Abstract
Background: Acute Respiratory Distress Syndrome (ARDS) is caused by non-cardiogenic pulmonary edema and occurs in critically ill patients. It is one of the fatal complications observed among severe COVID-19 cases managed in intensive care units (ICU). Supportive lung-protective ventilation and prone positioning remain the mainstay interventions. Purpose: We describe the severity of ARDS, clinical outcomes, and management of ICU patients with laboratory-confirmed COVID-19 infection in multiple Saudi hospitals. Methods: A multicenter retrospective cohort study was conducted of critically ill patients who were admitted to the ICU with COVID-19 and developed ARDS. Results: During our study, 1154 patients experienced ARDS: 591 (51.2%) with severe, 415 (36.0%) with moderate, and 148 (12.8%) with mild ARDS. The mean sequential organ failure assessment (SOFA) score was significantly higher in severe ARDS with COVID-19 (6 ± 5, p = 0.006). Kaplan–Meier survival analysis showed COVID-19 patients with mild ARDS had a significantly higher survival rate compared to COVID-19 patients who experienced severe ARDS (p = 0.023). Conclusion: ARDS is a challenging condition complicating COVID-19 infection. It carries significant morbidity and results in elevated mortality. ARDS requires protective mechanical ventilation and other critical care supportive measures. The severity of ARDS is associated significantly with the rate of death among the patients.
Keywords:
COVID-19; Acute Respiratory Distress Syndrome; ARDS; outcome; mortality; survival; risk factors 1. Introduction
COVID-19 disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which originated in China, has caused a global outbreak that has affected more than 70 million people worldwide [1]. SARS-CoV-2 associated pneumonia can be acquired in the community or in individuals with comorbidities [2,3]. The severity and mortality are particularly high in these patients [4]. Given the high mortality rates, the complexity of the care given to patients with COVID-19-associated respiratory distress syndrome (CARDS) has increased significantly worldwide. CARDS is ARDS due to SARS-CoV-2, and therefore follows the same characteristics of ARDS based on the Berlin definition [5]. Patients who are suffering hypoxemia are classified as mild (PaO2/FIO2 ≤ 300 mm Hg), moderate (PaO2/FIO2 ≤ 200 mm Hg), or severe (PaO2/FIO2 ≤ 100 mm Hg) [5] ARDS. The increasing number of reports on this condition globally has helped us to understand the respiratory mechanics and ventilatory management, targeted to optimize the care for these patients [6,7,8,9,10,11]. Due to the increasing number of patients with COVID-19, hospitals had to work under tremendous pressure and often with limited resources to provide the best possible care to these individuals. The observations of heterogeneity in the clinical course and the various features of CARDS have led to the development of different management strategies [12,13,14].
The severity of the SARS-CoV-2 infection depends on the nature of the virus and the lack of effective therapy [15,16,17,18]. In addition, individuals with CARDS have higher mortality due to refractory hypoxemia, septic shock, and multi-organ failure [19]. In a recent study, it has been demonstrated that the two most common comorbidities in COVID-19 cases was hypertension (severe 33.4 vs. 21.6% non-severe) followed by diabetes (severe 14.4% vs 9% non-severe) [20]. However, when it came to severe cases, it was estimated that CARDS occurred at a high incidence rate of 41.1% vs. 3% in non-severe cases. Acute kidney injury and shock in these severe cases were higher as well at 16.4% vs. 2.2% non-severe, and 19.9% vs. 4.1% in non-severe cases, respectively. The mortality rate was high at 30.3% in these severe cases [20].
Some of the studies suggest that about 20% of patients with SARS-CoV-2 experience high disease severity and require hospitalization [21,22]. Among the hospitalized patients, it is seen that over a quarter percentile [26%] eventually end up in the intensive care unit (ICU) [23]. Evidence on the efficacy of prone positioning while on a ventilator [14], pharmacological interventions with pulmonary vasodilators [24], and neuromuscular blocking agents [25,26,27] for COVID-19 patients with ARDS is limited, and is based on anecdotal observations and data on the outcomes are conflicting. For those who fail these interventions, extracorporeal membrane oxygenation (ECMO) is suggested as a rescue strategy (27). Therefore, observational studies are a reasonable alternative to randomized clinical trials. Hence, this study aims to investigate the clinical characteristics and outcomes of COVID-19 patients with ARDS in Saudi Arabia.
2. Methods
2.1. Study Design
A multicenter, retrospective, cohort study was conducted and included patients who had positive tests by reverse transcriptase–polymerase chain reaction (RT-PCR) for SARS-CoV-2 during 2020 from ICUs in Saudi Arabia. The diagnosis was confirmed, and severity was determined using the Berlin definition [5]. This study obtained an ethical clearance from the Institutional Review Board “REDACTED”. The study was performed in accordance with the Declaration of Helsinki. Unique patient codes were issued to each study participant to maintain anonymity and confidentiality throughout the study.
2.2. Population
A total of 1154 COVID-19 positive case who developed ARDS were included following the inclusion criteria. The following inclusion criteria were utilized: an ICU patient with confirmed SARS-CoV-2 infection, potentially requiring mechanical ventilation support, has respiratory arrest, organ failure, or potentially requiring continuous renal replacement therapy (CRRT), and has an acidosis and decreased level of consciousness. As this was a retrospective study, no informed consent was deemed necessary, and data were de-identified for the use of this publication.
2.3. Data Collection
Clinical data were collected utilizing standardized data collection forms [Microsoft Word and Excel forms (Microsoft Corp., Redmond, WA, USA)] following ICU admission. The recorded data included the following: age, sex, smoking status, medical comorbidities, and mode of respiratory support (invasive mechanical ventilation, non-invasive mechanical ventilation, and oxygen mask), the use of extracorporeal membrane oxygenation (ECMO), and prone positioning. The number of patients who died, had been discharged, and were still admitted in the ICU as of the end of the study period, were recorded, and the ICU length of stay was also determined.
2.4. Statistical Analysis
Descriptive variables were expressed as a percentage, mean and standard deviation (SD), or median and interquartile range (IQR), as appropriate. Variables across groups using Kruskal–Wallis or one-way Analysis of Variance (ANOVA) for numerical and categorical variables were compared. We assessed the relationship between ARDS severity and discontinuation from mechanical ventilation, ICU discharge, and mortality on day 28. Time to event curves were plotted using the Kaplan–Meier method and analyzed with the log-rank test and univariable Cox regression analysis. The chi-square test was used to determine the association of concomitant medication, heart failure, and liver failure with the course of ARDS severity in COVID-19 positive patients. Lastly, a one-way ANOVA was used to compare the transaminase levels among mild, moderate, and severe ARDS.
3. Results
The data from a total of 1154 COVID-19 positive patients who developed ARDS shows a resulting high prevalence rate of 78.5% where patients had a mean age of 55.9 ± 15.1 years and a mean body mass index (BMI) of 30.1 ± 6.8 kg/m2. In relation to ARDS, 591 (51.2%) had severe, 415 (36.0%) had moderate, and 148 (12.8%) had mild ARDS. Socio-demographic characteristics of the study population are shown in Table 1, including sex, nationality, and smoking habit; neither of them showed a significant difference in relation to the severity of ARDS. Most patients, 847 (73.4%) were males and half of them, 576 (49.9%) were Saudi nationals. All participants were critically ill; 111 (9.6%) of them had co-existing bronchial asthma and 92 (8%) had chronic kidney disease (CKD), with a significant association between severity of ARDS and the co-existence of bronchial asthma and CKD (p-values = 0.006 and 0.027, respectively). The mean sequential organ failure assessment (SOFA) score was significantly higher in COVID-19 patients with severe ARDS (6 ± 5, p-value 0.006) compared to mild and moderate ARDS cases.

Table 1.
Baseline characteristics (n = 1154).
In Table 2, non-invasive respiratory support of high-flow nasal cannula (HFNC) was needed for 219 (37%) of the severe ARDS patients, which was more than the mild and moderate ARDS groups. Similarly, bilevel positive airway pressure (BiPAP) was needed more in severe cases of ARDS (n = 96, 16.2%) when compared to mild and moderate cases. Further, invasive mechanical endotracheal tube (ETT) ventilation was needed by a total of 642 (55.6%) patients, the majority of them suffering from severe ARDS (n = 346, 58.5%).

Table 2.
Interventions during ICU stay (n = 1154).
Table 3 shows some patients that developed medical complications including pulmonary embolism, deep vein thrombosis, and stroke which were observed more in the severe ARDS group, compared to mild and moderate ARDS cases. A longer length of stay in the ICU was observed among patients with severe ARDS, compared to mild and moderate cases. The overall ICU mortality in COVID-19 complicated with ARDS was 488 (42.3%); 273 (46.2%) mortalities were in the severe ARDS group, 169 (40.7%) in the moderate group, and 46 (31.1%) in the mild ARDS group were observed. In the same table, more than half of the patients who received steroid therapy (n = 446, 65.5%) were from the severe ARDS group.

Table 3.
Overall patients’ outcomes (n = 1154).
Multiple logistic regression analysis is shown in Table 4 and revealed: (a) males infected with COVID-19 were more likely than COVID-19 females to develop ARDS (OR 1.165, 95% CI 0.871–1.557; p = 0.303). (b) COVID-19 patients of an older age [≥60 years old] were significantly more likely to develop ARDS (OR 0.987, 95% CI 0.978–0.995; p = 0.002). (c) COVID-19 patients who required high frequency oscillatory ventilation (HFOV) (OR 2.311, 95% CI 1.692–3.155; p = 0.0001), or invasive mechanical ventilation (IMV) (OR 1.660, 95% CI 1.291–2.133; p = 0.0001) were significantly more likely to develop ARDS. Survival analysis applying the Kaplan—Meier curve revealed COVID-19 patients with mild ARDS to have a significantly higher survival rate, compared to those who suffered severe ARDS, p-value 0.03. Figure 1.

Table 4.
Multiple logistic regression comparing the presence of ARDS predicted by demographic and clinical characteristics of patients (n = 1154).
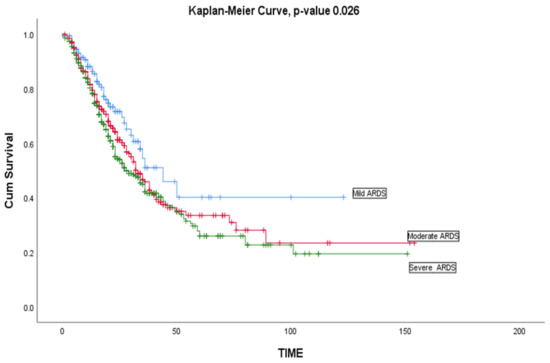
Figure 1.
Kaplan—Meier curve survival of critically ill patients with COVID-19 infection based on ARDS severity (mild ARDS, moderate ARDS, and severe ARDS) (n = 1154).
Multiple chi-square tests shown in Table 5 revealed a significant association between favipiravir and severity of ARDS (p = < 0.00001, 95% CI). A significant association was also seen between Toclizumab and course of severity of ARDS in COVID-19 positive patients (p = 0.049, 95% CI). Furthermore, the association of heart failure and chronic liver disease with the course of severity of ARDS did not show any significance (Table 6). Comparing the liver enzymes Aspartate transaminase (AST) and Alanine transaminase (ALT) (maximum levels shown in Table 7) using a one-way ANOVA showed there was no significant difference between the mild, moderate, and severe ARDS in COVID-19 positive patient groups (see Table 8 and Table 9, respectively).

Table 5.
Association a between medications and the course of ARDS severity (n = 1154).

Table 6.
Association between comorbidities and the course of ARDS severity (n = 1154).

Table 7.
Highest levels of AST and ALT enzyme (n = 1154).

Table 8.
Comparing levels of AST enzyme per ASDS status (n = 1154).

Table 9.
Comparing levels of ALT enzyme per ARDS status (n = 1154).
4. Discussion
The aim of our study was to assess the characteristics and outcomes of ADRS associated with COVID-19 in Saudi hospitals at the time of ICU admission. Our results showed that many patients were males (n = 847, 73.4%) and half of them were Saudi nationals (n = 576, 49.9%) with a mean age of 55.9 ± 15.1 years and a mean body mass index (BMI) of 30.1 ± 6.8 kg/m2. All participants were critically ill. In our study, the male gender predominated the development of ARDS in SARS-CoV-2 patients. Similar findings were suggested in most of the reports from South Korea and Iceland [26,28]. Males infected with COVID-19 were associated with more than a twofold higher risk of suffering ARDS compared to females [29].
In addition, patients with severe ARDS requiring mechanical ventilation are predisposed to acute kidney injury owing to the presence of acute lung injury in them [25,30]. In COVID-19 patients, acute kidney injuries are common clinical presentations affecting approximately 20–40% of patients admitted to ICU [31,32] and about 20% of patients admitted to an ICU with COVID-19 require renal replacement therapy at a median of 15 days from illness onset [33].
The risk factors and clinical characteristics associated with clinical outcomes in COVID-19 pneumonia patients who developed ARDS after admission were reported in this study. The presence of any coexisting illness was more common among patients with severe disease than among those with non-severe disease (38.7% vs. 21.0%) that was reported in China [2]. Consistent with previous reports, we found the development of ARDS in SARS-CoV-2 patients was high in the presence of comorbidities such as bronchial asthma and chronic kidney disease [34,35]. Patients with a predisposition to smoking were surprisingly found to be able to reduce the severity of CARDS owing to the inflection of the innate macrophage responses by nicotine which is an active component of cigarettes [36]. However, in our study it was found that smoking had no significant correlation with the severity of CARDS.
The management of CARDS is highly modulated by the severity of the condition. During the COVID-19 pandemic, in patients with moderate to severe CARDS, oxygenation was provided by the high-flow nasal cannula O2 method (HFNO) [37]. The advantage of this method is that it provides the patients with higher inspired oxygenation levels while it can also provide larger lung volume through the production of a low level of continuous positive airway pressure (CPAP) [38]. A retrospective study on 104 COVID-19 patients with moderate to severe hypoxemia showed that more than half (64%) of them were treated using the HFNO method and avoided the need for mechanical ventilation, thereby reducing the mortality in that group to 2.9% when compared to the 14.4% mortality in the group that required intubation [39]. However, in a multicenter trial on the use of HFNO therapy on ARDS patients, it was seen that although the method reduced the 90-day mortality of patients by 50%, it made no difference in the need for mechanical ventilation [40].
Continuous positive airway pressure (CPAP) or non-invasive positive pressure ventilation (NIPPV) methods have gained prominence during the COVID pandemic, especially in places where there has been a lack of proper ICU infrastructure [41]. All these non-invasive (NIV) methods have been shown to have a significant risk of failure in a global LUNGSAFE study [42]. It is seen that 22.2% of mild ARDS patients, 42% of moderate ARDS, and 47% of severe ARDS patients failed when the abovementioned non-invasive methods were used.
Based on the Berlin definition, stages of mild, moderate, and severe ARDS were associated with increased mortality and increased median duration of mechanical ventilation in the survivors [5]. In severe CARDS patients, invasive respiratory support—which includes endotracheal intubation and mechanical ventilation—is often recommended for patients with persistent respiratory distress or worsening of respiratory hypoxemia (SpO2 < 92%, which is similar to the respiratory management seen in the usual ARDS patients [43,44,45]. The main objective of mechanical ventilation is focused on the strategy to prevent atelectasis-recruitment and tidal-inflation stress, which can lead to ventilation-induced lung injury [46]. The most important measure is the use of low-tidal volumes to minimize atelectasis [47].
In our cohort study, about (n = 642, 56%) of patients initially received non-invasive methods of respiratory support, and (n = 669, 58%) of patients received invasive mechanical ventilation, which was less than that reported in observational studies from China [2], New York [48], Washington state [49], and Italy [50] (67%, 79%, 71%, and 88%), respectively. Our study demonstrated the SOFA score at admission. The results found a total SOFA score of 6 ± 4 with a mortality rate of 46% for severe ARDS patients and a SOFA score of 5 ± 3 for both mild and moderate cases with a mortality rate of 41 % and 31%, respectively. When compared with other studies, the United Arab Emirates reported a SOFA score of ICU patients of 4 (3–7) with a mortality rate of 22% [51]. In the European study of COVID-19, ICU group patients had a SOFA score of 5 (3–8) [52]. In a Spanish study, critically ill patients had a SOFA score of 5 (3–7), and the overall mortality rate was 31% [53]. A SOFA score of 4 (2–8) at admission was reported in a prospective multicenter study of COVID-19 critical care patients that was conducted in China [54].
Corticosteroids have significant anti-inflammatory and antifibrotic properties in relieving pulmonary and systemic injury in ARDS patients [55]. Several studies have found that corticosteroids can improve the clinical condition of patients suffering from severe acute respiratory syndrome [56,57]. One large randomized controlled trial from the United Kingdom (RECOVERY) showed that treating COVID-19 patients with intravenous corticosteroids (dexamethasone) for 10 days improved the survival [58]. The incidence of death was higher in the control group (41.4%) when compared to patients receiving dexamethasone (29.3%) among patients who were undergoing invasive mechanical ventilation. Among patients who received oxygen with no invasive mechanical ventilation, the death rate among the control group (26.2%) was again higher when compared to the dexamethasone group (23.3%). However, further trials need to be run to see if these results can be replicated and a further understanding of the side effects of corticosteroid use needs to be investigated. In addition, the effectiveness of corticosteroids in viral ARDS remains controversial [59,60]. Since the syndrome’s first clinical diagnosis, corticosteroids have been the most used drugs [61]. Clinicians commonly used adjunctive corticosteroids to decrease hyperinflammation in COVID-19 patients, particularly those with critical disease [2,62]. Our results showed that severe ARDS patients received corticosteroids at (n = 292, 65.5%), which is higher than what was reported in a European study, where only 46% of the severe ARDS patients received corticosteroids [52].
In our study, the incidence rate of pneumothorax has been found to vary between 5.8% and 7% in patients with mild to severe ARDS due to COVID-19. The pneumothorax incidence rate from our study is in line with the Özdemir et al. study that reported a 7.5% incidence among patients in the ICU who underwent intubation following the diagnosis of COVID-19 pneumonia [40,63], and is less than the incidence of pneumothorax of 10% that was reported in a Chinese study. We report a higher incidence rate of spontaneous pneumothorax (6.9%) in severe SARS-CoV-2 patients treated due to ARDS compared to the rates reported by one study in Hong Kong (1.3%) [64].
Venous thromboembolism is common in patients receiving COVID-19 treatment for ARDS, and it is associated with a high mortality rate [65]. Patients with COVID-19 manifest a hypercoagulable state, often resulting in deep vein thrombosis or pulmonary embolism [66]. Our results outline that the occurrence of pulmonary embolisms varies from 1.4% to 3.2 % depending on the severity of ARDS. One prospective cohort reported only 1.3% of pulmonary embolisms in critically ill patients [67], which is in contrast to the high prevalence of pulmonary embolisms reported (16.7%) in a cohort of patients admitted to the ICU for hypoxemic acute respiratory failure due to COVID-19 [66]. Pulmonary embolism occurrence in COVID-19 patients who suffered ARDS when matched with non-COVID-19 patients who never experienced ARDS was observed to be high (11.7% vs. 2.1%, p < 0.008) despite prophylactic anticoagulation [66].
The Kaplan—Meier median duration of survival was 9 days. The observed median length of stay was 8 days for mild ARDS cases, 9 days for moderate ARDS cases, and 10 days for severe ARDS cases. Survival analysis applying the Kaplan—Meier curve revealed COVID-19 patients with mild ARDS have a significantly higher survival rate compared to those with severe ARDS, (p-value 0.023) which is consistent with an American study [68].
The severity of CARDS patients varies greatly, with no standardized effective therapies [69,70]. Unfortunately, during the period of this study, no definite drug was available for CARDS that was capable of reducing either short-term or long-term mortality [71]. Thus, apart from corticosteroids, the study examined the association of some concomitant medications used for the treatment of COVID-19 with the course of severity of ARDS. Only Favipiravir and Tocilizumab showed a significant association with the course of severity of ARDS in COVID-19 positive patients. Though not directly used for CARDS management, favipiravir was reported to show a higher viral clearance in a preliminary double-blinded randomized controlled trial with 57 hospitalized COVID-19 patients, where assessment of chest X-rays showed remarkable improvement of pneumonia patients receiving Favipiravir [72]. Similarly, the efficacy of Tocilizumab was demonstrated through several studies on COVID-19 positive patients with ARDS. A study on 79 patients with severe COVID-19 pneumonia and worsening acute respiratory failure (ARF), showed that Tocilizumab treatment may be effective in COVID-19 patients with severe respiratory impairment receiving non-invasive ventilation [70]. In yet another study, Tocilizumab for the treatment of mechanically ventilated patients with COVID-19, included 154 patients, and showed an association of Tocilizumab with a lower mortality despite higher superinfection occurrence. Furthermore, as COVID-19 causes severe respiratory and multiorgan failure, including liver damage and elevated transaminase levels, this study examined the association of heart failure and liver damage to the course of severity of ARDS in COVID-19 positive patients. There was no significant association of neither heart failure nor liver damage with the severity of ARDS in COVID-19 positive patients. Though there are multiple studies suggesting patients with heart failure (HF) are at a higher risk of severe disease and mortality with COVID-19 [73], reports on the association of heart failure to the severity of ARDS in COVID-19 positive patients are limited. Additionally, liver-related comorbidities were not common among the patients with COVID-19 pneumonia and their frequency was about 3% [74]. Very low expression of the ACE2 receptor in hepatocytes and bile duct epithelium [75,76] possibly explain the small influence of viral infection in the liver [77]. Lastly, comparing the transaminase levels among mild, moderate, and severe ARDS patients with COVID-19 showed no significance. Analogous results were reported through a meta-analysis of 60 studies that concluded in critically ill and fatal cases, no difference was found in the prevalence of elevated ALT or AST [78].
This study has several strengths. First, our study represents one of the largest multicenter retrospective cohorts of patients with COVID-19 associated critical illness reported in Saudi Arabia. Second, our findings mirror the ongoing outbreak of COVID-19 in Saudi Arabia in addition to the data being collected using a large Ministry of Health electronic health record system. Lastly, data analyses were conducted with near-complete data.
5. Limitations
Though the present study was a multicenter study that included a large cohort, the retrospective nature of the study stalls the possibility of differences in the quality of care affecting the patients’ recovery which rises as a limitation of the study. As the ventilation management was not standardized, it could lead to data variability. Furthermore, analyzing vaccination history of the patient could have added insight on their immunity and resistance to severity of the disease. In addition, comparing COVID-19 ARDS with concurrent non-COVID-19 ARDS could have provided broader insights to CARDS management.
6. Conclusions
The study results revealed that ARDS is a challenging critical care condition complicating COVID-19 at a high rate, resulting in significant morbidity and mortality. ARDS requires protective mechanical ventilation and other critical care supportive measures. The severity of ARDS is associated significantly with the mortality rate among COVID-19 critically ill patients.
Author Contributions
Conceptualization, A.A.M.; validation, A.A.M. and S.A. (Saad Alhumaid); resources, A.A.M.; data curation, A.A.M., A.A.R., G.Y.A. and S.A. (Saad Alhumaid); writing—original draft preparation, L.L., J.S., K.A., W.N.A., A.A.M., A.W. and S.A. (Saad Alhumaid); writing—review and editing, S.A. (Sana Almahmoud), H.C., K.T., J.A.A.-T., J.M., L.H.A.-J. and F.M.A.; visualization, A.A.M.; supervision, A.A.R. and A.A.M.; project administration, A.A.M.; funding acquisition, A.A.M., S.A. (Saad Alhumaid), A.A.R., M.A., M.A.M., A.A., A.M.A., H.F.A., C.S. and S.A. (Sana Almahmoud). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study obtained approval from the Sulaiman Al Habib Medical Group Institutional Review Board (RC20.04.81). The study was performed in accordance with the Declaration of Helsinki.
Informed Consent Statement
Consent to participate was waived by the IRB committee due to the project’s descriptive nature (no new unapproved intervention). All patients’ information was anonymized, and the publication did not include images that may identify the person.
Data Availability Statement
Data available on request due to restrictions.
Acknowledgments
The authors would like to thank all the ICU doctors and nurses who fought against COVID-19 and supported this research.
Conflicts of Interest
All authors have no conflicts of interest to declare.
References
- WHO Coronavirus Disease (COVID-19) Dashboard; World Health Organization: Geneva, Switzerland, 2020. Available online: https://covid19.who.int/ (accessed on 1 June 2021).
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Domecq, J.P.; Lal, A.; Sheldrick, C.R.; Kumar, V.K.; Boman, K.; Bolesta, S.; Bansal, V.; Harhay, M.O.; Garcia, M.A.; Kaufman, M.; et al. Outcomes of Patients with Coronavirus Disease 2019 Receiving Organ Support Therapies: The International Viral Infection and Respiratory Illness Universal Study Registry. Crit. Care Med. 2021, 49, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Shrestha, G.S.; Khanal, S.; Sharma, S.; Nepal, G. COVID-19: Current Understanding of Pathophysiology. J. Nepal Health Res. Counc. 2020, 18, 351–359. [Google Scholar] [CrossRef]
- Zambon, M.; Vincent, J.L. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest 2008, 133, 1120–1127. [Google Scholar] [CrossRef]
- Sedhai, Y.R.; Yuan, M.; Ketcham, S.W.; Co, I.; Claar, D.D.; McSparron, J.I.; Prescott, H.C.; Sjoding, M.W. Validating Measures of Disease Severity in Acute Respiratory Distress Syndrome. Ann. Am. Thorac Soc. 2021, 18, 1211–1218. [Google Scholar] [CrossRef]
- Gajic, O.; Dabbagh, O.; Park, P.K.; Adesanya, A.; Chang, S.Y.; Hou, P.; Anderson, H., 3rd; Hoth, J.J.; Mikkelsen, M.E.; Gentile, N.T.; et al. Early identification of patients at risk of acute lung injury: Evaluation of lung injury prediction score in a multicenter cohort study. Am. J. Respir. Crit. Care Med. 2011, 183, 462–470. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.S.; Lal, C.V.; Li, J.D.; Lou, X.Y.; Viera, L.; Abdallah, T.; King, R.W.; Sethi, J.; Kanagarajah, P.; Restrepo-Jaramillo, R.; et al. The neutrophil chemoattractant peptide proline-glycine-proline is associated with acute respiratory distress syndrome. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 315, L653–L661. [Google Scholar] [CrossRef]
- Huang, D.; Ma, H.; Xiao, Z.; Blaivas, M.; Chen, Y.; Wen, J.; Guo, W.; Liang, J.; Liao, X.; Wang, Z.; et al. Diagnostic value of cardiopulmonary ultrasound in elderly patients with acute respiratory distress syndrome. BMC Pulm. Med. 2018, 18, 136. [Google Scholar] [CrossRef]
- Cherian, S.V.; Kumar, A.; Akasapu, K.; Ashton, R.W.; Aparnath, M.; Malhotra, A. Salvage therapies for refractory hypoxemia in ARDS. Respir. Med. 2018, 141, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.; Chen, L.; Lu, C.; Zhang, W.; Xia, J.A.; Sklar, M.C.; Du, B.; Brochard, L.; Qiu, H. Lung Recruitability in COVID-19-associated acute respiratory distress syndrome: A Single-Center Observational Study. Am. J. Respir. Crit. Care Med. 2020, 201, 1294–1297. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Al Mutair, A.; Al Mutairi, A.; Alhumaid, S.; Maaz Abdullah, S.; Zia Zaidi, A.R.; Rabaan, A.A.; Al-Omari, A. Examining and investigating the impact of demographic characteristics and chronic diseases on mortality of COVID-19: Retrospective study. PLoS ONE 2021, 16, e0257131. [Google Scholar] [CrossRef]
- Al Mutair, A.; Al Mutairi, A.; Zaidi, A.R.Z.; Salih, S.; Alhumaid, S.; Rabaan, A.A.; Al-Omari, A. Clinical Predictors of COVID-19 Mortality Among Patients in Intensive Care Units: A Retrospective Study. Int. J. Gen. Med. 2021, 14, 3719–3728. [Google Scholar] [CrossRef]
- Almoosa, Z.; Saad, M.; Qara, S.; Mustafa, M.; Mansour, A.; Alshab, D.; Alhashem, J.; ALKhawajah, S.; Alkhalifah, S.; ALmarzooq, M.; et al. Favipiravir versus standard of care in patients with severe COVID-19 infections: A retrospective comparative study. J. Infect Public Health 2021, 14, 1247–1253. [Google Scholar] [CrossRef]
- Alhumaid, S.; Al Mutair, A.; Al Alawi, Z.; Al Salman, K.; Al Dossary, N.; Omar, A.; Alismail, M.; Al Ghazal, A.M.; Jubarah, M.B.; Al Shaikh, H. Clinical features and prognostic factors of intensive and non-intensive 1014 COVID-19 patients: An experience cohort from Alahsa, Saudi Arabia. Eur. J. Med. Res. 2021, 26, 47. [Google Scholar] [CrossRef]
- Sun, P.; Qie, S.; Liu, Z.; Ren, J.; Li, K.; Xi, J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: A single arm meta-analysis. J. Med. Virol. 2020, 92, 612–617. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Deng, H.; Ou, C.; Liang, J.; Wang, Y.; Jiang, M.; Li, S. Clinical symptoms, comorbidities and complications in severe and non-severe patients with COVID-19: A systematic review and meta-analysis without cases duplication. Medicine 2020, 99, e23327. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Young, B.E.; Ong, S.W.X.; Kalimuddin, S.; Low, J.G.; Tan, S.Y.; Loh, J.; Ng, O.T.; Marimuthu, K.; Ang, L.W.; Mak, T.M.; et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA 2020, 323, 1488–1494. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020, 46, 854–887. [Google Scholar] [CrossRef] [Green Version]
- Coronavirus Disease-19: The First 7755 Cases in the Republic of Korea. Osong Public Health Res. Perspect. 2020, 11, 85–90, Erratum in Osong Public Health Res. Perspect. 2020, 11, 146. [CrossRef] [Green Version]
- Gudbjartsson, D.F.; Helgason, A.; Jonsson, H.; Magnusson, O.T.; Melsted, P.; Norddahl, G.L.; Saemundsdottir, J.; Sigurdsson, A.; Sulem, P.; Agustsdottir, A.B.; et al. Spread of SARS-CoV-2 in the Icelandic Population. N. Engl. J. Med. 2020, 382, 2302–2315. [Google Scholar] [CrossRef]
- Henry, B.M. COVID-19, ECMO, and lymphopenia: A word of caution. Lancet Respir. Med. 2020, 8, e24. [Google Scholar] [CrossRef]
- Coronavirus Disease-19: Summary of 2370 Contact Investigations of the First 30 Cases in the Republic of Korea. Osong Public Health Res. Perspect. 2020, 11, 81–84. [CrossRef] [Green Version]
- Hoste, E.A.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef]
- Hirsch, J.S.; Ng, J.H.; Ross, D.W.; Sharma, P.; Shah, H.H.; Barnett, R.L.; Hazzan, A.D.; Fishbane, S.; Jhaveri, K.D. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020, 98, 209–218. [Google Scholar] [CrossRef]
- Zahid, U.; Ramachandran, P.; Spitalewitz, S.; Alasadi, L.; Chakraborti, A.; Azhar, M.; Mikhalina, G.; Sherazi, A.; Narh, J.T.; Khattar, P. Acute kidney injury in COVID-19 patients: An inner city hospital experience and policy implications. Am. J. Nephrol. 2020, 51, 786–796. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Xu, W.; Sun, N.-N.; Gao, H.-N.; Chen, Z.-Y.; Yang, Y.; Ju, B.; Tang, L.-L. Risk factors analysis of COVID-19 patients with ARDS and prediction based on machine learning. Sci. Rep. 2021, 11, 2933. [Google Scholar] [CrossRef] [PubMed]
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Patidar, R.; Younis, K.; Desai, P.; Hosein, Z.; Padda, I.; Mangat, J.; Altaf, M. Comorbidity and its impact on patients with COVID-19. SN Compr. Clin. Med. 2020, 2, 1069–1076. [Google Scholar] [CrossRef]
- Kloc, M.; Ghobrial, R.M.; Kubiak, J.Z. How nicotine can inhibit cytokine storm in the lungs and prevent or lessen the severity of COVID-19 infection? Immunol. Lett. 2020, 224, 28–29. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, W.; Li, J.; Shu, W.; Duan, J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann. Intensive Care 2020, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, M. High-flow nasal cannula oxygen therapy in adults. J. Intensive Care 2015, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.; Gangemi, A.; Marron, R.; Chowdhury, J.; Yousef, I.; Zheng, M.; Mills, N.; Tragesser, L.; Giurintano, J.; Gupta, R.; et al. Retrospective analysis of high flow nasal therapy in COVID-19-related moderate-to-severe hypoxaemic respiratory failure. BMJ Open Respir. Res. 2020, 7, e000650. [Google Scholar] [CrossRef]
- Frat, J.-P.; Thille, A.W.; Mercat, A.; Girault, C.; Ragot, S.; Perbet, S.; Prat, G.; Boulain, T.; Morawiec, E.; Cottereau, A. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N. Engl. J. Med. 2015, 372, 2185–2196. [Google Scholar] [CrossRef] [Green Version]
- Winck, J.C.; Ambrosino, N. COVID-19 pandemic and non invasive respiratory management: Every Goliath needs a David. An evidence based evaluation of problems. Pulmonology 2020, 26, 213–220. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Madotto, F.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Bumbasirevic, V.; Piquilloud, L.; et al. Noninvasive Ventilation of Patients with Acute Respiratory Distress Syndrome. Insights from the LUNG SAFE Study. Am. J. Respir. Crit. Care Med. 2017, 195, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Bhimraj, A.; Morgan, R.L.; Shumaker, A.H.; Lavergne, V.; Baden, L.; Cheng, V.C.; Edwards, K.M.; Gandhi, R.; Muller, W.J.; O’Horo, J.C.; et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin. Infect. Dis. 2020, ciaa478. [Google Scholar] [CrossRef]
- Papazian, L.; Aubron, C.; Brochard, L.; Chiche, J.-D.; Combes, A.; Dreyfuss, D.; Forel, J.-M.; Guérin, C.; Jaber, S.; Mekontso-Dessap, A.; et al. Formal guidelines: Management of acute respiratory distress syndrome. Ann. Intensive Care 2019, 9, 69. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit. Care Med. 2017, 45, 486–552. [Google Scholar] [CrossRef]
- Beitler, J.R.; Malhotra, A.; Thompson, B.T. Ventilator-induced Lung Injury. Clin. Chest Med. 2016, 37, 633–646. [Google Scholar] [CrossRef]
- Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar]
- Cummings, M.J.; Baldwin, M.R.; Abrams, D.; Jacobson, S.D.; Meyer, B.J.; Balough, E.M.; Aaron, J.G.; Claassen, J.; Rabbani, L.E.; Hastie, J.; et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 2020, 395, 1763–1770. [Google Scholar] [CrossRef]
- Arentz, M.; Yim, E.; Klaff, L.; Lokhandwala, S.; Riedo, F.X.; Chong, M.; Lee, M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA 2020, 323, 1612–1614. [Google Scholar] [CrossRef] [Green Version]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, A.; Hamed, F.; Saleh, K.; Abduljawad, B.; Mallat, J. ICU outcomes of COVID-19 critically ill patients: An international comparative study. Anaesth. Crit. Care Pain Med. 2020, 39, 487–489. [Google Scholar] [CrossRef]
- COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med. 2021, 47, 60–73. [Google Scholar] [CrossRef]
- Ferrando, C.; Mellado-Artigas, R.; Gea, A.; Arruti, E.; Aldecoa, C.; Bordell, A.; Adalia, R.; Zattera, L.; Ramasco, F.; Monedero, P.; et al. Patient characteristics, clinical course and factors associated to ICU mortality in critically ill patients infected with SARS-CoV-2 in Spain: A prospective, cohort, multicentre study. Rev. Esp. Anestesiol. Reanim. 2020, 67, 425–437. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, D.; Fu, S.; Zhang, J.; Yang, X.; Xu, L.; Xu, J.; Wu, Y.; Huang, C.; Ouyang, Y.; et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: A cross-sectional study. Crit. Care 2020, 24, 219. [Google Scholar] [CrossRef]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory action of glucocorticoids—New mechanisms for old drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef] [Green Version]
- Peiris, J.S.; Chu, C.M.; Cheng, V.C.; Chan, K.S.; Hung, I.F.; Poon, L.L.; Law, K.I.; Tang, B.S.; Hon, T.Y.; Chan, C.S.; et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: A prospective study. Lancet 2003, 361, 1767–1772. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Zhang, F.; Xu, M.; Huang, K.; Zhong, W.; Cai, W.; Yin, Z.; Huang, S.; Deng, Z.; Wei, M.; et al. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. J. Med. Microbiol. 2003, 52, 715–720. [Google Scholar] [CrossRef]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- Villar, J.; Confalonieri, M.; Pastores, S.M.; Meduri, G.U. Rationale for Prolonged Corticosteroid Treatment in the Acute Respiratory Distress Syndrome Caused by Coronavirus Disease 2019. Crit. Care Explor. 2020, 2, e0111. [Google Scholar] [CrossRef] [Green Version]
- Russell, C.D.; Millar, J.E.; Baillie, J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020, 395, 473–475. [Google Scholar] [CrossRef] [Green Version]
- Ashbaugh, D.G.; Bigelow, D.B.; Petty, T.L.; Levine, B.E. Acute respiratory distress in adults. Lancet 1967, 2, 319–323. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Du, C.; Zhang, Y.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özdemir, S.; Bilgi, D.Ö.; Köse, S.; Oya, G. Pneumothorax in patients with coronavirus disease 2019 pneumonia with invasive mechanical ventilation. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-H.; Duan, J.; Han, X.; Liu, X.; Zhou, J.; Wang, X.; Zhu, L.; Mou, H.; Guo, S. High incidence and mortality of pneumothorax in critically Ill patients with COVID-19. Heart Lung 2021, 50, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Sihoe, A.D.; Wong, R.H.; Lee, A.T.; Lau, L.S.; Leung, N.Y.; Law, K.I.; Yim, A.P. Severe acute respiratory syndrome complicated by spontaneous pneumothorax. Chest 2004, 125, 2345–2351. [Google Scholar] [CrossRef] [Green Version]
- Llitjos, J.F.; Leclerc, M.; Chochois, C.; Monsallier, J.M.; Ramakers, M.; Auvray, M.; Merouani, K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020, 18, 1743–1746. [Google Scholar] [CrossRef]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef]
- Lim, W.; Meade, M.; Lauzier, F.; Zarychanski, R.; Mehta, S.; Lamontagne, F.; Dodek, P.; McIntyre, L.; Hall, R.; Heels-Ansdell, D.; et al. Failure of anticoagulant thromboprophylaxis: Risk factors in medical-surgical critically ill patients*. Crit. Care Med. 2015, 43, 401–410. [Google Scholar] [CrossRef]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345–1355. [Google Scholar] [CrossRef]
- Menzella, F.; Fontana, M.; Salvarani, C.; Massari, M.; Ruggiero, P.; Scelfo, C.; Barbieri, C.; Castagnetti, C.; Catellani, C.; Gibellini, G.; et al. Efficacy of tocilizumab in patients with COVID-19 ARDS undergoing noninvasive ventilation. Crit. Care 2020, 24, 589. [Google Scholar] [CrossRef]
- Matera, M.G.; Rogliani, P.; Calzetta, L.; Cazzola, M. Pharmacological management of COVID-19 patients with ARDS (CARDS): A narrative review. Respir. Med. 2020, 171, 106114. [Google Scholar] [CrossRef]
- Rahman, S.M.A.; Kabir, A.; Abdullah, A.B.M.; Alam, M.B.; Azad, K.A.K.; Miah, M.T.; Mowla, S.G.M.; Deb, S.R.; Amin, M.R.; Asaduzzaman, M.; et al. Safety and efficacy of favipiravir for the management of COVID-19 patients: A preliminary randomized control trial. Clin. Infect. Pract. 2022, 15, 100145. [Google Scholar] [CrossRef]
- Zhang, Y.; Coats, A.J.S.; Zheng, Z.; Adamo, M.; Ambrosio, G.; Anker, S.D.; Butler, J.; Xu, D.; Mao, J.; Khan, M.S.; et al. Management of heart failure patients with COVID-19: A joint position paper of the Chinese Heart Failure Association & National Heart Failure Committee and the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 941–956. [Google Scholar]
- Mantovani, A.; Beatrice, G.; Dalbeni, A. Coronavirus disease 2019 and prevalence of chronic liver disease: A meta-analysis. Liver Int. 2020, 40, 1316–1320. [Google Scholar] [CrossRef] [Green Version]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Bahardoust, M.; Heiat, M.; Khodabandeh, M.; Karbasi, A.; Bagheri-Hosseinabadi, Z.; Ataee, M.H.; Seidalian, N.; Babazadeh, A.; Agah, S.; Abyazi, M.A. Predictors for the severe coronavirus disease 2019 (COVID-19) infection in patients with underlying liver disease: A retrospective analytical study in Iran. Sci. Rep. 2021, 11, 3066. [Google Scholar] [CrossRef]
- Grace, J.A.; Herath, C.B.; Mak, K.Y.; Burrell, L.M.; Angus, P.W. Update on new aspects of the renin-angiotensin system in liver disease: Clinical implications and new therapeutic options. Clin. Sci. 2012, 123, 225–239. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Yang, Y.; Gao, D.; Xu, Y.; Gu, J.; Liu, P. Is liver involvement overestimated in COVID-19 patients? A meta-analysis. Int. J. Med. Sci. 2021, 18, 1285–1296. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).