Abstract
Introduction: Current treatments for patients with stage III non-small-cell lung cancer (NSCLC) are not sufficiently personalized, resulting in suboptimal outcomes and high mortality rates. The Developing Circulating and Imaging Biomarkers Towards Personalized Radiotherapy in Lung Cancer (VIGILANCE) study employs innovative health technologies to collect a range of clinical data and features. This includes longitudinal analyses of cell-free and circulating tumor DNA from blood samples and radiomic features extracted from standard-of-care imaging. Additionally, patient-reported outcome measures will be collected to capture patients’ symptoms and quality of life. This will provide invaluable insight into the patient experience during and after radiotherapy. We aim to evaluate whether the data, including patient-reported outcomes, can serve as biomarkers to refine treatment strategies, improve post-treatment follow-up and provide patients with realistic outcome predictions. Key endpoints include the following: (1) assessing whether baseline ctDNA status and its early on-treatment dynamics can identify patients with radioresistant disease who could benefit from treatment intensification; (2) determining whether post-radiotherapy ctDNA clearance can predict benefit from consolidation durvalumab, potentially sparing ctDNA-negative patients from unnecessary immunotherapy; and (3) developing integrated models combining novel ctDNA and radiomic biomarkers to distinguish between radiation fibrosis and tumor recurrence and to predict survival. We adopt a pragmatic approach by recruiting patients receiving standard-of-care treatments in a real-world setting. In addition, most of the clinical data is already routinely collected in our center, except for the blood tests for cell-free and circulating tumor DNA analysis. Methods and analysis: This is a single-center, prospective, exploratory, longitudinal, follow-up study, recruiting patients with stage III NSCLC undergoing standard-of-care curative-intent radiotherapy (with or without systemic therapy). Data collection spans from baseline to during radiotherapy and is extended up to 1 year following radiotherapy. The longitudinal analysis aims to describe and characterize dynamic changes in the collected features and assess their utility as prognostic and response biomarkers. Trial registration number: NCT06086574.
1. Introduction
Lung cancer is the leading cause of cancer mortality worldwide and costs the UK economy GBP 2.4 billion annually [1,2]. Non-small-cell lung cancer (NSCLC) is the most common subtype, representing approximately 85% of all patients diagnosed with lung cancer in the UK. About a third of patients with NSCLC present with stage III disease, which equates to approximately 8000 patients per year in England and Wales [3]. Stage III disease refers to locally advanced tumors that show no evidence of metastatic disease at the time of diagnosis (see Figure 1).
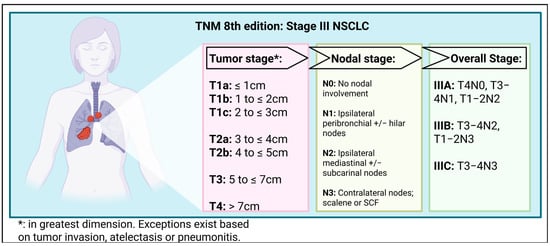
Figure 1.
Tumor Node Metastasis 8th edition—Stage III NSCLC [4]. TNM, tumor, node and metastasis; NSCLC, non-small-cell lung cancer; SCF, supra-clavicular fossa. Created in BioRender. Horne, A. (2025) https://BioRender.com/xw2j7vi (accessed on 7 November 2025).
Despite having potentially curative disease, only 60% of patients diagnosed with stage III NSCLC with good performance status (PS) are offered curative-intent treatment in England and Wales [3]. Consequently, outcomes are poor, with 85% of patients dying within 5 years of diagnosis [5]. The reasons for the poor uptake of curative-intent treatments are not fully understood; however, contributing factors include perceived low success rates and concerns about treatment-related side effects. In addition, clinical and logistical factors play a role, such as clinician bias, training issues and the distance to and accessibility of specialized care facilities [6,7].
Even when offered, curative treatments typically follow a one-size-fits-all approach, guided largely by patient fitness and tumor characteristics [8]. For patients with operable tumors, multimodality surgical treatments are the standard of care (SOC). However, most patients are not considered for resection due to fitness, comorbidities or tumor location. Patients not suitable for a surgical approach are assessed for radiotherapy-based treatments. Outcomes are improved by combining radiotherapy with chemotherapy. Patients who remain of good PS and respond to initial treatment are offered consolidation immunotherapy [9].
Multimodality treatments are resource-intensive and can cause significant acute and long term toxicities, lead to reduced quality of life (QOL) and contribute to patient morbidity and mortality. Furthermore, not all patients respond to treatment, and there is a need to identify responders and non-responders. Following treatment, patients are routinely followed up with interval computed tomography (CT) scans. Treatment failure is identified when macroscopic disease is evident on imaging. By this stage, patients are frequently symptomatic, and salvage options are usually limited. In addition, the interpretation of CT scans performed post-radiotherapy can be challenging. Radiotherapy scar tissue can evolve and change over several years and can be difficult to discriminate with residual tumor. This uncertainty often results in additional investigations and causes significant patient distress.
Biomarkers, a Solution?
Biomarkers are crucial in the realization of personalized medicine. They are defined as measurable and reproducible features attributable to a patient or tumor that can enhance diagnosis, identify optimum treatment and predict outcomes (see Table A1 for a glossary of terminology) [10]. They arise from a diverse range of biological systems and include molecular, histological, radiomic-based or physiological features [11]. To be clinically useful, biomarkers must be robustly validated, have a clearly defined role, demonstrate cost-effectiveness and be grounded within a transparent evidence base. Several biomarkers have been successfully integrated into the management of metastatic NSCLC [12]. Previously, chemotherapy regimens were offered, with marginal improvements in overall survival (OS). In addition, they were associated with high rates of toxicity. Currently, tumors are subtyped based on molecular features, including driver mutation status and programmed death-ligand 1 (PD-L1) expression. This information is used to select bespoke drug treatments, such as tyrosine kinase inhibitors (TKIs) or immunotherapy. This more personalized approach has resulted in patients receiving drug treatments that are life-prolonging and associated with low rates of toxicity, and they are therefore highly acceptable to patients.
Despite extensive research, there is a paucity of similar biomarkers to inform radiotherapy treatments in patients with lung cancer. There are many potential applications summarized in Table 1, and these include enhancing treatment decision-making, promoting the uptake of curative-intent therapies and improving clinical outcomes [10]. Currently, quantitative imaging features, including tumor volume and the maximum Standardized Uptake Value (SUVmax), a positron emission tomography–CT (PET-CT) scan measure of radioactive contrast in tissue, are used to give an indication as to the expected response to treatment. However, they cannot be considered as lung cancer biomarkers because there are no agreed thresholds for defining risk or agreed guidance as to how they might inform clinical decision-making.

Table 1.
Potential applications of biomarkers in lung cancer radiotherapy.
Tumor PD-L1 expression and epidermal growth factor receptor (EGFR) status are molecular features that have been used to guide systemic anti-cancer therapy (SACT) decisions in conjunction with lung cancer radiotherapy. Based on an ad hoc analysis of the PACIFIC trial, the European Medicines Agency (EMA) and National Institute for Health and Care Excellence (NICE) have recommended the use of durvalumab in patients with PD-L1 ≥ 1% [9,13]. In contrast, the US Food and Drug Administration (FDA) has reviewed the same evidence and recommended durvalumab in all patients regardless of PD-L1 expression [14]. These contradictory decisions were discussed by a panel of international lung cancer experts, who concluded that there is insufficient evidence regarding not offering durvalumab to patients with PD-L1 < 1% and that further research is required [15].
Further guidance has been provided for patients with EGFR-mutant NSCLC. The European Society for Medical Oncology (ESMO) has recommended that patients with EGFR-mutant tumors, regardless of PD-L1, should not be routinely offered consolidation durvalumab [16]. This recommendation is based on the understanding that these tumors are generally immunotherapy-resistant. In addition, the LAURA trial has recently reported improved progression-free survival (PFS) in patients with EGFR-mutated stage III NSCLC treated with consolidation osimertinib after concurrent chemoradiotherapy (CCRT) when compared to patients who received CCRT alone [17]. OS data is awaited.
Study rationale: There is a need for reliable, clinically meaningful and noninvasive biomarkers to guide lung cancer management and improve outcomes. The VIGILANCE study collects a range of clinical data and features across different research disciplines at multiple timepoints and integrates distinct health technologies for analysis. This includes analyzing cell-free and circulating tumor DNA (cf and ctDNA) from blood samples and radiomic features from SOC imaging. Additionally, electronic patient-reported outcome measures (ePROMs) collect patient symptoms, functional status and QOL data. The background and rationale for the inclusion of each of these features or potential biomarkers are now discussed. Table A2 summarizes key knowledge gaps regarding these features in relation to lung cancer radiotherapy.
Circulating tumor DNA analysis: cfDNA refers to the fragments of DNA that are located outside of cells within blood or tissue fluid samples. Tumor-originated cfDNA is known as ctDNA. DNA is released from cells through apoptosis, necrosis and active secretion [18]. Various targeted and untargeted approaches are available for detecting, isolating, quantifying and sequencing DNA. Targeted approaches focus on predefined regions of the genome, such as specific genes, mutations or methylation patterns. Approaches include droplet digital polymerase chain reaction (PCR); Beads, Emulsion, Amplification and Magnetics (BEAMing); targeted panel detection methods and targeted methylation assays. Untargeted approaches use a more comprehensive approach to analyze more of the available genome. Approaches include whole genome and whole exome sequencing methods and untargeted methylation assays. Analysis methods are becoming increasingly sensitive and show significant potential as a cancer biomarker [19].
Much of the published ctDNA research in patients with lung cancer undergoing radiotherapy is retrospective, analyzes blood samples from mixed stage and pathology patient groups or is prospective but involves small patient numbers acquired from limited timepoints. Despite these limitations, landmark retrospective studies using a targeted panel detection method called cancer personalized profiling by deep sequencing (Capp-SEQ) have been published [20,21]. The results of these studies demonstrate that ctDNA detection is a sensitive measure of minimal residual disease (MRD) and an early marker of treatment failure that preceded radiographic relapse by approximately five months. This information could help distinguish between evolving radiotherapy-induced fibrosis and tumor relapse during follow-up. The findings also suggested that ctDNA detection could identify patients who are most likely to benefit from consolidation durvalumab. Patients with undetectable ctDNA post-radiotherapy did not benefit from durvalumab. In patients with detectable ctDNA post-radiotherapy, the dynamics in serial samples during durvalumab treatment were informative. Patients whose ctDNA levels decreased benefited from durvalumab, while those with increasing levels appeared to predict treatment failure.
The largest prospective series in the curative-intent radiotherapy setting supported previous findings [22]. A total of 139 patients with stage IIB-III NSCLC undergoing CCRT underwent blood sampling for ctDNA analysis. Blood samples were acquired at multiple timepoints including at baseline, during radiotherapy (once 40 Gray was delivered), at the end of radiotherapy and every 3–6 months thereafter. Patients who achieved ctDNA clearance during or after radiotherapy experienced improved survival compared to those who did not. Also, patients with negative ctDNA after radiotherapy did not appear to benefit from consolidation durvalumab.
Other prospective studies have analyzed blood samples acquired during the first week of radiotherapy [23,24,25,26]. Despite recruiting a small number of patients, a temporary increase in the concentration of ctDNA was detected in some patients. The significance of this increase is unknown, but it could represent radiosensitivity, be a measure of early cell death and be an early predictor of treatment outcome. Additionally, sequencing this DNA could be used to identify tumor genomic information. This would be useful if solid organ biopsy is not feasible due to patient fitness or tumor position. It might also shorten time to definitive treatment if pathological information could be acquired from a blood test. This is currently being explored in a study focusing on patients with lung cancer receiving stereotactic ablative radiotherapy (SABR) [27].
Although still a novel technology, interventional studies are underway integrating ctDNA analysis to risk-stratify patients and guide decisions on whether to offer consolidation therapies following curative-intent radiotherapy for NSCLC (See Table 2 for a summary of some of the key studies in progress).

Table 2.
Summary of key radiotherapy studies in progress using ctDNA to risk-stratify patients.
The VIGILANCE study uses the cfDNA methylation profiling workflow (T7-MBD-seq) developed by the Cancer Research United Kingdom (CRUK) Manchester Institute and National Cancer Biomarker Center based in Manchester. ctDNA is distinguished from normal background cfDNA in blood samples based on the presence of tumor-specific somatic mutations and epigenetic alterations, such as the hypermethylation of CpG islands. Compared to other ctDNA detection methods, this analysis is relatively lower-cost and offers high sensitivity [32,33].
This technique has previously been used to analyze blood samples taken from patients with stage I-IV small-cell lung cancer (SCLC) [34]. A correlation between the concentration of ctDNA and OS was demonstrated, and DNA sequencing was able to distinguish between the different SCLC molecular subtypes. It has also been used to produce a tissue-of-origin classifier to support the diagnosis of patients with cancer of unknown primary [35]. To our knowledge, we are the first research group using a cfDNA methylation assay to analyze the blood samples acquired from patients undergoing radiotherapy for NSCLC.
Radiomics: Patients with lung cancer undergo multiple scans, with the aim of identifying, characterizing and measuring primary lung, nodal and metastatic lesions. This includes diagnostic thorax/abdomen CT and whole-body PET-CT scans. They also undergo radiotherapy planning 4D-CT, daily cone-beam CT during radiotherapy and post-treatment follow-up thorax/abdomen CT scans. SOC imaging is assessed by a radiologist who reports qualitative features, such as tethering or air bronchograms, and simple quantitative features, such as dimension or volume.
Since images are three-dimensional grayscale datasets, they can be analyzed to extract texture-, intensity- and spatial-based features that describe the distribution of voxel data. This workflow is known as radiomic analysis [36]. It is highly automated and uses algorithm-based platforms to efficiently extract thousands of features from numerous scans. Changes in features over time can be tracked between scans—so-called delta-radiomics. Extracted features can then undergo statistical analysis against a chosen outcome or clinical parameter. The most strongly associated subset of features forms a ‘radiomic signature’ and can be integrated into predictive models. These objective, data-driven features have the potential to complement traditional radiologist assessments as clinically useful biomarkers.
Numerous radiomic studies have been published that analyze CT scans acquired from patients with NSCLC treated with radiotherapy. However, due to methodological limitations, these studies have yet to translate into clinical impact. Review articles have been published that summarize the key limitations of radiomic studies [37,38,39]. They include heterogenous populations and treatments, the limited use of biological data and the absence of external validation cohorts or prospective sampling.
Guidance, including from the Image Biomarker Standardization Initiative (IBSI) and Radiomic Quality Score (RQS) version 1.0, describes how to perform key aspects of radiomic research [36,40,41]. Such guidance should be used to support the production of high-quality research. An example of high-quality research is a recent study that used an actuarial deep learning architectural model to build tumor control and pneumonitis risk models [42]. These models integrated radiomic PET-CT features with biological data, including serum cytokines and microRNA. The deep learning models outperformed those built with traditional probability methods and were externally validated using a cohort from the RTOG0617 study.
Radiomic research remains an area of active interest due to its noninvasive nature, high level of automation and potential cost-effectiveness. However, radiomic research remains exploratory, and there are currently no widely accepted radiomic features to guide treatment decisions. To our knowledge, no interventional studies are underway using radiomic features to tailor the management of patients with lung cancer. Consequently, prospective studies that align its design and methodology with radiomic guidelines are urgently required. Only then will a robust and meaningful evidence base be produced to inform interventional studies.
Patient-reported Outcome Measures: Patient-reported outcome measures (PROMs) are validated questionnaires that capture patients’ perspectives on their health status, QOL, functional status and symptom burden [43]. They are primarily used as outcome measures in clinical trials in recognition that clinician-assigned measures, such as PS and Common Terminology Criteria for Adverse Events (CTCAE) criteria, are subjective and prone to bias. Different questionnaires have been produced for use in patients with lung cancer. This includes the European Organization For Research and Treatment of Cancer (EORTC) Core QOL-30 (QLQ-C30) which integrates questions to capture functional status, symptoms and QOL [44]. Focused questionnaires have also been developed. These include the symptom-focused EORTC lung cancer 13 questionnaire (LC-13) and the functional status EORTC EuroQol-5D questionnaire [45]. The EuroQol-5D questionnaire is validated for economic assessment and is used to calculate quality-adjusted life years (QALYs). These questionnaires have been validated across different populations and settings to ensure they are reliable and responsive [46].
There is interest in extending the role of PROMs. Research has demonstrated that baseline PROM results are an independent predictor of survival in patients with stage I-IV lung cancer, outperforming clinician-assigned scores such as Eastern Cooperative Oncology Group (ECOG) PS [47,48]. Other retrospective research has analyzed longitudinal PROM data in patients with stage III NSCLC [49]. In this research, 80% of the patients received curative-intent radiotherapy, whilst the other patients received palliative SACT or best supportive care. To ensure patient scores were not influenced by the scan results, they were asked to complete questionnaires prior to their clinic consultations. There was a significant deterioration in PROM scores at the time of cancer progression. The results suggested that PROMs could have a role in identifying patients at risk of progressing prior to being confirmed on imaging.
Prospective exploratory data that describes the patient experience during and after radiotherapy (with or without SACT) is now required. This qualitative data would play a crucial role in improving treatment decision-making by describing the patient experience during different treatments. In addition, it could be used to predict clinical outcomes and enhance follow-up protocols. Additional research is required to assess the optimum timing and method of PROM collection and to ensure they are not overly burdensome for patients.
2. Materials and Methods
Study design and setting: The VIGILANCE study is a single-center, prospective, longitudinal, follow-up study that is taking place at The Christie NHS Foundation Trust, Manchester. It was developed by a multidisciplinary group from the Manchester Cancer Research Center (MCRC) and the National Cancer Biomarker Center, University of Manchester. The group includes oncologists, molecular biologists, biostatisticians, bio-informaticians and public–patient representatives. Primary funding is provided through the CRUK Clinical Academic Training Program within the MCRC. Additional funding has been secured from a Rosetrees Trust Intermediate Project Award and from AstraZeneca.
Research aims and objectives: The VIGILANCE study has several exploratory aims, and these are summarized in Table A2. Core aims include describing how PROs, imaging and ctDNA features assessed using different health technologies change during and after radiotherapy. Additional aims include assessing whether these features could be used as a biomarker to predict prognosis, treatment response and sensitivity to consolidation durvalumab. Based on these aims, the core research objectives include, but are not limited to, the following:
- To describe ctDNA concentration and gene mutations at baseline, during and up to 1 year following the completion of radiotherapy.
- To describe changes in radiomic features at baseline, during and up to 1 year following the completion of radiotherapy.
- To describe changes in PROMs at baseline, during and up to 1 year following the completion of radiotherapy.
- To describe the associations between features and changes in features over time, e.g., the association of radiomic features with changes in ctDNA concentration.
- To develop a predictive model using baseline and longitudinal ctDNA and radiomic features to predict benefit from consolidation durvalumab.
- To develop a prognostic model using baseline and longitudinal ctDNA data, radiomic features and PROMs to predict survival and tumor control rates.
Study population: The target population is patients diagnosed with stage III NSCLC undergoing curative-intent radiotherapy. This includes patients receiving radiotherapy alone, sequential CRT (SCRT) and CCRT with or without consolidation durvalumab. This study recruited 60 patients over an 18-month recruitment period. Eligibility criteria are summarized in Table 3.

Table 3.
Eligibility criteria for the VIGILANCE study.
Recruitment and enrolment: Patients suitable for entry into this study will be identified by screening clinic and treatment lists. The recruitment period spans from March 2023 to October 2024. Eligible patients will be provided with a patient information sheet and given at least 24 h to consider participation prior to consenting. Each patient will be followed for one year after the completion of radiotherapy, with a projected study end date of September 2028.
Patient and public involvement: A patient and public involvement (PPI) representative was involved throughout the design of this study. They were involved in helping to write and review the study protocol, funding application to the Rosetrees Trust and patient-facing documents. Their insight was invaluable in ensuring this study was of interest and acceptable to patients. They provided feedback on the number and timing of blood tests, as well as the need for any additional hospital visits. Their support will also be sought during the study write-up and in the preparation of the lay summary.
Standard-of-care treatment: All treatments delivered are SOC, and patients participating in interventional studies are ineligible.
Radiotherapy: Radiotherapy treatments are planned with four-dimensional CT (4DCT) scans with intravenous contrast. Patients with tumors located superior to the aortic arch will be scanned with their arms by their side in a 5-point thermoplastic immobilization shell. Patients with tumors below the aortic arch will be scanned with their arms above their head using an external immobilization device. Tumor and organ at risk contouring will be performed by a trained radiation oncologist as per local protocol and International Commission on Radiation Units and Measurements (ICRU) 62 guidance [50]. A motion-adapted gross tumor volume (maGTV) will be outlined using the maximum intensity projection (MIP) and checked on the different phases of the 4DCT. maGTV will include primary tumor and involved lymph nodes (those considered involved on diagnostic CT, PET-CT or biopsy/cytology). A 5 mm isotropic margin will be added to the maGTV to create the internal target volume (ITV), and an additional 5 mm isotropic margin will expand the ITV to form the planned treatment volume (PTV).
Treatment is delivered using intensity-modulated radiotherapy (IMRT) or volumetric-modulated arc therapy (VMAT) and image guidance provided by daily cone-beam CT scans. Radiotherapy prescriptions are based on guidelines from the EORTC and Royal College of Radiologists [51,52]. Patients treated with radical radiotherapy alone or SCRT commonly receive a hypofractionated regimen of 55 Gray in 20 daily fractions over 4 weeks. For those undergoing CCRT, treatment typically consists of 60–66 Gray in 30–33 daily fractions over 6 to 6.5 weeks.
Systemic anti-cancer therapies: Patients who are assessed as suitable for chemotherapy will receive sequential or concurrent treatment. For sequential treatment, up to 4 cycles of platinum-doublet chemotherapy are offered prior to radiotherapy. For concurrent treatment, 2 cycles of cisplatin and etoposide are offered unless there is contraindication to cisplatin. In such a situation, weekly carboplatin and paclitaxel is offered. Patients receiving concurrent therapy are considered for consolidation durvalumab if their tumor has a PD-L1 expression >1% (or PD-L1 unknown), ECOG PS is 0–1 and end of radiotherapy CT scan shows no evidence of progression.
Study and imaging data collection: Table 4 summarizes the baseline and longitudinal data that will be collected. The Clinical Outcomes and Data Unit will export study data from the hospital system where it is collected as part of routine care. The sources of data include the patient administration system (Careflow), the electronic patient record (EPR) (in-house system using standardized e-forms to collect structured information), the radiotherapy oncology information system (Mosaiq) and the electronic SACT prescribing system (iQemo). Investment in organizing and structuring healthcare data facilitates highly practical approaches to research data collection and transfer, enabling clinical studies to proceed efficiently. The study data is collected as per the trial scheme (Figure 2). All data is anonymized to a trial ID at the point of extraction to ensure confidentiality. Data quality and completeness will be assessed via the manual curation of a subset of patients.

Table 4.
A table summarizing the data that will be collected during the VIGILANCE study.
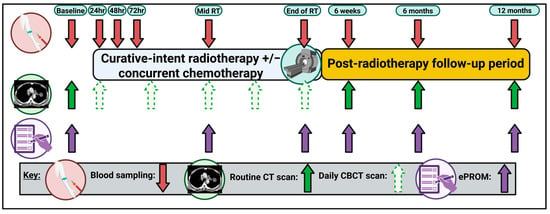
Figure 2.
The VIGILANCE study schema. hr, hour; CT, computerized tomography, CBCT; cone-beam computerized tomography; ePROM, electronic patient-reported outcome measure. Created in BioRender. Horne, A. (2025) https://BioRender.com/s5khurc (accessed on 7 November 2025).
Blood samples for ctDNA analysis are taken concurrently with SOC blood tests whenever possible, aiming to minimize patient discomfort and reduce hospital visits. Samples are collected in accordance with local procedures, policies, protocols and Good Clinical Practice (GCP) standards and are stored on site at the biomarker center until analysis.
ePROMs will be acquired using the existing MyChristie-MyHealth platform that is routinely used for all patients treated at the hospital [53]. Patients receive a text message or email prompt to complete a questionnaire that contains questions relevant to their tumor type and treatment. Patients can complete the questionnaire at home using a smart device or computer. It takes approximately 10 min to complete the questionnaire. The completed questionnaires are automatically filed in the EPR, allowing the clinical team to easily review them. This study uses the EuroQol-5D and adapted-REQUITE lung questionnaires to record patient functional status, symptoms and QOL [54]. As previously described, the EuroQol-5D questionnaire is used for economic evaluation. The adapted-REQUITE questionnaire demonstrates improved performance and stronger patient preference when compared to similar questionnaires [54]. Support for completing the form is available from the study team and research radiographers. Paper questionnaires are available for patients who are unable to engage with technology.
Imaging data is accessed via Query-Retrieve to the Greater Manchester Picture Archiving and Communications system (PACS), with radiotherapy planning and image guidance data exported by the hospital research radiographer team. All imaging data is stored in standard DICOM/DICOM-RT format with anonymization performed automatically via the Conquest DICOM version 2.0.0 open-source package [55]. Radiomic features will be extracted from images using PyRadiomics version 3.1.0rc2.post5, an open-source IBSI-compliant software package. The primary tumor and any nodal metastases involved will be analyzed as separate regions of interest. Texture-based features (e.g., homogeneity, correlation and joint entropy of the gray-level co-occurrence matrix), intensity-based features (e.g., entropy and energy) and spatial-based features (e.g., surface area, volume, surface-to-volume ratio and sphericity) will be extracted. The radiomic analysis will follow a previously described pipeline that consolidates guidance from the IBSI and RQS to ensure transparency and best practice [41].
Statistical analysis plan: PFS will be defined as the time from the start of radiotherapy to the date of progression or death. It will be calculated using the Kaplan–Meier method, and patients will be censored without progression at the time of the last imaging during follow-up. OS will be similarly defined from the start of radiotherapy to the date of death. Tumor control rates will be calculated at annual intervals and will be defined as the proportion of patients without evidence of disease progression at 12, 24, 36 months, etc., after the start of radiotherapy.
The demographic, clinical and experimental data will be analyzed to address the study objectives (see Research aims and objectives section within 2. Materials and Methods). Patients will be assessed as one cohort and as groups separated by the treatment received. First, a descriptive analysis to summarize baseline demographic and clinical characteristics and missing data will be performed. Secondly, longitudinal trends in ctDNA, radiomic features and ePROM data over the study period will be described. This includes summarizing the data graphically using spaghetti and line plots. Given the ordinal nature of the ePROM data, descriptive summaries will present the frequency and proportion of patients selecting each response at each timepoint. A mixed-effect model analysis will be performed to allow for fixed effect variables, such as time and treatment, to be assessed alongside categorical random effects, such as patient factors. Additionally, a generalized estimating equation will be used to summarize correlations in features across different timepoints. Time-to-event analysis, using the Cox proportional hazards model, will be performed to calculate the association between longitudinal changes in features, such as changes in ctDNA concentration, and outcome [56]. Similarly, time-to-deterioration analysis will be applied to the ePROM data [57]. Outcomes include OS, time to treatment failure and benefit from consolidation durvalumab.
Other analyses will assess correlations between continuous features with scatter plots and Pearson’s coefficient, while categorical associations will be assessed with Chi-squared or Fisher’s exact tests. In addition, we will explore a joint longitudinal time-to-event analysis, which will reduce bias in parameter estimation and increase the efficiency of statistical inference, as recommended by the Journal of Clinical Oncology’s statistical team [58]. Finally, we will perform a two-stage analysis of all pre-treatment markers (radiomics, ePROMs, ctDNA, maGTV, etc.) for their association with the outcome of interest, such as tumor control or PFS. Due to the large number of variables, a combination of dimensional reduction and regression techniques (e.g., LASSO) will be applied to reduce redundancy and identify robust predictors. Subsequently, univariate analysis will identify candidate variables which will then be entered into multivariate Cox models for final selection.
As this is a single-center, prospective study, we expect a low proportion of missing biomarker data. The type and extent of missing data will be recorded and described. Different imputation methods, such as last observation carried forward, will be considered to address gaps in the biomarker data.
Study ethics and patient safety: The VIGILANCE study was approved by the Northwest—Preston Research Ethics Committee on 21 October 2022 (IRAS Project ID 277065; Protocol Number CCR5165; REC Reference 20/EE/0155). The University of Manchester is the study sponsor and has organized indemnification. This study is being conducted in accordance with GCP and the Declaration of Helsinki regulations. In addition, it is registered on the US National Library of Medicine study database (ClinicalTrials.gov NCT:06086574) and receives support from the NIHR Clinical Research Network (CPMS 54111).
Given that patients receive SOC treatment, there are minimal risks to patients who participate. There is a measured risk of causing self-limiting bruises and discomfort from blood withdrawal. This small risk will be reduced by combining blood withdrawal for study bloods with SOC bloods where possible. There is no additional radiation exposure to justify as all imaging and radiotherapy treatments are SOC. The additional PROMs performed during this study do not pose any risk to the patients. Adverse events will be recorded and reported in accordance with local standard operating procedures.
Genetic analyses of tumor material that identifies a clinically significant result will be recorded in the patients’ notes and discussed with the patients’ primary oncologist. This is to ensure its relevancy is considered and that there is a plan to discuss the result with the patient.
Dissemination and open science: Both positive and negative results will be disseminated to the scientific community. This will include presentations at local, national and international conferences and manuscripts in high-impact, open access, peer-reviewed journals. To maximize impact, PPI support will be sought to ensure data is understandable and relevant. Anonymized datasets will be made available in public databases and repositories. This aligns this research with key principles of open science, promotes information sharing and supports the development of collaborative networks. It also promotes a culture of research transparency by allowing our methods and results to be validated and scrutinized by other research groups.
3. Discussion
Presently, radiotherapy-based treatments for patients with stage III NSCLC follow a largely standardized approach, resulting in unacceptably poor clinical outcomes. The VIGILANCE study is designed to address this critical gap by prospectively evaluating novel biomarker technologies that could be transformative for patient care. This study explores several scenarios with direct clinical implications.
A primary question is how ctDNA status should influence initial management. Emerging data suggests that a high baseline ctDNA burden is a negative prognostic factor (REF). If VIGILANCE supports this, baseline ctDNA could be used to risk-stratify patients. Those with high burden could be considered for more aggressive or novel treatment approaches, such as combination immunotherapy or targeted agents, to target potential micro-metastatic disease. Conversely, patients with low burden could represent a more favorable subgroup for which standard treatments are sufficient. This hypothesis is being tested in interventional trials like the APPROACH trial [29]. VIGILANCE will provide crucial real-world data to complement these randomized trials and inform strategies in a broader population.
Additionally, the study design, with deliberate frequent blood sampling during the first days of radiotherapy, will capture early ctDNA dynamics. A transient rise in ctDNA could reflect early radiation-induced cell death and be an indicator of tumor radiosensitivity [23,24,25]. For example, if an early decrease in ctDNA correlates with improved outcomes, it could serve as an early on-treatment response biomarker. This would allow for the novel possibility of response-adapted radiotherapy. For instance, patients showing rapid ctDNA clearance might be candidates for radiotherapy de-escalation to reduce toxicity, while those with a rising or persistent ctDNA signal could be identified early for treatment intensification.
The results could also be used to guide consolidation immunotherapy and identify patients who do not benefit from it. Retrospective data strongly suggest that post-radiotherapy ctDNA status is a predictive biomarker for durvalumab benefit [21,22]. VIGILANCE aims to prospectively validate this and inform future trials that assess whether consolidation durvalumab can be omitted in patients with undetectable ctDNA after chemoradiation. This would spare a subset of patients from immune-related toxicity and reduce healthcare costs. Conversely, patients with persistent post-radiotherapy ctDNA (indicating MRD) could be directed towards trials of novel consolidation treatments.
Beyond ctDNA, VIGILANCE integrates ctDNA analysis with radiomic features and PROM data, a combination that distinguishes it from other studies in the field. A robust signature built from these biomarkers that can reliably differentiate evolving radiation fibrosis from local recurrence would reduce patient anxiety and the need for invasive biopsies and intensive imaging schedules. Similarly, if early deterioration in specific ePROM domains predicts progression, as suggested previously [46], it could trigger earlier intervention and improved outcomes.
A key feature of this study is its pragmatic design, which includes patients treated with different radiotherapy fractionation schedules. This provides an opportunity for patients commonly excluded from clinical research to participate, enhancing the real-world applicability of the findings. However, we acknowledge that this, alongside the modest sample size inherent to an exploratory study, introduces heterogeneity and increases the risk of overfitting, limiting statistical power. Therefore, all associations between biomarkers will be considered exploratory, and any results will be treated as hypothesis-generating. Careful interpretation, including stratified analyses where feasible, will be employed to account for potential confounding.
Despite these limitations, the comparison of different biomarkers, such as correlations between ctDNA and radiomic features, will be crucial in understanding potential synergies and their association with clinical outcomes. Given the novelty and different costs associated with biomarker technologies, it is important to assess how different biomarkers can complement each other to improve performance whilst remaining cost-effective. The findings from VIGILANCE will directly inform the design of a subsequent, multi-site validation study, which will form part of a complementary PhD project. This future study will be appropriately powered to assess clinical utility and will be built upon a patient-centered approach with PPI input as its backbone.
Collaboration and multidisciplinary cooperation are central to the VIGILANCE study, offering opportunities for other research groups to access the dataset for the validation of their own biomarker research. Ultimately, the results are expected to significantly contribute to biomarker research and inform the design of future patient-centered validation and interventional studies.
Author Contributions
Conceptualization, A.H. and C.F.-F.; methodology, A.H., A.P., H.C., C.D., H.M., G.P. and C.F.-F.; writing—original draft preparation, A.H.; writing—review and editing, A.H., A.P., H.C., C.D., H.M., G.P. and C.F.-F.; visualization, A.H.; supervision, C.F.-F.; project administration, C.D.; funding acquisition, A.H., C.D., G.P. and C.F.-F. All authors have read and agreed to the published version of the manuscript.
Funding
Ashley Horne is supported by Cancer Research UK through funding to the Cancer Research UK Manchester Center [C147/A25254] and their Clinical Academic Training Program [C19941/A28707]. Funding for analysis was secured from a Rosetrees Trust Intermediate Project Award [PGS22/100103] and from AstraZeneca. Professor Faivre-Finn is supported by NIHR Manchester Biomedical Research Center and the CRUK Manchester Major Center.
Institutional Review Board Statement
Ethics approval was granted by the National Health Service Health Research Authority on 15 June 2020—IRAS Project ID 277065, Protocol Number CCR5165, REC Reference 20/EE/0155.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Both positive and negative results will be disseminated to the scientific community. This will include presentations at local, national and international conferences and manuscripts in high-impact, open access, peer-reviewed journals. To maximize impact, PPI support will be sought to ensure data is understandable and relevant. Anonymized datasets will be made available in public databases and repositories. This aligns this research with key principles of open science, promotes information sharing and supports the development of collaborative networks. It also promotes a culture of research transparency by allowing our methods and results to be validated and scrutinized by other research groups.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BEAMing | Beads, emulsion, amplification and magnetics |
| Capp-SEQ | Cancer personalized profiling by deep sequencing |
| CBCT | Cone-beam CT |
| CCRT | Concurrent chemoradiotherapy |
| cfDNA | Cell-free DNA |
| Core QOL-30 | QLQ-C30 questionnaire |
| CRT | Chemoradiotherapy |
| CRUK | Cancer Research United Kingdom |
| CT | Computed tomography |
| CTCAE | Common Terminology Criteria for Adverse Events |
| ctDNA | Circulating tumor DNA |
| ECOG | Eastern Cooperative Oncology Group |
| EDTA | Ethylenediaminetetraacetic acid |
| EGFR | Epidermal growth factor receptor |
| EGFRm | Epidermal growth factor receptor-mutant |
| EMA | European Medicines Agency |
| EORTC | European Organization for Research and Treatment of Cancer |
| EPR | Electronic patient record |
| ePROMs | Electronic PROMs |
| ESMO | European Society for Medical Oncology |
| FDA | Food and Drug Administration |
| GCP | Good clinical practice |
| IBSI | Image Biomarker Standardization Initiative |
| ICRU | International Commission on Radiation Units and Measurements |
| IMRT | Intensity-modulated radiotherapy |
| ITV | Internal target volume |
| LC-13 | Lung cancer thirteen questionnaire |
| maGTV | Motion-adapted gross tumor volume |
| MCRC | Manchester Cancer Research Center |
| MIP | Maximum intensity projection |
| MRD | Minimal residual disease |
| MRI | Magnetic resonance imaging |
| NICE | National Institute for Health and Care Excellence |
| NSCLC | Non-small-cell lung cancer |
| OS | Overall survival |
| PACS | Picture Archiving and Communications system |
| PCR | Polymerase chain reaction |
| PD-L1 | Programmed death-ligand one |
| PET-CT | Positron emission tomography–CT |
| PFS | Progression-free survival |
| PPI | Patient and public involvement |
| PROMs | Patient-reported outcome measures |
| PS | Performance status |
| PTV | Planned treatment volume |
| QALY | Quality-adjusted life year |
| QOL | Quality of life |
| RQS | Radiomic quality score |
| SABR | Stereotactic ablative radiotherapy |
| SACT | Systemic anti-cancer therapy |
| SCLC | Small-cell lung cancer |
| SCRT | Sequential chemoradiotherapy |
| SOC | Standard of care |
| SUV | Maximum standardized uptake value |
| TKI | Tyrosine kinase inhibitor |
| TNM | Tumor, node and metastasis |
| VIGILANCE | Developing Circulating and Imaging Biomarkers Towards Personalized Radiotherapy in Lung Cancer |
| VMAT | Volumetric-modulated arc therapy |
| 4DCT | Four-dimensional CT |
Appendix A

Table A1.
Biomarker definitions.
Table A1.
Biomarker definitions.
| Term | Definition |
|---|---|
| General terms: | |
| Biomarker | A measurable feature or process that can identify cancer or describe cancer behavior or processes. Traditionally, biomarkers were molecular or histological in origin. The definition has expanded to include other health technologies such as the radiography of physiological features. |
| Personalized medicine | The ability to provide tailor-made medical decisions and interventions to an individual patient. |
| Predictive biomarker | A feature that describes the probability that a patient will respond to an intervention, e.g., it predicts whether a patient will respond to consolidation immunotherapy. |
| Prognostic biomarker | A feature that describes the probability of a patient outcome, e.g., disease recurrence or overall survival. |
| Genomic terms: | |
| Circulating tumor cell (CTC) | Tumor cells isolated from blood. These cells can be analyzed for protein expression and enzyme function, and genetic material can be sequenced. |
| Circulating-free DNA (cfDNA) | DNA fragments found in blood or tissue fluid. They can originate from any cells within the body and can be idiopathic or pathological. DNA can be quantified and sequenced. |
| Circulating tumor DNA (ctDNA) | Tumor-originating DNA fragments found in blood or tissue fluid. DNA can be quantified and sequenced. Targeted and non-targeted approaches have been described and include the following: Targeted:
|
| DNA methylation | An epigenetic DNA change involving a methyl group attaching to the C-5 position of a cytosine ring. In cancer, abnormal hypermethylation is identified in gene promoter CpG islands and is recognized as a component of cancer development. |
| Driver mutation | A genetic alteration within a cancer cell that results in a growth advantage or promotes tumor formation. |
| Epigenetic alteration | An alteration to the DNA structure that does not involve a change in the nucleotide sequence. The main types are DNA methylation, histone modification and noncoding RNA action. |
| Genomic analysis | An analysis of DNA and genes to understand cancer development and evolution |
| Liquid biopsy | An analysis of tumor material isolated from blood or tissue fluid. Material includes circulating tumor DNA, circulating tumor cells and tumor extracellular vesicles. |
| Minimal residual disease (MRD) | Describes residual tumor cells or material following curative treatment. Residual disease may not be evident on imaging. |
| Tumor extracellular vesicles | Lipid bilayer particles released from tumor cells. They are involved in intercellular communication, including tumor cell growth and metastatic potential. |
| Tumor programmed death-ligand 1 (PD-L1) | A protein expressed in some tumors that inhibits the anti-tumor effect of T-cells. It is used as a biomarker for immunotherapy sensitivity. |
| Whole genome sequencing (WGS) | An analysis of the whole genome that describes the order of nucleotides. |
| Whole exome sequencing (WES) | An analysis of the protein-coding regions of the genome following the removal of noncoding introns. |
| Imaging-based terms: | |
| Cone-beam computed tomography (CBCT) scan | A medical imaging technique that is used for radiotherapy treatment positioning verification. |
| Quantitative imaging features | Measurable features, such as tumor dimension or volume, assessed on a scan. |
| Radiomics | A medical imaging technique that uses data characterization algorithms to extract many quantitative imaging features from a scan. These features can then be correlated with a biological feature or clinical outcome. The analysis of serial scans over multiple timepoints is known as delta-radiomics. |
| Qualitative imaging features | Descriptive features, such as pleural wall attachment or the presence of fibrosis, assessed on a patient’s imaging. They are otherwise known as semantic features. |
| 4-dimensional (4D)-CT scan | A method of CT imaging acquisition that captures the internal movement of a tumor and organs, such as due to patient breathing. |
| Patient-reported terms: | |
| Electronic patient-reported outcome measure (ePROM) | The electronic version of a PROM (see below) that is completed electronically, such as on a mobile device or tablet or online. |
| Patient-reported outcome (PRO) | A health outcome reported by a patient. It can include their experience of their own health, quality of life (QOL), symptoms and functional status. |
| Patient-reported outcome measure (PROM) | A standardized questionnaire completed by patients to record their health status, QOL, symptoms and functional status. |
DNA, deoxyribonucleic acid; PCR, polymerase chain reaction; RNA, ribonucleic acid; CT, computerized tomography.

Table A2.
Knowledge gaps and unmet needs with candidate biomarkers for the VIGILANCE study.
Table A2.
Knowledge gaps and unmet needs with candidate biomarkers for the VIGILANCE study.
| Biomarker Technology | Knowledge Gaps and Unmet Needs in Patients with NSCLC Treated with Radiotherapy +/− Systemic Therapy |
|---|---|
| ctDNA |
|
| Radiomics |
|
| PROMs |
|
NSCLC, non-small-cell lung cancer; ctDNA, circulating tumor DNA; PFS, progression-free survival; OS, overall survival; PROM, patient-reported outcome measure; CBCT, cone-beam CT scan; QOL, quality of life.
References
- International Agency for Research on Cancer. Lung Cancer Fact Sheet; World Health Organisation: Geneva, Switzerland, 2020; Available online: https://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf (accessed on 16 November 2021).
- Luengo-Fernandez, R.; Leal, J.; Gray, A.; Sullivan, R. Economic burden of cancer across the European Union: A population-based cost analysis. Lancet Oncol. 2013, 14, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Navani, N.; Conibear, J.; West, D. State of the Nation Report 2024; National Lung Cancer Audit: London, UK, 2024. [Google Scholar]
- Kalemkerian, G.P.; Donington, J.S.; Gore, E.M.; Ramalingam, S.S. (Eds.) TNM Classification for Lung Cancer. In Handbook of Lung Cancer and Other Thoracic Malignancies; Springer Publishing Company: New York, NY, USA, 2016; Available online: http://connect.springerpub.com/lookup/doi/10.1891/9781617052729.ap01 (accessed on 1 December 2021).
- Cancer Survival in England—Adults Diagnosed. Office for National Statistics. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/cancersurvivalratescancersurvivalinenglandadultsdiagnosed (accessed on 17 November 2021).
- Gillan, C.; Briggs, K.; Pazos, A.G.; Maurus, M.; Harnett, N.; Catton, P.; Wiljer, D. Barriers to accessing radiation therapy in Canada: A systematic review. Radiat. Oncol. Lond. Engl. 2012, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Jack, R.H.; Gulliford, M.C.; Ferguson, J.; Møller, H. Geographical inequalities in lung cancer management and survival in South East England: Evidence of variation in access to oncology services? Br. J. Cancer 2003, 88, 1025–1031. [Google Scholar] [CrossRef]
- Zer, A.; Ahn, M.-J.; Barlesi, F.; Bubendorf, L.; Ruysscher, D.D.; Garrido, P.; Gautschi, O.; Hendriks, L.E.; Jänne, P.A.; Kerr, K.M.; et al. Early and locally advanced non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2025, 36, 1245–1262. [Google Scholar] [CrossRef] [PubMed]
- Overview. Durvalumab for Treating Locally Advanced Unresectable Non-Small-Cell Lung Cancer After Platinum-Based Chemoradiation. Guidance. NICE. Available online: https://www.nice.org.uk/guidance/ta578 (accessed on 30 December 2021).
- Horne, A.; Harada, K.; Brown, K.D.; Chua, K.L.M.; McDonald, F.; Price, G.; Putora, P.M.; Rothwell, D.G.; Faivre-Finn, C. Treatment Response Biomarkers: Working Toward Personalized Radiotherapy for Lung Cancer. J. Thorac. Oncol. 2024, 19, 1164–1185. [Google Scholar] [CrossRef]
- O’Connor, J.P.B.; Aboagye, E.O.; Adams, J.E.; Aerts, H.J.W.L.; Barrington, S.F.; Beer, A.J.; Boellaard, R.; Bohndiek, S.E.; Brady, M.; Brown, G.; et al. Imaging biomarker roadmap for cancer studies. Nat. Rev. Clin. Oncol. 2017, 14, 169–186. [Google Scholar] [CrossRef]
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 339–357. [Google Scholar] [CrossRef]
- European Medicines Agency. Durvalumab European Public Assessment Report; European Medicine Agency: Amsterdam, The Netherlands, 2023; Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/imfinzi (accessed on 1 August 2023).
- US Food and Drug Administration. FDA Approves Durvalumab After Chemoradiation for Unresectable Stage III NSCLC; US Food and Drug Administration: Long Beach, CA, USA, 2018. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-durvalumab-after-chemoradiation-unresectable-stage-iii-nsclc (accessed on 1 August 2023).
- Peters, S.; Dafni, U.; Boyer, M.; De Ruysscher, D.; Faivre-Finn, C.; Felip, E.; Garrido, P.; Girard, N.; Guckenberger, M.; Haanen, J.; et al. Position of a panel of international lung cancer experts on the approval decision for use of durvalumab in stage III non-small-cell lung cancer (NSCLC) by the Committee for Medicinal Products for Human Use (CHMP). Ann. Oncol. 2019, 30, 161–165. [Google Scholar] [CrossRef]
- Passaro, A.; Leighl, N.; Blackhall, F.; Popat, S.; Kerr, K.; Ahn, M.J.; Arcila, M.E.; Arrieta, O.; Planchard, D.; de Marinis, F.; et al. ESMO expert consensus statements on the management of EGFR mutant non-small-cell lung cancer. Ann. Oncol. 2022, 33, 466–487. [Google Scholar] [CrossRef]
- Lu, S.; Kato, T.; Dong, X.; Ahn, M.-J.; Quang, L.-V.; Soparattanapaisarn, N.; Inoue, T.; Wang, C.-L.; Huang, M.; Yang, J.C.-H. Osimertinib after Chemoradiotherapy in Stage III EGFR-Mutated NSCLC. N. Engl. J. Med. 2024, 391, 585–597. [Google Scholar] [CrossRef]
- Zhong, R.; Gao, R.; Fu, W.; Li, C.; Huo, Z.; Gao, Y.; Lu, Y.; Li, F.; Ge, F.; Tu, H.; et al. Accuracy of minimal residual disease detection by circulating tumor DNA profiling in lung cancer: A meta-analysis. BMC Med. 2023, 21, 180. [Google Scholar] [CrossRef]
- Pellini, B.; Chaudhuri, A.A. Circulating Tumor DNA Minimal Residual Disease Detection of Non–Small-Cell Lung Cancer Treated With Curative Intent. J. Clin. Oncol. 2022, 40, 567–575. [Google Scholar] [CrossRef]
- Chaudhuri, A.A.; Chabon, J.J.; Lovejoy, A.F.; Newman, A.M.; Stehr, H.; Azad, T.D.; Khodadoust, M.S.; Esfahani, M.S.; Liu, C.L.; Zhou, L.; et al. Early Detection of Molecular Residual Disease in Localized Lung Cancer by Circulating Tumor DNA Profiling. Cancer Discov. 2017, 7, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Moding, E.J.; Liu, Y.; Nabet, B.Y.; Chabon, J.J.; Chaudhuri, A.A.; Hui, A.B.; Bonilla, R.F.; Ko, R.B.; Yoo, C.H.; Gojenola, L.; et al. Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small-cell lung cancer. Nat. Cancer 2020, 1, 176–183. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, J.-T.; Gao, X.; Chen, Z.-Y.; Yan, B.; Tan, P.-X.; Yang, X.-R.; Gao, W.; Gong, Y.; Tian, Z.; et al. Dynamic circulating tumor DNA during chemoradiotherapy predicts clinical outcomes for locally advanced non-small cell lung cancer patients. Cancer Cell 2023, 41, 1763–1773.e4. [Google Scholar] [CrossRef] [PubMed]
- Nygård, L.; Ahlborn, L.B.; Persson, G.F.; Chandrananda, D.; Langer, J.W.; Fischer, B.M.; Langer, S.W.; Gabrielaite, M.; Kjær, A.; Rosenfeld, N.; et al. Circulating cell free DNA during definitive chemo-radiotherapy in non-small cell lung cancer patients—Initial observations. PLoS ONE 2020, 15, e0231884. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.M.; Kim, Y.J.; Lee, H.Y.; Choi, C.; Ahn, W.-G.; Lee, T.; Pyo, H.; Park, J.H.; Park, D.; Park, W.-Y. Targeted Liquid Biopsy Using Irradiation to Facilitate the Release of Cell-Free DNA from a Spatially Aimed Tumor Tissue. Cancer Res. Treat. 2021, 54, 40–53. [Google Scholar] [CrossRef]
- Walls, G.M.; McConnell, L.; McAleese, J.; Murray, P.; Lynch, T.B.; Savage, K.; Hanna, G.G.; de Castro, D.G. Early circulating tumour DNA kinetics measured by ultra-deep next-generation sequencing during radical radiotherapy for non-small cell lung cancer: A feasibility study. Radiat. Oncol. 2020, 15, 132. [Google Scholar] [CrossRef]
- MacManus, M.; Kirby, L.; Blyth, B.; Banks, O.; Martin, O.A.; Yeung, M.M.; Plumridge, N.; Shaw, M.; Hegi-Johnson, F.; Siva, S.; et al. Early circulating tumor DNA dynamics at the commencement of curative-intent radiotherapy or chemoradiotherapy for NSCLC. Clin. Transl. Radiat. Oncol. 2023, 43, 100682. [Google Scholar] [CrossRef]
- Lawson Health Research Institute. Detection of Circulating Tumor DNA After Stereotactic Ablative Radiotherapy in Patients with Unbiopsied Lung Tumors; ClinicalTrials.gov: Bethesda, MD, USA, 2023; Report No.: NCT05921474. Available online: https://clinicaltrials.gov/study/NCT05921474 (accessed on 1 January 2024).
- Mohamed, I. A Phase II Study of Circulating Tumor DNA Directed Consolidation Durvalumab (MEDI4736) Following Induction and Concurrent Durvalumab with SABR for Stage I NSCLC. SCION: SABR and Checkpoint Inhibition of NSCLC; ClinicalTrials.gov: Bethesda, MD, USA, 2021; Report No.: NCT04944173. Available online: https://clinicaltrials.gov/ct2/show/NCT04944173 (accessed on 30 March 2023).
- Guangdong Association of Clinical Trials. ctDNA to Guide Treatment Decisions After Almonertinib Induction Therapy for EGFR-Mutation-Positive Unresectable Stage III Non-Small Cell Lung Cancer in the MDT Diagnostic Model: An Open, Multicenter, Phase II Clinical Study; ClinicalTrials.gov: Bethesda, MD, USA, 2021; Report No.: NCT04841811. Available online: https://clinicaltrials.gov/ct2/show/NCT04841811 (accessed on 30 March 2023).
- Study Details. Adjuvant ctDNA-Adapted Personalized Treatment in Early Stage NSCLC (ADAPT-E); ClinicalTrials.gov: Bethesda, MD, USA, 2021; Report No.: NCT04585477. Available online: https://clinicaltrials.gov/study/NCT04585477 (accessed on 10 July 2024).
- Study Details. Personalized Escalation of Consolidation Treatment Following Chemoradiotherapy and Immunotherapy in Stage III NSCLC in Stage III NSCLC; ClinicalTrials.gov: Bethesda, MD, USA, 2021; Report No.: NCT04585490. Available online: https://clinicaltrials.gov/study/NCT04585490 (accessed on 10 July 2024).
- Keller, L.; Belloum, Y.; Wikman, H.; Pantel, K. Clinical relevance of blood-based ctDNA analysis: Mutation detection and beyond. Br. J. Cancer 2021, 124, 345–358. [Google Scholar] [CrossRef]
- Liang, R.; Li, X.; Li, W.; Zhu, X.; Li, C. DNA methylation in lung cancer patients: Opening a “window of life” under precision medicine. Biomed. Pharmacother. 2021, 144, 112202. [Google Scholar] [CrossRef]
- Chemi, F.; Pearce, S.P.; Clipson, A.; Hill, S.M.; Conway, A.-M.; Richardson, S.A.; Kamieniecka, K.; Caeser, R.; White, D.J.; Mohan, S.; et al. cfDNA methylome profiling for detection and subtyping of small cell lung cancers. Nat. Cancer 2022, 3, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- A cfDNA Methylation-Based Tissue-of-Origin Classifier for Cancers of Unknown Primary. Nature Communications. Available online: https://www.nature.com/articles/s41467-024-47195-7 (accessed on 22 August 2024).
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Walls, G.M.; Osman, S.O.S.; Brown, K.H.; Butterworth, K.T.; Hanna, G.G.; Hounsell, A.R.; McGarry, C.K.; Leijenaar, R.T.H.; Lambin, P.; Cole, A.J.; et al. Radiomics for Predicting Lung Cancer Outcomes Following Radiotherapy: A Systematic Review. Clin. Oncol. 2021, 34, E107–E122. [Google Scholar] [CrossRef]
- Fornacon-Wood, I.; Faivre-Finn, C.; O’Connor, J.P.B.; Price, G.J. Radiomics as a personalized medicine tool in lung cancer: Separating the hope from the hype. Lung Cancer 2020, 146, 197–208. [Google Scholar] [CrossRef]
- Kothari, G.; Korte, J.; Lehrer, E.J.; Zaorsky, N.G.; Lazarakis, S.; Kron, T.; Hardcastle, N.; Siva, S. A systematic review and meta-analysis of the prognostic value of radiomics based models in non-small cell lung cancer treated with curative radiotherapy. Radiother. Oncol. 2021, 155, 188–203. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Horne, A.; Abravan, A.; Fornacon-Wood, I.; O’Connor, J.P.B.; Price, G.; McWilliam, A.; Faivre-Finn, C. Mastering CT-based radiomic research in lung cancer: A practical guide from study design to critical appraisal. Br. J. Radiol. 2025, 98, 653–668. [Google Scholar] [CrossRef]
- Cui, S.; Ten Haken, R.K.; El Naqa, I. Integrating Multiomics Information in Deep Learning Architectures for Joint Actuarial Outcome Prediction in Non-Small Cell Lung Cancer Patients After Radiation Therapy. Int. J. Radiat. Oncol. 2021, 110, 893–904. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. JNCI J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Fayers, P.; Aaronson, N.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A. The EORTC QLQ-C30 Scoring Manual, 3rd ed.; European Organisation for Research and Treatment of Cancer: Brussels, Belgium, 2001. [Google Scholar]
- Balestroni, G.; Bertolotti, G. EuroQol-5D (EQ-5D): An instrument for measuring quality of life. Monaldi Arch. Chest Dis. Arch. Monaldi Mal. Torace 2012, 78, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Winstanley, J.; Kenny, P.; Viney, R.; Zapart, S.; Boyer, M. Validity, Reliability and Responsiveness of the EORTC QLQ-C30 and the EORTC QLQ-LC13 in Australians with Early Stage Non-Small Cell Lung Cancer. 2008. Available online: https://www.semanticscholar.org/paper/Validity%2C-reliability-and-responsiveness-of-the-and-Winstanley-Kenny/5ee7f74ed80832b3044c4ddd7de52235726949cb (accessed on 19 June 2024).
- Jacot, W.; Colinet, B.; Bertrand, D.; Lacombe, S.; Bozonnat, M.-C.; Daurès, J.-P.; Pujol, J.-L. Quality of life and comorbidity score as prognostic determinants in non-small-cell lung cancer patients. Ann. Oncol. 2008, 19, 1458–1464. [Google Scholar] [CrossRef]
- Montazeri, A.; Milroy, R.; Hole, D.; McEwen, J.; Gillis, C.R. Quality of life in lung cancer patients: As an important prognostic factor. Lung Cancer 2001, 31, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Real-World Outcomes in Patients with Unresected Stage III Non-Small Cell Lung Cancer—ProQuest. Available online: https://www.proquest.com/docview/2174251496?accountid=12253&parentSessionId=m%2B6px1RoRy7vdQNTAQZzAuyQpwhaL2MoSIQPkUfR%2BPw%3D&pq-origsite=primo (accessed on 28 December 2021).
- ICRU Report 62, Prescribing, Recording and Reporting Photon Beam Therapy (Supplement to ICRU 50)—ICRU. Available online: https://www.icru.org/report/prescribing-recording-and-reporting-photon-beam-therapy-report-62/ (accessed on 20 June 2024).
- Royal College of Radiologists. Radiotherapy for Lung Cancer: RCR Consensus Statements; Royal College of Radiologists: London, UK, 2020; Available online: https://www.rcr.ac.uk/our-services/all-our-publications/clinical-oncology-publications/radiotherapy-for-lung-cancer-rcr-consensus-statements/ (accessed on 7 November 2025).
- De Ruysscher, D.; Faivre-Finn, C.; Moeller, D.; Nestle, U.; Hurkmans, C.W.; Le Péchoux, C.; Belderbos, J.; Guckenberger, M.; Senan, S. European Organization for Research and Treatment of Cancer (EORTC) recommendations for planning and delivery of high-dose, high precision radiotherapy for lung cancer. Radiother. Oncol. 2017, 124, 1–10. [Google Scholar] [CrossRef]
- Payne, A.; Horne, A.; Bayman, N.; Blackhall, F.; Bostock, L.; Chan, C.; Coote, J.; Eaton, M.; Fenemore, J.; Gomes, F.; et al. Patient and clinician-reported experiences of using electronic patient reported outcome measures (ePROMs) as part of routine cancer care. J. Patient-Rep. Outcomes 2023, 7, 42. [Google Scholar] [CrossRef]
- Jordan, T.; Nuamek, T.; Fornacon-Wood, I.; Califano, R.; Coote, J.; Harris, M.; Mistry, H.; Taylor, P.; Woolf, D.; Faivre-Finn, C. A study demonstrating users’ preference for the adapted-REQUITE patient-reported outcome questionnaire over PRO-CTCAE® in patients with lung cancer. Front. Oncol. 2024, 14, 1328871. [Google Scholar] [CrossRef]
- Van Herk, M. Conquest DICOM Software, Version 2.0.0; GitHub; University of Manchester: Manchester, UK, 2024; Available online: https://github.com/marcelvanherk/Conquest-DICOM-Server (accessed on 7 November 2025).
- Emmerson, J.; Brown, J.M. Understanding Survival Analysis in Clinical Trials. Clin. Oncol. 2021, 33, 12–14. [Google Scholar] [CrossRef]
- Fornacon-Wood, I.; Nuamek, T.; Hudson, E.M.; Kendall, J.; Absolom, K.; O’Hara, C.; Palmer, R.; Price, G.; Velikova, G.; Yorke, J.; et al. Analyzing patient-reported outcome data in oncology care. Int. J. Radiat. Oncol. Biol. Phys. 2024, 121, 1115–1124. [Google Scholar] [CrossRef]
- Ibrahim, J.G.; Chu, H.; Chen, L.M. Basic Concepts and Methods for Joint Models of Longitudinal and Survival Data. J. Clin. Oncol. 2010, 28, 2796–2801. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).