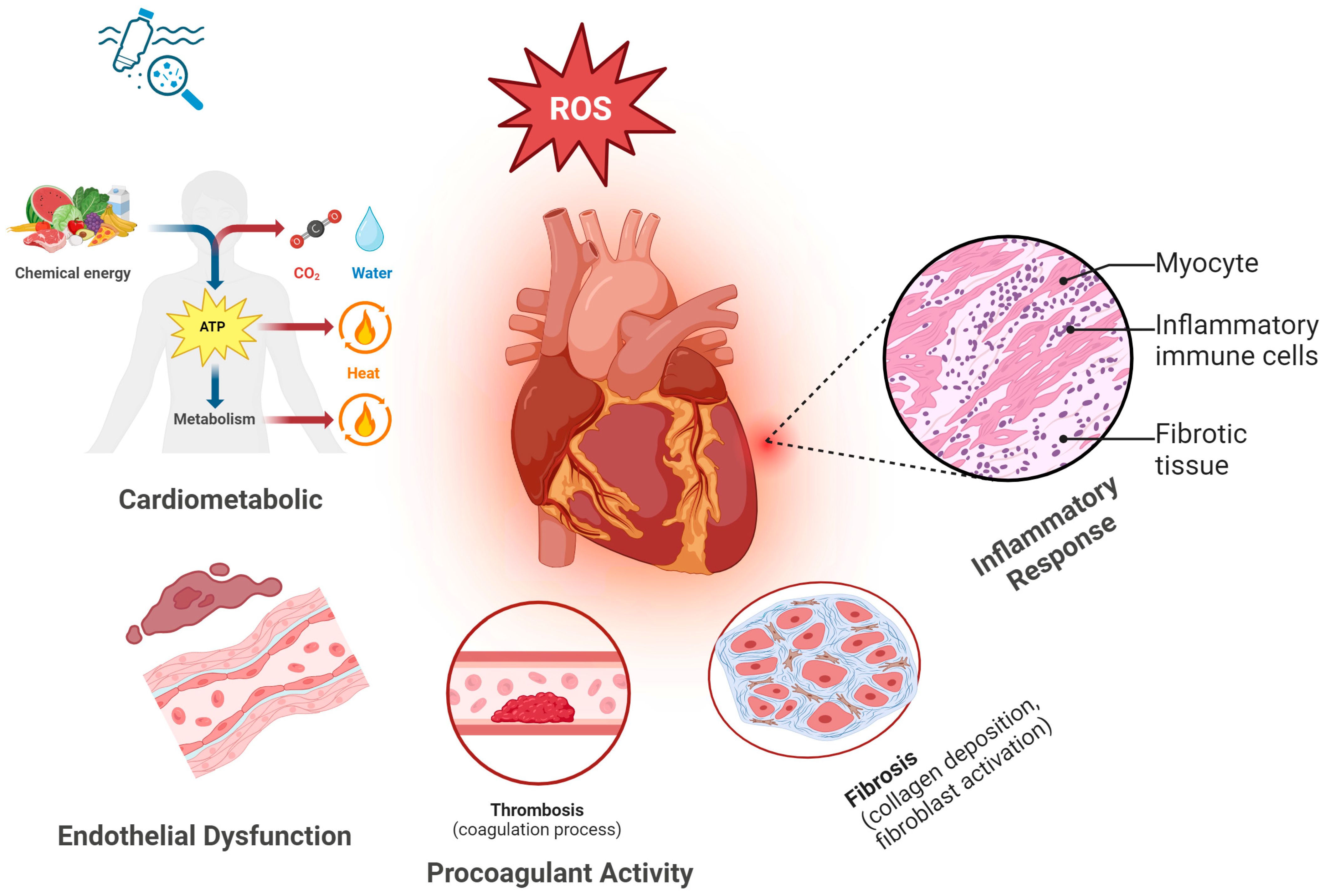
4.4. Cardiovascular System Response to MPs
4.4.1. Inflammatory Response
4.4.2. Oxidative Stress
4.4.3. Endothelial Dysfunction
4.4.4. Procoagulant Activity
4.4.5. Potential Cardiometabolic Implications
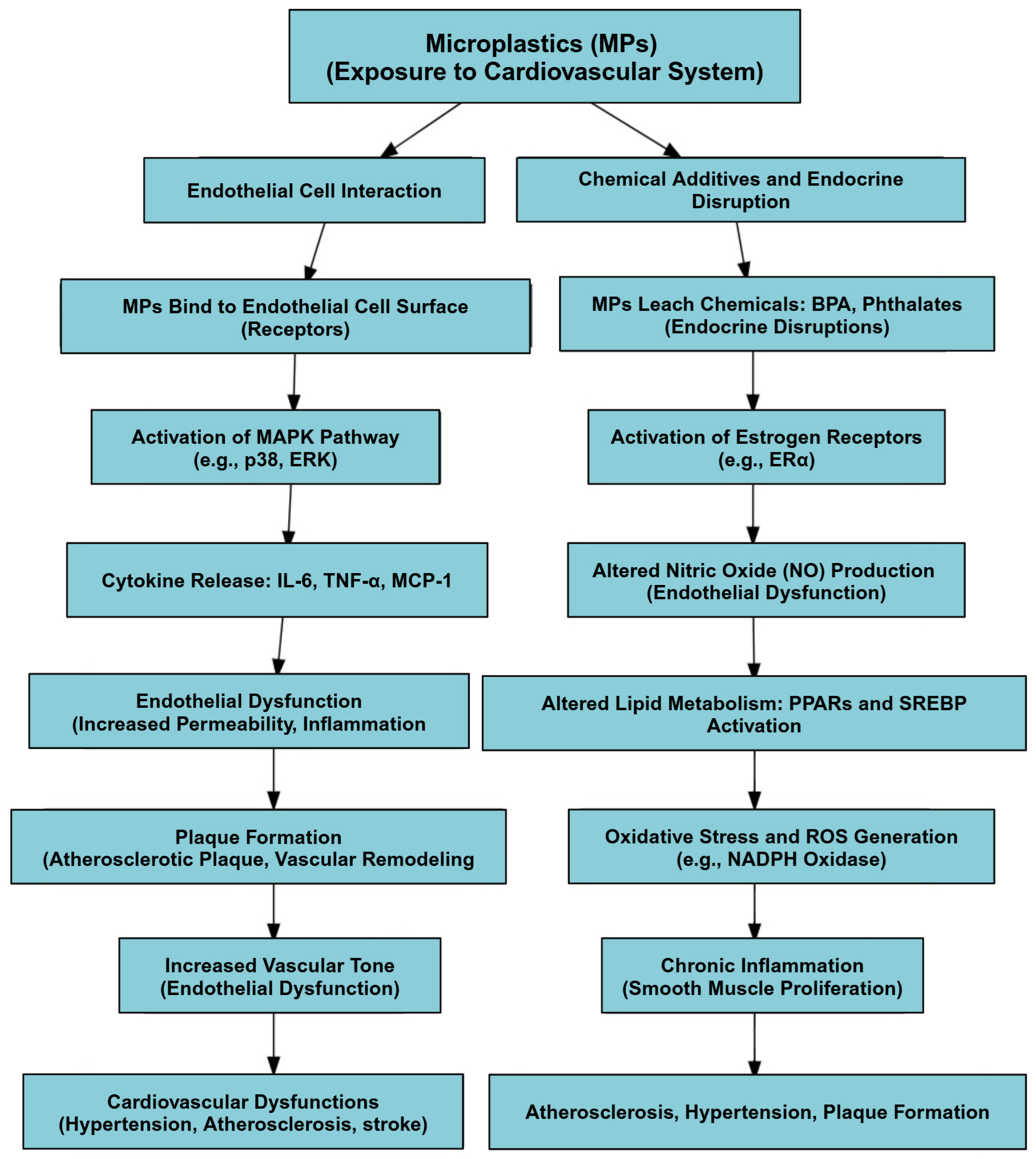
4.4.6. Mechanism of Action of MPs in the Cardiovascular System
4.5. Neurotoxicity of the Nervous System in Response to MPs
4.6. Endocrine System Disruption in Response to MPs
4.7. Carcinogenic Potential of MPs
4.8. Reproductive System Response to MPs
4.9. Skin and Dermal System
4.10. Scenarios with Negligible Health Impacts and Adaptive Responses
4.10.1. Scenarios with Negligible Health Impacts
4.10.2. Adaptive Responses to MP Exposure
5. Regulations on MPs and Health Protection
6. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ziani, K.; Ioniță-Mîndrican, C.-B.; Mititelu, M.; Neacșu, S.M.; Negrei, C.; Moroșan, E.; Drăgănescu, D.; Preda, O.-T. Microplastics: A Real Global Threat for Environment and Food Safety: A State of the Art Review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Adhikary, S.; Bhattacharya, S.; Agarwal, R.; Ganguly, A.; Nanda, S.; Rajak, P. The Alarming Link between Environmental Microplastics and Health Hazards with Special Emphasis on Cancer. Life Sci. 2024, 355, 122937. [Google Scholar] [CrossRef] [PubMed]
- Bamigboye, O.; Alfred, M.O.; Bayode, A.A.; Unuabonah, E.I.; Omorogie, M.O. The Growing Threats and Mitigation of Environmental Microplastics. Environ. Chem. Ecotoxicol. 2024, 6, 259–268. [Google Scholar] [CrossRef]
- Sharma, S.; Bhardwaj, A.; Thakur, M.; Saini, A. Understanding Microplastic Pollution of Marine Ecosystem: A Review. Environ. Sci. Pollut. Res. 2024, 31, 41402–41445. [Google Scholar] [CrossRef]
- Talukdar, A.; Kundu, P.; Bhattacharya, S.; Dutta, N. Microplastic Contamination in Wastewater: Sources, Distribution, Detection and Remediation through Physical and Chemical-Biological Methods. Sci. Total Environ. 2024, 916, 170254. [Google Scholar] [CrossRef]
- Lamichhane, G.; Acharya, A.; Marahatha, R.; Modi, B.; Paudel, R.; Adhikari, A.; Raut, B.K.; Aryal, S.; Parajuli, N. Microplastics in Environment: Global Concern, Challenges, and Controlling Measures. Int. J. Environ. Sci. Technol. 2023, 20, 4673–4694. [Google Scholar] [CrossRef]
- Jiang, B.; Kauffman, A.E.; Li, L.; McFee, W.; Cai, B.; Weinstein, J.; Lead, J.R.; Chatterjee, S.; Scott, G.I.; Xiao, S. Health Impacts of Environmental Contamination of Micro- and Nanoplastics: A Review. Environ. Health Prev. Med. 2020, 25, 29. [Google Scholar] [CrossRef]
- Ghosh, S.; Sinha, J.K.; Ghosh, S.; Vashisth, K.; Han, S.; Bhaskar, R. Microplastics as an Emerging Threat to the Global Environment and Human Health. Sustainability 2023, 15, 10821. [Google Scholar] [CrossRef]
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Shubham; Das, S.; et al. Impacts of Plastic Pollution on Ecosystem Services, Sustainable Development Goals, and Need to Focus on Circular Economy and Policy Interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Yuan, Z.; Nag, R.; Cummins, E. Human Health Concerns Regarding Microplastics in the Aquatic Environment—From Marine to Food Systems. Sci. Total Environ. 2022, 823, 153730. [Google Scholar] [CrossRef] [PubMed]
- Lga, B.; A, D.V.; Brbo, L.; Ak, L.; L, G. Marine Microplastic Debris: An Emerging Issue for Food Security, Food Safety and Human Health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef]
- De-la-Torre, G.E. Microplastics: An Emerging Threat to Food Security and Human Health. J. Food Sci. Technol. 2020, 57, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, A.; Molinero, N.; Reinosa, J.J.; Alcolea-Rodriguez, V.; Portela, R.; Bañares, M.A.; Fernández, J.F.; Moreno-Arribas, M.V. PET Microplastics Affect Human Gut Microbiota Communities during Simulated Gastrointestinal Digestion, First Evidence of Plausible Polymer Biodegradation during Human Digestion. Sci. Rep. 2022, 12, 528. [Google Scholar] [CrossRef] [PubMed]
- Y, D.; Y, Z.; B, L.; H, R. Tissue Accumulation of Microplastics in Mice and Biomarker Responses Suggest Widespread Health Risks of Exposure. Sci. Rep. 2017, 7, srep46687. [Google Scholar] [CrossRef]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene Microplastics Induce Gut Microbiota Dysbiosis and Hepatic Lipid Metabolism Disorder in Mice. Sci. Total Environ. 2018, 631–632, 449–458. [Google Scholar] [CrossRef]
- Zolotova, N.; Dzhalilova, D.; Tsvetkov, I.; Makarova, O. Influence of Microplastics on Morphological Manifestations of Experimental Acute Colitis. Toxics 2023, 11, 730. [Google Scholar] [CrossRef]
- Saha, S.C.; Saha, G. Effect of Microplastics Deposition on Human Lung Airways: A Review with Computational Benefits and Challenges. Heliyon 2024, 10, e24355. [Google Scholar] [CrossRef]
- Prata, J.C. Airborne Microplastics: Consequences to Human Health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of Microplastics in Human Placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Halfar, J.; Čabanová, K.; Vávra, K.; Delongová, P.; Motyka, O.; Špaček, R.; Kukutschová, J.; Šimetka, O.; Heviánková, S. Microplastics and Additives in Patients with Preterm Birth: The First Evidence of Their Presence in Both Human Amniotic Fluid and Placenta. Chemosphere 2023, 343, 140301. [Google Scholar] [CrossRef] [PubMed]
- Vincoff, S.; Schleupner, B.; Santos, J.; Morrison, M.; Zhang, N.; Dunphy-Daly, M.M.; Eward, W.C.; Armstrong, A.J.; Diana, Z.; Somarelli, J.A. The Known and Unknown: Investigating the Carcinogenic Potential of Plastic Additives. Environ. Sci. Technol. 2024, 58, 10445–10457. [Google Scholar] [CrossRef] [PubMed]
- IARC Monographs Volume 121: Styrene, Styrene-7,8-Oxide, and Quinolone. Available online: https://www.iarc.who.int/news-events/iarc-monographs-meetings-volume-121-styrene-styrene-78-oxide-and-quinolone (accessed on 13 October 2024).
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Rochman, C.M. Microplastics Research-from Sink to Source. Science 2018, 360, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Gündoğdu, S. Contamination of Table Salts from Turkey with Microplastics. Food Addit. Contam. Part A 2018, 35, 1006–1014. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Jadhav, E.B.; Sankhla, M.S.; Bhat, R.A.; Bhagat, D.S. Microplastics from Food Packaging: An Overview of Human Consumption, Health Threats, and Alternative Solutions. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100608. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic Contamination of Tap Water, Beer, and Sea Salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A First Overview of Textile Fibers, Including Microplastics, in Indoor and Outdoor Environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in Air: Are We Breathing It In? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Phoenix, V.; Roux, G.L.; Jiménez, P.D.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric Transport and Deposition of Microplastics in a Remote Mountain Catchment. Nat. Geosci. 2019, 12, 339. [Google Scholar] [CrossRef]
- Zhou, Y.; Ashokkumar, V.; Amobonye, A.; Bhattacharjee, G.; Sirohi, R.; Singh, V.; Flora, G.; Kumar, V.; Pillai, S.; Zhang, Z.; et al. Current Research Trends on Cosmetic Microplastic Pollution and Its Impacts on the Ecosystem: A Review. Environ. Pollut. 2023, 320, 121106. [Google Scholar] [CrossRef] [PubMed]
- Bikiaris, N.; Nikolaidis, N.F.; Barmpalexis, P. Microplastics (MPs) in Cosmetics: A Review on Their Presence in Personal-Care, Cosmetic, and Cleaning Products (PCCPs) and Sustainable Alternatives from Biobased and Biodegradable Polymers. Cosmetics 2024, 11, 145. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, Y.; Xu, H. Trojan Horse in the Intestine: A Review on the Biotoxicity of Microplastics Combined Environmental Contaminants. J. Hazard. Mater. 2022, 439, 129652. [Google Scholar] [CrossRef]
- Akyildiz, S.H.; Fiore, S.; Bruno, M.; Sezgin, H.; Yalcin-Enis, I.; Yalcin, B.; Bellopede, R. Release of Microplastic Fibers from Synthetic Textiles during Household Washing. Environ. Pollut. 2024, 357, 124455. [Google Scholar] [CrossRef]
- Polystyrene Microplastics Induce Activation and Cell Death of Neutrophils through Strong Adherence and Engulfment. J. Hazard. Mater. 2024, 480, 136100. [CrossRef]
- Shang, Q.; Wu, H.; Wang, K.; Zhang, M.; Dou, Y.; Jiang, X.; Zhao, Y.; Zhao, H.; Chen, Z.-J.; Wang, J.; et al. Exposure to Polystyrene Microplastics during Lactational Period Alters Immune Status in Both Male Mice and Their Offspring. Sci. Total Environ. 2024, 951, 175371. [Google Scholar] [CrossRef]
- Rajendran, D.; Chandrasekaran, N. Journey of Micronanoplastics with Blood Components. RSC Adv. 2023, 13, 31435–31459. [Google Scholar] [CrossRef]
- Lee, Y.; Cho, J.; Sohn, J.; Kim, C. Health Effects of Microplastic Exposures: Current Issues and Perspectives in South Korea. Yonsei Med. J. 2023, 64, 301–308. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gao, J.; Yu, H.; Su, H.; Yang, Y.; Cao, Y.; Zhang, Q.; Ren, Y.; Hollert, H.; Shi, H.; et al. An Emerging Role of Microplastics in the Etiology of Lung Ground Glass Nodules. Environ. Sci. Eur. 2022, 34, 25. [Google Scholar] [CrossRef]
- Lu, K.; Zhan, D.; Fang, Y.; Li, L.; Chen, G.; Chen, S.; Wang, L. Microplastics, Potential Threat to Patients with Lung Diseases. Front. Toxicol. 2022, 4, 958414. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Bai, X.; Li, K.; Zhang, G.; Zhang, M.; Hu, M.; Huang, Y. Human Exposure to Ambient Atmospheric Microplastics in a Megacity: Spatiotemporal Variation and Associated Microorganism-Related Health Risk. Environ. Sci. Technol. 2024, 58, 3702–3713. [Google Scholar] [CrossRef]
- Sun, A.; Wang, W.-X. Human Exposure to Microplastics and Its Associated Health Risks. Environ. Health 2023, 1, 139–149. [Google Scholar] [CrossRef]
- Andrade, B.; Jara-Gutiérrez, C.; Paz-Araos, M.; Vázquez, M.C.; Díaz, P.; Murgas, P. The Relationship between Reactive Oxygen Species and the cGAS/STING Signaling Pathway in the Inflammaging Process. Int. J. Mol. Sci. 2022, 23, 15182. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, L.; Pang, Y. cGAS-STING Pathway in Pathogenesis and Treatment of Osteoarthritis and Rheumatoid Arthritis. Front. Immunol. 2024, 15, 1384372. [Google Scholar] [CrossRef]
- Zhou, J.; Zhuang, Z.; Li, J.; Feng, Z. Significance of the cGAS-STING Pathway in Health and Disease. Int. J. Mol. Sci. 2023, 24, 13316. [Google Scholar] [CrossRef]
- Shao, X.; Ding, Z.; Zhou, W.; Li, Y.; Li, Z.; Cui, H.; Lin, X.; Cao, G.; Cheng, B.; Sun, H.; et al. Intrinsic Bioactivity of Black Phosphorus Nanomaterials on Mitotic Centrosome Destabilization through Suppression of PLK1 Kinase. Nat. Nanotechnol. 2021, 16, 1150–1160. [Google Scholar] [CrossRef]
- Sangkham, S.; Faikhaw, O.; Munkong, N.; Sakunkoo, P.; Arunlertaree, C.; Chavali, M.; Mousazadeh, M.; Tiwari, A. A Review on Microplastics and Nanoplastics in the Environment: Their Occurrence, Exposure Routes, Toxic Studies, and Potential Effects on Human Health. Mar. Pollut. Bull. 2022, 181, 113832. [Google Scholar] [CrossRef]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Turner, A.; Kelly, F.J.; Dominguez, A.O.; Jaafarzadeh, N. Distribution and Potential Health Impacts of Microplastics and Microrubbers in Air and Street Dusts from Asaluyeh County, Iran. Environ. Pollut. 2019, 244, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Ayseli, M.T.; Cetinkaya, T. Chapter 5—A Review of the Association of Air Pollution on Pregnant Health. In Diseases and Health Consequences of Air Pollution; Dehghani, M.H., Karri, R.R., Vera, T., Hassan, S.K.M., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 109–144. ISBN 978-0-443-16080-6. [Google Scholar]
- Facciolà, A.; Visalli, G.; Pruiti Ciarello, M.; Di Pietro, A. Newly Emerging Airborne Pollutants: Current Knowledge of Health Impact of Micro and Nanoplastics. Int. J. Environ. Res. Public Health 2021, 18, 2997. [Google Scholar] [CrossRef] [PubMed]
- Warheit, D.B.; Hart, G.A.; Hesterberg, T.W.; Collins, J.J.; Dyer, W.M.; Swaen, G.M.H.; Castranova, V.; Soiefer, A.I.; Kennedy, G.L. Potential Pulmonary Effects of Man-Made Organic Fiber (MMOF) Dusts. Crit. Rev. Toxicol. 2001, 31, 697–736. [Google Scholar] [CrossRef]
- Li, Y.; Shi, T.; Li, X.; Sun, H.; Xia, X.; Ji, X.; Zhang, J.; Liu, M.; Lin, Y.; Zhang, R.; et al. Inhaled Tire-Wear Microplastic Particles Induced Pulmonary Fibrotic Injury via Epithelial Cytoskeleton Rearrangement. Environ. Int. 2022, 164, 107257. [Google Scholar] [CrossRef]
- Charlton-Howard, H.S.; Bond, A.L.; Rivers-Auty, J.; Lavers, J.L. ‘Plasticosis’: Characterising Macro- and Microplastic-Associated Fibrosis in Seabird Tissues. J. Hazard. Mater. 2023, 450, 131090. [Google Scholar] [CrossRef]
- Morawska, L.; Buonanno, G. The Physics of Particle Formation and Deposition during Breathing. Nat. Rev. Phys. 2021, 3, 300–301. [Google Scholar] [CrossRef]
- Eschenbacher, W.L.; Kreiss, K.; Lougheed, M.D.; Pransky, G.S.; Day, B.; Castellan, R.M. Nylon Flock–Associated Interstitial Lung Disease. Am. J. Respir. Crit. Care Med. 1999, 159, 2003–2008. [Google Scholar] [CrossRef]
- Turcotte, S.E.; Chee, A.; Walsh, R.; Grant, F.C.; Liss, G.M.; Boag, A.; Forkert, L.; Munt, P.W.; Lougheed, M.D. Flock Worker’s Lung Disease: Natural History of Cases and Exposed Workers in Kingston, Ontario. Chest 2013, 143, 1642–1648. [Google Scholar] [CrossRef]
- Danso, I.K.; Woo, J.-H.; Lee, K. Pulmonary Toxicity of Polystyrene, Polypropylene, and Polyvinyl Chloride Microplastics in Mice. Molecules 2022, 27, 7926. [Google Scholar] [CrossRef]
- S, A.; B, T.; E, L.; C, O.; A, T.; K, S.; A, S.; I, O.; A, K.; B, N. The Respiratory Effects of Occupational Polypropylene Flock Exposure. Eur. Respir. J. 2005, 25, 110–117. [Google Scholar] [CrossRef]
- Waring, R.H.; Harris, R.M.; Mitchell, S.C. Plastic Contamination of the Food Chain: A Threat to Human Health? Maturitas 2018, 115, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, S.; Xu, H. Effects of Microplastic and Engineered Nanomaterials on Inflammatory Bowel Disease: A Review. Chemosphere 2023, 326, 138486. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of Different Shapes of Microplastics Initiates Intestinal Injury and Gut Microbiota Dysbiosis in the Gut of Zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, B.; Raffael, B.; Angers-Loustau, A.; Gilliland, D.; Kestens, V.; Petrillo, M.; Rio-Echevarria, I.M.; den Eede, G.V. Review of Micro- and Nanoplastic Contamination in the Food Chain. Food Addit. Contam. Part A 2019, 36, 639–673. [Google Scholar] [CrossRef]
- Osman, A.I.; Hosny, M.; Eltaweil, A.S.; Omar, S.; Elgarahy, A.M.; Farghali, M.; Yap, P.-S.; Wu, Y.-S.; Nagandran, S.; Batumalaie, K.; et al. Microplastic Sources, Formation, Toxicity and Remediation: A Review. Environ. Chem. Lett. 2023, 21, 2129–2169. [Google Scholar] [CrossRef]
- Origin and Fate of Dietary Nanoparticles and Microparticles in the Gastrointestinal Tract. J. Autoimmun. 2010, 34, J226–J233. [CrossRef]
- Fackelmann, G.; Sommer, S. Microplastics and the Gut Microbiome: How Chronically Exposed Species May Suffer from Gut Dysbiosis. Mar. Pollut. Bull. 2019, 143, 193–203. [Google Scholar] [CrossRef]
- Deng, Y.; Yan, Z.; Shen, R.; Wang, M.; Huang, Y.; Ren, H.; Zhang, Y.; Lemos, B. Microplastics Release Phthalate Esters and Cause Aggravated Adverse Effects in the Mouse Gut. Environ. Int. 2020, 143, 105916. [Google Scholar] [CrossRef]
- Ivleva, N.P. Chemical Analysis of Microplastics and Nanoplastics: Challenges, Advanced Methods, and Perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef]
- Sofield, C.E.; Anderton, R.S.; Gorecki, A.M. Mind over Microplastics: Exploring Microplastic-Induced Gut Disruption and Gut-Brain-Axis Consequences. Curr. Issues Mol. Biol. 2024, 46, 4186–4202. [Google Scholar] [CrossRef]
- Niu, H.; Liu, S.; Jiang, Y.; Hu, Y.; Li, Y.; He, L.; Xing, M.; Li, X.; Wu, L.; Chen, Z.; et al. Are Microplastics Toxic? A Review from Eco-Toxicity to Effects on the Gut Microbiota. Metabolites 2023, 13, 739. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wei, F.; Qiu, S.; Xing, B.; Hou, J. Polystyrene Microplastics Trigger Adiposity in Mice by Remodeling Gut Microbiota and Boosting Fatty Acid Synthesis. Sci. Total Environ. 2023, 890, 164297. [Google Scholar] [CrossRef] [PubMed]
- Bastyans, S.; Jackson, S.; Fejer, G. Micro and Nano-Plastics, a Threat to Human Health? Emerg. Top. Life Sci. 2022, 6, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Shum, T.-F.; Wang, L.; Chiou, J. Impact of Plasticizer on the Intestinal Epithelial Integrity and Tissue-Repairing Ability within Cells in the Proximity of the Human Gut Microbiome. Int. J. Environ. Res. Public Health 2023, 20, 2152. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, D.; Zhan, J.; Liu, X.; Li, P.; Ma, X.; Hou, H.; Wang, P. Polystyrene Microplastics Induce Kidney Injury via Gut Barrier Dysfunction and C5a/C5aR Pathway Activation. Environ. Pollut. 2024, 342, 122909. [Google Scholar] [CrossRef]
- Aleman, R.S.; Moncada, M.; Aryana, K.J. Leaky Gut and the Ingredients That Help Treat It: A Review. Molecules 2023, 28, 619. [Google Scholar] [CrossRef]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef]
- Demarquoy, J. Microplastics and Microbiota: Unraveling the Hidden Environmental Challenge. World J. Gastroenterol. 2024, 30, 2191–2194. [Google Scholar] [CrossRef]
- Markiewicz, M.; Richard, E.; Marks, N.; Ludwicka-Bradley, A. Impact of Endothelial Microparticles on Coagulation, Inflammation, and Angiogenesis in Age-Related Vascular Diseases. J. Aging Res. 2013, 2013, 734509. [Google Scholar] [CrossRef]
- Lovren, F.; Verma, S. Evolving Role of Microparticles in the Pathophysiology of Endothelial Dysfunction. Clin. Chem. 2013, 59, 1166–1174. [Google Scholar] [CrossRef]
- Yee, M.S.-L.; Hii, L.-W.; Looi, C.K.; Lim, W.-M.; Wong, S.-F.; Kok, Y.-Y.; Tan, B.-K.; Wong, C.-Y.; Leong, C.-O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Wang, X.; Fan, Z.; Mao, W.; Shi, Y.; Wu, Y.; Liu, T.; Xu, Z.; Wang, H.; Chen, H. Polystyrene Microplastics Facilitate Renal Fibrosis through Accelerating Tubular Epithelial Cell Senescence. Food Chem. Toxicol. 2024, 191, 114888. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, S.; Liu, Q.; Wei, J.; Jin, Y.; Wang, X.; Zhang, L. Polystyrene Microplastics Cause Cardiac Fibrosis by Activating Wnt/β-Catenin Signaling Pathway and Promoting Cardiomyocyte Apoptosis in Rats. Environ. Pollut. 2020, 265, 115025. [Google Scholar] [CrossRef] [PubMed]
- Persiani, E.; Cecchettini, A.; Ceccherini, E.; Gisone, I.; Morales, M.A.; Vozzi, F. Microplastics: A Matter of the Heart (and Vascular System). Biomedicines 2023, 11, 264. [Google Scholar] [CrossRef]
- Das, A. The Emerging Role of Microplastics in Systemic Toxicity: Involvement of Reactive Oxygen Species (ROS). Sci. Total Environ. 2023, 895, 165076. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Dey, A.; Mondal, S.; Gautam, M.K. Environmental Microplastics and Nanoplastics: Effects on Cardiovascular System. Toxicol. Anal. Et Clin. 2024, 36, 145–157. [Google Scholar] [CrossRef]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and Intestinal Effects of Nano- and Microplastics: A Review of the Literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef]
- Xie, X.; Wang, K.; Shen, X.; li, X.; Wang, S.; Yuan, S.; Li, B.; Wang, Z. Potential Mechanisms of Aortic Medial Degeneration Promoted by Co-Exposure to Microplastics and Lead. J. Hazard. Mater. 2024, 475, 134854. [Google Scholar] [CrossRef]
- Lomonaco, T.; Persiani, E.; Biagini, D.; Gisone, I.; Ceccherini, E.; Cecchettini, A.; Corti, A.; Ghimenti, S.; Francesco, F.D.; Castelvetro, V.; et al. Type-Specific Inflammatory Responses of Vascular Cells Activated by Interaction with Virgin and Aged Microplastics. Ecotoxicol. Environ. Saf. 2024, 282, 116695. [Google Scholar] [CrossRef]
- Umamaheswari, S.; Priyadarshinee, S.; Kadirvelu, K.; Ramesh, M. Polystyrene Microplastics Induce Apoptosis via ROS-Mediated P53 Signaling Pathway in Zebrafish. Chem. Biol. Interact. 2021, 345, 109550. [Google Scholar] [CrossRef]
- Shaito, A.; Aramouni, K.; Assaf, R.; Parenti, A.; Orekhov, A.; El Yazbi, A.; Pintus, G.; Eid, A.H. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front. Biosci. 2022, 27, 105. [Google Scholar] [CrossRef] [PubMed]
- Osto, E.; Cosentino, F. Chapter 22—The Role of Oxidative Stress in Endothelial Dysfunction and Vascular Inflammation. In Nitric Oxide, 2nd ed.; Ignarro, L.J., Ed.; Academic Press: San Diego, CA, USA, 2010; pp. 705–754. ISBN 978-0-12-373866-0. [Google Scholar]
- Kadac-Czapska, K.; Ośko, J.; Knez, E.; Grembecka, M. Microplastics and Oxidative Stress—Current Problems and Prospects. Antioxidants 2024, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Choi, D.; Han, S.; Jung, S.Y.; Choi, J.; Hong, J. Potential Toxicity of Polystyrene Microplastic Particles. Sci. Rep. 2020, 10, 7391. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Fulgenzi, G.; Graciotti, L.; Spadoni, T.; D’Onofrio, N.; Scisciola, L.; Grotta, R.L.; Frigé, C.; et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N. Engl. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef]
- Poredos, P.; Poredos, A.V.; Gregoric, I. Endothelial Dysfunction and Its Clinical Implications. Angiology 2021, 72, 604–615. [Google Scholar] [CrossRef]
- Godo, S.; Shimokawa, H. Endothelial Functions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef]
- Gou, D.; Deng, J.-Y.; Tang, Q.-P.; Lu, J.; Bao, L.; Liu, Y.; Pei, D.-S. Elucidating the Underlying Toxic Mechanisms of Nanoplastics on Zebrafish Hematological and Circulatory Systems. Environ. Sci. Nano 2024, 11, 3900–3917. [Google Scholar] [CrossRef]
- Vishalakshi, G.J.; Hemshekhar, M.; Sandesha, V.D.; Prashanth, K.S.; Jagadish, S.; Paul, M.; Kemparaju, K.; Girish, K.S. Bisphenol AF Elevates Procoagulant Platelets by Inducing Necroptosis via RIPK1-Inflammasome Axis. Toxicology 2021, 454, 152742. [Google Scholar] [CrossRef]
- Wu, D.; Feng, Y.; Wang, R.; Jiang, J.; Guan, Q.; Yang, X.; Wei, H.; Xia, Y.; Luo, Y. Pigment Microparticles and Microplastics Found in Human Thrombi Based on Raman Spectral Evidence. J. Adv. Res. 2023, 49, 141–150. [Google Scholar] [CrossRef]
- Liang, J.; Ji, F.; Abdullah, A.L.B.; Qin, W.; Zhu, T.; Tay, Y.J.; Li, Y.; Han, M. Micro/Nano-Plastics Impacts in Cardiovascular Systems across Species. Sci. Total Environ. 2024, 942, 173770. [Google Scholar] [CrossRef]
- Meneguzzi, A.; Fava, C.; Castelli, M.; Minuz, P. Exposure to Perfluoroalkyl Chemicals and Cardiovascular Disease: Experimental and Epidemiological Evidence. Front. Endocrinol. 2021, 12, 706352. [Google Scholar] [CrossRef] [PubMed]
- Symeonides, C.; Aromataris, E.; Mulders, Y.; Dizon, J.; Stern, C.; Barker, T.H.; Whitehorn, A.; Pollock, D.; Marin, T.; Dunlop, S. An Umbrella Review of Meta-Analyses Evaluating Associations between Human Health and Exposure to Major Classes of Plastic-Associated Chemicals. Ann. Glob. Health 2024, 90, 52. [Google Scholar] [CrossRef] [PubMed]
- Prattichizzo, F.; Ceriello, A.; Pellegrini, V.; La Grotta, R.; Graciotti, L.; Olivieri, F.; Paolisso, P.; D’agostino, B.; Iovino, P.; Balestrieri, M.L.; et al. Micro-Nanoplastics and Cardiovascular Diseases: Evidence and Perspectives. Eur. Heart J. 2024, 45, 4099–4110. [Google Scholar] [CrossRef] [PubMed]
- Florance, I.; Chandrasekaran, N.; Gopinath, P.M.; Mukherjee, A. Exposure to Polystyrene Nanoplastics Impairs Lipid Metabolism in Human and Murine Macrophages in Vitro. Ecotoxicol. Environ. Saf. 2022, 238, 113612. [Google Scholar] [CrossRef]
- Wang, B.; Liang, B.; Huang, Y.; Li, Z.; Zhang, B.; Du, J.; Ye, R.; Xian, H.; Deng, Y.; Xiu, J.; et al. Long-Chain Acyl Carnitines Aggravate Polystyrene Nanoplastics-Induced Atherosclerosis by Upregulating MARCO. Adv. Sci. 2023, 10, 2205876. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, J.; Huang, Q.; Xie, Y.; Wu, R.; Zhong, J.; Deng, H. Multi-Omics Analysis Reveals Size-Dependent Toxicity and Vascular Endothelial Cell Injury Induced by Microplastic Exposure in Vivo and in Vitro. Environ. Sci.: Nano 2022, 9, 663–683. [Google Scholar] [CrossRef]
- Yin, K.; Wang, Y.; Zhao, H.; Wang, D.; Guo, M.; Mu, M.; Liu, Y.; Nie, X.; Li, B.; Li, J.; et al. A Comparative Review of Microplastics and Nanoplastics: Toxicity Hazards on Digestive, Reproductive and Nervous System. Sci. Total Environ. 2021, 774, 145758. [Google Scholar] [CrossRef]
- Solleiro-Villavicencio, H.; Gomez-De León, C.T.; Del Río-Araiza, V.H.; Morales-Montor, J. The Detrimental Effect of Microplastics on Critical Periods of Development in the Neuroendocrine System. Birth Defects Res. 2020, 112, 1326–1340. [Google Scholar] [CrossRef]
- Prüst, M.; Meijer, J.; Westerink, R.H.S. The Plastic Brain: Neurotoxicity of Micro- and Nanoplastics. Part. Fibre Toxicol. 2020, 17, 24. [Google Scholar] [CrossRef]
- Liu, X.; Yang, H.; Yan, X.; Xu, S.; Fan, Y.; Xu, H.; Ma, Y.; Hou, W.; Javed, R.; Zhang, Y. Co-Exposure of Polystyrene Microplastics and Iron Aggravates Cognitive Decline in Aging Mice via Ferroptosis Induction. Ecotoxicol. Environ. Saf. 2022, 233, 113342. [Google Scholar] [CrossRef]
- Paing, Y.M.M.; Eom, Y.; Song, G.B.; Kim, B.; Choi, M.G.; Hong, S.; Lee, S.H. Neurotoxic Effects of Polystyrene Nanoplastics on Memory and Microglial Activation: Insights from in Vivo and in Vitro Studies. Sci. Total Environ. 2024, 924, 171681. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Zhang, Y.; Zhao, H.; Zeng, T.; Zhao, X. Polystyrene Nanoplastics Penetrate across the Blood-Brain Barrier and Induce Activation of Microglia in the Brain of Mice. Chemosphere 2022, 298, 134261. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Stommel, E.W.; Torres-Jardón, R.; Hernández-Luna, J.; Aiello-Mora, M.; González-Maciel, A.; Reynoso-Robles, R.; Pérez-Guillé, B.; Silva-Pereyra, H.G.; Tehuacanero-Cuapa, S.; et al. Alzheimer and Parkinson Diseases, Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis Overlapping Neuropathology Start in the First Two Decades of Life in Pollution Exposed Urbanites and Brain Ultrafine Particulate Matter and Industrial Nanoparticles, Including Fe, Ti, Al, V, Ni, Hg, Co, Cu, Zn, Ag, Pt, Ce, La, Pr and W Are Key Players. Metropolitan Mexico City Health Crisis Is in Progress. Front. Hum. Neurosci. 2024, 17, 1297467. [Google Scholar] [CrossRef]
- Peters, A. Ambient Air Pollution and Alzheimer’s Disease: The Role of the Composition of Fine Particles. Proc. Natl. Acad. Sci. USA 2023, 120, e2220028120. [Google Scholar] [CrossRef]
- Rai, P.K.; Sonne, C.; Brown, R.J.C.; Younis, S.A.; Kim, K.-H. Adsorption of Environmental Contaminants on Micro- and Nano-Scale Plastic Polymers and the Influence of Weathering Processes on Their Adsorptive Attributes. J. Hazard. Mater. 2022, 427, 127903. [Google Scholar] [CrossRef]
- Imparato, C.; Bifulco, A.; Silvestri, B.; Vitiello, G. Recent Advances in Endocrine Disrupting Compounds Degradation through Metal Oxide-Based Nanomaterials. Catalysts 2022, 12, 289. [Google Scholar] [CrossRef]
- Surana, D.; Gupta, J.; Sharma, S.; Kumar, S.; Ghosh, P. A Review on Advances in Removal of Endocrine Disrupting Compounds from Aquatic Matrices: Future Perspectives on Utilization of Agri-Waste Based Adsorbents. Sci. Total Environ. 2022, 826, 154129. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z.; Yusoff, F.M.; Praveena, S.M.; Harun, R. Drinking Water Consumption and Association between Actual and Perceived Risks of Endocrine Disrupting Compounds. NPJ Clean Water 2022, 5, 1–10. [Google Scholar] [CrossRef]
- Domenech, J.; Marcos, R. Pathways of Human Exposure to Microplastics, and Estimation of the Total Burden. Curr. Opin. Food Sci. 2021, 39, 144–151. [Google Scholar] [CrossRef]
- Gallo, F.; Fossi, C.; Weber, R.; Santillo, D.; Sousa, J.; Ingram, I.; Nadal, A.; Romano, D. Marine Litter Plastics and Microplastics and Their Toxic Chemicals Components: The Need for Urgent Preventive Measures. Environ. Sci. Eur. 2018, 30, 13. [Google Scholar] [CrossRef]
- Baj, J.; Dring, J.C.; Czeczelewski, M.; Kozyra, P.; Forma, A.; Flieger, J.; Kowalska, B.; Buszewicz, G.; Teresiński, G. Derivatives of Plastics as Potential Carcinogenic Factors: The Current State of Knowledge. Cancers 2022, 14, 4637. [Google Scholar] [CrossRef] [PubMed]
- Zarus, G.M.; Muianga, C.; Brenner, S.; Stallings, K.; Casillas, G.; Pohl, H.R.; Mumtaz, M.M.; Gehle, K. Worker Studies Suggest Unique Liver Carcinogenicity Potential of Polyvinyl Chloride Microplastics. Am. J. Ind. Med. 2023, 66, 1033–1047. [Google Scholar] [CrossRef] [PubMed]
- Brynzak-Schreiber, E.; Schögl, E.; Bapp, C.; Cseh, K.; Kopatz, V.; Jakupec, M.A.; Weber, A.; Lange, T.; Toca-Herrera, J.L.; del Favero, G.; et al. Microplastics Role in Cell Migration and Distribution during Cancer Cell Division. Chemosphere 2024, 353, 141463. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, X.; Huang, R.; Tang, C.; Hu, C.; Ning, P.; Wang, F. Cytotoxicity and Genotoxicity of Polystyrene Micro- and Nanoplastics with Different Size and Surface Modification in A549 Cells. Int. J. Nanomed. 2022, 17, 4509–4523. [Google Scholar] [CrossRef]
- Ke, G.; Jt, H.; Zi, K.; T, H.; Qa, S. Exposure of Human Lung Cells to Polystyrene Microplastics Significantly Retards Cell Proliferation and Triggers Morphological Changes. Chem. Res. Toxicol. 2021, 34, 1069–1081. [Google Scholar] [CrossRef]
- Yang, S.; Cheng, Y.; Chen, Z.; Liu, T.; Yin, L.; Pu, Y.; Liang, G. In Vitro Evaluation of Nanoplastics Using Human Lung Epithelial Cells, Microarray Analysis and Co-Culture Model. Ecotoxicol. Environ. Saf. 2021, 226, 112837. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, W.; Tang, H.; Wang, P.; Zhang, Y.; Liu, S.; Qiu, J.; Chen, H.; Wang, L.; Wang, R.; et al. Microplastics Exposure Causes the Senescence of Human Lung Epithelial Cells and Mouse Lungs by Inducing ROS Signaling. Environ. Int. 2024, 185, 108489. [Google Scholar] [CrossRef]
- Kumar, N.; Delu, V.; Ulasov, I.; Kumar, S.; Singh, R.K.; Kumar, S.; Shukla, A.; Patel, A.K.; Yadav, L.; Tiwari, R.; et al. Pharmacological Insights: Mitochondrial ROS Generation by FNC (Azvudine) in Dalton’s Lymphoma Cells Revealed by Super Resolution Imaging. Cell Biochem. Biophys. 2024, 82, 873–883. [Google Scholar] [CrossRef]
- Park, E.-J.; Han, J.-S.; Park, E.-J.; Seong, E.; Lee, G.-H.; Kim, D.-W.; Son, H.-Y.; Han, H.-Y.; Lee, B.-S. Repeated-Oral Dose Toxicity of Polyethylene Microplastics and the Possible Implications on Reproduction and Development of the next Generation. Toxicol. Lett. 2020, 324, 75–85. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Li, G.; Xiong, Y.; Zhang, Y.; Zhang, M. The Hidden Threat: Unraveling the Impact of Microplastics on Reproductive Health. Sci. Total Environ. 2024, 935, 173177. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Ma, S.; Sun, Z.; Wang, Z. Microplastics May Be a Significant Cause of Male Infertility. Am. J. Men’s Health 2022, 16, 15579883221096549. [Google Scholar] [CrossRef]
- Lin, W.; Luo, H.; Wu, J.; Liu, X.; Cao, B.; Liu, Y.; Yang, P.; Yang, J. Polystyrene Microplastics Enhance the Microcystin-LR-Induced Gonadal Damage and Reproductive Endocrine Disruption in Zebrafish. Sci. Total Environ. 2023, 876, 162664. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Wu, S.; Wei, G. Adverse Effects of Microplastics and Nanoplastics on the Reproductive System: A Comprehensive Review of Fertility and Potential Harmful Interactions. Sci. Total Environ. 2023, 903, 166258. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, H.E.; Jørgensen, N.; Toppari, J. Semen Quality in the 21st Century. Nat. Rev. Urol. 2017, 14, 120–130. [Google Scholar] [CrossRef]
- Sychrová, E.; Yawer, A.; Labohá, P.; Basu, A.; Dydowiczová, A.; Virmani, I.; Babica, P.; Sovadinová, I. In Vitro Testicular Toxicity of Environmentally Relevant Endocrine-Disrupting Chemicals: 2D vs. 3D Models of Prepubertal Leydig TM3 Cells. Environ. Toxicol. Pharmacol. 2022, 93, 103869. [Google Scholar] [CrossRef]
- Amran, N.H.; Zaid, S.S.M.; Mokhtar, M.H.; Manaf, L.A.; Othman, S. Exposure to Microplastics during Early Developmental Stage: Review of Current Evidence. Toxics 2022, 10, 597. [Google Scholar] [CrossRef]
- Zurub, R.E.; Cariaco, Y.; Wade, M.G.; Bainbridge, S.A. Microplastics Exposure: Implications for Human Fertility, Pregnancy and Child Health. Front. Endocrinol. 2023, 14, 1330396. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, M.; Zhang, H.; Zhang, C.; Feng, L.; Hu, J.; Gao, Y.; Yuan, X.-Z.; Zhao, Y.; Zhao, H.; et al. Lactating Exposure to Microplastics at the Dose of Infants Ingested during Artificial Feeding Induced Reproductive Toxicity in Female Mice and Their Offspring. Sci. Total Environ. 2024, 949, 174972. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Lu, L.; Zheng, M.; Zhang, X.; Tian, H.; Wang, W.; Ru, S. Polystyrene Microplastics Cause Tissue Damages, Sex-Specific Reproductive Disruption and Transgenerational Effects in Marine Medaka (Oryzias melastigma). Environ. Pollut. 2019, 254, 113024. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, Y.; Wang, S.; Xie, J.; Han, Q.; Chen, M. Comparing the Effects of Polystyrene Microplastics Exposure on Reproduction and Fertility in Male and Female Mice. Toxicology 2022, 465, 153059. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef] [PubMed]
- Y, W.; X, X.; G, J. Microplastics Exposure Promotes the Proliferation of Skin Cancer Cells but Inhibits the Growth of Normal Skin Cells by Regulating the Inflammatory Process. Ecotoxicol. Environ. Saf. 2023, 267, 115636. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Li, R.; Li, Z.; Wang, D. Health Risk of Human Exposure to Microplastics: A Review. Environ. Chem. Lett. 2024, 22, 1155–1183. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, M.; Feng, Z.; Wang, Z.; Lv, M.; Chang, J.; Chen, L.; Wang, C. Human Microplastics Exposure and Potential Health Risks to Target Organs by Different Routes: A Review. Curr. Pollut. Rep. 2023, 9, 468–485. [Google Scholar] [CrossRef]
- Shi, X.; Wang, X.; Peng, L.; Chen, Y.; Liu, C.; Yang, Q.; Wu, K. Associations between Polybrominated Diphenyl Ethers (PBDEs) Levels in Adipose Tissues and Female Menstrual Cycle and Menstrual Bleeding Duration in Shantou, China. Environ. Pollut. 2022, 301, 119025. [Google Scholar] [CrossRef]
- Yurchenko, A.A.; Rajabi, F.; Braz-Petta, T.; Fassihi, H.; Lehmann, A.; Nishigori, C.; Wang, J.; Padioleau, I.; Gunbin, K.; Panunzi, L.; et al. Genomic Mutation Landscape of Skin Cancers from DNA Repair-Deficient Xeroderma Pigmentosum Patients. Nat. Commun. 2023, 14, 2561. [Google Scholar] [CrossRef]
- Alimba, C.G.; Faggio, C.; Sivanesan, S.; Ogunkanmi, A.L.; Krishnamurthi, K. Micro(Nano)-Plastics in the Environment and Risk of Carcinogenesis: Insight into Possible Mechanisms. J. Hazard. Mater. 2021, 416, 126143. [Google Scholar] [CrossRef]
- Bhuyan, M.S. Effects of Microplastics on Fish and in Human Health. Front. Environ. Sci. 2022, 10, 827289. [Google Scholar] [CrossRef]
- Li, Y.; Tao, L.; Wang, Q.; Wang, F.; Li, G.; Song, M. Potential Health Impact of Microplastics: A Review of Environmental Distribution, Human Exposure, and Toxic Effects. Environ. Health 2023, 1, 249–257. [Google Scholar] [CrossRef]
- Han, S.; Bang, J.; Choi, D.; Hwang, J.; Kim, T.; Oh, Y.; Hwang, Y.; Choi, J.; Hong, J. Surface Pattern Analysis of Microplastics and Their Impact on Human-Derived Cells. ACS Appl. Polym. Mater. 2020, 2, 4541–4550. [Google Scholar] [CrossRef]
- Kukkola, A.; Chetwynd, A.J.; Krause, S.; Lynch, I. Beyond Microbeads: Examining the Role of Cosmetics in Microplastic Pollution and Spotlighting Unanswered Questions. J. Hazard. Mater. 2024, 476, 135053. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Deng, B.; Kang, Z.; Araujo, P.; Mjøs, S.A.; Liu, R.; Lin, J.; Yang, T.; Qu, Y. Tissue Accumulation of Polystyrene Microplastics Causes Oxidative Stress, Hepatopancreatic Injury and Metabolome Alterations in Litopenaeus vannamei. Ecotoxicol. Environ. Saf. 2023, 256, 114871. [Google Scholar] [CrossRef] [PubMed]
- Goodman, K.E.; Hua, T.; Sang, Q.-X.A. Effects of Polystyrene Microplastics on Human Kidney and Liver Cell Morphology, Cellular Proliferation, and Metabolism. ACS Omega 2022, 7, 34136–34153. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.-B.; Won, E.-J.; Kang, H.-M.; Lee, M.-C.; Hwang, D.-S.; Hwang, U.-K.; Zhou, B.; Souissi, S.; Lee, S.-J.; Lee, J.-S. Microplastic Size-Dependent Toxicity, Oxidative Stress Induction, and p-JNK and p-P38 Activation in the Monogonont Rotifer (Brachionus koreanus). Environ. Sci. Technol. 2016, 50, 8849–8857. [Google Scholar] [CrossRef]
- Jin, Y.; Xia, J.; Pan, Z.; Yang, J.; Wang, W.; Fu, Z. Polystyrene Microplastics Induce Microbiota Dysbiosis and Inflammation in the Gut of Adult Zebrafish. Environ. Pollut. 2018, 235, 322–329. [Google Scholar] [CrossRef]
- von Moos, N.; Burkhardt-Holm, P.; Köhler, A. Uptake and Effects of Microplastics on Cells and Tissue of the Blue Mussel Mytilus Edulis L. after an Experimental Exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar] [CrossRef]
- Kiessling, T.; Hinzmann, M.; Mederake, L.; Dittmann, S.; Brennecke, D.; Böhm-Beck, M.; Knickmeier, K.; Thiel, M. What Potential Does the EU Single-Use Plastics Directive Have for Reducing Plastic Pollution at Coastlines and Riversides? An Evaluation Based on Citizen Science Data. Waste Manag. 2023, 164, 106–118. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, G.; Ahmed, U.; Ahmad, M.A. Impact of Microplastics on Human Health: Risks, Diseases, and Affected Body Systems. Microplastics 2025, 4, 23. https://doi.org/10.3390/microplastics4020023
Abbas G, Ahmed U, Ahmad MA. Impact of Microplastics on Human Health: Risks, Diseases, and Affected Body Systems. Microplastics. 2025; 4(2):23. https://doi.org/10.3390/microplastics4020023
Chicago/Turabian StyleAbbas, Ghulam, Usama Ahmed, and Muhammad Arsalan Ahmad. 2025. "Impact of Microplastics on Human Health: Risks, Diseases, and Affected Body Systems" Microplastics 4, no. 2: 23. https://doi.org/10.3390/microplastics4020023
APA StyleAbbas, G., Ahmed, U., & Ahmad, M. A. (2025). Impact of Microplastics on Human Health: Risks, Diseases, and Affected Body Systems. Microplastics, 4(2), 23. https://doi.org/10.3390/microplastics4020023






