Abstract
We have kinetically estimated the enzymatic redox reaction at the horseradish peroxidase (HRP)-modified electrode combined with ionic liquids by adding N-(2-methoxythethyl)-N-methylpyrrolidinium bis(trifluoromethane sulfonyl)imide (MEMPTFSI) to HRP/carbon paste (CP)/Ketjenblack EC600JC (EC). The fluctuation of the steady-state reduction current of HRP at the HRP/CP-modified electrode progressively increased as the applied potential was lowered. The enzymatic redox reaction with hydrogen peroxide as a substrate at the HRP/CP/EC/MEMPTFSI-modified electrode and the HRP/CP-modified electrode could be correlated by the Michaelis–Menten equation. The Michaelis constant of the enzymatic redox reaction at the HRP/CP/EC/MEMPTFSI-modified electrode was the same as that at the HRP/CP-modified electrode. On the other hand, the turnover number of the enzymatic redox reaction at the HRP/CP/EC/MEMPTFSI-modified electrode was six times larger than that at the HRP/CP-modified electrode. Consequently, the specificity constant of the enzymatic redox reaction at the HRP/CP/EC/MEMPTFSI-modified electrode was much higher than that at the HRP/CP-modified electrode.
1. Introduction
To reduce emissions of greenhouse gases such as CO2, bioprocesses have received attention as a potential alternative to conventional chemical processes, which require large amounts of energy and non-renewable resources such as oil [1]. Bioprocesses generally include enzymes, which are biocatalysts that exhibit high biological activity and specificity under mild conditions, and they have been widely used in applications such as synthesis, sensing, and fuel cells [1,2,3,4,5]. Specifically, the oxidoreductase family of enzymes is useful for designing biotransformations and biosensors [6,7,8,9,10,11,12,13,14,15]. Oxidoreductases can be used to catalyze polymerization, coupling, hydroxylation, and oxygen-transfer reactions, as well as to convert CO2 into fuel. Moreover, by detecting hydrogen peroxide or other peroxides, oxidoreductases can be used as molecular-recognition elements in biosensors for diagnosis, analysis, environmental protection, and security surveillance.
Enzymes, used as catalysts and recognition elements in biotransformations and biosensors, are typically immobilized by attaching themselves to various insoluble carriers. This stabilizes the enzymes and allows for their recycling [16,17,18]. Various immobilization methods can be used to fabricate biocatalyst electrodes [19]. Carbon-paste (CP) electrodes have been widely investigated because of their ease of fabrication. Enzymes are generally incorporated into CP by mixing them with a CP oil consisting of uniformly sized graphite particles and paraffin oil [20,21,22]. We have reported that the electrochemical activity of enzymes tends to be inhibited by the impeded electron transfer and low accessibility of mediators because enzymes are embedded within electrodes by insulating paraffin oil [21]. On the other hand, enzyme-modified electrodes receiving the electrons from electrode directly for the redox reaction of enzymes usually belong to the direct electron transfer (DET). A significant challenge with direct electron transfer (DET) enzyme-modified electrodes is the difficulty in transferring electrons from the electrode to the enzyme’s redox sites, as these sites are typically deeply embedded within the enzyme’s core [23].
Ionic liquids, which are salts in the liquid state at room temperature, have increasingly attracted attention as an innovative non-aqueous medium for chemical processes, because of their lack of vapor pressure, high thermal and chemical stability, excellent ionic conductivity, wide potential window, and high polarity [24,25]. In addition, the activity of enzymes is highly accelerated in water-immiscible ionic liquids, and the thermal stability of enzymes is dramatically enhanced by adding ionic liquids to an aqueous solution containing enzymes [26,27,28,29,30]. We discovered that incorporating enzymes into CP with ionic liquids effectively promotes the redox reaction of mediators at the horseradish peroxidase (HRP)-modified electrode under irreversible electrochemical reaction conditions [31]. Furthermore, to apply the property of oxidoreductase-modified electrodes composed with ionic liquids to biotransformations and biosensors, it is important to kinetically investigate the enzymatic redox reaction at the oxidoreductase-modified electrode combined with ionic liquids [32].
In this study, we have kinetically estimated the enzymatic redox reaction with oxidoreductases immobilized on the electrode consisting of carbon paste (CP), Ketjenblack EC600JC (EC), and ionic liquid. We have used HRP as an oxidoreductase because its structure, functions, and properties have been well known [33]. As an ionic liquid, we have used N-(2-methoxythethyl)-N-methylpyrrolidinium bis(trifluoromethane sulfonyl)imide (MEMPTFSI), which is hydrophobic and has a high ionic conductivity [24]. Moreover, since MEMPTFSI has (CF3SO2)2N− as an anion instead of a carboxylate anion that binds to the sixth coordination position of Fe(III) in HRP, a substrate such as hydrogen peroxide is considered to be easily accessible to the active site of HRP [34,35].
2. Materials and Methods
2.1. Materials
Horseradish peroxidase (HRP) (100 units/mg) and 67 mM phosphate-buffered solution (pH 7.0) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Room-temperature ionic liquid N-(2-methoxythethyl)-N-methylpyrrolidinium bis(trifluoromethane sulfonyl)imide (MEMPTFSI) was purchased from Kanto Chemical Co., Inc. (Tokyo, Japan). Ketjenblack (EC600JC: EC) was purchased from Lion Specialty Chemicals Co., Ltd. (Tokyo, Japan). Carbon-paste oil (CPO: CP) prepared by mixing uniformly sized graphite powder and paraffin oil was purchased from BAS (Tokyo, Japan). Hydrogen peroxide (H2O2) (27% w/w aq. Soln., stab.) was purchased from Alfa Aesar (Heysham, UK). All other reagents were of an analytical grade and were used as received without further purification.
2.2. Preparation of HRP-Modified Electrode
The HRP/CP/EC/MEMPTFSI-modified electrode and the HRP/CP-modified electrode were prepared as follows. First, HRP/CP/EC/MEMPTFSI and HRP/CP mixtures were prepared by thoroughly mixing each component in a mortar at weight ratios of 1.00:9.45:1.23:8.32 and 1.00:19, respectively. The mixtures were then packed into a carbon-paste electrode hole (1.6 mm inner diameter, 3 mm outer diameter, 3 mm depth) to prepare the HRP/CP/EC/MEMPTFSI-modified electrode and the HRP/CP-modified electrode. After packing, electrode surfaces were smoothed with a piece of copy paper before electrochemical measurements were performed.
All prepared electrodes were stored at 4 °C in a refrigerator when not in use. All enzyme-modified electrodes were also soaked in a phosphate-buffered solution (67 mM, pH 7.0) for 3 h before electrochemical measurements. The storage stability of enzyme-modified electrodes was perfectly maintained after one month.
2.3. Instruments
Electrochemical experiments were performed using a three-electrode system comprising an HRP-modified electrode as a working electrode, a platinum wire as a counter electrode, and a saturated Ag/AgCl electrode (sat. KCl) as a reference electrode. Unless stated otherwise, a phosphate-buffered solution (67 mM, pH 7.0) was used as an aqueous electrolytic solution. All phosphate-buffered solutions were purged with nitrogen gas for at least 30 min prior to recording chronoamperograms, and a nitrogen atmosphere was maintained throughout the experiment.
All measurements were carried out in an 8 mL electrochemical cell (model VB3; EC Frontier, Kyoto, Japan) at room temperature (25 °C) under a constant stirring condition within an electrically shielded box. The electrochemical cell was connected to a potentiostat/galvanostat (Princeton Applied Research model 263A; EG&G Instruments, Princeton, NJ, USA). To investigate the chronoamperometric response performance, the HRP-modified electrode was polarized toward the negative direction at a difference potential (vs. Ag/AgCl). After the procedures, both the HRP/CP-modified electrode and the HRP/CP/EC/MEMPTFSI-modified electrode were polarized at −0.4 V (vs. Ag/AgCl) for the enzymatic redox reaction with H2O2 as a substrate.
3. Results
3.1. Chronoamperometric Response Performance at the HRP/CP-Modified Electrode under Applied Potential Control
Most enzymes are easily denatured and inactivated under various physical and chemical stresses due to the disruption of weak interactions, including ionic bonds, hydrogen bonds, and hydrophobic interactions, which are prime determinants of enzyme tertiary structures [32]. Enzymes tend to be denatured and inactivated under external stress such as an electric field [36]. Accordingly, to prevent HRP denaturation and deactivation, it is preferable to use HRP when the applied reduction potential is applied toward the more positive potential direction.
In order to investigate the influence of the applied potential on the stability of HRP at the HRP/CP-modified electrode, the chronoamperometric experiment was carried out at the HRP/CP-modified electrode under the applied potential control. After soaking the HRP/CP-modified electrode in a 67 mM phosphate-buffered solution (pH 7.0) for about 3 h, the chronoamperometry was carried out at different applied potentials (vs. Ag/AgCl) in 2 mL of 67 mM phosphate-buffered solution (pH 7.0) saturated by nitrogen gas under a constant stirring condition. The time-dependence of steady-state currents was examined at regular voltage intervals, with the applied potential ranging from 0.15 V to −0.8 V (vs. Ag/AgCl). Figure 1a shows the chronoamperograms of the HRP/CP-modified electrode under different applied potentials from 0.15 V to −0.8 V (vs. Ag/AgCl) at regular voltage intervals of 0.05 V. Figure 1b shows a plot of the average steady-state currents obtained from 80 to 100 s in each chronoamperogram (Figure 1a) against the applied potential (E).
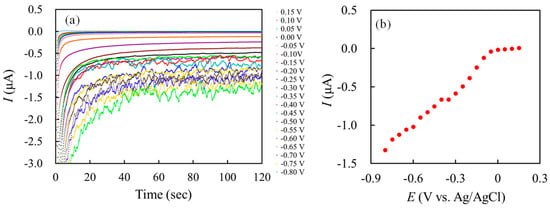
Figure 1.
(a) The chronoamperograms of the HRP/CP-modified electrode under different applied potentials from 0.15 V to −0.8 V (vs. Ag/AgCl) at regular voltage intervals of 0.05 V. (b) A plot of average steady-state currents obtained from 80 to 100 s in each chronoamperogram versus the applied potential.
As shown in Figure 1a, the fluctuation of the steady-state reduction current became progressively greater as the applied potential became lower. The steady-state reduction currents almost remained at invariance with a decrease in the applied potential until around 0 V vs. Ag/AgCl, and gradually increased with decreasing the applied potential, as shown in Figure 1b.
HRP belongs to an oxidoreductase that catalyzes enzymatic redox reactions with coenzymes and/or mediators [21,23,31]. Furthermore, HRP as an oxidoreductase can carry out the redox reactions without coenzymes and/or mediators via DET from the electrodes [21,23]. Consequently, the redox reaction of HRP at the HRP/CP-modified electrode can be carried out via the DET, as follows [21,23,37].
[HRP(FeIII)]Ferric enzyme + e− + H+ ↔ [HRP(FeII) − H+]Ferrous enzyme
The immobilization of enzymes on the surface of carriers strongly affects the performance of enzymes [18]. For instance, when HRP molecules are arranged on the electrode surface in either a side-on or end-on orientation (as depicted in Scheme 1), the distance between the iron heme group of HRP (the active site) and the electrode surface is shorter in a side-on orientation than in an end-on orientation. Since the iron heme group of HRP is encapsulated by a thick shell of insulating protein, which impedes the smooth transfer of electrons [21,35], the DET between the surface of electrodes and the iron heme groups of HRP is facilitated in a side-on orientation compared to an end-on orientation. The reduction reaction of HRP on the HRP/CP-modified electrode occurs via the DET between the surface of electrodes and the iron heme groups of HRP (Equation (1)). Hence, the reduction reaction of HRP in a side-on orientation is more efficient than that in an end-on orientation. Additionally, these variations in distance could also influence the applied potential needed for electron movement.
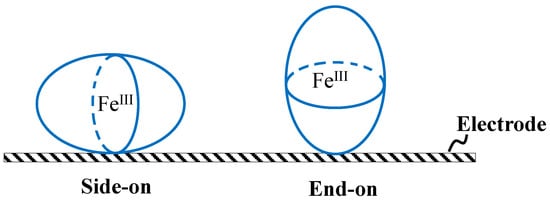
Scheme 1.
Arrangement of HRP molecules arranged on the electrode surface in either a side-on or end-on orientation.
When the applied potential was applied toward the more negative potential direction, the lower the applied potential, the greater the reduction overpotential, which is the additional potential needed to drive a reaction at a certain rate beyond the thermodynamic requirement [38]. Generally, when the overpotential is small, the diffusion of the reactants becomes the rate-determining step, so the current increases as the overpotential increases. On the other hand, when the overpotential is large, the movement of electrons becomes the rate-determining step, so the current is limited by the applied potential. Therefore, this implies that the greater reduction overpotential may enhance the reduction reaction of oxidant [HRP(FeIII)]Ferric enzyme in the forward-reaction process of Equation (1). However, as shown in Figure 1a, the exposure of HRP under the greater reduction overpotential resulted in the fluctuation of the steady-state redaction current. These results indicate that the destabilization of HRP may occur under greater reduction overpotential. Furthermore, the reduction peak in cyclic voltammograms of HRP on the HRP/CP-modified electrode were about −0.3 ~ −0.5 V (vs. Ag/AgCl) dependent on the scan rates in an irreversible electrochemical system [21]. Accordingly, the applied potential on the HRP-modified electrode was set at −0.4 V vs. Ag/AgCl for the enzymatic redox reaction with H2O2 as a substrate in the following chronoamperometric experiment.
3.2. Current Behavior of the HRP/CP/EC/MEMPTFSI-Modified Electrode by Injecting H2O2
Figure 2a,b show the typical plots of current versus time for the HRP/CP-modified electrode and the HRP/CP/EC/MEMPTFSI-modified electrode upon successive injections of 100 μL H2O2 (0.01 M) at regular time intervals (about 100 s). After soaking the HRP/CP-modified electrode and the HRP/CP/EC/MEMPTFSI-modified electrode in a 67 mM phosphate-buffered solution (pH 7.0) for about 3 h, the chronoamperometry was carried out on the two enzyme-modified electrodes separately at a constant applied potential (−0.4 V vs. Ag/AgCl) in a 2 mL of 67 mM phosphate-buffered solution (pH 7.0) saturated by nitrogen gas under a constant stirring condition. The HRP/CP-modified electrode and the HRP/CP/EC/MEMPTFSI-modified electrode rapidly responded to successive increments of substrate (H2O2) at an applied potential of −0.4 V (vs. Ag/AgCl), approaching steady-state current responses within 10 to 20 s. On the other hand, as shown in Figure 2a,b, the addition of hydrogen peroxide to a CP electrode or a CP/EC/MEMPTFSI electrode without an enzyme did not result in any changes in the reduction current. Accordingly, the result indicates that the immobilized HRP generates the electrode response.
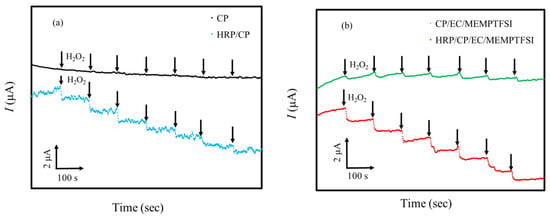
Figure 2.
The typical current–time recording with successive additions of H2O2 at regular time intervals. (a) Current–time recording lines for the CP electrode (black line) and the HRP/CP-modified electrode (blue line). (b) Current–time recording lines for) the CP/EC/MEMPTFSI electrode (green line) and the HRP/CP/EC/MEMPTFSI-modified electrode (red lined).
The reaction rate (v) of redox reaction is given by [38]
where I is the steady-state current, F is the Faraday constant, and n is the stoichiometric number of electrons consumed in the electrode reaction.
A baseline correction was applied to the steady-state current used in Equation (2), which was correlated with the H2O2 reduction reaction at both the HRP/CP/EC/MEMPTFSI-modified electrode and HRP/CP-modified electrode. Furthermore, the n value equals two in Equation (2), as explained below. In the case of the HRP-modified electrode, by adding H2O2 to an aqueous electrolytic solution, [HRP(FeIII)]Ferric enzyme of HRP-modified electrodes soaked in the aqueous electrolytic solution was firstly oxidated into compound I, accompanied by the reduction of H2O2 in the following way [16,23,39].
HRP[FeIII]Ferric enzyme + H2O2 → compound I + H2O
compound I + e− + H+ → compound II (fast)
compound II + e− + H+ → HRP[FeIII]Ferric enzyme
Compound I is reduced by receiving an electron provided directly through the electrode and produces compound II. Reaction (4) is a fast electrochemical reaction process, then compound II further receives an electron from the electrode and a proton ion to return to an original form of [HRP(FeIII)]Ferric enzyme. Therefore, the total electrons consumed in reactions (4) to (5) on the HRP-modified electrode for the reduction reactions of compound I and compound II to [HRP(FeIII)]Ferric enzyme are two electrons.
According to reactions (3) to (5), when the chronoamperometry carried out at an appropriated reduction overpotential as the applied potential, HRP at the HRP/CP/EC/MEMPTFSI-modified electrode and the HRP/CP-modified electrode can be regenerated sufficiently. According to the relationship between the stability of the steady-state current and the applied potential mentioned above, the applied potential was set at −0.4 V (vs. Ag/AgCl), which could supply sufficient electrons to make HRP carry out the enzymatic reduction reaction steadily.
3.3. Kinetic Behavior of the HRP/CP/EC/MEMPTFSI-Modified Electrode
Figure 3a,b show the Lineweaver–Burk plot of the enzymatic redox reaction using H2O2 at the HRP/CP-modified electrode and the HRP/CP/EC/MEMPTFSI-modified electrode, respectively. The Lineweaver–Burk plot exhibits the relationship between 1/v and 1/[S], as shown in the following equation [32,40]:
where v is the reaction rate, Vmax is the apparent maximum reaction rate, [S] is the concentration of substrate, and KM is the apparent Michaelis constant. Additionally, Vmax is as follows:
where kcat represents the turnover number and [E]0 is the overall enzyme concentration. Since the lines of the HRP/CP-modified electrode and the HRP/CP/EC/MEMPTFSI-modified electrode had the correlation constants (r2) of 0.99 and 0.95, respectively, a satisfactory linearity was shown. Therefore, it is concluded that the enzymatic redox reaction using H2O2 in the present study obeys the following Michaelis–Menten-type kinetics. Firstly, KM and Vmax are obtained from Figure 3a,b using Equation (6). Then, by substituting the obtained Vmax value into Equation (7), the value of kcat can be obtained.
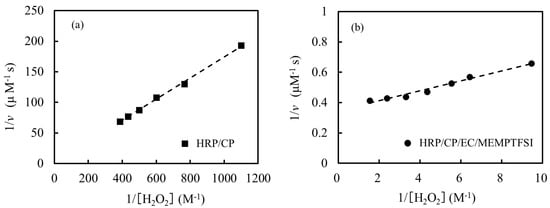
Figure 3.
Lineweaver–Burk plot for the concentrations of H2O2 at (a) the HRP/CP-modified electrode (■) and (b) the HRP/CP/EC/MEMPTFSI-modified electrode (●), respectively.
Table 1 shows the kinetic parameters of the enzymatic redox reaction using H2O2 as a substrate. The KM value of the enzymatic redox reaction at the HRP/CP/EC/MEMPTFSI-modified electrode was the same as that at the HRP/CP-modified electrode. The KM value represents the substrate concentration at which the enzymatic reaction rate is half of its maximum rate [32,40]. The smaller the KM value, the higher the affinity between the enzyme and the substrate. Accordingly, it is suggested that the affinity between the HRP and the H2O2 in the enzymatic redox reaction at the HRP/CP/EC/MEMPTFSI-modified electrode is the same as that at the HRP/CP-modified electrode. On the other hand, the kcat value of the enzymatic redox reaction at the HRP/CP/EC/MEMPTFSI-modified electrode was six times larger than that at the HRP/CP-modified electrode. Since kcat is moles of substrates that can be converted by one mole of enzymes per second, the larger the kcat value, the higher the activity [32]. Consequently, the kcat/KM value for the enzymatic redox reaction at the HRP/CP/EC/MEMPTFSI-modified electrode was six times higher than that at the HRP/CP-modified electrode. kcat/KM is the specificity constant and corresponds on the catalytic efficiency. This implies that HRP at the HRP/CP/EC/MEMPTFSI-modified electrode is able to more effectively accelerate the reaction and acts as a superior catalyst compared to that at the HRP/CP-modified electrode. This could be attributed to various factors, such as the materials used in the HRP/CP/EC/MEMPTFSI-modified electrode, which could provide more active sites for the reaction, enhance the electron transfer, or lower the energy barrier of the reaction. When enzymes come into contact with an immobilizing surface by adsorption, enzymes may change their conformation and become denatured [18]. Ionic liquids tend to enhance the stability of enzymes [26,27,28,29,30]. Moreover, since ionic liquids have high ionic conductivity, the addition of ionic liquids to enzyme-modified electrodes can greatly improve the electron transfer and the electroactive surface area [31]. These results indicate that the HRP/CP/EC/MEMPTFSI-modified electrode is much more favorable for use in biotransformation and biosensors because of the high catalytic efficiency.

Table 1.
Kinetic parameters of enzymatic redox reaction at HRP-modified electrode with or without ionic liquids.
4. Conclusions
We have demonstrated that the enzymatic redox reaction using H2O2 as a substrate at the HRP-modified electrode with ionic liquids is efficiently enhanced, compared to that at the HRP-modified electrode without ionic liquids. The destabilization of HRP occurred under a higher reduction overpotential. The enzymatic redox reaction at the HRP/CP/EC/MEMPTFSI-modified electrode and the HRP/CP-modified electrode could be correlated by the Michaelis–Menten equation. The affinity of HRP for H2O2 in the enzymatic redox reaction was similar at both the HRP/CP/EC/MEMPTFSI-modified and HRP/CP-modified electrodes. On the other hand, the turnover number of the enzymatic redox reaction at the HRP/CP/EC/MEMPTFSI-modified electrode was superior to that at the HRP/CP-modified electrode. Accordingly, since the catalytic efficiency of the enzymatic redox reaction at the HRP/CP/EC/MEMPTFSI-modified electrode was much higher than that at the HRP/CP-modified electrode, it is suggested that the HRP/CP/EC/MEMPTFSI-modified electrode is more suitable for biotransformations such as polymerization, coupling, hydroxylation, oxygen-transfer reactions, and biosensors for diagnosis, analysis, environmental protection, and security surveillance.
Author Contributions
Y.N.: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing—Original Draft, Writing—Review and Editing, Visualization, Supervision. T.K.: Conceptualization, Methodology, Resources. H.N.: Conceptualization, Methodology, Writing—Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting this study are available from the authors upon reasonable request.
Conflicts of Interest
Authors Yasuko Noritomi and Takashi Kuboki were employed by the company Toshiba Corporation. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Heinzle, E.; Biwer, A.P.; Cooney, C.L. Development of Sustainable Bioprocesses, Modeling and Assessment; John Wiley & Sons, Ltd.: Chichester, UK, 2007. [Google Scholar]
- Buchholz, K.; Kasche, V.; Bornscheuer, U.T. Biocatalyst and Enzyme Technology, 2nd ed.; Wiley-Blackwell: Weinheim, Germany, 2012. [Google Scholar]
- Silwana, B.; Horst, C.V.D.; Iwuoha, E.; Somerset, V.; Environ, J. Amperometric determination of cadmium, lead, and mercury metal ions using a novel polymer immobilised horseradish peroxidase biosensor system. Sci. Health A Tox Hazard Subst. Environ. Eng. 2014, 49, 1501–1511. [Google Scholar] [CrossRef]
- Leech, D.; Kavanagh, P.; Schuhmann, W. Enzymatic fuel cells: Recent progress. Electrochim. Acta 2012, 84, 223–234. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Lopes, G.R.; Pinto, D.C.G.A.; Silva, A.M. Horseradish peroxidase (HRP) as a tool in green chemistry. RSC Adv. 2014, 4, 37244–37265. [Google Scholar] [CrossRef]
- Reda, T.; Plugge, C.M.; Abram, N.J.; Hirst, J. Reversible interconversion of carbon dioxide and formate by an electroactive enzyme. Proc. Natl. Acad. Sci. USA 2008, 105, 10654–10658. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, F.A.; Hirst, J. Reversibility and efficiency in electrocatalytic energy conversion and lessons from enzymes. Proc. Natl. Acad. Sci. USA 2011, 108, 14049–14051. [Google Scholar] [CrossRef] [PubMed]
- Bassegoda, A.; Madden, C.; Wakerley, D.W.; Reisner, E.; Hirst, J. Reversible interconversion of CO2 and formate by a molybdenum containing formate dehydrogenase. J. Am. Chem. Soc. 2014, 136, 5473–15476. [Google Scholar] [CrossRef]
- Marpani, F.; Pinelo, M.; Meyer, A.S. Enzymatic conversion of CO2 to CH3OH via reverse dehydrogenase cascade biocatalysis: Quantitative comparison of efficiencies of immobilized enzyme systems. Biochem. Eng. J. 2017, 127, 217–228. [Google Scholar] [CrossRef]
- Sultana, S.; Sahoo, P.C.; Martha, S.; Parida, K. A review of harvesting clean fuels from enzymatic CO2 reduction. RSC Adv. 2016, 6, 44170–44194. [Google Scholar] [CrossRef]
- Rayalu, S.; Yadav, R.; Wanjari, S.; Prabhu, C.; Musthnoori, S.C.; Labhasetwar, N.; Satyanarayanan, T.; Kotwal, S.; Wate, S.R.; Hong, S.G.; et al. Nanobiocatalysts for carbon capture, sequestration and valorisation. Top Catal. 2012, 55, 1217–1230. [Google Scholar] [CrossRef]
- Ji, X.Y.; Su, Z.G.; Wang, P.; Ma, G.G.; Zhang, S.P. Tethering of nicotinamide adenine dinucleotide inside hollow nanofibers for high-yield synthesis of methanol from carbon dioxide catalyzed by coencapsulated multienzymes. ACS Nano 2015, 9, 4600–4610. [Google Scholar] [CrossRef] [PubMed]
- Addo, P.K.; Arechederra, R.L.; Waheed, A.; Shoemaker, J.D.; Sly, W.S.; Minteer, S.D. Methanol production via bioelectrocatalytic reduction of carbon dioxide: Role of carbonic anhydrase in improving electrode performance. Electrochem. Solid State Lett. 2011, 14, E9–E13. [Google Scholar] [CrossRef]
- Schlager, S.; Dibenedetto, A.; Aresta, M.; Apaydin, D.H.; Dumitru, L.M.; Neugebauer, H.; Sariciftci, N.S. Biocatalytic and bioelectrocatalytic approaches for the reduction of carbon dioxide using enzymes. Energy Technol. 2017, 5, 812–821. [Google Scholar] [CrossRef]
- Komori, K.; Tatsuma, T.; Sakai, Y. Direct electron transfer kinetics of peroxidase at edge plane sites of cup-stacked carbon nanofibers and their comparison with single walled carbon nanotubes. Langmuir 2016, 3, 9163–9170. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Noritomi, H. Biochar: Characterization and Applications in Enzyme Technology; Generis Publishing: Durham, NC, USA, 2022; ISBN 979-8-88676-250-1. [Google Scholar]
- Kano, K.; Ikeda, T. Fundamentals and practices of mediated bioelectrocatalysis. Anal. Sci. 2000, 16, 1013–1021. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Cepra, G. Thermal stabilization of enzymes immobilized within carbon paste electrodes. Anal. Chem. 1997, 69, 3124–3127. [Google Scholar] [CrossRef] [PubMed]
- Noritomi, Y.; Kuboki, T.; Noritomi, H. Estimation of immobilized horseradish peroxidase in a low salt concentration for an irreversible electrochemical system. Results Chem. 2020, 2, 100055. [Google Scholar] [CrossRef]
- Taliene, V.R.; Ruzgas, T.; Razumas, V.; Kulys, J. Chronoamperometric and cyclic voltammetric study of carbon paste electrodes using ferricyanide and ferrocenemonocarboxylic acid. J. Electroanal. Chem. 1994, 372, 85–89. [Google Scholar] [CrossRef]
- Freire, R.S.; Pessoa, C.A.; Mello, L.D.; Kubota, L.T. Direct electron transfer: An approach for electrochemical biosensors with higher selectivity and sensitivity. J. Braz. Chem. Soc. 2003, 14, 230–243. [Google Scholar] [CrossRef]
- Yue, K.; Zhai, C.X.; Gu, S.N.; He, Y.Y.; Yeo, J.J.; Zhou, G.W. Performance-enhanced lithium metal batteries through ionic liquid based electrolytes and mechanism research derived by density functional theory calculation. Electrochim. Acta 2021, 368, 137535. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T. Ionic liquids as tool to improve enzymatic organic synthesis. Chem. Rev. 2017, 117, 10567–10607. [Google Scholar] [CrossRef]
- Noritomi, H.; Nishida, S.; Kato, S. Protease-catalyzed esterification of amino acid in water-miscible ionic liquid. Biotechnol. Lett. 2007, 29, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Noritomi, H.; Suzuki, K.; Kikuta, M.; Kato, S. Catalytic activity of α-chymotrypsin in enzymatic peptide synthesis in ionic liquids. Biochem. Eng. J. 2007, 47, 27–30. [Google Scholar] [CrossRef]
- Noritomi, H.; Minamisawa, K.; Kamiya, R.; Kato, S. Thermal stability of proteins in the presence of aprotic ionic liquids. J. Biomed. Sci. Eng. 2011, 4, 94–99. [Google Scholar] [CrossRef]
- Noritomi, H.; Chiba, H.; Kikuta, M.; Kato, S. How can aprotic ionic liquids affect enzymatic enantioselectivity. J. Biomed. Sci. Eng. 2013, 6, 954–959. [Google Scholar] [CrossRef]
- Noritomi, Y.; Kuboki, T.; Noritomi, H. Promotion of the redox reaction at horseradish peroxidase modified electrode combined with ionic liquids under irreversible electrochemical conditions. Results Chem. 2022, 4, 100666. [Google Scholar] [CrossRef]
- Bailey, J.E.; Ollis, D.F. Biochemical Engineering Fundamentals, 2nd ed.; McGraw-Hill: New York, NY, USA, 1986. [Google Scholar]
- Dunford, H.B.; Stillman, J.S. On the function and mechanism of action of peroxidases. Coord. Chem. Rev. 1976, 19, 187–251. [Google Scholar] [CrossRef]
- Das, D.; Dasgupta, A.; Das, P.K. Improved activity of horseradish peroxidase (HRP) in ‘specifically designed’ ionic liquid. Tetrahedron Lett. 2007, 48, 5635–5639. [Google Scholar] [CrossRef]
- Gajhede, M.; Schuller, D.J.; Henriksen, A.; Smith, A.T.; Poulos, T.L. Crystal structure of horseradish peroxidase C at 2.15 Å resolution. Nat. Struct. Biol. 1997, 4, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Bekard, I.; Dunstan, D.E. Electric field induced changes in protein conformation. Soft Matter 2014, 10, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Berglund, G.I.; Carlsson, G.H.; Smith, A.T.; Szöke, H.; Henriksen, A.; Hajdu, J. The catalytic pathway of horseradish peroxidase at high resolution. Nature 2002, 417, 463–468. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2001; pp. 7+21. [Google Scholar]
- Ruzgas, T.; Csoregi, E.; Emneus, J.; Gorton, L.; Marko-Varga, G. Peroxidase-modified electrodes: Fundamentals and application. Anal. Chim. Acta 1996, 330, 123–138. [Google Scholar] [CrossRef]
- Fersht, A. Structure and Mechanism in Protein Science: A Guide to Enzyme Catalysis and Protein Folding; W. H. Freeman and Company: New York, NY, USA, 1999. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).