Abstract
Lignin is one of the important components of lignocellulosic cell walls, which endows plant cell walls with rigidity and strength and protects them from microbial invasion. The presence of lignin is thought to hinder the conversion of biomass to bioenergy, so understanding enzyme-lignin interactions is very important in order to reduce the inhibition of lignin and improve the hydrolysis yields. Conversion of lignocellulosic raw materials into bioethanol is divided into pretreatment, enzymatic hydrolysis, and fermentation. In this paper, both pretreatment and enzymatic hydrolysis of lignocellulose are described in detail. Finally, the reasons why lignin hinders enzymatic hydrolysis efficiency, mainly from forming spatial barriers and interacting with cellulase, are discussed, and the influencing factors and mechanisms of action of cellulase hydrolysis are explored with a view to targeted regulation of lignin structure to improve lignocellulosic saccharification.
1. Introduction
Industrialization and population growth have caused a significant growth in energy consumption, which cannot be met by fossil fuel reserves. At the same time, fossil fuels are also non-sustainable and pose many environmental pollution problems [1]. Therefore, the focus of energy research has begun to shift to bioenergy, and bioethanol, biomethane, and bio-oil are all clean energy alternatives to fossil fuels [2]. Among them, the production process of bioethanol mainly includes pretreatment of lignocellulosic feedstock, enzymatic hydrolysis, and fermentation. Lignocellulosic feedstock is a complex macromolecular material composed of hemicellulose, cellulose, and lignin [3], which is renewable, low-cost, and easily available. Lignocellulose can be used as raw material to produce bioethanol in order to reduce dependence on fossil fuels and relieve energy pressure.
One of the vital components of plant cell walls is lignin, which gives rigidity and strength to plant cell walls and protects them from microbial attack [4]. Therefore, lignocellulosic raw materials are recalcitrant and pose a great challenge to subsequent enzymatic hydrolysis and biorefining processes. The presence of lignin, which is structurally complex and highly resistant, is considered an obstacle to the efficient conversion of biomass into bioethanol; inhibiting cellulase hydrolysis mainly manifests as a physical barrier and non-productive adsorption of enzymes [5]. Different sources of lignin and its structural units make the molecular structure of lignin differential, which affects the hydrolysis process of cellulose. In addition, functional groups such as methoxy, phenolic hydroxyl, carboxyl, and aliphatic hydroxyl groups of lignin can influence enzymatic hydrolysis’s efficiency through the interaction between enzymes and lignin.
On this basis, this paper reviews the research progress in the interaction mechanism between lignin and cellulase and the effects of the content, distribution, source, and structure of lignin on enzymatic hydrolysis. The results of this series of studies may provide methodological and theoretical support for the efficient and targeted regulation of lignocellulose.
2. Lignocellulose Raw Materials
Lignocellulosic materials consist mainly of 30–45% cellulose, 15–25% hemicellulose, and 15–25% lignin, which is intricately intertwined through covalent bonds to form resistant biocomposites [6]. Cellulose microfibrils (composed of ordered polymer chains containing tightly dense crystalline regions) are embedded in a matrix of lignin and hemicellulose, as in Figure 1 [7].
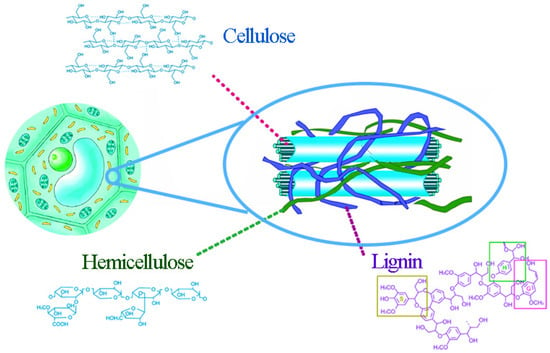
Figure 1.
Cellulose chains surrounded by hemicellulose and lignin; adapted from [7].
Cellulose is a water-insoluble chain polymer composed of glucose groups linked by β-1,4-glycosidic, and its degree of polymerization is up to 10,000. Hydroxyl groups of each glucose unit are more than sufficient to form interchain and intrachain hydrogen bonds, stabilize the molecular structure itself, and connect with adjacent molecules to form microfibrils [8]. A large number of intermolecular and intramolecular hydrogen bonds from a very complex three-dimensional hydrogen bond network structure of cellulose, and the intertwining of crystalline and non-crystalline regions, form the two-phase structure of cellulose, where the proportion of crystalline regions in cellulose is called the crystallinity [9]. The crystallization degree of cellulose shows different enzymatic hydrolysis conversions, and it has been shown that breaking the crystalline structure of cellulose into an amorphous structure can significantly increase the enzymatic hydrolysis conversion of cellulose [10].
Hemicellulose, which is closely bound to cellulose and lignin by hydrogen bonds and van der Waals forces, is mainly composed of two or more monosaccharides as non-homogeneous glycans, including xylose, mannose, arabinose, glucose, galactose, and their derivatives [11]. For different plants, the structure of hemicellulose may vary somewhat; usually hemicellulose in coniferous wood is dominated by hexosan and, in broad-leaved wood, by pentosan. Even in the same plant, hemicellulose content and composition vary considerably in bark, stems, roots, and branches [12]. Compared to cellulose, hemicellulose has a low molecular weight and a more amorphous region, making it more soluble and more susceptible to chemical attack [13].
Lignin is the only naturally renewable aromatic polymer mainly distributed in the secondary cell walls of plants [14]. Lignin is mainly composed of three hydroxycinnamic alcohols, cetearyl alcohol, and mustard alcohol, and each produces a corresponding type of lignin unit: syringyl structure (S), guaiacyl structure (G), and p-hydroxyphenyl structure (H) (as shown in Figure 2) [15]. Lignin polymers are mainly composed of these three structural units through ether bonds, C-C bonds, e.g., β-O-4, β-β, β-5, α-O-4, 4-O-5, etc.
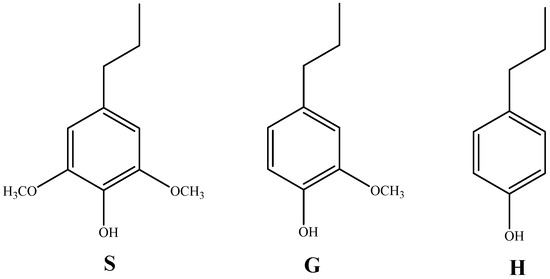
Figure 2.
The three monomers of lignin (p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S); adapted from [15].
The lignin content and structure vary greatly from lignocelluloses to lignocelluloses, e.g., coniferous wood contains 25–31% lignin and contains only G structural units. In contrast, broadleaf wood (including G and S units) contains 16–24% lignin. For herbaceous species, the lignin content is approximately 16–21%, and all three structural units are included [16]. Therefore, according to the structural units contained, lignin is classified as G-type (coniferous lignin), GS-type (broadleaf lignin), and GSH-type (graminaceous lignin).
3. Efficient Saccharification of Lignocellulosic Materials
3.1. Pretreatment of Lignocellulosic Materials
The complexity of plant cell wall microstructure, the variety of chemical composition, and the heterogeneity of ingredient distribution constitute the resistance of lignocellulosic biomass to biological and chemical deconstruction [17]. Therefore, an appropriate and cost-effective pretreatment technology is essential to improve the conversion of fibrous feedstocks into biofuels. Pretreatment, which reduces the recalcitrance of woody fibrous feedstocks by altering their structural or chemical composition, is playing a more and more important impact on biomass utilization with the introduction of the concept of the biorefinery, which is capable of both improving the sugar conversion of cellulosic fractions and recovering hemicellulose and lignin for reuse [18,19]. A variety of pretreatment methods, including physical, chemical, biological, or a group of these methods, have been extensively researched and developed in the last decades. The original feature of woody biomass and the influence of the following pretreatment has a prominent effect on the development of substrate properties, which, conversely, determine the effect of enzymatic hydrolysis, as shown in Figure 3 [20].
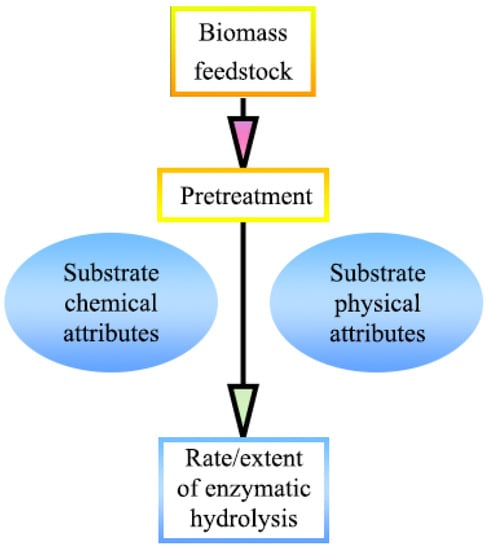
Figure 3.
The original characteristics of woody biomass and the effect of pretreatment on enzymatic hydrolysis; adapted from [20].
The physical method mainly uses mechanical methods to decrease the particle size of raw material particles, decrease the crystallinity, and increase the specific surface area without adding any chemical or biological reagents. In order to decrease the particle size of lignocellulose, improve the accessibility of enzymes to cellulose, and shorten the reaction time of enzymatic hydrolysis of glucose, Wu et al. [21] used an intermittent ball mill to pretreat the enzymatic hydrolysis slurry of lignocellulose. Later, in the research of Silva et al. [22], they found that the degradation rate of wheat straw could be improved by reducing the grain size of wheat straw by ball mill, and it could be an effective pretreatment method because similar glucose yield could be obtained compared with steam blast pretreatment. Sun et al. [23] treated the wheat with freeze–thaw pretreatment, which resulted in the formation of transverse and longitudinal cracks, which increased the pore size while breaking the hydrogen bonds in the crystalline region and increased the maximum enzymatic efficiency of hemicellulose and cellulose to 57.06% and 70.66%, respectively.
Chemical pretreatment solubilized the lignocellulosic biomass by breaking the covalent cross-linkage bonds and reduced the crystallinity of cellulose by breaking the lignin–cellulose linkage. Dilute acid pretreatment is a relatively common way in which hemicellulose is easily converted to soluble sugars during pretreatment, and the reduction of hemicellulose can significantly promote the enzymatic hydrolysis of pretreated biomass. In addition, hydrothermal pretreatment and organosolv pretreatment are also commonly used in industry. In the study by De Oliveira et al. [24], they pretreated sugarcane with dilute sulfuric acid to selectively remove hemicellulose, and, after pretreatment, the porosity of the cell wall was reduced and the conversion of glucan by cellulase hydrolysis was observed. On the other hand, in the experiment of Zhu et al. [25], they established a new pretreatment process, sulfite pretreatment, for spruce and red pine under the conditions of 8–10% and 1.8–3.7% of sodium bisulfite and sulfuric acid addition, respectively, to achieve nearly full hemicellulose separation, and the enzymatic hydrolysis conversion was able to exceed 90% after 48 h of enzymatic hydrolysis. In addition, Krishnan et al. [26] pretreated sugarcane bagasse and sugarcane leaves under alkaline conditions, and ammonia fiber burst pretreatment (AFEX) improved enzyme accessibility to cellulose during hydrolysis by breaking ether bonds and lignin–carbohydrate complexes, and the maximum glucan conversion of sugarcane bagasse and sugarcane leaf bagasse after AFEX pretreatment can reach about 85%. It is worth mentioning that hydrothermal pretreatment increases the accessibility of cellulose and minimizes the generation of inhibition products. For example, hydrothermal pretreatment has a great impact on the structure of biomass of Brazilian duckweed (L. punctata) and its conversion to bioethanol [27].
Biological pretreatment is a green, safe, and inexpensive pretreatment with the advantages of low energy requirements, simple operating conditions and equipment, no inhibitor formation, and no need to recycle chemicals after pretreatment. In the experiment of Sahae et al. [28], white rot fungus was used to pretreat corn stover, and the results showed that the pretreatment of white rot fungus could effectively improve the enzymatic hydrolysis ability of corn stover. da Silva et al. [29] used wood-rotting fungi to pretreat sugarcane bagasse and found that the selective white-rot fungus Ceriporiopsis subvermispora selectively degrades lignin in lignocellulosic biomass, and was able to retain a large proportion of the glucan fraction and augment its conversion to enzymatic hydrolysis. In addition, Asgher et al. [30] pretreated sugarcane bagasse with ligninolytic enzymes, and the lignin removal was up to 33%, and the yield of cellulose enzymatic hydrolysis reached 72.9%.
3.2. Cellulase Hydrolysis
Enzymatic hydrolysis of lignocellulosic biomass is a non-homogeneous biocatalytic process. The current enzymatic hydrolysis process focuses on the hydrolysis of cellulose into glucose because hemicellulose contains different types of glycans and requires different types of enzymes for hydrolysis, which is a complex procedure [31]. The degradation of cellulose to glucose requires the synergistic action of three cellulases (exo-glucanase CBH, endo-glucanase EG, and cellobiose enzyme BG) [32].
Cellulases are composed of three parts: the binding domain, the catalytic domain, and the connecting bridge [33]. The structural domain of cellulase allows cellulase to adsorb to the surface of the substrate, and the amount of adsorption will directly affect the efficiency of cellulase hydrolysis. After adsorption, the cellulose is hydrolyzed by the three major components of cellulase. The mechanism of cellulase hydrolysis of cellulose is broadly based on three theoretical hypotheses: the fragmentation theory, the primary reaction hypothesis, and the synergistic theory [34], and, so far, researchers generally agree that cellulase degrades the substrate through the synergistic theory. During enzymatic hydrolysis, EG randomly breaks the β-1,4-glycosidic bond in the internal noncrystalline region of the cellulose polysaccharide chain, resulting in new ends; subsequently, CBH acts on the reducing and nonreducing ends to produce cellobiose, after which BG hydrolyzes the cellobiose to glucose [35].
4. Factors Influencing the Hydrolysis of Cellulase
The enzymatic effect of lignocellulose is usually influenced by many factors of the hydrolysis process, such as enzymatic conditions, cellulose accessibility, and the coating of cellulose by lignin.
The effect of enzymatic hydrolysis conditions on cellulose sugar conversion is mainly reflected in the hydrolysis temperature, hydrolysis concentration, pH, etc. In terms of hydrolysis temperature, Ooshima et al. [36] adsorbed cellulase onto microcrystalline cellulose under different temperature conditions, and the results showed that cellulose and cellulase showed different binding capacities at different temperatures, and the adsorption of cellulase decreased with the increase in temperature. In terms of hydrolysis concentration, Kinnarinen et al. [37] found in their study that the enzymatic hydrolysis conversion increases with increasing cellulase concentration, but when the concentration reaches a certain level, the hydrolysis rate stops increasing. In addition to the above two aspects, pH also affects the adsorption between substrate components and cellulase by changing the surface charge. Du et al. [38] studied the adsorption of cellulase on the substrate corn cob under different pH conditions and found that cellulase adsorbed more readily at pH < 4.8, i.e., under acidic conditions, and desorbed more readily under alkaline or neutral conditions.
Hydrolysis of cellulose occurs only when cellulase is adsorbed on the cellulose [39]. Therefore, enhancing the affinity of cellulase on cellulose substrates plays an important role in increasing the efficiency of cellulase hydrolysis, and the accessible surface district of cellulose is a momentous factor in determining the rate and extent of cellulose–cellulase adsorption. To increase the accessibility of the substrate to cellulase, Hou et al. [40] pretreated rice straw feedstock with a renewable lysine ionic liquid–water mixture, and the pretreated rice straw showed a significant increase in its surface area and pore volume, with glucose yields of up to 81% after enzymatic hydrolysis. It was also found that the surface area of corn straw increased 26.6-fold and the pore volume increased 30-fold after pretreatment with ionic liquid, and the enzymatic hydrolysis rate and the sugar yield of the treated corn straw were increased [41].
In addition, cellulase hydrolysis is also affected by substrates, such as the crystallinity of cellulose and the spatial hindrance of lignin and hemicellulose. Crystallinity is considered an important substrate characteristic that affects enzyme hydrolysis. The structure of cellulose macromolecules has both amorphous and crystalline regions, and the strong hydrogen bonding makes the enzymatic hydrolysis rate in the crystalline region 3–30 times lower than in the amorphous region [42]. Several studies have shown that high crystallinity is not favorable for the enzymatic hydrolysis of cellulose [43]. For example, in the experiment of Cheng et al. [44], they prepared cellulose from potato pulp and corn stover to contrast their enzymatic hydrolysis and physicochemical properties performance. The cellulose from potato pulp had lower crystallinity and was able to provide higher enzymatic accessibility compared to cellulose from corn stover. Similarly, Satari et al. [45] pretreated different types of wood (elm and pine) with NMMO solution, related their structural properties to the degree of enzymatic transformation, and found that cellulose with lower crystallinity was more conducive to enzymatic hydrolysis.
4.1. Mechanism of Lignin–Enzyme Interactions
Lignin restricts the enzymatic hydrolysis of lignocellulosic raw materials in the following two main ways: on the one hand, it forms a spatial barrier to limit the accessibility of cellulose; on the other hand, it interacts with cellulase, and ineffective adsorption occurs [46,47].
The unproductive adsorption between lignin and cellulase relies on three main driving forces: hydrophobic, hydrogen bonding interactions, and electrostatic.
Lignin is naturally hydrophobic, while the structure of cellulase also contains hydrophobic groups such as tyrosine and tryptophan, and cellulase is adsorbed to lignin due to hydrophobic interaction once it is dispersed in water. Lignin can adsorb cellulase, and hydrophobic interaction is considered to be the primary driving force [48]. For example, the cellulose binding domain in Cel7B has a higher hydrophobicity compared to Cel7A, so Cel7B is more likely to adsorb on lignin [49]. In this area of research, Hodgson et al. [50] compared the contact angles of lignin and cellulose and found that when both are present, cellulase is preferentially adsorbed by lignin due to its higher hydrophobicity.
Electrostatic interaction has a significant impact on the adsorption of lignin and cellulase. Lu et al. [51] investigated the adsorption of cellulase through liquid hot water pretreatment of corn stover lignin and its mechanism and found that lignin from pretreated corn stover exhibited a higher surface charge compared to lignin from untreated corn stover due to electrostatic gravitational forces that enhanced the cellulase and lignin interaction forces. In addition, it has been shown that changes in pH also affect the surface charge. In the study by Lou et al. [52], they found, through their study, that the surface charge of lignin increases with increasing pH, leading to a significant increase in its hydrophilicity and reducing the ineffective adsorption of cellulase. They investigated the mechanism by which the enzymatic saccharification of lignocellulose is significantly enhanced at elevated pH values of 5.5–6.0. As shown in Figure 4, the increase in pH value significantly increases the surface charge of lignin, which becomes more hydrophilic and promotes electrostatic interactions between cellulase and lignin.
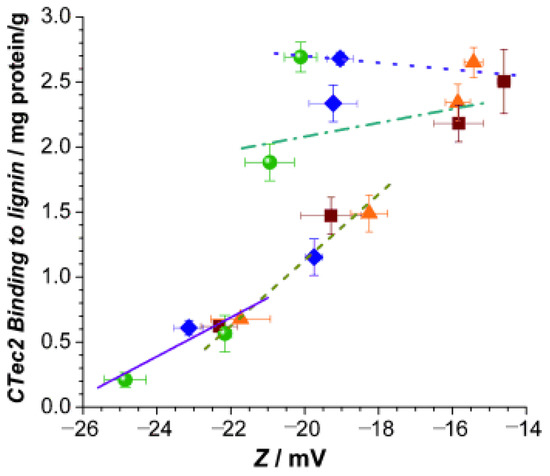
Figure 4.
Correlations between lignin surface charge (Zeta potential, Z) and nonspecific CTec2 binding to the four hydrolysis lignin residues at different pH. ▪ DA; ▴ SP-B2; ⧫ SP-B4; • SP-B6; pH 4.5 y = −0.026x + 2.18, r2 = 0.65; pH 4.8 y = 0.053x + 3.14, r2 = 0.40; pH 5.5 y = 0.253x + 6.19, r2 = 0.94; pH 6.0 y = 0.150x + 3.99, r2 = 0.90; adapted from [52].
Hydrogen bonding refers to the interaction between hydrogen atoms on the surface functional groups of lignin with enzymes. To some extent, it has been hypothesized and confirmed that the phenolic hydroxyl groups of lignin are the main causes of enzyme adsorption [53]. Amination modification of lignin can decrease the content of phenolic hydroxyl groups in lignin and alleviate the negative effect of lignin on enzymatic hydrolysis.
In the experiments of Mou et al. [54], by aminating lignin with dimethylamine and diethylenetriamine, they proved that the introduction of a certain amount of amine groups can reduce the surface charge and hydrophobicity of lignin, and can also greatly reduce the adsorption of cellulase by lignin. Under optimal conditions, the adsorption reduction is 96.32% (Figure 5). In addition, adding surfactants can alleviate the inhibition of lignin on cellulase hydrolysis. Zhang et al. [55] found, in their study, that the addition of the surfactant polyethylene glycol can have a facilitating effect on cellulase hydrolysis due to the formation of hydrogen bonds between the ether–oxygen bond in polyethylene glycol and the phenolic hydroxyl group of lignin, thereby reducing the interaction of lignin with cellulase. However, the relative contribution of hydrogen bonding in lignin–enzyme interactions is unclear on account of the lack of studies. Due to the interaction of individual factors, further studies are needed.
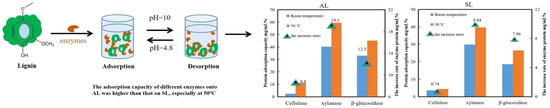
Figure 5.
Using eucalyptus lignin as raw material, the dynamic adsorption differences in different enzymes and enzyme components were studied, and the effect of lignin on the enzymatic activities of different enzymes and enzyme components was determined; adapted from [54].
Spatial site hindrance of lignin is another important factor that impedes cellulase hydrolysis in addition to non-productive adsorption. Djajadi et al. [56] showed that hydrothermal pretreatment of gram lignin impeded cellulase hydrolysis because lignin acted as a physical barrier that prevented the cellulose from being entered by enzymes, rather than through non-productive adsorption to the enzyme. Moreover, Donaldson et al. [57] found that alkali pretreatment affected the redistribution of lignin in the fiber cell wall to the fiber surface, creating a physical barrier to the linking of cellulose and cellulase, thus negatively affecting cellulase hydrolysis.
4.2. Effect of Lignin Functional Groups on Cellulase Hydrolysis
The lower the lignin content, the higher the enzymatic hydrolysis efficiency, as shown in Figure 6. Lignin contains a number of functional groups, such as aliphatic hydroxyl groups, phenolic hydroxyl groups, carboxyl groups, and methoxy groups. The combination of enzymes and lignin cannot produce products, which is largely attributed to the interaction between these functional groups and cellulase.
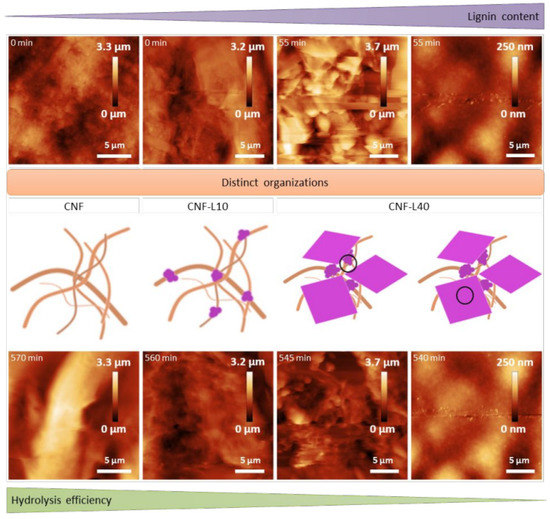
Figure 6.
Schematic representation of the main conclusions regarding Cellic CTec 2 efficiency towards substrate with increasing lignin content and distinct structural organization; adapted from [58].
The phenolic and aliphatic hydroxyl groups in lignin promote the adsorption between lignin and cellulase by forming hydrogen bonds with hydroxyl and carbonyl groups in cellulase, and in the study of Huang et al. [59], it can be observed that the higher the content of the phenolic hydrocarbon group, the stronger the adsorption affinity and binding force. In a modification of lignin, hydroxypropylation inhibits the free phenolic hydroxyl group in lignin, thus significantly reducing its inhibitory effect during the process of enzymatic hydrolysis [60]. Pan et al. [61] found that chemical blockage of free phenolic hydroxyl groups by hydroxypropylation greatly removed the inhibitory effect of lignin, and that interference caused by phenolic hydroxyl groups is probably more important than physical barriers and nonspecific adsorption in the inhibition of cellulase digestion by lignin.
Carboxyl groups were able to lower the inhibitory influence of lignin on substrate enzyme hydrolysis by improving the hydrophilic and negative charge effects of lignin. Palonen et al. [62] demonstrated that an increase in carboxyl group content in lignin can lead to a decrease in its non-specific adsorption by cellulase. Subsequently, Ying et al. [63], in their study on the influence of modified alkali lignin on cellulase–lignin interactions and enzyme hydrolysis efficiency, found that alkali pretreatment combined with carboxylation treatment increased the carboxyl content of lignin and was able to significantly enhance the efficiency of cellulase hydrolysis.
The sulfonic acid group of sulfonated lignin could increase the hydrophilicity of lignin and decrease the non-specific binding of cellulase to lignin [64]. In the experiment of Wu et al. [65], it can be found that the introduction of the sulfonic acid group in lignin was able to enhance the hydrophilicity of lignin and reduce the degree of binding to the enzyme while enhancing the hydrolysis of cellulose. It has proved that the addition of sulfonated lignin (SL) to the enzymatic hydrolysis system results in the formation of SL–cellulase complexes between SL and cellulase, which enhances the electrostatic repulsion among the substrate lignin and cellulase and hinders its adsorption by cellulase [66]. Del Rio et al. [67] found, through a study, that the substrate sulfonation weakened the ineffective adsorption of lignin from the substrate to the enzyme during enzymatic hydrolysis, thus increasing the yield of cellulase hydrolysis.
5. Conclusions and Outlook
Lignin has a highly polymeric and complex structure whose presence imparts rigidity to lignocellulose, thus preventing the degradation of polysaccharides in the structure by microorganisms and enzymes. Because of the complex physicochemical properties of lignin and enzymes, the underlying principles involving the interaction between lignin and enzymatic transformation are uncertain. In addition, the added value of lignin is of great value for the comprehensive utilization of biomass, carbohydrates, and other lignin components. However, its comprehensive utilization needs to eliminate the interference of non-lignin factors, which still needs to be further explored. This paper summarizes in detail the methods of lignin modification and the mechanism of its enzymatic conversion of biomass. In summary, the modification of lignin to obtain lignin with specific properties has become an important means to improve the adsorption capacity of cellulase and cellulose and enhance the hydrolysis of cellulose.
Future work can consider the preparation of lignin samples with different unit compositions and the use of advanced techniques, such as dissipative quartz crystal microanalytical balances, to perform in situ, real-time studies of lignin with different structural units, and the dynamic behavior of cellulose complexes in enzymatic adsorption and/or enzymatic hydrolysis, under the exclusion of other interfering factors (i.e., identical conditions except for the different lignin structures), to gain insight into the internal relationship between lignin structure and the lignin–cellulase interaction. This paper provides technical and theoretical support for the improvement of existing pretreatment methods and the growth of fresh efficient pretreatment methods, and helps to enhance the efficiency of lignocellulosic enzymatic digestion and realize the industrialization of lignocellulose-based bioethanol conversion. It also helps to enhance the enzymatic efficiency of lignocellulose and realize the industrial production of bioethanol from lignocellulose.
Author Contributions
Conceptualization, W.W. and P.L.; methodology, L.H.; software, Y.W.; validation, L.Z. and J.L.; investigation, P.L.; resources, L.H.; data curation, P.L.; writing—original draft preparation, W.W.; writing—review and editing, Y.J.; project administration, Y.J.; funding acquisition, Y.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 31730106, 32271797.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Devi, A.; Singh, A.; Bajar, S.; Pant, D.; Din, Z.U. Ethanol from lignocellulosic biomass: An in-depth analysis of pre-treatment methods, fermentation approaches and detoxification processes. J. Environ. Chem. Eng. 2021, 9, 105798. [Google Scholar] [CrossRef]
- Göncü, B.; Gülşen, H.; Hoşgün, E.Z. Bioethanol production from pistachio (Pistacia vera L.) shells applying ozone pretreatment and subsequent enzymatic hydrolysis. Environ. Technol. 2021, 42, 2438–2446. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.N.; Sun, S.L.; Cao, X.F.; Sun, R.C. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Verma, A.; Singhania, R.R.; Varjani, S.; Di Dong, C.; Patel, A.K. Current understanding of the inhibition factors and their mechanism of action for the lignocellulosic biomass hydrolysis. Bioresour. Technol. 2021, 332, 125042. [Google Scholar] [CrossRef]
- Hao, X.; Xu, F.; Zhang, J. Effect of pretreatments on production of xylooligosaccharides and monosaccharides from corncob by a two-step hydrolysis. Carbohydr. Polym. 2022, 285, 119217. [Google Scholar] [CrossRef]
- Lynd, L.R.; Weimer, P.J.; van Zyl, W.H.; Pretorius, I.S. Microbial cellulose utilization: Fundamentals and biotechnolog. Microbiol. Mol. Biol. R 2002, 66, 506–577. [Google Scholar] [CrossRef]
- Hasanov, I.; Raud, M.; Kikas, T. The Role of Ionic Liquids in the Lignin Separation from Lignocellulosic Biomass. Energies 2020, 13, 4864. [Google Scholar] [CrossRef]
- Liu, K.; Xu, T.; Du, H.S.; Zheng, T.; Liu, H.Y.; Zhang, M.; Liu, W.; Zhang, R.; Li, H.M.; Si, C.L. Lignin-based electrodes for energy storage application. Carbohyd. Polym. 2021, 259, 117740. [Google Scholar] [CrossRef]
- Sun, L.; Han, J.; Wu, J.; Huang, W.; Li, Y.; Mao, Y.; Wang, L.; Wang, Y. Cellulose pretreatment with inorganic salt hydrate: Dissolution, regeneration, structure and morphology. Ind. Crop. Prod. 2022, 180, 114722. [Google Scholar] [CrossRef]
- Chen, X.; Xiong, L.; Li, H.; Zhang, L.; Yuan, G.; Chen, X.; Wang, C.; Chen, X. The inhibitory effect of xylan on enzymatic hydrolysis of cellulose is dependent on cellulose ultrastructure. Cellulose 2020, 27, 4417–4428. [Google Scholar] [CrossRef]
- Yu, H.; Wang, J.; Yu, J.-X.; Wang, Y.; Chi, R.-A. Adsorption performance and stability of the modified straws and their extracts of cellulose, lignin, and hemicellulose for Pb2+: pH effect. Arab. J. Chem. 2020, 13, 9019–9033. [Google Scholar] [CrossRef]
- Khan, M.U.; Ahring, B.K. Lignin degradation under anaerobic digestion: Influence of lignin modifications—A review. Biomass-Bioenergy 2019, 128, 105325. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van Den Bosch, S.; Koelewijn, S.-F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Rana, M.; Park, J.-H. Advancement in technologies for the depolymerization of lignin. Fuel Process. Technol. 2018, 181, 115–132. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, J.; Wang, K.; Wang, B.; Sun, S.; Lin, X.; Song, L.; Wu, A.; Li, H. Characterization of Lignin Structures in Phyllostachys edulis (Moso Bamboo) at Different Ages. Polymers 2020, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.Q.; Shiung, L.S.; Wu, Y.J.; Ge, S.B.; Wu, J.L.; Cai, L.P.; Huang, Z.H.; van Le, Q.; Sonne, C.; Xia, C.L. Simultaneous removal of nitrate and sulfate using an up-flow three-dimensional biofilm electrode reactor: Performance and microbial response. Bioresour. Technol. 2020, 324, 124631. [Google Scholar] [CrossRef]
- Cai, J.; Wang, Y.; Liu, J.; Zhang, X.; Li, F. Pretreatment enhanced structural disruption, enzymatic hydrolysis, fermentative hydrogen production from rice straw. Int. J. Hydrogen Energy 2022, 47, 11778–11786. [Google Scholar] [CrossRef]
- Li, P.; Yang, C.; Jiang, Z.; Jin, Y.; Wu, W. Lignocellulose pretreatment by deep eutectic solvents and related technologies: A review. J. Bioresour. Bioprod. 2023, 8, 33–44. [Google Scholar] [CrossRef]
- Sun, L.L.; Yue, Z.; Sun, S.C.; Sun, S.N.; Yuan, X.F.; Tong, Q.; Jia, L. Exploration of deep eutectic solvent-based biphasic system for furfural production and enhancing enzymatic hydrolysis: Chemical, topochemical, and morphological changes. Bioresour. Technol. 2022, 352, 127074. [Google Scholar] [CrossRef]
- Chandra, R.P.; Bura, R.; Mabee, W.E.; Berlin, A.; Pan, X.; Saddler, J.N. Substrate pretreatment: The key to effective enzymatic hydrolysis of lignocellulosics? Biofuels 2007, 108, 67–93. [Google Scholar]
- Wu, Y.; Ge, S.; Xia, C.; Mei, C.; Kim, K.-H.; Cai, L.; Smith, L.M.; Lee, J.; Shi, S.Q. Application of intermittent ball milling to enzymatic hydrolysis for efficient conversion of lignocellulosic biomass into glucose. Renew. Sustain. Energy Rev. 2021, 136, 110442. [Google Scholar] [CrossRef]
- Silva, G.G.D.; Couturier, M.; Berrin, J.-G.; Buléon, A.; Rouau, X. Effects of grinding processes on enzymatic degradation of wheat straw. Bioresour. Technol. 2012, 103, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Deng, Y.; Li, S.; Xu, W.; Liu, G. Enhanced efficiency of enzymatic hydrolysis of wheat straw via freeze–thaw pretreatment. Environ. Sci. Pollut. Res. 2022, 29, 56696–56704. [Google Scholar] [CrossRef]
- Santos, V.T.D.O.; Siqueira, G.; Milagres, A.M.F.; Ferraz, A. Role of hemicellulose removal during dilute acid pretreatment on the cellulose accessibility and enzymatic hydrolysis of compositionally diverse sugarcane hybrids. Ind. Crop. Prod. 2018, 111, 722–730. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Pan, X.J.; Wang, G.S.; Gleisner, R. Sulfite pretreatment (SPORL) for robust enzymatic saccharification of spruce and red pine. Bioresour. Technol. 2009, 100, 2411–2418. [Google Scholar] [CrossRef]
- Krishnan, C.; Sousa, L.D.C.; Jin, M.; Chang, L.; Dale, B.E.; Balan, V. Alkali-based AFEX pretreatment for the conversion of sugarcane bagasse and cane leaf residues to ethanol. Biotechnol. Bioeng. 2010, 107, 441–450. [Google Scholar] [CrossRef]
- Souto, L.R.F.; da Silva, I.F.; Ninow, J.L.; Collins, S.R.; Elliston, A.; Waldron, K.W. Effect of hydrothermal pre-treatment on duckweed (Landoltia punctata) biomass for simultaneous saccharification and fermentation process. Biomass-Bioenergy 2019, 127, 105259. [Google Scholar] [CrossRef]
- Saha, B.C.; Qureshi, N.; Kennedy, G.J.; Cotta, M.A. Biological pretreatment of corn stover with white-rot fungus for improved enzymatic hydrolysis. Int. Biodeterior. Biodegrad. 2016, 109, 29–35. [Google Scholar] [CrossRef]
- da Silva, M.A.; Ferraz, A. Biological pretreatment of sugarcane bagasse with basidiomycetes producing varied patterns of biodegradation. Bioresour. Technol. 2017, 225, 17–22. [Google Scholar]
- Asgher, M.; Ahmad, Z.; Iqbal, H.M.N. Alkali and enzymatic delignification of sugarcane bagasse to expose cellulose polymers for saccharification and bio-ethanol production. Ind. Crop. Prod. 2013, 44, 488–495. [Google Scholar] [CrossRef]
- Ni, J.; Wang, H.; Chen, Y.; She, Z.; Na, H.; Zhu, J. A novel facile two-step method for producing glucose from cellulose. Bioresour. Technol. 2013, 137, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, R.H.; Pan, Z.L. Investigation of adsorption kinetics and isotherm of cellulase and beta-glucosidase on lignocellulosic substrates. Biomass. Bioenerg. 2016, 91, 1–9. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, B.; Chen, H.; Wu, W.; Wu, S.; Jin, Y.; Xiao, H. Recent advances in understanding the effects of lignin structural characteristics on enzymatic hydrolysis. Biotechnol. Biofuels 2021, 14, 205. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chi, C.; Gong, Y.; Li, H.; Wu, Y. Research progress in the fractal kinetics of lignocellulose enzymatic hydrolysis. China Pulp Pap. 2015, 34, 62–67. [Google Scholar]
- Bhat, S.; Owen, E.; Bhat, M.K. Isolation and Characterisation of a Major Cellobiohydrolase (S8) and a Major Endoglucanase (S11) Subunit from the Cellulosome of Clostridium thermocellum. Anaerobe 2001, 7, 171–179. [Google Scholar] [CrossRef]
- Ooshima, H.; Sakata, M.; Harano, Y. Adsorption of cellulase fromTrichoderma viride on cellulose. Biotechnol. Bioeng. 1983, 25, 3103–3114. [Google Scholar] [CrossRef]
- Kinnarinen, T.; Häkkinen, A. Influence of enzyme loading on enzymatic hydrolysis of cardboard waste and size distribution of the resulting fiber residue. Bioresour. Technol. 2014, 159, 136–142. [Google Scholar] [CrossRef]
- Du, R.Y.; Su, R.X.; Li, X.; Tantai, X.W.; Liu, Z.H.; Yang, J.F.; Qi, W.; He, Z.M. Controlled adsorption of cellulase onto pretreated corncob by pH adjustment. Cellulose 2012, 19, 371–380. [Google Scholar] [CrossRef]
- Gabhane, J.; Kumar, S.; Sarma, A. Effect of glycerol thermal and hydrothermal pretreatments on lignin degradation and enzymatic hydrolysis in paddy straw. Renew. Energy 2020, 154, 1304–1313. [Google Scholar] [CrossRef]
- Hou, X.-D.; Li, N.; Zong, M.-H. Significantly enhancing enzymatic hydrolysis of rice straw after pretreatment using renewable ionic liquid–water mixtures. Bioresour. Technol. 2013, 136, 469–474. [Google Scholar] [CrossRef]
- Li, C.; Cheng, G.; Balan, V.; Kent, M.S.; Ong, M.; Chundawat, S.P.; Sousa, L.D.; Melnichenko, Y.B.; Dale, B.E.; Simmons, B.A.; et al. Influence of physico-chemical changes on enzymatic digestibility of ionic liquid and AFEX pretreated corn stover. Bioresour. Technol. 2011, 102, 6928–6936. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part II: Fundamentals of different pre-treatments to increase the enzymatic digestibility of lignocellulose. Biofuels Bioprod. Biorefining 2012, 6, 561–579. [Google Scholar] [CrossRef]
- Chundawat, S.P.S.; Bellesia, G.; Uppugundla, N.; Sousa, L.D.C.; Gao, D.; Cheh, A.M.; Agarwal, U.P.; Bianchetti, C.M.; Phillips, J.G.N.; Langan, P.; et al. Restructuring the Crystalline Cellulose Hydrogen Bond Network Enhances Its Depolymerization Rate. J. Am. Chem. Soc. 2011, 133, 11163–11174. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Hu, X.; Gu, Z.; Hong, Y.; Li, Z.; Li, C. Characterization of physicochemical properties of cellulose from potato pulp and their effects on enzymatic hydrolysis by cellulase. Int. J. Biol. Macromol. 2019, 131, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Satari, B.; Karimi, K.; Molaverdi, M. Structural features influential to enzymatic hydrolysis of cellulose-solvent-based pretreated pinewood and elmwood for ethanol production. Bioprocess Biosyst. Eng. 2017, 41, 249–264. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, S.; Xie, J. Evaluation of the effects of isolated lignin on enzymatic hydrolysis of cellulose. Enzym. Microb. Technol. 2017, 101, 44–50. [Google Scholar] [CrossRef]
- Saini, J.K.; Patel, A.K.; Adsul, M.; Singhania, R.R. Cellulase adsorption on lignin: A roadblock for economic hydrolysis of biomass. Renew. Energy 2016, 98, 29–42. [Google Scholar] [CrossRef]
- Pareek, N.; Gillgren, T.; Jönsson, L.J. Adsorption of proteins involved in hydrolysis of lignocellulose on lignins and hemicelluloses. Bioresour. Technol. 2013, 148, 70–77. [Google Scholar] [CrossRef]
- Costaouëc, T.L.; Pakarinen, A.; Várnai, A.; Puranen, T.; Viikari, L. The role of carbohydrate binding module (CBM) at high substrate consistency: Comparison of Trichoderma reesei and Thermoascus aurantiacus Cel7A (CBHI) and Cel5A (EGII). Bioresour. Technol. 2013, 143, 196–203. [Google Scholar] [CrossRef]
- Hodgson, K.T.; Berg, J.C. The effect of surfactants on wicking flow in fiber networks. J. Colloid Interface Sci. 1988, 121, 22–31. [Google Scholar] [CrossRef]
- Lu, X.; Zheng, X.; Li, X.; Zhao, J. Adsorption and mechanism of cellulase enzymes onto lignin isolated from corn stover pretreated with liquid hot water. Biotechnol. Biofuels 2016, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.M.; Zhu, J.Y.; Lan, T.Q.; Lai, H.R.; Qiu, X.Q. pH-induced lignin surface modification to reduce nonspecific cellulase binding and enhance enzymatic saccharification of lignocelluloses. ChemSusChem 2013, 6, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Fritz, C.; Ferrer, A.; Salas, C.; Jameel, H.; Rojas, O.J. Interactions between Cellulolytic Enzymes with Native, Autohydrolysis, and Technical Lignins and the Effect of a Polysorbate Amphiphile in Reducing Nonproductive Binding. Biomacromolecules 2015, 16, 3878–3888. [Google Scholar] [CrossRef] [PubMed]
- Mou, H.; Wu, X.; Huang, J.; Liu, Y.; Fan, H. Eucalyptus lignin modification for dynamic adsorption with lignocellulose-degradation enzymes dependent on pH values. Ind. Crop. Prod. 2021, 169, 113650. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Xu, X.M.; Zhang, Y.Y.; Li, J.F. Effect of adding surfactant for transforming lignocellulose into fermentable sugars during biocatalysing. Biotechnol. Bioproc. E 2011, 16, 930–936. [Google Scholar] [CrossRef]
- Djajadi, D.T.; Jensen, M.M.; Oliveira, M.; Jensen, A.; Thygesen, L.G.; Pinelo, M.; Glasius, M.; Jørgensen, H.; Meyer, A.S. Lignin from hydrothermally pretreated grass biomass retards enzymatic cellulose degradation by acting as a physical barrier rather than by inducing nonproductive adsorption of enzymes. Biotechnol. Biofuels 2018, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, L.A.; Wong, K.K.Y.; Mackie, K.L. Ultrastructure of steam-exploded wood. Wood Sci. Technol. 1988, 22, 103–114. [Google Scholar] [CrossRef]
- Lambert, E.; Aguié-Béghin, V.; Dessaint, D.; Foulon, L.; Chabbert, B.; Paës, G.; Molinari, M. Real Time and Quantitative Imaging of Lignocellulosic Films Hydrolysis by Atomic Force Microscopy Reveals Lignin Recalcitrance at Nanoscale. Biomacromolecules 2018, 20, 515–527. [Google Scholar] [CrossRef]
- Huang, C.; He, J.; Min, D.; Lai, C.; Yong, Q. Understanding the Nonproductive Enzyme Adsorption and Physicochemical Properties of Residual Lignins in Moso Bamboo Pretreated with Sulfuric Acid and Kraft Pulping. Appl. Biochem. Biotechnol. 2016, 180, 1508–1523. [Google Scholar] [CrossRef]
- Yang, Q.; Pan, X. Correlation between lignin physicochemical properties and inhibition to enzymatic hydrolysis of cellulose. Biotechnol. Bioeng. 2015, 113, 1213–1224. [Google Scholar] [CrossRef]
- Pan, X. Role of Functional Groups in Lignin Inhibition of Enzymatic Hydrolysis of Cellulose to Glucose. J. Biobased Mater. Bioenergy 2008, 2, 25–32. [Google Scholar] [CrossRef]
- Palonen, H.; Viikari, L. Role of oxidative enzymatic treatments on enzymatic hydrolysis of softwood. Biotechnol. Bioeng. 2004, 86, 550–557. [Google Scholar] [CrossRef]
- Ying, W.; Shi, Z.; Yang, H.; Xu, G.; Zheng, Z.; Yang, J. Effect of alkaline lignin modification on cellulase–lignin interactions and enzymatic saccharification yield. Biotechnol. Biofuels 2018, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.S.; Wang, W.X.; Wang, Y.; Jin, Y.C. Complete Dissolution of Ball-Milled Masson Pine Using an Aqueous Sodium Hydroxide Solvent. Bioresources 2016, 10, 7361–7371. [Google Scholar] [CrossRef]
- Wu, J.; Chandra, R.P.; Takada, M.; Liu, L.-Y.; Renneckar, S.; Kim, K.H.; Kim, C.S.; Saddler, J.N. Enhancing Enzyme-Mediated Cellulose Hydrolysis by Incorporating Acid Groups onto the Lignin during Biomass Pretreatment. Front. Bioeng. Biotechnol. 2020, 8, 608835. [Google Scholar] [CrossRef]
- Zheng, W.; Lan, T.; Li, H.; Yue, G.; Zhou, H. Exploring why sodium lignosulfonate influenced enzymatic hydrolysis efficiency of cellulose from the perspective of substrate–enzyme adsorption. Biotechnol. Biofuels 2020, 13, 1–12. [Google Scholar] [CrossRef]
- Del Rio, L.F.; Chandra, R.P.; Saddler, J.N. The effects of increasing swelling and anionic charges on the enzymatic hydrolysis of organosolv-pretreated softwoods at low enzyme loadings. Biotechnol. Bioeng. 2011, 108, 1549–1558. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).