Abstract
Background/Objectives: Genetic abnormalities play a pivotal role in patient risk stratification, therapeutic decision-making, and elucidating the disease pathogenesis in hematological malignancies. In multiple myeloma (MM) and acute myeloid leukemia (AML)/myelodysplastic syndrome (MDS), numerous recurring genetic aberrations are well documented. Fluorescence in situ hybridization (FISH) is a cornerstone of clinical diagnostics for detecting these abnormalities. Conventionally, FISH assesses up to two biomarkers, with one or two circles per slide, but this approach faces challenges when cancer cell yields are limited, particularly in post-treatment follow-up specimens. Methods: To overcome this limitation, we developed a multi-well method, enabling the simultaneous testing of multiple biomarkers on a single microscopic slide. This study included 53 MM and 129 AML/MDS cases. Results: With a cohort of 182 patients, 1016 FISH assays performed on multi-well slides accurately detected diagnostic genetic aberrations previously identified by karyotyping and/or FISH, achieving a sensitivity and specificity of 100%. The use of multi-well slides achieved up to a 2.5-fold increase in the number of wells per slide while achieving more than a 3-fold reduction in the reagent volume compared to traditional methods. This advancement leverages distinct FISH signal patterns to strategically combine biomarkers within multiple wells, suitable for specimens from diagnosis, follow-ups, and relapses, regardless of the cancer cell quantity. Conclusions: The multi-well approach enhances the accessibility to comprehensive biomarker analysis, reducing both the processing time and costs. Beyond MM and AML/MDS, this technique holds promise for use with other hematological malignancies with limited sample volumes, offering an efficient, cost-effective solution for precision diagnostics.
1. Introduction
Genetic abnormalities play a pivotal role in patient risk stratification, therapeutic decision-making, and elucidating the disease pathogenesis in hematological malignancies. Numerous recurring genetic aberrations are well documented in multiple myeloma (MM) [1,2,3,4,5,6,7] and acute myeloid leukemia (AML)/myelodysplastic syndrome (MDS) [8,9,10,11,12,13,14].
MM is a malignant condition arising from the uncontrolled proliferation of plasma cells, classified as a plasma cell neoplasm [4,15]. Frequent genetic alterations in MM include structural variants (SVs), such as translocations affecting the IGH and MYC loci; copy number variants (CNVs), including gains at 1q, losses at 17p (del(17p)), and losses at 13q (del(13q)); ploidy variations like hyperdiploidy or hypodiploidy; and mutations in genes such as those within the RAS signaling pathway, BRAF, FAM46C, DIS3, and TP53 [3,4,7,10,16]. Trisomies or hyperdiploid states and IGH-related translocations or fusions are classified as initiating events in MM, whereas gains at 1q, del(17p) with TP53 mutations, and MYC translocations or fusions are regarded as later events tied to disease advancement [2,4]. For risk assessment in MM, the Revised International Staging System integrates high-risk genetic markers—such as del(17p), t(4;14), involving IGH::FGFR3/NSD2, and t(14;16), involving IGH::MAF—and serum indicators [17,18]. Additional alterations, such as t(14;20), involving IGH::MAFB, and MYC translocations or fusions, are viewed as possible indicators of an elevated risk of progression in MM [1,2,16]. High-risk markers are usually associated with aggressive disease and a poor response to standard therapies, which may prompt clinicians to consider intensified treatments or clinical trials [2,4,16].
AML and MDS are recognized as myeloid malignancies [8]. Beyond their typical clinical features, morphological characteristics, immunophenotypic changes, and gene mutations, recurrent CNVs—including deletions like 5q, 7q, or 20q, monosomy 5 or 7, and trisomy 8—and SVs such as KMT2A gene rearrangements are frequently observed in these malignancies [8,10,14]. CNVs and SVs play a pivotal role in AML and MDS [8,9,10,11,12,13,14]. For instance, deletions like 5q or 7q and monosomy 7 often disrupt critical tumor suppressor genes, leading to uncontrolled cell growth and a poor prognosis, particularly in MDS and therapy-related AML, often necessitating aggressive interventions like stem cell transplantation [19,20]. Trisomy 8, another common CNV, is associated with intermediate-risk AML and may contribute to disease progression by altering the gene dosage [21]. Similarly, SVs such as KMT2A gene rearrangements, frequently seen in AML, result in the production of fusion proteins that drive leukemogenesis by dysregulating gene expression and promoting aggressive disease behavior [22]. These alterations serve as diagnostic markers, guide risk stratification, and inform therapeutic decisions, making their detection essential for personalized patient management.
Fluorescence in situ hybridization (FISH) is a critical tool in clinical diagnostics for patients with hematological malignancies [23,24,25]. This technique uses fluorescently labeled DNA probes to detect specific chromosomal abnormalities—such as CNVs, translocations, and SVs—directly in interphase cells or metaphase chromosomes. Unlike conventional karyotyping, which requires the division of cells and may miss subtle or cryptic alterations, FISH offers high sensitivity and specificity, making it invaluable for diagnosis, risk stratification, and treatment planning in these diseases. FISH’s ability to rapidly detect these changes in interphase cells—without requiring metaphase spreads—makes it especially useful when the sample quality is poor or cell division is sparse, as is common in post-treatment AML/MDS specimens, and is particularly advantageous in MM, where the plasma cell proliferation is often low. For MM, these alterations are detected using an MM-specific FISH panel, often applied to CD138+ plasma cells isolated from bone marrow samples, which can eliminate other blood-forming cells using various enrichment approaches [25,26,27]. These enrichment strategies rely on tagging plasma cells with specific monoclonal antibodies, such as CD138, followed by separation via immunomagnetic techniques or fluorescence-activated cell sorting [24,25,26,28,29,30]. Among these options, immunomagnetic cell sorting with anti-CD138 antibodies has emerged as the favored approach [24,26], distinguished by its cost-effectiveness and reduced demand for advanced technical expertise relative to fluorescence-activated cell sorting. With its robust efficacy, CD138+ FISH enables monitoring using post-treatment samples, even when cancer cell yields are limited, ensuring the accurate tracking of the disease burden [31].
FISH’s clinical utility stems from its speed, specificity, and ability to target predefined abnormalities, providing results within days compared to the weeks required for karyotyping or NGS. This rapid turnaround is crucial for timely decision-making in acute hematological malignancies like AML. In MM, AML, and MDS, FISH bridges the gap between cytogenetics and molecular diagnostics, delivering actionable insights into chromosomal abnormalities that drive disease behavior. Its precision in detecting prognostic markers, adaptability to small or post-treatment samples, and efficiency in clinical workflows make it indispensable for personalized patient care. Therefore, FISH is a cornerstone of clinical diagnostics for detecting chromosome abnormalities in these diseases.
While FISH is cost-effective compared to genome-wide sequencing for detecting recurrent chromosomal abnormalities, each FISH biomarker probe typically ranges from USD 75 to USD 225 per patient test in the U.S., depending on the target, supplier, and manufacturer-recommended protocol. FISH panels for hematological malignancies often include multiple probes—for instance, a standard AML/MDS panel typically features five probes, while an MM panel includes at least seven. Traditionally, FISH is limited to assessing up to two biomarkers per slide, requiring multiple slides to complete a full panel. This approach poses challenges, including the need for additional slides and the risk of an insufficient cell yield, especially in post-treatment follow-up samples with limited or low cancer cell counts. Innovations like multi-well slides may address these limitations, enhancing FISH’s practicality and advancing its role in resource-constrained settings.
The Johns Hopkins Hospital Cancer Cytogenomics Laboratory conducted a proof-of-principle study to validate the use of multi-well slides—designed with smaller wells—for analyzing CD138+ plasma cells with the MM FISH protocol and fresh bone marrow/blood samples with the AML/MDS FISH protocol in patients with MM and AML/MDS. The objective of this study was to evaluate the feasibility, adaptability, and resource efficiency of FISH for detecting chromosomal abnormalities in these patients using multi-well slides in a clinical diagnostic setting.
2. Materials and Methods
2.1. The Patient Cohort in This Study
This study comprised 182 consecutive bone marrow or peripheral blood specimens referred to the Johns Hopkins Hospital between 1 August 2024 and 28 February 2025 (Table 1). The study cohort comprised 53 MM cases subjected to 371 FISH assays and 129 AML/MDS cases subjected to 645 FISH assays, totaling 1016 FISH assays. Samples underwent routine diagnostic evaluation, including histomorphologic assessment, flow cytometry, FISH, conventional chromosome analysis, a gene fusion assay, a targeted next-generation sequencing assay, and/or an optical genome mapping test. Leukemias were classified according to the World Health Organization criteria based on clinical, morphologic, immunophenotypic, cytogenetic, and molecular genetic features [32].

Table 1.
Specimen types in this study.
2.2. AML/MDS FISH Testing on Multi-Well Slides
FISH was performed on interphase nuclei from direct cultures using disease-specific probe panels according to the manufacturer’s protocol, as previously described [11]. The AML/MDS FISH panel included the probes 5p15.2 (D5S23, D5S721), 5q31 (EGR1), 7cen (D7Z1), 7q31 (D7S522), 8cen (D8Z2), 11q23 (KMT2A) (Abbott Molecular, Inc., Des Plaines, IL, USA), and 20q12 (D20S108) and 20q13.12 (D20S150) (Cytocell Ltd., Cambridge, UK) (Table 2).

Table 2.
FISH probe panels utilized in this study.
FISH was performed using a multi-well slide featuring twelve 6 mm wells, in contrast to a conventional slide with one to two 15 mm wells. Approximately 3 µL of the specimen was dispensed into each strategically spaced well (see Figure 1a). The slides were baked at 90 °C for 10 min, followed by the addition of 1.5 µL of the probe to each well. The slides were then covered with either a single 22 mm × 50 mm coverslip or multiple 12 mm circular coverslips. Hybridization was performed overnight using the LSI Thermobrite program (melt at 73 °C for 2 min, hybridize at 37 °C overnight), followed by a 2 min 73 °C post-hybridization wash with a buffer (0.4× SSC/0.3% NP-40) and two 2 min room temperature washes (2× SSC/0.1% NP-40) and the application of 4 µL of DAPI III. Two technologists, blinded to each other’s results, each independently evaluated 100 nuclei per probe (200 nuclei in total per probe) using fluorescence microscopy on a Zeiss Axioscope system (Carl Zeiss Microscopy, LLC, Oberkochen, Germany). The analysis was performed using CytoVision software, version 7.7. A specimen was deemed abnormal if the results exceeded the laboratory-established cutoffs for each probe set, determined during clinical laboratory validation. The cutoff values were calculated based on the probability of a false positive, derived from the binomial distribution using the β-inverse function with a 95% confidence level.
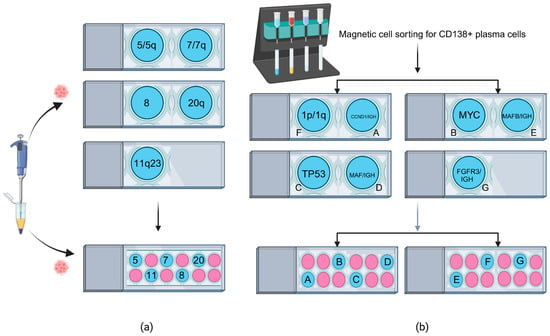
Figure 1.
FISH on multi-well slides. (a) AML/MDS FISH test comparison. The top panel shows standard FISH performed on conventional slides, requiring three separate slides to complete an AML/MDS panel test. The bottom panel demonstrates FISH on a multi-well slide, enabling the entire AML/MDS panel to be conducted on a single slide. (b) MM FISH test comparison. FISH was performed on CD138+ plasma cells isolated via magnetic cell sorting. The top panel shows standard FISH on conventional slides, requiring four separate slides to complete an MM panel test. The bottom panel demonstrates FISH on two multi-well slides, enabling the entire MM panel to be conducted using fewer slides than the standard method.
2.3. CD138 MM FISH Testing on Multi-Well Slides
FISH was performed on CD138+ plasma cells. CD138 cell selection was performed on bone marrow samples via manual isolation, utilizing CD138 microbeads and an autoMACS magnetic column from Miltenyi Biotec (Gaithersburg, MD, USA), as previously described [5]. FISH for plasma cell neoplasms/multiple myeloma includes probes for CDKN2C/CKS1B at 1p32/1q21, IGH/CCND1 at 14q32 and 11q13, and IGH/FGFR3 at 14q32 and 4p16 (Abbott Molecular, Inc., Des Plaines, IL, USA), TP53 at 17p13, IGH/MAF at 14q32 and 16q23, and IGH/MAFB at 14q32 and 20q12 (Cytocell Ltd., 3-4 Technopark, Newmarket Road, Cambridge, UK), and MYC at 8q24.21 (ZytoVision GmbH, Fischkai 1, D-27572 Bremerhaven, Germany) (Table 2). MM FISH tests on multi-well slides followed a methodology similar to that described above for AML/MDS FISH tests on multi-well slides. However, the MM FISH panel utilized two slides, designated “Slide 1” and “Slide 2”, to accommodate the seven probes (see Figure 1b). The analysis and reporting of the multiple myeloma (MM) FISH panel closely mirrored those of the AML/MDS FISH panel, with a notable exception. In contrast to the AML/MDS panel, which assessed 200 nuclei per probe, the MM FISH panel involved two technologists, blinded to each other’s results, who each independently evaluated 25 CD138-enriched nuclei per probe, resulting in a total of 50 CD138+ nuclei per probe. A specimen was deemed abnormal if the results exceeded the laboratory-established cutoffs for each probe set, determined during clinical laboratory validation. The cutoff values were calculated based on the probability of a false positive, derived from the binomial distribution using the β-inverse function with a 95% confidence level.
2.4. Statistical Calculations
The sensitivity, specificity, and accuracy of FISH performed on multi-well slides for the tested loci were evaluated against standard FISH/karyotyping using MedCalc statistical software (https://www.medcalc.org/calc/diagnostic_test.php, last accessed 28 February 2025).
3. Results
FISH on multi-well slides was successfully conducted for a cohort of 182 patients, encompassing a total of 1016 individual FISH assays. This included 371 FISH assays performed on CD138+-enriched cells from 53 MM patients and 645 FISH assays for 129 AML/MDS patients. When evaluated against diagnostic genetic aberrations previously confirmed by karyotyping and/or standard FISH, the multi-well slide FISH method demonstrated a sensitivity and specificity of 100% in parallel comparisons with standard FISH/karyotyping. Furthermore, by reducing the number of slides, probe volume (e.g., a 70% decrease from 5.0 µL to 1.5 µL per test), and processing time, this approach enables laboratories to maintain or even increase their diagnostic output with fewer resources.
3.1. AML/MDS FISH Testing on Multi-Well Slides
For a cohort of 129 patients with AML or MDS, FISH was conducted on multi-well slides using 645 individual probes to identify diagnostic genetic aberrations. Of the samples analyzed, 24 (18.6%) exhibited abnormalities based on the results from the tested probes. The most frequent aberrations involved 7cen/7q alterations, primarily monosomy 7 and 7q deletions, observed in 10 cases (7.8%). These were followed by eight cases (6.2%) with 5q deletions/monosomy 5 or 20q deletions; six cases (4.7%) with trisomy 8; and two cases (1.6%) with KMT2A gene rearrangements at 11q23. Additional findings included KMT2A amplification in four cases (3.1%), KMT2A loss in two cases (1.6%), and a 5p gain in one case (0.8%). These results are comprehensively presented in Table 3 and visually represented in Figure 2.

Table 3.
Results of multi-well slide FISH analysis for 129 AML/MDS cases.
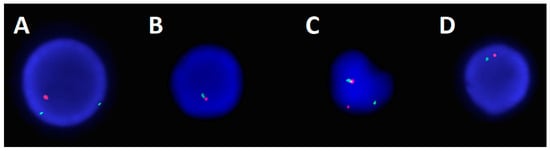
Figure 2.
FISH images from the AML/MDS panel analysis. (A) Obtained utilizing a copy number probe set for chromosome 5, where green represents 5p15.2 and orange represents 5q31, an image of an interphase cell displaying a pattern of 5q deletion (1O 2G). (B) Obtained utilizing a copy number probe set for chromosome 20, where green represents 20q13.1 and red represents 20q12, an image of an interphase cell displaying a pattern of 20q loss (1R 1G). (C) Obtained utilizing a break-apart probe set for chromosome 11, where the green represents the centromeric portion of KMT2A and the orange represents the telomeric portion of KMT2A, an image of an interphase cell displaying a pattern of split/rearranged KMT2A (1O 1G 1F). (D) Obtained utilizing a copy number probe set for chromosome 7, where green represents centromere 7 and orange represents 7q31, an image of an interphase cell displaying a pattern of monosomy 7 (1O 1G).
3.2. CD138 MM FISH Testing on Multi-Well Slides
For a cohort of 53 patients with MM, FISH was performed on multi-well slides using 371 individual probes to detect diagnostic genetic aberrations. Of the samples evaluated, 24 (45.3%) displayed abnormalities based on the results from the tested probes. The most prevalent alteration was a 1q gain, detected in eleven cases (20.8%), whereas the least frequent was an IGH::MAF fusion, noted in only one case (1.9%). These findings, consistent with clinically relevant MM genetic markers, are comprehensively detailed in Table 4 and visually illustrated in Figure 3.

Table 4.
Results of multi-well slide FISH analysis using CD138+ cells in 53 MM cases.
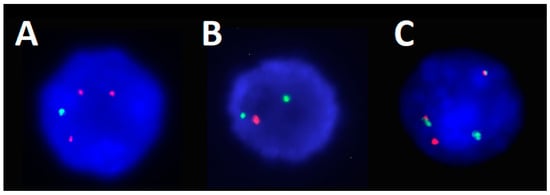
Figure 3.
FISH images from the CD138+ MM panel analysis. (A) Obtained utilizing a copy number probe set for chromosome 1, where green represents CDKN2C at 1p32 and orange represents CKS1B at 1q21, an image of an interphase cell displaying a pattern of 1q gain with the loss of 1p (3O 1G). (B) Obtained utilizing a copy number probe set for chromosome 17, where orange represents centromere 17 and red represents TP53 at 17p13, an image of an interphase cell displaying a pattern of 17p/TP53 deletion (1R 2G). (C) Obtained utilizing a dual fusion probe set for chromosomes 14 and 16, where green represents IGH at centromere 14q32 and red represents MAF at 16q23, an image of an interphase cell displaying a pattern of IGH::MAF dual fusion (1R 1G 2F).
4. Discussion
The performance of FISH on multi-well slides represents a significant evolution of traditional FISH methodology, introducing an innovative platform that enhances clinical cytogenetics laboratories’ capabilities. Unlike standard FISH, which relies on larger, single-well slides for hybridization, this approach employs slides with multiple smaller wells, each capable of hosting independent probe reactions. The current study examining MM and AML/MDS FISH panels—encompassing 182 patients (53 with MM and 129 with AML/MDS)—demonstrates multi-well FISH’s potential as a scalable, efficient alternative to conventional practices. With a demonstrated diagnostic accuracy of 100% sensitivity and specificity across 1016 individual probes (371 for MM, 645 for AML/MDS), this method offers compelling advantages in a clinical diagnostic setting. These benefits align with the growing demand for high-throughput, resource-efficient testing in precision oncology, particularly for diseases like MM and AML/MDS, where rapid, reliable genetic profiling informs treatment decisions (e.g., risk stratification using TP53 deletions or IGH/FGFR3 translocations).
4.1. Advantages of Performing FISH on Multi-Well Slides for Leukemia Patients
In this study, the implementation of multi-well slides in MM and AML/MDS FISH panels proved successful, maintaining diagnostic accuracy while offering several advantages over conventional FISH methods.
4.1.1. Enhanced Cell Concentration and Technologist Time Savings
The reduced well size of multi-well slides significantly increases the concentration of target cells—such as CD138+-enriched plasma cells in MM or scarce cancer cells in AML patients’ post-treatment samples—within a confined area. This design elevates the density of nuclei available for scoring, minimizing the time and effort technologists expend to locate and evaluate an adequate number of cells. Consequently, this facilitates the more consistent and reliable detection of genetic aberrations, particularly in challenging low-yield samples, where cell scarcity often complicates analysis. Enhanced diagnostic reliability improves patient outcomes by ensuring the accurate identification of clinically actionable abnormalities.
Moreover, the reduction in the analysis time optimizes laboratory workflows by decreasing the manual effort required per test. This efficiency boosts the throughput, enabling technologists to process a higher volume of cases within the same timeframe. Alternatively, it frees up the capacity for technologists to tackle more intricate tasks, such as interpreting rare or subtle genetic aberrations, troubleshooting atypical hybridization patterns, or refining protocols for quality control. In high-throughput clinical cytogenetics labs, where hundreds to thousands of FISH tests are performed annually, these time savings translate into substantial productivity gains. Faster turnaround times enhance service delivery, while the increased capacity supports the lab’s ability to meet rising clinical demands efficiently, ultimately reducing operational bottlenecks and improving resource allocation to meet the need for both routine and complex diagnostic evaluations.
4.1.2. Reduced Probe Volume (Cost Savings) and Optimized Resource Utilization
Multi-well slides require significantly less of the probe per test—approximately 3.5 µL less than the 5 µL used in traditional FISH, representing a 70% reduction. Given that FISH probe costs typically range from USD 75 to USD 225 per patient, this reduction translates to a per-test expense decrease to USD 22.5 to USD 76.5 with multi-well slides, compared to USD 75–USD 225 with traditional methods, depending on the probe price. Multiple wells, each designed to accommodate a distinct probe, enable laboratories to perform multiple hybridizations on a single slide, maximizing the efficiency of each probe vial. This optimization increases the number of tests per vial, reducing the per-test costs and extending the probe inventory longevity.
Furthermore, although multi-well slides have a higher per-slide cost than standard single- or dual-well formats, their use in our AML/MDS panel led to a USD 0.98 reduction in the slide cost per panel, resulting in total savings of USD 126.42 across tests for 129 AML/MDS cases in our study cohort. In high-throughput clinical cytogenetics labs, where hundreds to thousands of tests are processed annually, these savings compound substantially, lowering operational expenses and enhancing budget efficiency when using costly FISH probes, such as those targeting chromosomal aberrations in MM or AML/MDS.
The multi-well slide format further optimizes resource utilization by reducing the number of slides required per FISH panel, streamlining workflows, and minimizing equipment demands. For example, the MM FISH panel, comprising seven probes (e.g., for CDKN2C/CKS1B at 1p32/1q21, TP53 at 17p13, MYC at 8q24.21, and IGH translocations including t(4;14), t(11;14), t(14;16), and t(14;20)), can be consolidated on two multi-well slides, instead of requiring four standard slides (with two wells) as in traditional FISH. Similarly, the AML/MDS panel, with five probes targeting key aberrations (e.g., 5p/5q, 7cen/7q, trisomy 8, KMT2A at 11q23, and 20q), fits on a single multi-well slide, compared to multiple slides when using conventional methods. This consolidation reduces the physical footprint of each test, decreasing the burden on critical equipment like the ThermoBrite hybridization system. By using fewer slides, the approach frees up instrument capacity, shortens processing times, and enhances the batch processing efficiency, eliminating repetitive setup cycles and accelerating turnaround times.
4.2. Potential Pitfalls of Performing FISH on Multi-Well Slides and Future Improvements
The implementation of FISH on multi-well slides introduces significant advantages but also presents potential challenges for clinical adoption that warrant consideration. This section evaluates these potential pitfalls and proposes actionable improvements to ensure diagnostic reliability.
4.2.1. Potential for Cross-Contamination
The compact design of multi-well slides, with wells in close proximity to each other, elevates the risk of probe or cell carryover between adjacent wells, particularly during the hybridization step. Although this study observed no instances of cross-contamination, such an event could lead to false positives or mask low-frequency aberrations. In this study, 1.5 µL of the probe was added to each well, and the wells were then covered with a single 22 mm × 50 mm coverslip for overnight hybridization. While effective in this controlled setting, the use of a single large coverslip may allow for probe diffusion or leakage across wells under less optimal conditions, such as in cases with uneven sealing or an excessive probe volume.
To mitigate this risk, replacing the single 22 mm × 50 mm coverslip with individual 10 mm circular coverslips for each well would physically isolate probes, reducing the potential for cross-well diffusion during hybridization. Sealing each coverslip with a non-reactive adhesive (e.g., rubber cement) could further enhance containment. Furthermore, refining pipetting techniques—such as using precision micropipettes with hydrophobic tips—could minimize the carryover during probe application.
4.2.2. Learning Curve, Limited Well Size, and Cell Capacity
Adopting multi-well slides necessitates the training of technologists so they can adapt to a modified setup and a scoring process slightly distinct from that of traditional FISH. Unlike conventional slides, which use a larger surface area for probe application and cell spreading, multi-well slides require the precise pipetting of small probe volumes (e.g., 1.5 µL) into confined wells and subsequent scoring within a reduced field of view. In this study, technologists successfully adapted to these changes, achieving 100% concordance with standard methods, but initial training was essential. This included optimizing the drying time of cell pellets, mastering accurate probe pipetting to avoid overflow, applying coverslips, and adjusting microscopy techniques. To mitigate the potential risk of evaporation in multi-well slides due to small reagent volumes, enhanced sealing or humidity control could be incorporated to maintain sample integrity during high-temperature probe hybridizations and washes. While the learning curve is moderate—requiring only a few hours to days of practice depending on prior experience—it may temporarily slow workflows during implementation, particularly in labs with a high staff turnover.
The smaller well size of multi-well slides, a key feature enabling probe consolidation and greater resource efficiency, imposes constraints on the cell capacity and requires careful sample preparation. Each well must contain an optimal cell concentration to ensure sufficient nuclei for reliable FISH analysis, a challenge that varies with samples’ cellularity. For low-cellularity samples—such as those from AML patients post-treatment with reduced leukemic blasts or MM patients with limited CD138+ plasma cells after magnetic cell sorting—the cell pellet is typically divided equally across wells. In this study, this approach proved effective, leveraging the wells’ ability to concentrate sparse cells into a scorable density, enhancing the detection of genetic aberrations. However, for high-cellularity samples—such as AML specimens at initial diagnosis with abundant blasts (>50% of cells)—using the undiluted original specimen risks overcrowding, leading to overlapping nuclei that obscure scoring. Here, diluting the sample to an ideal concentration before dispensing it into wells is critical to maintain clarity and accuracy.
4.3. The Adoption of FISH on Multi-Well Slides in a Clinical Diagnostic Setting
The adoption of FISH on multi-well slides represents a transformative advancement in clinical diagnostics, as validated by this proof-of-principle study in AML/MDS and MM. The multi-well slide setup integrates seamlessly with existing laboratory protocols, requiring only minor procedural adjustments—such as adapting to smaller probe volumes and well-specific scoring—while significantly optimizing resource utilization. Crucially, this method leverages standard laboratory equipment (e.g., FISH systems, fluorescence microscopes) that is already in place, eliminating the need for substantial capital investment in new infrastructure or specialized automation systems (e.g., BioDot’s CellWriter systems (https://www.biodot.com/applications/fish, accessed on 20 April 2025), Leica’s Bond RX Biosystems (https://www.leicabiosystems.com/us/ihc-ish/ihc-ish-instruments/bond-rx/, accessed on 20 April 2025), etc.). In this study, multi-well slides were successfully used for AML/MDS and MM FISH panels, consolidating multiple probes (e.g., five for AML/MDS, seven for MM) on fewer slides with no compromise in performance. This versatility demonstrates the technology’s adaptability across diverse cytogenetic assays, making it scalable for laboratories of all sizes, from small-scale facilities to high-throughput clinical cytogenetics labs. By enhancing the operational flexibility and maintaining workflow efficiency without requiring costly upgrades, multi-well slide FISH offers a cost-effective, accessible solution that aligns with resource-conscious diagnostic environments.
The strategic advantage of using multi-well slides lies in their resource efficiency in a clinical diagnostic setting. This approach reduces the number of slides, probe volume, and processing time, enabling laboratories to maintain or enhance their diagnostic output with fewer resources. Such efficiency aligns with lean operational models, minimizing waste and potentially expanding the capacity for additional services, such as broader diagnostic panels or research initiatives. For example, consolidating multiple hybridizations on a single slide decreases the physical and temporal footprint of each test, facilitating a higher throughput and improving service delivery, particularly in resource-constrained settings where equipment and reagent costs are limiting factors.
The reliability of multi-well slides is substantiated by their alignment with established clinical standards. This study evaluated FISH using multi-well slides in patients with AML/MDS and MM, achieving complete concordance with the available standard FISH and karyotyping results in a clinical diagnostic setting. This equivalence confirms that multi-well slides provide diagnostic accuracy comparable to that of traditional methods, detecting critical aberrations with no compromise in sensitivity or specificity. Consequently, multi-well slide FISH serves as a direct replacement for conventional FISH, requiring only initial validation and minimal technologist retraining focused primarily on adapting to the multi-well format, rather than extensive procedural revisions. This ease of integration, paired with its proven performance, establishes multi-well slide FISH as a practical, efficient solution for modern clinical diagnostics, enabling faster turnaround times and bolstering laboratories’ capacity to meet escalating clinical demands. Future developments in multi-well slide design, such as slides with even smaller wells, could further reduce reagent volumes while maintaining diagnostic accuracy, potentially enhancing the resource efficiency of FISH protocols in clinical diagnostic settings.
5. Conclusions
This study represents the first application of CD138+ MM FISH and AML/MDS FISH testing using a multi-well slide approach in a clinical diagnostic setting. The multi-well method improves access to comprehensive biomarker analysis by reducing the processing time and costs. Beyond its utility in MM and AML/MDS, this technique shows potential for use with other hematological malignancies with limited sample volumes, providing an efficient and cost-effective solution for precision diagnostics in these conditions.
Author Contributions
Conceptualization, F.T., M.K. and Y.S.Z.; methodology, F.T., M.K., V.S., J.G., A.N., L.M., P.L. and Y.S.Z.; validation, F.T., M.K., V.S., J.G., A.N., L.M., P.L. and Y.S.Z.; formal analysis, F.T., M.K., V.S., J.G., A.N., L.M., P.L. and Y.S.Z.; data curation, W.M., B.P. and M.P.; writing—original draft preparation, F.T. and Y.S.Z.; writing—review and editing, F.T., M.K., V.S., J.G., A.N., L.M., P.L. and Y.S.Z.; visualization, F.T., M.K. and Y.S.Z.; supervision, F.T., M.K. and Y.S.Z.; project administration, F.T., M.K. and Y.S.Z.; funding acquisition, F.T., M.K. and Y.S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
No outside funding. The Johns Hopkins Cytogenomics Laboratory is an academic laboratory supported by the Johns Hopkins School of Medicine Department of Pathology.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Johns Hopkins School of Medicine (protocol code: IRB00310100; date of approval: 5 August 2022).
Informed Consent Statement
The requirement for patient consent was waived due to this being a retrospective study.
Data Availability Statement
The dataset used for the current study is available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to acknowledge the efforts of the cytogenetics technologists and laboratory technicians of the Johns Hopkins Cytogenomics Laboratory.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AML | Acute myeloid leukemia |
| CD38+ | CD38 enrichment |
| CNV | Copy number variant |
| del | Deletion |
| FISH | Fluorescence in situ hybridization |
| MDS | Myelodysplastic syndrome |
| MM | Multiple myeloma |
| SV | Structural variant |
| t | Translocation |
References
- Abdallah, N.H.; Binder, M.; Rajkumar, S.V.; Greipp, P.T.; Kapoor, P.; Dispenzieri, A.; Gertz, M.A.; Baughn, L.B.; Lacy, M.Q.; Hayman, S.R.; et al. A simple additive staging system for newly diagnosed multiple myeloma. Blood Cancer J. 2022, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Cardona-Benavides, I.J.; de Ramon, C.; Gutierrez, N.C. Genetic Abnormalities in Multiple Myeloma: Prognostic and Therapeutic Implications. Cells 2021, 10, 336. [Google Scholar] [CrossRef]
- Clarke, S.E.; Fuller, K.A.; Erber, W.N. Chromosomal defects in multiple myeloma. Blood Rev. 2024, 64, 101168. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.S.; Klausner, M.; Ghabrial, J.; Stinnett, V.; Long, P.; Morsberger, L.; Murry, J.B.; Beierl, K.; Gocke, C.D.; Xian, R.R.; et al. A comprehensive approach to evaluate genetic abnormalities in multiple myeloma using optical genome mapping. Blood Cancer J. 2024, 14, 78. [Google Scholar] [CrossRef]
- Saxe, D.; Seo, E.J.; Bergeron, M.B.; Han, J.Y. Recent advances in cytogenetic characterization of multiple myeloma. Int. J. Lab. Hematol. 2019, 41, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Rustad, E.H.; Yellapantula, V.D.; Glodzik, D.; Maclachlan, K.H.; Diamond, B.; Boyle, E.M.; Ashby, C.; Blaney, P.; Gundem, G.; Hultcrantz, M.; et al. Revealing the impact of structural variants in multiple myeloma. Blood Cancer Discov. 2020, 1, 258–273. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Garcia-Manero, G. Myelodysplastic syndromes: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2023, 98, 1307–1325. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef]
- Jiang, L.; Pallavajjala, A.; Huang, J.; Haley, L.; Morsberger, L.; Stinnett, V.; Hardy, M.; Park, R.; Ament, C.; Finch, A.; et al. Clinical Utility of Targeted Next-Generation Sequencing Assay to Detect Copy Number Variants Associated with Myelodysplastic Syndrome in Myeloid Malignancies. J. Mol. Diagn. 2021, 23, 467–483. [Google Scholar] [CrossRef]
- Halper-Stromberg, E.; Stinnett, V.; Morsberger, L.; Pallavajjala, A.; Levis, M.J.; DeZern, A.E.; Lei, M.; Phan, B.; Xian, R.R.; Gocke, C.D.; et al. 1q jumping translocation as a biomarker in myeloid malignancy: Frequently mutated genes associated with bad prognosis and low survival. Exp. Hematol. Oncol. 2024, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.J.; Murry, J.B.; Morsberger, L.A.; Klausner, M.; Chen, S.; Gocke, C.D.; McCallion, A.S.; Zou, Y.S. Ring Chromosomes in Hematological Malignancies Are Associated with TP53 Gene Mutations and Characteristic Copy Number Variants. Cancers 2023, 15, 5439. [Google Scholar] [CrossRef]
- Snaith, O.; Poveda-Rogers, C.; Laczko, D.; Yang, G.; Morrissette, J.J.D. Cytogenetics and genomics of acute myeloid leukemia. Best Pract. Res. Clin. Haematol. 2024, 37, 101533. [Google Scholar] [CrossRef]
- Palumbo, A.; Anderson, K. Multiple myeloma. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.J.; Green, D.J.; Kwok, M.; Lee, S.; Coffey, D.G.; Holmberg, L.A.; Tuazon, S.; Gopal, A.K.; Libby, E.N. Diagnosis and Management of Multiple Myeloma: A Review. JAMA 2022, 327, 464–477. [Google Scholar] [CrossRef]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- D’Agostino, M.; Cairns, D.A.; Lahuerta, J.J.; Wester, R.; Bertsch, U.; Waage, A.; Zamagni, E.; Mateos, M.V.; Dall’Olio, D.; van de Donk, N.; et al. Second Revision of the International Staging System (R2-ISS) for Overall Survival in Multiple Myeloma: A European Myeloma Network (EMN) Report Within the HARMONY Project. J. Clin. Oncol. 2022, 40, 3406–3418. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, S.; Mascarenhas, J.; Steensma, D.P. Loss of 5q in myeloid malignancies—A gain in understanding of biological and clinical consequences. Blood Rev. 2021, 46, 100735. [Google Scholar] [CrossRef]
- Mori, M.; Kubota, Y.; Durmaz, A.; Gurnari, C.; Goodings, C.; Adema, V.; Ponvilawan, B.; Bahaj, W.S.; Kewan, T.; LaFramboise, T.; et al. Genomics of deletion 7 and 7q in myeloid neoplasm: From pathogenic culprits to potential synthetic lethal therapeutic targets. Leukemia 2023, 37, 2082–2093. [Google Scholar] [CrossRef]
- Hemsing, A.L.; Hovland, R.; Tsykunova, G.; Reikvam, H. Trisomy 8 in acute myeloid leukemia. Expert. Rev. Hematol. 2019, 12, 947–958. [Google Scholar] [CrossRef]
- Hernandez-Sanchez, A.; Gonzalez, T.; Sobas, M.; Strang, E.; Castellani, G.; Abaigar, M.; Valk, P.J.M.; Villaverde Ramiro, A.; Benner, A.; Metzeler, K.H.; et al. Rearrangements involving 11q23.3/KMT2A in adult AML: Mutational landscape and prognostic implications—A HARMONY study. Leukemia 2024, 38, 1929–1937. [Google Scholar] [CrossRef]
- Kokate, P.; Dalvi, R.; Koppaka, N.; Mandava, S. Prognostic classification of MDS is improved by the inclusion of FISH panel testing with conventional cytogenetics. Cancer Genet. 2017, 216–217, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Muddasani, R.; Orlowski, R.Z.; Abruzzo, L.V.; Qazilbash, M.H.; You, M.J.; Wang, Y.; Zhao, M.; Chen, S.; Glitza, I.C.; et al. Plasma cell enrichment enhances detection of high-risk cytogenomic abnormalities by fluorescence in situ hybridization and improves risk stratification of patients with plasma cell neoplasms. Arch. Pathol. Lab. Med. 2013, 137, 625–631. [Google Scholar] [CrossRef]
- Ross, F.M.; Avet-Loiseau, H.; Ameye, G.; Gutierrez, N.C.; Liebisch, P.; O’Connor, S.; Dalva, K.; Fabris, S.; Testi, A.M.; Jarosova, M.; et al. Report from the European Myeloma Network on interphase FISH in multiple myeloma and related disorders. Haematologica 2012, 97, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, L.; Biggerstaff, J.S.; Chapman, D.B.; Scott, J.M.; Johnson, K.R.; Ghirardelli, K.M.; Fritschle, W.K.; Martinez, D.L.; Bennington, R.K.; de Baca, M.E.; et al. Detection of genomic abnormalities in multiple myeloma: The application of FISH analysis in combination with various plasma cell enrichment techniques. Am. J. Clin. Pathol. 2011, 136, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Panakkal, V.; Rana, S.; Rathore, S.; Anshu, A.; Balakrishnan, A.; Singh, C.; Jandial, A.; Sachdeva, M.U.S.; Varma, N.; Lad, D.; et al. The success rate of interphase fluorescence in situ hybridization in plasma cell disorders can be improved using unconventional sources of plasma cells. Int. J. Lab. Hematol. 2022, 44, 157–162. [Google Scholar] [CrossRef]
- Gagnon, M.F.; Midthun, S.M.; Fangel, J.A.; Schuh, C.M.; Luoma, I.M.; Pearce, K.E.; Meyer, R.G.; Ailawadhi, S.; Arribas, M.J.; Braggio, E.; et al. Superior detection rate of plasma cell FISH using FACS-FISH. Am. J. Clin. Pathol. 2024, 161, 60–70. [Google Scholar] [CrossRef]
- Ha, J.; Cho, H.; Lee, T.G.; Shin, S.; Chung, H.; Jang, J.E.; Kim, S.J.; Cheong, J.W.; Lee, S.T.; Kim, J.S.; et al. Cytogenetic testing by fluorescence in situ hybridization is improved by plasma cell sorting in multiple myeloma. Sci. Rep. 2022, 12, 8287. [Google Scholar] [CrossRef]
- Christensen, T.; Deng, W.; McMahill, B.; Schappert, J.; Liu, W.; Saleki, R.; Zou, Y.S. Utilization of magnetic-activated cell sorting and high-density single nucleotide polymorphism microarrays improves diagnostic yield and prognostic value in clinical testing for patients with multiple myeloma and normal routine chromosome study. Acta Haematol. 2014, 132, 233–236. [Google Scholar] [CrossRef]
- Smith, D.; Stephenson, C.; Percy, L.; Lach, A.; Chatters, S.; Kempski, H.; Yong, K. Cohort analysis of FISH testing of CD138(+) cells in relapsed multiple myeloma: Implications for prognosis and choice of therapy. Br. J. Haematol. 2015, 171, 881–883. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board (Ed.) Haematolymphoid Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2024; Volume 11, Available online: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Haematolymphoid-Tumours-2024 (accessed on 23 April 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).