Abstract
Almost a year after the declaration of the pandemic due to the SARS-CoV-2 virus which causes the COVID-19 disease and the need to contain the progression and treatment, the promising option was designing an effective and safe vaccine to reach a state of massive immunity. The first vaccine approved was the one produced by Pfizer–BioNTech, and its application started in December 2020. Within days of the first applications, 0.2% of adverse events were reported. Herein, a series of 26 cases with the manifestation of adverse events related to the application of the first dose of the BNT162b2 vaccine from Pfizer–BioNTech in healthcare workers from Mexico. Of these cases, only five patients were classified with a certainty of anaphylaxis; two of them presented seizures, and their management is described individually. After the examination of all the cases, the symptoms were resolved. In Mexico and around the globe, the vaccination process continues, and the report of possible AEFIs is still needed to contribute to the pharmacovigilance of this new vaccine and improve its safety profile.
1. Introduction
Due to the classification of the COVID-19 disease caused by the SARS-CoV-2 virus as a pandemic in March 2020 by the World Health Organization (WHO) [1] and the alarming number of millions of infected people, the vast majority of governments around the globe had to propose drastic measures to guarantee the population’s safety, and the entire scientific community started to look for effective treatments to contain disease progression. An example of this was the RECOVERY Study (Randomised Evaluation of COVID-19 Therapy) [2,3], and the SOLIDARITY international clinical trial supported by the WHO [4].
However, despite the increased number of clinical trials related to COVID-19 treatment and the multiple combinations of therapeutic schemes with or without supporting evidence [5], the most promising option, like in any infectious disease, was the design of an effective and safe vaccine preventing the virus spread and able to facilitate a state of mass immunity.
In the COVID-19 pandemic scenario, the proposed design of the different vaccine options included monoclonal antibodies, antiviral and antigenic proteins, peptides, and modified viruses [6]. The first vaccine able to demonstrate superior effectiveness compared to others was BNT162b2 by Pfizer–BioNTech. This vaccine was produced by a transcription of one single messenger RNA (mRNA) chain from a DNA template with information encoding for the SARS-CoV-2 protein S (Spike protein) [7]. This vaccine could produce neutralizing antibodies and prevent the joining between the Spike protein and the ACE-2 receptor to avoid viral spread in the body.
In November 2020, this vaccine (BNT162b2 by Pfizer-BioNTech) reported its phase 3 clinical trial results, showing a 95% effectiveness after 28 days of the first dose administration. Among the advantages demonstrated and unlike the other vaccines (ChadoX1 NCoV-19), ethnic and racial diversity and a wide age range (16 to over 65 years) stand out [8]. After the evaluation of their results, the FDA and the Advisory Committee on Immunization Practices issued their recommendations for the application of the BNT162B2 vaccines at the end of 2020. The initial doses were authorized for health workers and nursing homes [9].
However, a few days after the first applications, 0.2% of adverse events were reported (4393 out of 1,893,369 applications), of which only 21 cases (0.4%) were severe anaphylactic reactions, and 7 of these were patients with a history of anaphylaxis from other causes. The rest of the cases corresponded to local pain, headache, and redness in the application site [10,11]. Even though all the reports were of total and satisfactory recovery, the main regulatory entities issued recommendations and absolute contraindications for the vaccine application. One important recommendation is to have an adequate system to identify and treat anaphylactic reactions, especially within the first 30 min in which these events have been observed. In addition, if the reaction is not clear, the absolute recommendation is to apply the Brighton Scoring System to classify and treat the allergic reaction related to the vaccine [12].
The vaccination process in Mexico was presented in the Política Nacional de Vacunación contra el Virus SARS-CoV-2 para la Prevención de la COVID-19 en México (National Vaccination Policy Against the SARS-CoV-2 Virus for the prevention of COVID-19 in Mexico) [13], where five stages of vaccination were included. The first one, from December 2020 to February 2021, was focused on healthcare workers, the second, from February to April 2021, was focused on adults over 60 years old, the third, from April to May 2021, focused on adults between 50 and 59 years old, the fourth, from May to June 2021, focused on adults between 40 and 49 years old, and the last one, from June 2021 to March 2022, focused on the rest of the population. This policy included a mandatory observation period of at least 30 min after the application of the vaccine to identify and treat the possible Adverse Event Following Immunization (AEFI) on time [13].
The epidemiological surveillance for the identification and notification of AEFIs in Mexico is based on the Manual de Procedimientos Estandarizados para la Vigilancia Epidemiológica de Eventos Supuestamente Atribuibles a la Vacunación o Inmunización (Manual of Standardized Procedures for Epidemiological Surveillance of Events Supposedly Attributable to Vaccination or Immunization). AEFI surveillance is passive, which is why the event is detected spontaneously by those who identify it in the healthcare institution or by notification of the patient. The notification must be made immediately from the system designed for this purpose at each of the levels of the notification network [14].
From 24 December 2020 to 30 December 2022, the last report on AEFIs in Mexico documented a total of 38,757 AEFIs, of which 97.01% were non-serious events that occur more frequently in females; severe AEFIs occur more frequently in women over 60 years of age. In this same report, it was described that for every 1000 applied doses of BNT162b2 by Pfizer–BioNTech, there were 0.55 AEFIs, the most common being headaches, pain/tenderness at the application site, and asthenia. These data are similar to those of the healthcare workers population, with 99.11% of non-serious AEFI events during the vaccination process [15]. Also, García-Grimshaw et al. described that about 65.1% of AEFI reported in Mexico were mostly not serious, neurological symptoms [16].
Despite the existence of the pharmacovigilance system, for the notification and prevention of AEFI, experts recommend the application of various methods of monitoring and surveillance to increase the security of the used vaccines and to detect new possible effects occurring, perhaps, due to the vaccination process [17,18,19]. In Mexico, the management or evolution of these effects has not been fully documented. For that reason, the objective of this communication is to present 26 possible AEFIs after the application of BNT162b2 by Pfizer–BioNTech that required hospital care in the intensive care unit for at least 2 h.
2. Materials and Methods
In January 2021, the vaccination process in Mexico was focused on healthcare workers who responded to the needs derived from the COVID-19 pandemic and according to the guidelines established in the Política Nacional de Vacunación contra el Virus SARS-CoV-2 para la Prevención de la COVID-19 en México (National Vaccination Policy Against the SARS-CoV-2 Virus for the prevention of COVID-19 in Mexico). The distribution of vaccines was concentrated in hospital institutions where the immunization was performed. Following this guideline, each immunized healthcare worker was followed for a period of at least 30 min after immunization, where possible AEFIs were detected by a specialized nurse with an inventory of possible symptoms (pain and sensibility in the application site, headaches, asthenia, and increase in blood pressure) observed after the vaccine application. This process was conducted under the Passive Surveillance (PS) framework. If no symptoms were observed in that period, the healthcare worker returned to their usual activities, with the following instruction: If they present any symptoms related to AEFI, they must inform and start the monitoring.
According to the vaccination planning, 650 first doses were considered to have been applied in two days in the Hospital General de San Juan del Río located in San Juan del Río, Queretaro, Mexico, a second-level hospital. Of these 650 doses, only 545 were applied due to the appearance of 26 possible AEFIs. A total of 5/26 patients with AEFI cases had to stay in the hospital for 24 h, and 1/5 of those had to stay at the Intensive Care Unit (ICU) (Figure 1).
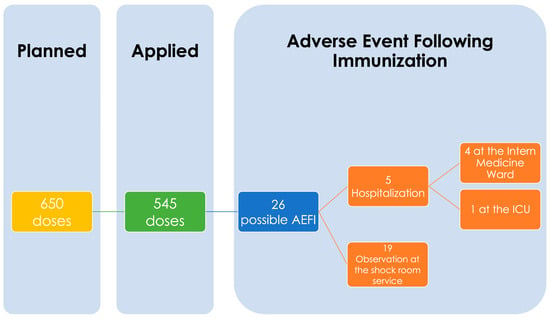
Figure 1.
Possible Cases of Adverse Event Following Immunization (AEFI). Flowchart.
At the time of reporting any of the possible symptoms related to the vaccination, the patients were directed to the shock room set up for the vaccination process, where the following data were recorded: sex, age, comorbidities such as chronic diseases and history of allergic reaction, symptoms experienced after vaccination and the time of symptoms onset. After that, the Brighton Scoring System used to classify allergic reactions was applied. The Brighton Scoring System is based on the definition of cases based on the diagnostic certainty and major and minor criteria associated with the application of vaccines that can be observed. The score was classified into 5 categories: Levels 1 to 3 as events which meet the case definition of anaphylaxis; Level 4 as a reported anaphylaxis with insufficient evidence to meet the case definition; and Level 5 as a not a case of anaphylaxis [12].
All patients with a possible case of AEFI signed an informed consent form before the registration of their data, expressing their willingness to share their data in the biomedical literature, guaranteeing their anonymity and adequate clinical care.
3. Results
In 545 applications of the first doses of BNT162b2 by Pfizer–BioNTech, 26 cases with a probable AEFI event were identified, which represents 4.77% of the doses applied in the hospital centre. Of the AEFI reported, 80.77% corresponded to female persons. The average age was 33.62 years, reporting only one case of a person older than 60 years. Among the pathological antecedents, 23.08% had a history of cardiovascular comorbidities; in a lesser proportion, antecedents of respiratory, mental, and endocrine diseases were reported (3.85% each one), as well as congenital syndromes. Also, 53.85% reported a history of allergies to drugs (38.46%) and food (19.23%). The self-reported allergies to drugs were allergies to antibiotics like penicillin, quinolone and fluoroquinolones, cephalosporin, tetracyclines, and non-steroidal anti-inflammatory drugs.
AEFI events appeared in a range from 0 to 18 h after immunization (mean: 81 min). In three patients, the symptoms appeared immediately. The symptoms most experienced by the patients were hypertensive crisis, rash, pruritus, headache, dizziness, and tachycardia. In addition to these, two cases of seizures were observed, as well as pain at the application site, nausea, anxiety, fever, and sweating.
According to the Brighton classification, 5 patients (19.23%) were classified as cases with a higher level of certainty of anaphylaxis (Level 1), 7 (26.92%) with a moderate level of certainty of anaphylaxis (Level 2), and 14 (53.85%) as cases that did not meet the definition criteria for anaphylaxis (Level 5).
All AEFI cases were discharged within a maximum time of 24 h according to the evolution and reduction in symptoms. Of the 26 cases, only 5 remained hospitalized for 24 h, 4 in the internal medicine service and 1 in the ICU. The rest were discharged when the presence of symptoms decreased, 42.31% in 2 h, 30.77% in 6 h, and 7.69% in 12 h (Table 1).

Table 1.
Characteristics of Patients with AEFIs, symptoms, Brighton criteria, symptom onset, and outcome.
Patients with rash were treated with diphenhydramine 50 mg intravenously as a single dose and discharged in less than 12 h with oral antihistamine and alarm data that included the new appearance of the rash, >10 cm erythema at the application site, localized muscle weakness and neurological disorders. Patients with headaches were treated with acetaminophen 500 mg−1g and ketorolac 30 mg intravenously and were discharged with an analgesic prescription. Diazepam 5 mg was administered intramuscularly to one case (number 13) of a patient who presented generalized partial seizures; at least the use of epinephrine was not needed.
Of the 26 AEFIs identified after immunization, the presence of seizures in response to vaccination was observed in two cases. Both cases were classified into Level 1 in the Brighton Criteria (higher level of certainty of anaphylaxis). These cases and their treatment are described in the next section.
3.1. Clinical Case 1
A 28-year-old female patient presented with an allergy to penicillin and trimethoprim/sulfamethoxazole diagnosed in childhood. Forty minutes after the application of the vaccine, she presented a rash on the ears, neck, and arms. Diphenhydramine 50 mg was administered intravenously, so the rash eased in a few minutes. One hour after the vaccine application, she presented a witnessed tonic seizure manifested by abnormal arm extension, mydriasis, and eye deviation upwards and to the left for 20 s with hypoxemia when the oxygen saturation by pulse oximetry (SpO2) was 84%. Therefore, an oxygen mask with a reservoir bag was used, which increased SpO2 to 98%. The patient presented post-ictal status for 30 min with the following vital signs: blood pressure (BP) 156/90 mmHg, heart rate (HR) 138 beats per minute (bpm), and respiratory rate (RR) 32 breaths per minute. Later, she regained alertness without neurological deficit or complications. Two hours later, she maintained BP: 130/87 mmHg, HR: 106 bpm, RR: 22, and SpO2: 100%. She was kept under observation with a headache and was discharged after 24 h (Table 1, Case 1).
3.2. Clinical Case 2
A 28-year-old male patient presented with a history of asthma and bronchiectasis, was treated with inhaled bronchodilators, was allergic to pineapple and unspecified contact with pollen. Five minutes after applying the vaccine, he presented simple tonic–clonic partial seizures in the upper and lower right limbs with decreased alertness (from simple to complex), with alertness recovery 30 s after diazepam 10 mg was administered intramuscularly. He remained under observation and presented muscle weakness on the right body hemisphere that resolved in 30 min. Later, he presented wheezing and 88% oxygen saturation by pulse oximetry, which required the administration of bronchodilators and supplemental oxygen. The patient was discharged hassle-free after 24 h (Table 1, Case 13).
4. Discussion
A total of 4.77% of the vaccination doses applied (26 cases) in the healthcare centre were identified as possible AEFI; of these cases, only 12 were classified as Level 1 or 2 in the Brighton Criteria with some level of certainty of anaphylaxis. Most affected people were female people, younger, and with a history of allergies to drugs and food, with systemic, dermatological, cardiovascular, and neurological symptoms the first to be described in the progression of the response. Due to the number of cases with a reaction event to vaccination in the Hospital General de San Juan del Río during the first vaccination day (January 2021), the process was stopped to guarantee healthcare for those who required it during and after the vaccine application. The immunization process was continued after the successful resolution of the previous events.
Vaccination in Mexico began in December 2020 with the Pfizer–BioNTech BNT162b2 vaccine. Healthcare workers were the first group to be vaccinated. By the end of 2020, 11.1 AEFIs were observed per million doses of the Pfizer–BioNTech vaccine, and 71% of these occurred within 15 min of vaccination [5]. Since these adverse effects were identified, the FDA issued a recommendation asserting that the application of the Pfizer–BioNTech vaccine should be avoided in people with a known history of severe allergic reactions to any component of this vaccine. Also, a warning was issued to people with a history of alterations and immune diseases who could experience a major risk of AEFIs after the vaccination. This recommendation came from the first PS after vaccination started [20]. These recommendations were also applied in the Política Nacional de Vacunación contra el Virus SARS-CoV-2 para la Prevención de la COVID-19 en México (National Vaccination Policy Against the SARS-CoV-2 Virus for the prevention of COVID-19 in Mexico).
Currently, 133,972,266 doses of COVID-19 vaccines have been applied in Mexico, of which 35,874,667 were from Pfizer–BioNTech with a rate of 0.55 AEFI per 1000 doses [15], and around the globe, different research groups have reported the frequency of AEFIs due to the Pfizer–BioNTech vaccine application. In Lebanon, 0.99 cases per 1000 doses administrated was reported [21], while in Vietnam, the proportion of AEFI after the first dose was 24.4% [22], and in The Netherlands, the percentage was 40.76% after the first dose. This percentage is lower than that of other vaccine brands (AstraZeneca, Janssen, and Moderna) [23].
Despite the frequency of AEFIs observed, the current and available evidence about COVID-19 vaccination supports immunization as a key to controlling the pandemic [24].
In our PV, we observed the typical symptoms reported after the first doses of any COVID-19 vaccine: headaches, pain/tenderness at the application site, and asthenia, symptoms more frequently observed in the female sex, a situation that has been previously observed in different studies. Green et al. showed that the risk of adverse events (allergic, local, neurological reactions and any adverse event) following the first dose was higher in females without age correlation [25], and the mechanisms underlying this situation are not fully understood, but some authors hypothesized that the high frequency of adverse events in females can be explained by a stronger immediate response to the antigen modulated through the innate immune system [26,27], the role of the testosterone in depressing immune response [28,29], and the localization of the ACE2 and ANG-II receptor type 2 in the X chromosome [30,31].
According to our observation, 53.85% of the individuals who experienced possible AEFIs had a history of allergy to drugs or foods. In this line, Nittner-Marszalska et al. reported that individuals with a history of allergy are at greater risk of experiencing adverse effects after immunization with the Pfizer–BioNTech vaccine [32]. The authors hypothesized that this observation could be explained by a lower reactivity threshold of effector cells to non-IgE activating factors and the activation of other cell populations.
Along the same line, most of the adverse reactions like allergies are not attributed to the vaccine’s active ingredient and could occur due to the excipients such as the polyethylene glycol (PEG) which was added in the Pfizer–BioNTech vaccine to stabilize the nanoparticle containing the mRNA [33]. PEG is also found in several products on the market, including drug and beauty products such as lotions or lubricants [34]. However, the diagnosis of PEG allergy is rare, consistent with the low rate of anaphylaxis cases after the Pfizer–BioNTech or Moderna vaccine application, even when there are reports of anaphylaxis with confirmed PEG allergy [35]. Despite the recommendation to avoid the Pfizer–BioNTech vaccine in individuals with an allergy to vaccine ingredients or PEG, some authors have reported that one individual with a history of severe PEG anaphylaxis could safely receive the dose of Pfizer–BioNTech with pre-treatment [36].
Another mechanism related to adverse effects, widely described in PEG-containing nano-drugs, is CARPA (complement-activation-related pseudo-allergy). These reactions are immediate and IgE-independent with characteristic symptoms of type I hypersensitivity reactions [37]. A mechanism is proposed where the first step is the binding of antibodies to liposomes, which causes a complement activation and a release of anaphylatoxins that manifest their stimulatory effects on innate immune cells that mediate an allergic reaction and secrete vasoactive and inflammatory mediators responsible for hypersensitivity symptoms [38].
The first adverse effects reported in our PV were cutaneous. They were characterized by exanthema, hives, and in some patients the criteria for anaphylaxis were not met, so epinephrine was not used as an initial treatment [39]. According to the CDC guidelines, the treatment of skin lesions must be performed with symptomatic administration of antihistamines and the application of topical steroids [24]. The EAACI guidelines for anaphylaxis treatment also recommend the use of H1 antihistamines as the third line of treatment to improve cutaneous symptoms; another recommendation is a concern about the use of corticosteroids; their effects can reduce the risk of respiratory symptoms in the late phase of anaphylaxis [25]. It is important to highlight the differences in the treatment to control the anaphylactic reaction. In our observations, it was found that the use of antihistaminic drugs may be the correct therapy for the management of the anaphylactic reaction associated with immunization with the Pfizer–BioNTech vaccine. This could be justified due to the fact that some skin reactions cannot be explained by immunologically mediated mechanisms, such as dizziness, nausea, or tachycardia, and according to current definitions, they are not considered real allergic reactions, since an immunological mechanism that justifies clinical manifestations has not been identified [40].
Most adverse reactions to medications or vaccines are frequently considered by health providers as allergic reactions; however, according to recent reports, the anaphylaxis index is 1.31 (95% CI, 0.90–1.84) per million doses of vaccines [21]. It is not possible to determine the type of reaction that occurred in our patients, although by the time of appearance of the lesions, in a few minutes, it can be inferred that the mechanism is related to type I hypersensitivity since, although skin lesions have also been reported for type IV hypersensitivity, these are retarded [22]. The presence of urticaria as a reaction that occurs in the first 4 h has been reported by some authors. Out of 55 cases, 30 (55%) presented urticaria with the BNT162b2 vaccine, and 25 (45%) with the mRNA1273 vaccine; in this case series, one patient had cholinergic urticaria, and the adverse reaction was triggered by heat rather than by reaction to the vaccine [23].
Esquivel-Valerio et al. described the adverse events after the application of diverse vaccines authorized in Mexico in a population with rheumatological diseases. They observed 70.2% of pain in the injection site, 34.7% of fatigue, 30.6% of headache and 29.3% of muscle pain. Unlike our reported cases, there were no rash or urticarial reactions. The report is not specific about the previous treatment of rheumatic disease [26].
The AEFIs reported in our PV are consistent with the results in the literature, but the presence of symptoms such as dizziness, nausea, tachycardia, and hypertensive crisis could be explained by the anxiety caused by the application of new treatments such as the vaccine for the prevention of COVID-19 [41].
After applying the first dose of these vaccines, if an associated severe adverse effect has occurred, it is necessary to assess the risk of applying the second dose without preparation. A thorough review by an allergy and immunology specialist is proposed to be performed, especially in individuals with a high risk of AEFIs.
In Mexico and around the globe, vaccines from different producers are still needed to guarantee pandemic control. For that reason, the pharmacovigilance of all kinds of vaccines is required, not just to improve their safety, but also to increase the willingness to vaccination.
It is appropriate to recognize that the descriptions offered here come from an observational study in the PV framework, and due to these characteristics, there are some limitations that must be discussed: (1) this report comes from AEFIs that occurred in a healthcare population which is more prone to communicate possible events related to the vaccine. This situation is also related to the second limitation: (2) the ages of the individuals reporting AEFIs are similar to the ages of patients in previous studies conducted in healthcare workers, which reduced the external validity of the observations [42,43,44]. (3) Due to the characteristic of the PV, it was impossible to identify the data from those who did not report an AEFI and assess the risks of those that reported an AEFI due to some characteristic such as allergy and COVID-19 disease history (vaccine allergy). Despite the limitations in this work, all the symptoms reported by the individuals who experienced AEFIs were confirmed by a healthcare worker in order to reduce the bias of reporting. We reached consensus to classify the possible anaphylaxis due to the vaccine based on the Brighton criteria, which was improved to adhere to the current evidence, the US National Institute of Allergy and Infectious Disease/Food Allergy Anaphylaxis Network (NIAID/FAAN) and the World Allergy Organization (WAO) standards [45]. Also, this work is one of a few reporting on healthcare workers from Mexico, and it contributed to vaccine safety evaluation.
5. Conclusions
The Pfizer–BioNTech COVID-19 vaccine presented uncommon adverse reactions in this case series, but these adverse reactions were successfully resolved. It is mandatory to continue the pharmacovigilance of each vaccine developed to control the COVID-19 pandemic to improve the safety profile of each vaccine, which will increase the willingness to vaccination in the population.
Author Contributions
O.R.P.-N. and C.D.A.-B. contributed to the conception of the idea, the coordination, collected the data and manuscript review. E.D.-T. contributed to collecting the data, coordination, and manuscript review. M.d.L.M.-M. contributed to collecting and reviewing the data. Ú.M. contributed to the literature search and R.A.-P. contributed to the conception of the idea, literature search, manuscript preparation, and manuscript review. O.R.P.-N., C.D.A.-B., E.D.-T., L.D.C.-O., M.d.L.M.-M., Ú.M., R.A.-P. and M.E.P.-P. reviewed and accepted the last version of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study complied with the premises established in the Declaration of Helsinki for research on human beings and is in accordance with the Guide for Health Research during Disasters, Emergencies, or Epidemics outbreaks (CIOM, 2016). The nature of this study guarantees minimal risk and respects the granting of informed consent by each research person. The participants were informed of the characteristics of the study as well as the use of the data obtained and the researchers.
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Data is available on request to the corresponding authors due to ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lake, M.A. What we know so far: COVID-19 current clinical knowledge and research. Clin. Med. 2020, 20, 124–127. [Google Scholar] [CrossRef]
- Duncan, A.; Halim, D.; El Kholy, K. The RECOVERY trial: An analysis and reflection two years on. Eur. J. Intern. Med. 2022, 105, 111–112. [Google Scholar] [CrossRef]
- Normand, S.-L.T. The RECOVERY Platform. N. Engl. J. Med. 2021, 384, 757–758. [Google Scholar] [CrossRef]
- World Health Organization (WHO). “Solidarity” Clinical Trial for COVID-19 Treatments. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-COVID-19-treatments (accessed on 15 August 2023).
- Pearson, H. How COVID broke the evidence pipeline. Nature 2021, 593, 182–185. [Google Scholar] [CrossRef]
- Chung, J.Y.; Thone, M.N.; Kwon, Y.J. COVID-19 vaccines: The status and perspectives in delivery points of view. Adv. Drug Deliv. Rev. 2021, 170, 1–25. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: What do we know about the late stage vaccine candidates? BMJ 2020, 371, m4576. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: Pfizer and BioNTech submit vaccine for US authorisation. BMJ 2020, 371, m4552. [Google Scholar] [CrossRef]
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine—United States, December 14–23, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 46. [Google Scholar] [CrossRef] [PubMed]
- Baum, S.G. Allergic Reactions to the Pfizer-BioNTech and Moderna COVID-19 Vaccines: Early Reports. NEJM J. Watch 2021, 2021. [Google Scholar] [CrossRef]
- Rüggeberg, J.U.; Gold, M.S.; Bayas, J.-M.; Blum, M.D.; Bonhoeffer, J.; Friedlander, S.; de Souza Brito, G.; Heininger, U.; Imoukhuede, B.; Khamesipour, A.; et al. Anaphylaxis: Case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2007, 25, 5675–5684. [Google Scholar] [CrossRef] [PubMed]
- Gobierno de México. Política Nacional Rectora de Vacunación Contra el SARS-CoV-2 para la Prevención de la COVID-19 en México. Documento Rector; Gobierno de México: México, 2021; p. 39. [Google Scholar]
- Gobierno de México. Manual de Procedimientos Estandarizados para la Vigilancia Epidemiológica de Eventos Supuestamente Atribuibles a la Vacunación o Inmunización (ESAVI); Gobierno de México: México, 2021; p. 56. [Google Scholar]
- Secretaría de Salud, México. Reporte ESAVI COVID-19-Julio 2021; Secretaría de Salud: Acapulco, México, 2021; p. 13. [Google Scholar]
- García-Grimshaw, M.; Ceballos-Liceaga, S.E.; Hernández-Vanegas, L.E.; Núñez, I.; Hernández-Valdivia, N.; Carrillo-García, D.A.; Michel-Chávez, A.; Galnares-Olalde, J.A.; Carbajal-Sandoval, G.; Del Mar Saniger-Alba, M.; et al. Neurologic adverse events among 704,003 first-dose recipients of the BNT162b2 mRNA COVID-19 vaccine in Mexico: A nationwide descriptive study. Clin. Immunol. 2021, 229, 108786. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, A.; Mitchell, J.; Barrett, J.; Sköld, H.; Taavola, H.; Erlanson, N.; Melgarejo-González, C.; Yue, Q.-Y. Global safety monitoring of COVID-19 vaccines: How pharmacovigilance rose to the challenge. Ther. Adv. Drug Saf. 2022, 13, 20420986221118972. [Google Scholar] [CrossRef]
- Lee, G.M.; Romero, J.R.; Bell, B.P. Postapproval Vaccine Safety Surveillance for COVID-19 Vaccines in the US. JAMA 2020, 324, 1937–1938. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Fact Sheet for Heatlhcare Providers Administering Vaccine. Emergency Use Authorization of the PfizerBioNTech COVID-19 Vaccine to Prevent Coronavirus Disease 2019; Food and Drug Administration, EUA: Silver Spring, MD, USA, 2021; p. 48. [Google Scholar]
- Zeitoun, A.; Hallit, S.; Chehade, S.; Ibrahim, A.; Helali, M.; Allam, C.; Karam, R. A 1-year analysis of adverse events following COVID-19 vaccination in Lebanon: A retrospective study. J. Pharm. Policy Pract. 2023, 16, 24. [Google Scholar] [CrossRef] [PubMed]
- Le, X.T.T.; Hoang, Q.L.; Ta, N.T.K.; Pham, Q.T.; Nguyen, T.T.; Phan, H.T.M.; Nguyen, T.V.; Le, H.T.T.; Nguyen, N.T.; Hoang, L.D.; et al. Common adverse events following immunization with the COVID-19 comirnaty vaccine (Pfizer-BioNTech) among adult population in Hanoi, Vietnam, 2021. Front. Trop. Dis. 2022, 3, 987698. [Google Scholar] [CrossRef]
- Kant, A.; Jansen, J.; van Balveren, L.; van Hunsel, F. Description of Frequencies of Reported Adverse Events Following Immunization Among Four Different COVID-19 Vaccine Brands. Drug Saf. 2022, 45, 319–331. [Google Scholar] [CrossRef]
- Papadakos, S.P.; Mazonakis, N.; Papadakis, M.; Tsioutis, C.; Spernovasilis, N. Pill versus vaccine for COVID-19: Is there a genuine dilemma? Ethics Med. Public Health 2022, 21, 100741. [Google Scholar] [CrossRef]
- Green, M.S.; Peer, V.; Magid, A.; Hagani, N.; Anis, E.; Nitzan, D. Gender Differences in Adverse Events Following the Pfizer-BioNTech COVID-19 Vaccine. Vaccines 2022, 10, 233. [Google Scholar] [CrossRef]
- Liu, T.; Balzano-Nogueira, L.; Lleo, A.; Conesa, A. Transcriptional Differences for COVID-19 Disease Map Genes between Males and Females Indicate a Different Basal Immunophenotype Relevant to the Disease. Genes 2020, 11, 1447. [Google Scholar] [CrossRef]
- Jacobsen, H.; Klein, S.L. Sex Differences in Immunity to Viral Infections. Front. Immunol. 2021, 12, 720952. [Google Scholar] [CrossRef] [PubMed]
- Trigunaite, A.; Dimo, J.; Jørgensen, T.N. Suppressive effects of androgens on the immune system. Cell. Immunol. 2015, 294, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Gubbels Bupp, M.R.; Jorgensen, T.N. Androgen-Induced Immunosuppression. Front. Immunol. 2018, 9, 794. [Google Scholar] [CrossRef] [PubMed]
- Foresta, C.; Rocca, M.S.; Di Nisio, A. Gender susceptibility to COVID-19: A review of the putative role of sex hormones and X chromosome. J. Endocrinol. Investig. 2021, 44, 951–956. [Google Scholar] [CrossRef]
- Ursin, R.L.; Shapiro, J.R.; Klein, S.L. Sex-biased Immune Responses Following SARS-CoV-2 Infection. Trends Microbiol. 2020, 28, 952–954. [Google Scholar] [CrossRef]
- Nittner-Marszalska, M.; Rosiek-Biegus, M.; Kopeć, A.; Pawłowicz, R.; Kosińska, M.; Łata, A.; Szenborn, L. Pfizer-BioNTech COVID-19 Vaccine Tolerance in Allergic versus Non-Allergic Individuals. Vaccines 2021, 9, 553. [Google Scholar] [CrossRef]
- Banerji, A.; Wickner, P.G.; Saff, R.; Stone, C.A.; Robinson, L.B.; Long, A.A.; Wolfson, A.R.; Williams, P.; Khan, D.A.; Phillips, E.; et al. mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 1423–1437. [Google Scholar] [CrossRef]
- Bigini, P.; Gobbi, M.; Bonati, M.; Clavenna, A.; Zucchetti, M.; Garattini, S.; Pasut, G. The role and impact of polyethylene glycol on anaphylactic reactions to COVID-19 nano-vaccines. Nat. Nanotechnol. 2021, 16, 1169–1171. [Google Scholar] [CrossRef]
- McSweeney, M.D.; Mohan, M.; Commins, S.P.; Lai, S.K. Anaphylaxis to Pfizer/BioNTech mRNA COVID-19 Vaccine in a Patient With Clinically Confirmed PEG Allergy. Front. Allergy 2021, 2, 715844. [Google Scholar] [CrossRef]
- Li, D.H.; Lee, E.; Song, C. Successful mRNA COVID-19 vaccination in a patient with a history of severe polyethylene glycol anaphylaxis. Allergy Asthma Clin. Immunol. 2022, 18, 57. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-H.; Stone, C.A.; Jakubovic, B.; Phillips, E.J.; Sussman, G.; Park, J.; Hoang, U.; Kirshner, S.L.; Levin, R.; Kozlowski, S. Anti-PEG IgE in anaphylaxis associated with polyethylene glycol. J. Allergy Clin. Immunol. Pract. 2021, 9, 1731–1733.e3. [Google Scholar] [CrossRef] [PubMed]
- Kozma, G.T.; Mészáros, T.; Vashegyi, I.; Fülöp, T.; Örfi, E.; Dézsi, L.; Rosivall, L.; Bavli, Y.; Urbanics, R.; Mollnes, T.E.; et al. Pseudo-anaphylaxis to Polyethylene Glycol (PEG)-Coated Liposomes: Roles of Anti-PEG IgM and Complement Activation in a Porcine Model of Human Infusion Reactions. ACS Nano 2019, 13, 9315–9324. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.; Nair, N. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine. JAMA 2021, 325, 780–781. [Google Scholar] [CrossRef]
- Karnes, J.H.; Miller, M.A.; White, K.D.; Konvinse, K.C.; Pavlos, R.K.; Redwood, A.J.; Peter, J.G.; Lehloenya, R.; Mallal, S.A.; Phillips, E.J. Applications of Immunopharmacogenomics: Predicting, Preventing, and Understanding Immune-Mediated Adverse Drug Reactions. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 463–486. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization; World Health Organization. Consultation Document for Case Definitions: Adverse Events of Special Interest and Adverse Events Following Immunization during COVID-19 Vaccine Introduction; Pan American Health Organization, EUA: Washington, DC, USA, 2021; p. 66. [Google Scholar]
- Dziedzic, A.; Riad, A.; Attia, S.; Klugar, M.; Tanasiewicz, M. Self-Reported Adverse Events of COVID-19 Vaccines in Polish Healthcare Workers and Medical Students. Cross-Sectional Study and Pooled Analysis of CoVaST Project Results in Central Europe. J. Clin. Med. 2021, 10, 5338. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Izquierdo, M.; Soler-Iborte, E.; de Rojas, J.P.; Pegalajar-García, M.D.; Gil-Villalba, A.; Ruiz-Villaverde, R.; Valero-Ubierna, M.D.C. Factors Associated with Adverse Reactions to BNT162b2 COVID-19 Vaccine in a Cohort of 3969 Hospital Workers. Vaccines 2022, 10, 15. [Google Scholar] [CrossRef]
- Ripabelli, G.; Tamburro, M.; Buccieri, N.; Adesso, C.; Caggiano, V.; Cannizzaro, F.; Di Palma, M.A.; Mantuano, G.; Montemitro, V.G.; Natale, A.; et al. Active Surveillance of Adverse Events in Healthcare Workers Recipients After Vaccination with COVID-19 BNT162b2 Vaccine (Pfizer-BioNTech, Comirnaty): A Cross-Sectional Study. J. Community Health 2022, 47, 211–225. [Google Scholar] [CrossRef]
- Gold, M.S.; Amarasinghe, A.; Greenhawt, M.; Kelso, J.M.; Kochhar, S.; Yu-Hor Thong, B.; Top, K.A.; Turner, P.J.; Worm, M.; Law, B. Anaphylaxis: Revision of the Brighton collaboration case definition. Vaccine 2023, 41, 2605–2614. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).