Responses of the Corylus avellana Colonized by the Tuber Melanosporum Mycorrhiza to Short-Term Rhizosphere Disturbance
Abstract
1. Introduction
2. Materials and Methods
2.1. Host Plant
2.2. Spore Inoculation
2.3. Estimation and Identification of Mycorrhization
2.4. Photosynthetic Activities
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zambonelli, A.; Iotti, M.; Hall, I. Current status of truffle cultivation: Recent results and future perspectives. Ital. J. Mycol. 2015, 44, 31–40. [Google Scholar]
- Merényi, Z.; Varga, T.; Hubai, A.G.; Pitlik, P.; Erős, Á.; Trappe, J.M.; Bratek, Z. Challenges in the delimitation of morphologically similar species: A case study of Tuber brumale agg. (Ascomycota, Pezizales). Mycol. Prog. 2017, 16, 613–624. [Google Scholar] [CrossRef]
- Cântar, I.C.; Merce, O.; Cadar, N. Expanding of T. melanosporum truffle in culture-case study. J. Hortic. For. Biotechnol. 2014, 18, 40–44. [Google Scholar]
- Hall, I.R.; Yun, W.; Amicucci, A. Cultivation of edible ectomycorrhizal mushrooms. Trends Biotechnol. 2003, 21, 433–438. [Google Scholar] [CrossRef]
- Chevalier, G.; Sourzat, P. Soils and Techniques for Cultivating Tuber melanosporum and Tuber aestivum in Europe. In Edible Ectomycorrhizal Mushrooms; Soil Biology; Zambonelli, A., Bonito, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volime 34. [Google Scholar] [CrossRef]
- Murat, C. Forty years of inoculating seedlings with truffle fungi: Past and future perspectives. Mycorrhiza 2015, 25, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Castaño, C.; Lindahl, B.D.; Alday, J.G.; Hagenbo, A.; Martínez de Aragón, J.; Parladé, J.; Pera, J.; Bonet, J.A. Soil microclimate changes affect soil fungal communities in a Mediterranean pine forest. New Phytol. 2018, 220, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Barreda, S.; Marco, P.; Martín-Santafé, M.; Tejedor-Calvo, E.; Sánchez, S. Edaphic and temporal patterns of T. melanosporum fruit body traits and effect of localized peat-based amendment. Sci. Rep. 2020, 10, 4422. [Google Scholar] [CrossRef]
- Fischer, C.R.; Oliach, D.; Bonet, J.A.; Colinas, C. Best Practices for Cultivation of Truffles; Forest Sciences Centre of Catalonia: Solsona, Spain; Yaşama Dair Vakıf: Antalaya, Turkey, 2017; 68p, ISBN 978-84-697-8163-0. [Google Scholar]
- Bonet, J.A.; Fischer, C.R.; Colinas, C. Cultivation of black truffle to promote reforestation and land-use stability. Agron. Sustain. Dev. 2006, 26, 69–76. [Google Scholar] [CrossRef]
- Olivera, A.; Fischer, C.R.; Bonet, J.A.; Martínez de Aragón, J.; Oliach, D.; Colinas, C. Weed management and irrigation are key treatments in emerging black truffle (T. melanosporum) cultivation. New For. 2011, 42, 227–239. [Google Scholar] [CrossRef]
- Andrés-Alpuente, A.; Sánchez, S.; Martín, M.; Aguirre, Á.J.; Barriuso, J.J. Comparative analysis of different methods for evaluating quality of Quercus ilex seedlings inoculated with T. melanosporum. Mycorrhiza 2014, 24, 29–37. [Google Scholar] [CrossRef]
- Garcia-Barreda, S.; Molina-Grau, S.; Reyna, S. Fertilization of Quercus seedlings inoculated with T. melanosporum: Effects on growth and mycorrhization of two host species and two inoculation methods. iForest-Biogeosci. For. 2016, 10, 267. [Google Scholar]
- Santelices, R.; Palfner, G. Controlled rhizogenesis and mycorrhization of hazelnut (C. avellana L.) cuttings with black truffle (T. melanosporum Vitt.). Chil. J. Agric. Res. 2010, 70, 204–212. [Google Scholar] [CrossRef]
- Pereira, G.; Palfner, G.; Chávez, D.; Suz, L.M.; Machuca, Á.; Honrubia, M. Using common mycorrhizal networks for controlled inoculation of Quercus spp. with T. melanosporum: The nurse plant method. Mycorrhiza 2013, 23, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Kentelky, E.; Lukács, Z.; Lunka, T.A.; Benedek, K.; Domokos, E.; Putnoky-Csicsó, B.; Szekely-Varga, Z. Mycorrhization of Corylus avellana L. and Quercus robur L. seedlings with Tuber aestivum Vittad. Sci. Pap. 2022, 66, 701–705. [Google Scholar]
- Nicoletti, R.; Petriccione, M.; Curci, M.; Scortichini, M. Hazelnut-associated bacteria and their implications in crop management. Horticulturae 2022, 8, 1195. [Google Scholar] [CrossRef]
- Splivallo, R.; Fischer, U.; Göbel, C.; Feussner, I.; Karlovsky, P. Truffles regulate plant root morphogenesis via the production of auxin and ethylene. Plant Physiol. 2009, 150, 2018–2029. [Google Scholar] [CrossRef] [PubMed]
- Splivallo, R.; Ottonello, S.; Mello, A.; Karlovsky, P. Truffle volatiles: From chemical ecology to aroma biosynthesis. New Phytol. 2011, 189, 688–699. [Google Scholar] [CrossRef]
- Chanclud, E.; Morel, J.B. Plant hormones: A fungal point of view. Mol. Plant Pathol. 2016, 17, 1289–1297. [Google Scholar] [CrossRef]
- Habtemariam, A.A.; Bratek, Z.; Gyulavári, P. Observations on mycorrhization of pecan seedlings with a European truffle. Rhizosphere 2021, 19, 100409. [Google Scholar] [CrossRef]
- Sillo, F.; Brunetti, C.; Marroni, F.; Vita, F.; dos Santos Nascimento, L.B.; Vizzini, A.; Mello, A.; Balestrini, R. Systemic effects of Tuber melanosporum inoculation in two Corylus avellana genotypes. Tree Physiol. 2022, 42, 1463–1480. [Google Scholar] [CrossRef]
- Angelini, P.; Bricchi, E.; Akhtar, M.S.; Properzi, A.; Fleming, J.L.E.; Tirillini, B.; Venanzoni, R. Isolation and identification of allelochemicals from ascocarp of Tuber species. Plant Soil Microbes 2016, 2, 225–252. [Google Scholar]
- Benucci, G.M.N.; Bonito, G.; Falini, L.B.; Bencivenga, M. Mycorrhization of Pecan trees (C. illinoinensis) with commercial truffle species: T. aestivum Vittad. and T. borchii Vittad. Mycorrhiza 2012, 22, 383–392. [Google Scholar] [CrossRef]
- Alvarado, P.; Manjon, J.L. A quantitative and molecular examination of T. melanosporum mycorrhizae in Quercus ilex seedlings from different suppliers in Spain. For. Syst. 2013, 22, 159–169. [Google Scholar] [CrossRef][Green Version]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant root growth, architecture and function. Plant Soil 2009, 321, 153–187. [Google Scholar] [CrossRef]
- Bonito, G.; Smith, M.E.; Brenneman, T.; Vilgalys, R. Assessing ectomycorrhizal fungal spore banks of truffle producing soils with pecan seedling trap-plants. Plant Soil 2012, 356, 357–366. [Google Scholar] [CrossRef]
- Varga, T.; Merényi, Z.; Bratek, Z.; Solti, Á. Mycorrhizal colonization by T. aestivum has a negative effect on the vitality of oak and hazel seedlings. Acta Biol. Szeged. 2014, 58, 49–53. [Google Scholar]
- Agerer, R.; Rambold, G. DEEMY—An Information System for Characterization and Determination of Ectomycorrhizae. München, Germany. 2004. Available online: http://www.deemy.de (accessed on 15 November 2024).
- Guevara-Guerrero, G.; Pacioni, G.; Leonardi, M.; Ocañas, F.G.; Hernández, R.G. Mycorrhizal Synthesis of Périgord Black Truffle (T. melanosporum) with Mexican Oak Species. Microbiol. Biotechnol. Lett. 2022, 50, 40–50. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee SJ, W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- LI-COR Biosciences. LI-6800 Portable Photosynthesis System, Software Version 2.0; LI-COR Biosciences: Lincoln, NE, USA, 2021. [Google Scholar]
- Marozzi, G.; Sánchez, S.; Benucci, G.M.N.; Bonito, G.; Falini, L.B.; Albertini, E.; Donnini, D. Mycorrhization of pecan (C. illinoinensis) with black truffles: T. melanosporum and T. brumale. Mycorrhiza 2017, 27, 303–309. [Google Scholar] [CrossRef]
- Donnini, D.; Benucci, G.M.; Bencivenga, M.; Falini, L.B. Quality assessment of truffle-inoculated seedlings in Italy: Proposing revised parameters for certification. For. Syst. 2014, 23, 385–393. [Google Scholar] [CrossRef]
- Bhavana, D.; Rashmi, A. Dynamics of soil nutrients and ecto-mycorrhizal symbionts in disturbed and undisturbed stands of Tropical Dry Deciduous Forest of Central India. Int. J. Pharma Bio Sci. 2013, 4, B-1077–B-1084. [Google Scholar]
- Falini, L.B.; Benucci, G.M.N.; Bencivenga, M.; Donnini, D. Mycorrhization level in truffle plants and presence of concurrent fungi. Acta Mycologica 2012, 47, 169–173. [Google Scholar] [CrossRef]
- Núñez, J.D.; Planelles, R.; Barreal, J.R.; de Omeñaca, J.S. The influence of the mycorrhization with black truffle in growth, gas exchange and mineral nutrition of P. halepensis. For. Syst. 2004, 13, 317–327. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, R.; Lu, B.; Guerin-Laguette, A.; He, X.; Yu, F. Mycorrhization of Q. mongolica seedlings by T. melanosporum alters root carbon exudation and rhizosphere bacterial communities. Plant Soil 2021, 467, 391–403. [Google Scholar] [CrossRef]
- Nardinia, A.; Salleo, S.; Tyree, M.T.; Vertovec, M. Influence of the ectomycorrhizas formed by Tuber melanosporum Vitt. on hydraulic conductance and water relations of Quercus ilex L. seedlings. Ann. For. Sci. 2000, 57, 305–312. [Google Scholar] [CrossRef]
- Itoo, Z.A.; Reshi, Z.A. The multifunctional role of ectomycorrhizal associations in forest ecosystem processes. Bot. Rev. 2013, 79, 371–400. [Google Scholar] [CrossRef]
- Holland, T.; Bowen, P.; Kokkoris, V.; Richards, A.; Rosa, D.; Hart, M. The effect of root pruning on the arbuscular mycorrhizal symbiosis in grapevine rootstocks. Chem. Biol. Technol. Agric. 2019, 6, 21. [Google Scholar] [CrossRef]
- Martins, A.; Casimiro, A.; Pais, M.S. Influence of mycorrhization on physiological parameters of micropropagated Castanea sativa Mill. plants. Mycorrhiza 1997, 7, 161–165. [Google Scholar] [CrossRef]

| Variable 1 vs. Variable 2 | Pearson’s r | p | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| Mycorrhization (%) vs. Seedling length (cm) | 0.371 * | 0.01 | 0.094 | 0.595 |
| Mycorrhization (%) vs. Collar diameter (mm) | 0.17 | 0.254 | −0.123 | 0.436 |
| Mycorrhization (%) vs. Root length (cm) | 0.179 | 0.228 | −0.114 | 0.444 |
| Seedling length (cm) vs. Collar diameter (mm) | 0.584 *** | <0.001 | 0.357 | 0.746 |
| Seedling length (cm) vs. Root length (cm) | 0.203 | 0.172 | −0.09 | 0.463 |
| Collar diameter (mm) vs. Root length (cm) | 0.312 * | 0.033 | 0.027 | 0.55 |
| Parameters | Mean ± SD | Minimum | Maximum | |||
|---|---|---|---|---|---|---|
| I | II | I | II | I | II | |
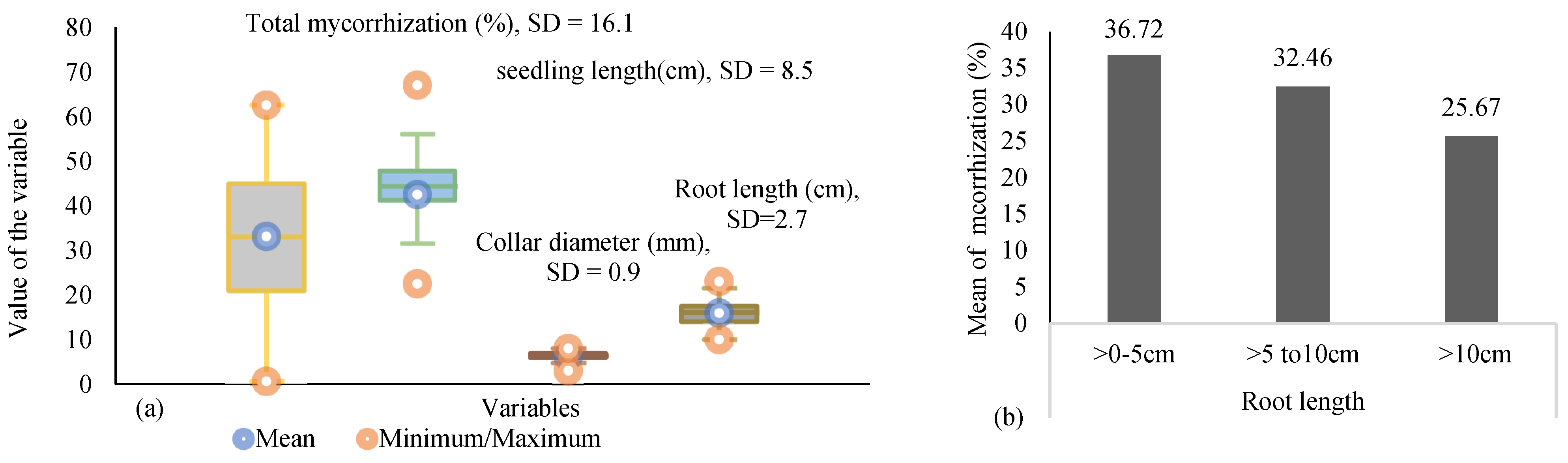
| Total mycorrhization (%) | 35.7 ± 18.5 | 37.7 ± 18.6 | 1.89 | 6.4 | 62.5 | 61.4 |
| Mycorrhization near to collar (0–5 cm) (%) | 37.9 ± 23.7 | 41.6 ± 20.5 | 0 | 8.3 | 70 | 70.2 |
| seedling length (cm) | 36.5 ± 9.5 | 46.6 ± 7.9 | 22.5 | 35.5 | 48.5 | 67 |
| Collar diameter (mm) | 5.9 ± 1.3 | 6.2 ± 0.4 | 3 | 5.7 | 7.8 | 7.1 |
| Root length (cm) | 16 ± 2.3 | 14.8 ± 2.3 | 13.5 | 12 | 21.5 | 19 |
| Fv/Fm | 0.8 ± 0.01 | 0.8 ± 0.05 | 0.8 | 0.8 | 0.8 | 0.8 |
| PhiPS2 | 0.1 ± 0.03 | 0.2 ± 0.02 | 0.06 | 0.2 | 0.2 | 0.2 |
| NPQ | 2.9 ± 0.4 | 2.6 ± 0.3 | 2.3 | 2.3 | 3.5 | 3.2 |
| E (mol m−2 s−1) | 0.0005 ± 0.0003 | 0.002 ± 0.0003 | 0.0002 | 0.001 | 0.001 | 0.002 |
| A (µmol m−2 s−1) | 3.03 ± 1.6 | 7.76 ± 0.9 | 0.9 | 6.6 | 6.2 | 9.2 |
| Gsw (mol m−2 s−1) | 0.03 ± 0.02 | 0.08 ± 0.02 | 0.007 | 0.06 | 0.06 | 0.1 |
| PhiCO2 (μmol CO2 μmol photons−1) | 0.009 ± 0.004 | 0.02 ± 0.005 | 0.005 | 0.01 | 0.02 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habtemariam, A.A.; Cseh, P.; Csizmár, M.; Fodor, F.; Bratek, Z. Responses of the Corylus avellana Colonized by the Tuber Melanosporum Mycorrhiza to Short-Term Rhizosphere Disturbance. Appl. Microbiol. 2025, 5, 133. https://doi.org/10.3390/applmicrobiol5040133
Habtemariam AA, Cseh P, Csizmár M, Fodor F, Bratek Z. Responses of the Corylus avellana Colonized by the Tuber Melanosporum Mycorrhiza to Short-Term Rhizosphere Disturbance. Applied Microbiology. 2025; 5(4):133. https://doi.org/10.3390/applmicrobiol5040133
Chicago/Turabian StyleHabtemariam, Akale Assamere, Péter Cseh, Mihály Csizmár, Ferenc Fodor, and Zoltán Bratek. 2025. "Responses of the Corylus avellana Colonized by the Tuber Melanosporum Mycorrhiza to Short-Term Rhizosphere Disturbance" Applied Microbiology 5, no. 4: 133. https://doi.org/10.3390/applmicrobiol5040133
APA StyleHabtemariam, A. A., Cseh, P., Csizmár, M., Fodor, F., & Bratek, Z. (2025). Responses of the Corylus avellana Colonized by the Tuber Melanosporum Mycorrhiza to Short-Term Rhizosphere Disturbance. Applied Microbiology, 5(4), 133. https://doi.org/10.3390/applmicrobiol5040133









