Abstract
Integrating bioluminescent organisms as passive lighting sources in the built environment is currently a hot topic. However, there are several limitations facing the implementation and up-scaling of these naturally bioluminescent organisms in the built environment on architectural and urban scales, such as the scale, sensitivity, enclosure, and difficulty of maintenance. Moreover, there are complex technicalities and operational aspects of conventional bioreactors that host these bioluminescent agents, especially in terms of managing their recharge and effluent, not to mention their high maintenance cost. The current work offers a sustainable, stand-alone, bioluminescent urban screen system employing Aliivibrio fischeri CECT 524 bioink on 3D-printed customized scaffolds as bioreceptive panel design based on a field-diffusion pattern to host the bioluminescent bacterial bioink. The field-diffusion pattern was employed thanks to its proven efficiency in entrapment of the various microbial cultures. Three different growth media were tested for culturing Aliivibrio fischeri CECT 524, including Luria Bertani Broth (LB), the Tryptone Soy Broth (TSB), and the standard Marine Broth (MB). The results revealed that the Marine Broth (MB) media achieved the highest bioluminescent intensity and duration. The maximum light emission typically in range of ~490 nm of blue–green light captured by a conventional reflex camera (human eye vision) was observed for 10 consecutive days in complete darkness after 3–10 s, at a room temperature of 25 °C. This was visible mainly at the thin curvilinear peaks of the 3D-printed field pattern. P1 achieved the highest performance in terms of visible blue–green light, and a duration of 10 days of active bioluminescence was achieved without the need for refilling, thanks to the high number of peaks and narrow wells at <0.5 cm of its field-diffusion pattern. This study proves the efficiency of this biomimetic pattern in terms of the bioreceptivity of the bioluminescent bacterial bioink. Furthermore, the proposed 3D-printed urban screens proved their economic sustainability in terms of affordability and their minimized production processes, in addition to their easy maintenance and recharge. These results qualify these 3D-printed bioluminescent urban screens for easy and decentralized adoption and application on an architectural and urban scale.
1. Introduction
Current research trajectories are focused on achieving sustainability through the intelligent usage of non-renewable energy resources and the development of novel alternative renewable energy systems. This has been one of the primary research focuses over the last 20 years and remains so to this day. Most sources of alternative renewable energy typically rely on centralized energy management, storage, and distribution systems, as seen in solar, wind, and hydroelectric power stations, each of which requires complex and non-eco-friendly manufactured parts and electronics that necessitate an expert to implement, operate, and harness their generated power. Although these systems may work for large-scale commercial architectural and urban applications, such as factories, the complexity of these systems hinders their domestic use on a small-scale, average user level for urban and architectural purposes. For example, one study (Atasu, et al., 2021) [1] reported a high cost of managing solar panel waste, imposing environmental and economic concerns in terms of managing these non-organic or eco-digestible wastes. The recycling cost of one panel was usually USD 20–USD 30, in comparison to the USD 1–USD 2 cost of sending that same panel to landfill. Similarly, Kuby Renewable Energy Ltd. (2019) [2] detailed the components of a standard solar panel, exhibiting their high energy demand for manufacturing their silicon semiconductors as well as their incorporation of hazardous chemicals that require strict control in the manufacturing process. Similarly, wind power is costly due to the need to build remotely located stations due to their high noise and threat to wildlife [3], and their efficiency in terms of power generation is also questionable [4]. This deficiency in the performance, sustainability, and economic competence of solar and wind power has mainly led to a shift toward searching for bioenergy solutions, for instance, modern solid and liquid bioenergy [5]. The trajectory of bioenergy research and applications incorporates various and expanding options by exploiting biomass in microbial fuel cells for generating light and/or electricity from non-pathogenic microbial strains. For example, in 2007–2008, the bacterial “Barcelona Biolamps” [6] employed the Aliivibrio fischeri bacterial strain in bioreactors to fully illuminate an apartment with living light, while Abdallah, et al., 2019 [7] employed a cathodic immobilized laccase-secreting, non-pathogenic fungal strain Aspergillus sydowii NYKA 510 in a single-chamber microbial fuel cell to generate 0.76 V, 380 mAm−2, 160 mWm−2, and 0.4 W at 2000 Ω, which was later employed in a self-sufficient lighting unit employing a system of two sets of four MFCs each, connected in series, for electricity generation. Despite the successful implantation of these bacteria- and fungi-powered devices, their non-photosynthetic characteristics have hindered the autonomous durability of these biobatteries or biolamps. This has led to the focus on algae-powered bioreactors for energy generation as an integral sustainable solution, where the benefits include not only the generation of sustainable, self-maintained, and self-sufficiently generated bioelectricity but also carbon dioxide mitigation, oxygen, and industrially valuable enzyme production and even the production of nutritious elements such as proteins [8]. Still, these liquid batteries did not solve the complexity of the above operation and maintenance problems. It might have complicated the issue by introducing these wet liquid battery systems that operate using living organisms that are sensitive to their environmental conditions. Added to this is their low energy production and the high cost of electrolyte membranes [9], adding further constraints to their architectural and urban applications, as reported in [10], with the criteria for the implementation of these bioactive liquid systems in architecture and urban applications taking into consideration their life cycle and maintenance rate and boosting their energy output in reasonable applications.
Therefore, a third research trajectory sought to implement the intrinsic nature of bio-emitted light, known as “bioluminescent” activity, at the material-scale level. For example, Mitiouchkina, et al., 2020 [11] engineered tobacco lines expressing a fungal bioluminescent system [12], which converts caffeic acid present in all plants into luciferin and reports self-sustained luminescence that is easily visible to the naked eye, in the search for integrating fluorescent and bioluminescent genes in plants. However, such endeavors did not consider architectural or urban applications by proposing the genetic modification of plants or trees that are growing or can be adopted on the urban scale. Therefore, an easier and more sustainable procedure was proposed in the “Genetic Barcelona Project” by applying genetic modification to lemon tree leaves to make them autonomously fluorescent in the first phase (2003–2006) in seven lemon trees, which was achieved by introducing the GFP gene into their DNA, offering a renewable, sustainable, autonomous, and passive source of urban lighting [13]. These genetically modified lemon trees were kept alive for 13 years and would still be alive today if it were not for the need to relocate the research work and its infrastructure. They remained actively fluorescent at night despite their trivial level of emitted light intensity, which could only be sensed in total darkness. These steps fueled research interest in biomanufacturing novel, advanced bioluminescent or fluorescent materials. For example, a group of researchers [14] explored the idea of making of a bio-luminescent micro-architecture, exploring the use of light-emitting bacteria as an architectural material by investigating the 3D printing of bioluminescent bacteria. The project employed cutting-edge 3D bioprinting technologies to create the bacterial culture and carrier media using a collaborative robot with a bespoke micro-dispenser to liberate the form of the medium at an architectural scale of fabrication. However, the feasibility of scaling-up the project to architectural-scale applications remains questionable due to the complex biomanufacturing processes, which can only be performed inside a laboratory under strict sterile conditions and with expert management of the sensitive bioprinting process, which itself imposes stress on the viability of the bacterial cells in the culture. This is due to the well-known rheological deferential effect pre-, during, and post-printing caused by the pressure at the 3D bioprinter extruder tip [15]. Additionally, the scale of the developed bacterial bioink is inapplicable, as the hydrogel base cannot sustain itself alone, especially when the number of 3D-printed layers becomes higher at the architectural scale, due to its poor rheological properties which were not measured in the reported project. In addition, there is a potential need for crosslinking this reported bioink to sustain its form post-printing, which might again affect the viability of the cells. And the possible contamination of this biocompatible hydrogel, which might lead to the coexistence of undesired bacterial and fungal pathogenic and harmful strains. Still, that is not everything; the feasibility of bioluminescent activity operation varies definitely across varied scales because of the spaces between the cells, oxygen, nutrient exposure, cell migration, and signaling. Similarly, Thomsen et al., 2020 and Tyse et al., 2022 [16,17] projects like “Bioluminescent micro-architectures: Imprimer la lumière”, which examined bioluminescent bacterial cultures as an architectural material, suffered from the same issues of the non-feasible scalability of the proposed materials and procedures on an architectural scale. Despite the research merits in terms of developing computational models for simulating the behavior, growth rates, and lifespan of the proposed bacterial materials and taking into consideration the ecosystem in which the light-emitting metabolisms take place, as well as their limited lifespan. However, there is a deviation between the computational simulations and the living cultures, where the most significant deviation between the simulation and real observations is seen in the first hours due to the lag phase, which the simulation does not consider. In addition, there are imperfections in the 3D-printed media compared to its twin 3D model, where the thickness of the extruded media is not consistent. Therefore, such speculations of unrealistic upscaling from the lab scale to the architectural scale must be reevaluated. Therefore, the current research aims to minimize the wide gap between research projects with their pilot-scale micro-models and the architectural scale by understanding the customization and application potential of this bioactive material.
The limitations of this 3D-printed bioluminescent biomaterial trajectory imply the need for customizing and testing these bioink materials in terms of their rheology, biocompatibility, cell viability, activity post-printing, cell migration, and behavior on an scale adequate for architectural applications before attempting to propose them for architectural applications [18]. This was investigated by (Estevez and Abdallah, 2024) [19], who developed a bio-cement composed of a self-mineralizing hydrogel with excellent encapsulation properties, cell compatibility, anti-fungal properties, and high calcium phosphate concentration as a bone-replacement and a bioactive self-mineralizing biomaterial that allows for scaling-up to architecture-scale applications with the possibility of incorporating living cells, thanks to its excellent encapsulating and rheological properties, offering a stand-alone structural material, expanding novel research aspects on studying the lifespan, proliferation, migration, and behavior of the encapsulated cells under the chronically mineralizing material and its time-deferential morphology. Furthermore, it opens vast potential for testing this hydrogel with a wide array of microbial and animal cell strains, since it is biocompatible and non-toxic to human cells, as reported in [19].
Engineered bioluminescent biomaterials, either incorporating a naturally bioluminescent or a GMO organism, are considered the best trajectory for implementing bioluminescent activity on the material-scale level. However, there are still several considerations to be addressed before introducing these bioactive materials into the built environment with direct contact with people.
For example, the specific chemical, physical, and physiological characteristics of the employed bioluminescent microbial strains must be understood, which vary between bacteria, fungi, and algae in terms of their bioluminescent activity stimuli, intensity, and duration. In addition, the maintenance and co-existence of these living materials must be carried out in a safe way for the bioactive agent and for users. Moreover, the limits of the physical translation and morphogenesis of these living materials in the built environment are crucial, as their physical properties will determine their physical existence and functionality within the environment. These aspects and more must still remain under extensive study before these bioluminescent materials can be implemented in the architectural and urban built environment. Therefore, scaffold structures can offer a possible durable and refillable physical transient between bacterial bioink and its environment of implementation, offering customized, sustainable encapsulation of bioactive bioluminescent organisms.
These scaffolds for bioluminescent bioink are borrowed from the field of tissue engineering, where living cells or bioinks are seeded on scaffolds that are both compatible with the cells’ behavior and provide a physical form for these cells to proliferate and differentiate, thereby creating the required tissue [20]. In addition, they offer easier, more reliable procedures and easier maintenance of these living cultures than bioreactor devices.
This customized scaffold design was proposed in the paper 3D-Printed Bioreceptive Tiles of Reaction–Diffusion (Gierer–Meinhardt Model) [21] by adopting a mixed-algae culture with varied algal strains, ranging from filamentous to unicellular, to form a dense mesh of a photosynthetic bioactive scaffold that sustains the existence of the bioluminescent unicellular algae resting on a 3D-printed PLA tile. Although the results of such methods were successful in preserving the life of the bioluminescent algae, the bioluminescent activity was trivial and could not be sensed with the naked eye. Thus, in the current work, the objective is to incorporate the bioluminescent bacterial strain Aliivibrio fischeri, which is more efficient in terms of its emitted bioluminescent light intensity and duration [22], in the form of a bioactive bioink layered on a PLA scaffold that will host the system. This approach facilitates the implementation of this system on both the architectural and urban scales, as it requires minimal one-step maintenance for recharging these bioluminescent tiles with new media, which is carried out by spraying new culture media slurry onto these panels. Moreover, these panels can be incorporated on the urban scale with varied options of enclosure by using ion-exchange membranes or dense polycarbonate glass.
Figure 1 exhibits a collection of research trajectories for obtaining bio-light developed since 2003 by Estévez, and Dollens., 2007. [13] and later by Abdallah, et al., 2019; Jaafari, et al., 2021; Estevez, and Abdallah, 2024 and Abdallah, and Estévez, 2023 [7,8,19,21] from 2019 to 2024. These involve using bioluminescent bioreactors, genetically modified plants with the GFP gene or with the A. vibrio genes responsible for bioluminescence, bioelectricity from fungi and algae in MFCs, bioluminescence panels obtained by immobilization of naturally bioluminescent unicellular marine algae entrapped in a mixed-filamentous-green-algae culture, and self-mineralized hydrogels with excellent encapsulation properties.
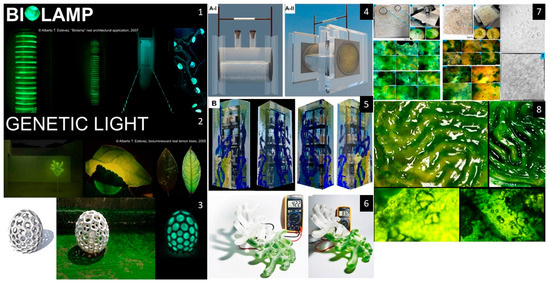
Figure 1.
Images from the authors of varied research trajectories on developing bio-light through natural and genetically modified bioluminescence and fluorescence activity, bioelectricity, and bioluminescence symbiosis on architectural and urban scaffolds: (1) © Alberto T. Estévez, Biolamps (Genetic Barcelona Project, 2nd phase, 2007–2010). Bioluminescent batteries applied in a Biolamp 2 (left), in a Skirting Board Biolamp (center), and in a Roots Biolamp (right); photo was made with a conventional reflex camera (human eye vision). (2) © Alberto T. Estévez, Genetic Barcelona Project, 1st phase, 2003–2006. Left, manifesto image, light from trees produced with GFP, green fluorescent protein. Genetic creation of bioluminescent plants for urban and domestic use. Comparison between a lemon tree leaf with GFP and another without GFP from the same lemon tree type. Center, photo by Alberto T. Estévez with a conventional reflex camera, and right, photo by Alberto T. Estévez and Josep Clotet with a special UV photo camera. (3) © Alberto T. Estévez, Barcelona Biodigital Pavilion, Barcelona, 2008–09. After a “bio-learning” process (in this case, from microscopic structure research on radiolarians and pollen), CAD-CAM technologies were applied for producing directly real 1:1 scale architecture. Left, digital drawing; center, real photo, directly digitally fabricated with CNC and actually installed on a large architectural scale; right, image of a table lamp with application of Biolamps for digital fabrication with a 3D printer on a small-object scale. (4) Lighting unit design utilizing ACMSC-MFC with A. sydowii NYKA 510. Two different views of an ACMSC-MFC modelled by [7] (A-I and A-II). (5) Mixed approach of patterned customized mass method for a lighting unit design utilizing an ACMSC-MFC system. (B) The final design of a self-sufficient lighting unit powered by ACMSC-MFC from different viewpoints, designed by [7], using Rhinoceros 3D+ Grasshopper. (6) A photosynthetic-algae-based MFC produced by [8], producing sufficient electrical current for domestic applications. (7,8) Three-dimensionally printed bioreceptive tiles as scaffolds hosting a mixed algal culture to create symbiosis between photosynthetic filamentous green algae and unicellular bioluminescent algae, exhibiting two reaction-diffusion-based customized patterns employed in bioreceptive tiles to immobilize the exact multi-scale lengths of the typically hosted algal strains, accompanied by microscopy images of the cultures immobilized on the tiles, showing the bioluminescent activity of the hosted bioluminescent algae strain Pyrocystis fusiformis [21].
Thus, the objective of the current work focuses primarily on bridging the gap between bioluminescent micro-materials, projects, and pilot-scale models and the realistic architectural- and urban-scale applications of these bioluminescent materials through employing a customized biomimetic bioreceptive pattern as a scaffold for bioluminescent bioink to create bioluminescent urban panels, which can be used as cladding at an urban and architectural scale. This can only be achieved by understanding the essential criteria and requirements of the architecture-scale applications to achieve a stand-alone, durable, enclosed, and sustainably maintained bioluminescent material system that employs these urban scaffolds. This study departs from the petri-scale level of bioluminescent bacterial architectures to fully integrated architectural-scale optimized scaffolds that can guarantee an extended and boosted living bioluminescent architectural materials. Thus, the current work will outline the experimental procedures conducted to fabricate a real-scale bioluminescent urban screen model composed of a replica of the screen unit, which is a 3D-printed tile hosting a bioluminescent bacterial culture. The methodology includes developing the biomimetic design of the bioreceptive pattern based on field-diffusion and 3D printing the recipient scaffold (the tile), optimization of the media for the Aliivibrio fischeri culture in terms of the best obtained bioluminescence visibility by the naked eye and the best duration, and the application and bioluminescence performance of the inoculated scaffolds with the bioluminescent bioink from the bacterial culture.
This study aims to prove the economic feasibility and competence of this system on the architectural and urban scales and to pave the way for the following step in this research project, which will involve measuring the spatial bioluminescence light intensity from various spatial points in an interior space and in an exterior space, to determine their efficiency as domestic and urban lights and their functional efficiency. This study is conducted as part of the research project, BioLumCity, funded by Fritz und Trude Fortmann Stiftung.
2. Materials and Methods
2.1. Biocompatible Design and 3D Printing of the Scaffold: The Bioreceptive Urban Screen Panel
The design concept of the urban screen panels is developed from the physiochemical field as a form of diffusion to facilitate the integration of a stain of augmented bioluminescent bacteria, Aliivibrio fischeri, in the built environment. The field attractors were chosen for the current design case. To explore different topologies in a gradient methodology, this study moved from localized cellular entrapment to smoother gradient topologies achieved by the field, as learned from nature. Two form-finding steps were employed: the first step employed diffusion-based generative design models to generate several design iterations for the bioreceptive tiles, while the second step optimized the form-finding equation parameters to develop a more continuous topology. The results of the design form-finding informed the third design phase of the algorithmic customization, where four additional design iterations were reconstructed from 2D to 3D. The software packages used were Rhinoceros 3D, Grasshopper, and Scripting in Python. Following this phase, the design iteration C2 was customized to be 3D-printed by adding transitional parallel topological steps (curves) to maximize the entrapment of the bacterial culture, which is crucial for sustaining the bacterial culture’s viability and augmenting its bioluminescent intensity and duration. The modified version of the C2 design model was 3D-printed as a bioreceptive tile measuring 18 × 18 × 0.5 cm and featuring a rectangular micro-texture grid of 0.1 × 0.1 cm. This was 3D-printed with a Felix Tec 4 singular-head 3D printer with translucent PLA at a 210 °C printing head temperature and a 70 °C printing bed temperature.
2.2. Optimizing the Culture Media of the Bioluminescent Bacteria Aliivibrio fischeri CECT 524 in Liquid and Solid Media
A total of 15 mL of Aliivibrio fischeri strain ES144/NCIMB1281 (Aliivibrio fischeri CECT 524) was obtained in freeze-dried form from CECT (Colección Española de Cultivos Tipo). Three different culture media were tested to enhance the bioluminescence activity of the Aliivibrio fischeri: Luria Bertani Broth (LB), LB Broth, and Lennox (Condalab) + Sodium Chloride AGR (Labkem). The sea salt concentrations in the LB media were varied by 15%, 20%, and 25%, tested both in a liquid culture and in petri culture of 10 cm plates mixed with agar (in triplicate). Tryptone Soy Broth (TSB) used dextrose as the carbon source, sodium chloride as the osmotic balance agent, and dipotassium phosphate as a buffering agent. The standard Marine Broth (MB) contained minerals and salts simulating seawater and peptone and yeast extract as nitrogen sources. The salt and mineral concentrations were adjusted to simulate the composition of seawater. Each of the three culture media was tested in liquid (150 mL/flask) and solid forms, mixed with agar–agar BAC, providing firmness and prolonging the bioluminescence of the organism. The petri dishes containing the medium and Aliivibrio fischeri CECT 524 were sealed in sterile conditions and incubated at room temperature (25 °C) to promote colony growth after 24 h post-inclusion. The bioluminescence activity was activated and lasted for seven days. Observations were made in a dark chamber for a week to monitor the bioluminescence. To determine the optimal medium for the Vibrio, the optical density (OD) was measured to compare the conditions and duration of the bioluminescence of the bacterial cultures under the three different media.
2.3. Optimizing the Culture Media of the Bioluminescent Urban Tiles Hosting Aliivibrio fischeri CECT 524 in Bioink
The optimized MB (Marine Broth) media were mixed with four different concentrations of MB with 100%, 50%, 40%, and 35% of agar–agar BAC to optimize the media-to-agar concentration ratio and to create a gel bioink for application on the urban screen panels (tiles), balancing the medium density based on the feedback of the bioluminescence activity test of the Aliivibrio fischeri CECT 524 culture on the tiles. The pH of the media was adjusted with 1M NaOH or KOH to pH 6, 7, and 8, and the media were autoclaved for 20 min at 121 °C and placed in a 47 °C water bath in triplicate to maintain the media at an optimal temperature, thereby preventing gelling. Aliivibrio fischeri CECT 524 was inoculated individually in each plate. Using a micropipette, 200 µL of glycerol stock (prepared with 50% Aliivibrio fischeri CECT 524 and 50% glycerol) and 20 mL of Marine Broth with agar–agar BAC at various percentages were added at 47 °C to avoid killing the organisms.
2.4. Inoculation of the Urban Tiles (3D Culture of Bioluminescent Aliivibrio fischeri CECT 524 in Sol-Gel Bioink on the 3D Printed Scaffolds)
Three 3D-printed urban screen panels (tiles) were cut into smaller pieces, each measuring 5 × 5 cm, into four distinct parts (P1, P2, P3, and P4), following a rectangular 90° grid to be contained within a 15 cm petri dish size and for the biocompatibility of the deferential topology of the tile design to be evaluated in terms of boosting the bioluminescence activity of the hosted bioluminescent bioink of the Aliivibrio fischeri CECT 524. The 3D panels were pre-cooled on a cold plate (−32 °C) and manually inoculated using a serological pipette with the Aliivibrio fischeri bioink, which had been previously cultured in Marine Broth (MB) with 40% agar at 37 °C to boost the bacterial cells’ anchorage first on the micro-textured 3D-printed panels, and then the agar medium was poured over the plates, ensuring uniform coverage of the structures. The medium was allowed to solidify before incubating the plates at 26 °C.
2.5. Three-Dimensionally Printed Urban Screen Panels’ Bioluminescence Activity
The cultured 3D-printed tiles (in parts) inoculated with the Aliivibrio fischeri + MB optimized media bioink were monitored in a dark chamber at room temperature and recorded using a LENS WIDE setup with the following settings: EV 0, ISO 4000, exposure time 20 s, F AUTO, and WB 2200. The inoculated 3D-printed panel parts (P1, P2, P3, and P4) were kept enclosed within the petri dish space without sealing. Light emission observations were conducted for up to 14 days post-inoculation to evaluate the intensity and durability of the bioluminescence.
3. Results
3.1. Three-Dimensionally Printed Biocompatible Scaffold: The Urban Screen Tile
The first design phase mixed the reaction diffusion polar-periodic pattern with the field gradient effect. However, the results of this phase were limited to localized cellular entrapment patterns rather than the required smooth gradient transition of a continuous topology. The resulting patterns of this first design phase suffered from discontinuities in their topologies and separated wells that hindered the bacterial culture’s chemotaxis behavior necessary for augmenting the bioluminescence intensity. The second design phase reached several design iterations of field patterns with a more continuous topology, as exhibited in Figure 2 (1,2). These results of field-continuous topology-based patterns informed the algorithmic customization design phase (Figure 2(3)), reaching design iteration C2 (Figure 2(4)), which was 3D-printed as a bioreceptive tile measuring 18 × 18 × 0.5 cm with a rectangular micro-texture grid measuring 0.1 × 0.1 cm, including the addition of transitional parallel topological steps (curves) (Figure 2(6)) to maximize the entrapment of the bacterial culture and to enable dense biofilm formation for sustaining the bacterial culture’s viability and augmenting its bioluminescence intensity and duration.
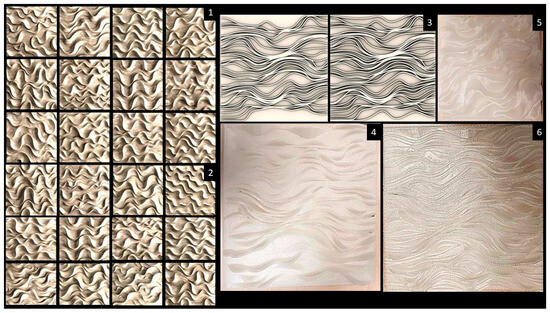
Figure 2.
The generative design process of field-topology-based bioreceptive tiles for immobilizing bioluminescent bacteria A. fischeri. (1,2) Twenty-six different design iterations originating from the second design phase, where the diffusion parameters were modified to create a smooth and open topological transition in the fields pattern. (3) The algorithmic customized design of the bioreceptive tile based on the field-continuous topology pattern developed in Rhinoceros 3D, Grasshopper, and Python, featuring a dense curvilinear pattern and a more continuous topology, which qualifies it as a bioreceptive surface for bioluminescent bacteria. (4) The 3D-printed bioreceptive tile design C2 and its further enhanced version with added curvilinear offsets for enhanced entrapment of the bacterial culture, as exhibited in (6) The two tiles were 3D-printed with translucent PLA with a micro-texture of a rectangular grid: each tile had dimensions of 18 × 18 × 0.5 cm. (5) Another iteration of C2, varying the diffusion parameters to fuse the curves into continuous topologies; this 3D-printed iteration was tested separately and will be exhibited in a future study.
Economical Competence and Enclosure and Assembly in the Built Environment
The bioreceptive tile design is suitable for mass production using affordable 3D printing tools and materials, requiring a moderate fabrication time of 4 h and a 300% increase in printing speed per tile. To reduce the printing time per tile by increasing the speed of the 3D printing process, thus proving the compatibility of the tile´s design with high definition, accurate printing settings and the high resolution and precision of the employed 3D printer were used in the current study. An average desktop FDM 3D printer, which costs EUR ~350 (Shenzhen Creality 3D Technology Co, Ltd. Shenzhen, China, https://www.creality.com/products/creality-ender-3-s1-pro-fdm-3d-printer), can be used to print an unlimited number of these tiles. The PLA filament used to print one tile costs EUR 4. While machine wear-out costs can be added to the overall cost of the tiles, which includes the electrical power and the effects of the 3D printing job on machine wear-out, this cost is defined by the manufacturer. It can be estimated as a percentage of the material cost or can be equal to the material cost. In the current work, the wear-out cost is defined as equal to the material cost due to the high printing speed wear-out effect. Therefore, the total production cost for one bioreceptive tile is EUR 8. In some cases, the designer´s cost can be added to the overall cost of the tile; however, in this work, only the actual production costs are presented, consistent with the purpose of the decentralized production of this bioreceptive tile for bioluminescence integration in the built environment.
On the other hand, the costs of culturing the bioluminescent bacteria strain Aliivibrio fischeri used in the current study are also not estimated from the production cost of the tiles, since it can be obtained from friendly microbiology labs in universities and research centers, and even if purchased, starter kit only costs EUR ~340 (including the media components; Fisher Scientific S.L. 28108-Alcobendas (Madrid, Spain), ESPAÑA. Thermo Fisher Scientific Inc., Waltham, MA, USA), which can be used to regenerate the bacterial culture unlimitedly.
Such bioreceptive tiles do not need to be in bioreactor containers, since the bioluminescent bacteria can grow and glow on solid media, as shown in the current work. Furthermore, these bioreceptive tiles can be used as cladding for any facade and can be enclosed by a wide array of membranous materials, such as ion-exchange membranes [23], to ensure their safe integration in the built environment. There are a variety of ion-exchange membranes that range in price from EUR 1.5 per 1m2 to EUR 500 (Fuel Cell Store, © 2025., Bryan, TX, USA). This production cost of the bioluminescent tile holds economic competence in comparison to the production and operation costs of a bioreactor, since for 3D printing a hermetic bioreactor with the same field pattern, the production cost per bioreactor tile will be multiplied by 6; (6 × EUR 4 = EUR 24), having the six sides of a simple cuboid or a box bioreactor. This leads to an increase in the overall production cost, considering the machine wear-out factor, which is estimated equally to the material cost, giving an overall cost of EUR 48. There is also the cost of auxiliary system components, including circulation and effluent, which consist of pipes, valves, and filters, and the complexity of discharging the effluent and recharging the system with fresh media. All these factors qualify the bioreceptive tiles developed in the current study as a more durable and easily manageable system than bioreactors.
A fully assembled 1:1 architectural-scale urban screen is exhibited in Figure 3, side by side with the details of the developed tile, featuring its square micro-texture as the building-block scaffold of this bioluminescent urban screen system.

Figure 3.
The urban screen system is designed for hosting the bioluminescent bacterial strain A. fischeri for integration in the built environment and in architectural and urban applications. (1) One 3D-printed bioreceptive tile of the bioluminescent urban screen, showing the varied topology between peaks and wells and interstitial spaces, as well as the micro-textured square grid for better anchorage of the hosted bacterial strain. (2) A fully assembled urban screen panel measuring 170 × 90 × 4 cm, incorporating the 3D-printed tiles with varied PLA filaments between translucent, transparent, and fluorescent, which will be tested for their effect on the bioluminescent activity of the hosted in A. fischeri and exhibited in a future study.
3.2. Optimized Culture Media of the Bioluminescent Bacteria Aliivibrio fischeri CECT 524
The Marine Broth (MB) yielded a higher growth rate of Aliivibrio fischeri CECT 524, with the bioink 3D culture showing visible bioluminescent emitted light but with fluctuations in absorbance, where a negative OD value at 18 h was recorded, probably due to nutrient consumption by the growing bacteria causing the solution to become clear (Figure 4, Table 1). From the different agar concentrations analyzed, the 40% to 50% agar concentration for the solid media applied to the urban screen panels achieved the best results in terms of enabling the bioluminescent chemotaxis behavior due to the enhanced mobility of A. fischeri at lower agar concentrations, which ensured better nutrient availability. No differences in bioluminescence expression were observed between NaOH and KOH used as pH adjustment agents applied to the bioink media on the urban screen panels.
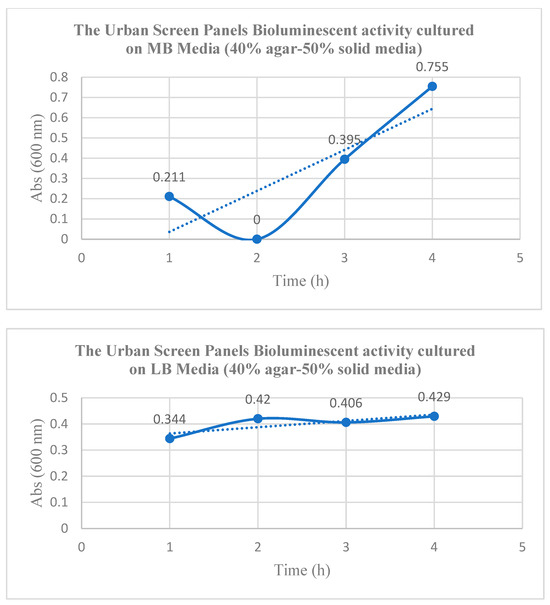
Figure 4.
The bioluminescence activity of the urban screen panels under MB media vs. LB media. (1) The urban screen panels’ bioluminescence activity when cultured on MB media (40% agar–50% solid media), exhibiting the highest maximum Abs as an indication of the A. fischeri bacterial culture density and activity when cultivated on Marine Broth reaching 0.755 Abs, despite the fluctuations on the second hour that is attributed to the lag phase. (2) The urban screen panels’ bioluminescence activity when cultured on LB media (40% agar–50% solid media) showing the highest reached Abs of 0.429 which is lower than in the case of Marine Broth Culture media. The unit Abs is absorbance, a quantity that measures how much light a substance absorbs at a particular wavelength. It is a logarithmic measure, often called optical density (Molecular Devices, 2024: https://www.moleculardevices.com/technology/absorbance, accessed on 5 September 2025), with higher Abs values indicating more light absorption by the sample and less light passing through. The solid lines is the estimated Absorbance (Abs) values in time (h), while the dotted lines is the deducted function representing the overall behavior of the bioluminescent bacterial culture on the tiles; which are a growth function in the first "The urban screen panels’ bioluminescence activity when cultured on MB media (40% agar–50% solid media)", and almost a fixed function in the second “The urban screen panels’ bioluminescence activity when cultured on LB media (40% agar–50% solid media)”.

Table 1.
The urban screen panels’ bioluminescence activity when cultured on MB vs. LB, as measured by absorbance at 600 nm at 5, 3, and 2 h, respectively.
The LB Broth media ranked second in in terms of the achieved culture growth and bioluminescence activity, with the optimal sea salt concentration achieved by 15 g/L sodium chloride AGR.
The TSB media recorded negative results.
3.3. Three-Dimensionally Printed Urban Screen Panels with Bioluminescence Activity
The best results in terms of the bioluminescence activity of the Aliivibrio fischeri CECT 524 was blue–green light at ~490 nm, which was more visible with the naked eye on the P1 part of the urban screen panel (tiles) (Figure 5), with a higher emission of light, enough to illuminate the tile surface space after only 3-10 s when placed in a dark room and with a 10-s exposure time captured with a standard digital camera. A longer duration of luminescence activity, lasting up to 10 days without refilling the growth medium, was demonstrated by the MB media slurry spray, in comparison to the other three parts (P2, P3, and P4), which lasted between 3 and 7 days of visible bioluminescence activity. It was observed that the maximum light emission was visible at the thin curvilinear peaks of the 3D-printed field patterns, as they were more durable in terms of their bioluminescence activity in comparison to the 3D surfaces of the wells and the projected strips, as exhibited in Figure 5, showing the density of the projected peaks that were >0.5 cm in height, the projected strips of ≤0.3 cm, and the wells at each of the four parts of the 3D-printed bioreceptive panel with its field-based pattern.
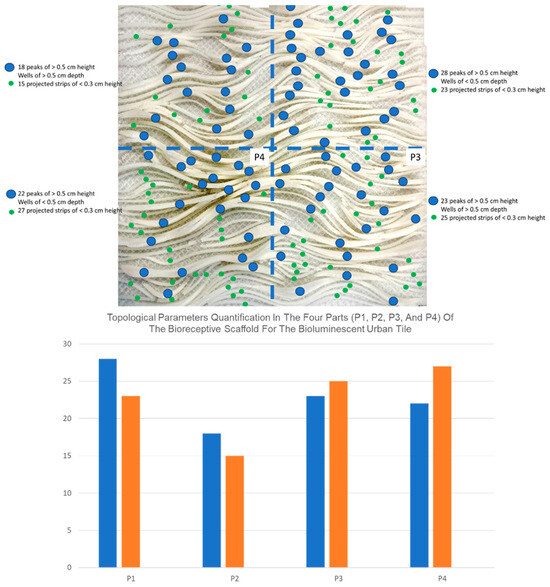
Figure 5.
the field-based pattern density and topological level distribution in each of the four parts of the original bioreceptive 3D-printed panel, serving as a scaffold for the bioluminescent bioink. In each of the four parts, the number of peaks > 0.5 cm, the projected strips of ≤0.3 cm, and the varied-size wells are exhibited to justify the best bioluminescent activity results obtained on P1. The blue dots represent the number of peaks, and the green dots represent the number of projected strips, while the wells were not assigned to a specific color since any interstitial spaces between the peaks or strips were considered wells. P1 excelled in terms of the number of peaks with 28 peaks that were higher than 0.5 cm, thanks to their short extension in comparison to the continuous peaks of P2. P1 also had tighter wells with widths of less than 0.5 cm, which enhances cell attachment and culture anchorage. Finally, the moderate number of projected strips in P1, combined with the tight wells and the high peaks, yielded the best bioluminescence durability and intensity in comparison to P2, P3, and P4.
As exhibited in Figure 5, the number of peaks was the primary parameter determining the optimal bio receptivity for the bioluminescent bacterial bioink, which yielded the highest intensity and durability of blue–green emitted light, lasting for 10 days without the need for refilling in the case of P1, thanks to the thinner layer of the bioluminescent bacterial bioink at these peaks compared to the wells and the strips. This facilitated better oxygenation and the shortest path for the bacterial cells to glide through the 3D culture of the bioink and demonstrate their collaborative bioluminescence behavior. Moreover, in P1, with the highest number of 28 peaks, the distribution of high peaks, with their short distances, increased the oxygen circulation, alongside the effect of the peaks in the topology, augmented by the interplay with the tighter wells and the moderate number of projected strips, i.e., 23 strips that are less than 0.3 cm in height. P4 ranked second in terms of bioluminescence efficiency thanks to its 22 peaks and tight wells. P3 came third, with 23 peaks that were higher than 0.5 cm. The wider wells of more than 0.5 cm in width hindered the bioluminescence chemotaxis behavior and affected the anchorage of the 3D culture to the scaffold, despite the large number of the projected strips. P2 came last, with a lower number of peaks and wider wells that hindered the anchorage of the 3D cell culture within the bioluminescent bioink, proving the effect of the scaffold´s geometry on the bioluminescence efficiency of the bioink. Figure 6 exhibits the culturing procedures for the cold-plate freezing of the 3D-printed tile pre-inoculation for sterilization and for sustaining the slurry culture added in step 2. Steps 3 and 4 exhibit P1 post-inoculation, and the blue–green light was emitted for 10 days without the need for refilling the media, proving the sustainability of this approach based on collaborating the customized geometrical design of the scaffold with the customized bioluminescent bioink. It also delivers lower maintenance costs and procedures than bioreactors and has self-sustaining structures, enabling architectural-and urban-scale applications.

Figure 6.
The bioluminescent urban screen culturing and bioluminescence activity: (1) Cold-plate freezing of the 3D-printed tile, divided into four parts, for sterilization and preparation for adding the slurry culture of the bioluminescent bioink. The seeding process was followed by covering it with the media in 5 layers. (2) The seeded urban panels’ parts cultured in petri dishes. (3) The P1 part of the urban screen panel seeded with the bioluminescent bacterial culture + media in agar. (4) The same part under a conventional digital camera with an exposure time of 10 s in a dark room, exhibiting the visible bioluminescence activity, which lasted for 10 days without refilling.
4. Discussion
The current work aims to scale-up the application of bioluminescent materials as architectural- and urban-scale functional materials to achieve sustainability. Going beyond the previous literature, which was limited to pilot-scale and petri plate research endeavors, to study the bioluminescent behavior of various naturally occurring or GMO-modified organisms [11,12,15,16,17]. Therefore, the current work’s novelty and significance lie in exploiting the optimized bioluminescent activity of A. fischeri culture as an architectural material with a real architecture- and urban-scale applications through the developed bioluminescent urban screens that can be used as cladding at an urban and architecture scale. This is achieved through the customized design of these bioreceptive tiles, which were developed from the physical and chemical fields that are expressed morphologically in nature on various scales through self-organization [24], deferential growth, or reaction–diffusion mechanisms [25]. The gradients present in the field patterns signify the gradual transfer (or travel) of force, where the force intensity expressed in the reaction of the topology form decreases congruently with the increase in distance moving away from the force point (attractor point, curve, or region). These gradients create a complex mix of topology, surface, and simple geometry, which can be conceived as a point cloud. This gradual transfer of force and its consequent gradient reaction of particles of self-organization in time and space is the most compatible approach when designing a bioreceptive surface for bioluminescent bacterial culture, facilitating the integration of augmented A. fischeri bioluminescent bacteria in the built environment. Unlike algae, fungi, and plants that are multicell and tissue-based organisms, bacteria do not have a unified corpus that augments their viability or sustains them across an extended topology. This limitation is reflected in the need for specific conditions for the bacteria to search for nutrients and keep their micro-community alive. Mainly, this involves the need for a continuous, motile topology to facilitate the gliding and swimming of bacteria towards nutrients [26,27,28]. In addition, these continuous topologies need to provide attachment to the bacterial cells in order to keep them attached to the bioreceptive surface and keep them almost embedded within it. Therefore, this was reflected in the bioreceptive tile design, incorporating high peaks, projected strips, and wells, translating this field-based pattern into a bioreceptive scaffold that facilitates the anchorage of the bacterial cells without hindering their motility. Moreover, this was also reflected in the seeding procedures of the bioluminescent bacterial bioink on the panels first to boost the culture attachability to the tiles and then covering it with the optimized media slurry to offer the nutrients required to maintain the culture as well as offering a refiling mechanism for these bioluminescent urban screens by spraying new media slurry every 10 days, as exhibited in the results of the bioluminescence activity durability testing of these tiles, a process which is more feasible in terms of maintenance than a typical bioreactor.
The field pattern design employed in the urban screen panels goes beyond the literature on developing customized bioreceptive surfaces for passive immobilization of specific strains, either one- or multi-scale length microbial strains, since Abdallah and Estevez, 2023 [21] employed a sharper and tighter gradient-based reaction diffusion mechanism pattern, where the optimum ratio between the hosted algal strain cells size and the niche size was 0.05% to 0.75%. The current work modifies this methodology by balancing a localized immobilization pattern technique with a continuous topology, since the A. fischeri bioluminescent bacteria requires motility and decreased distance travels between each agent in the culture to boost the emitted light intensity and duration of its bioluminescent activity, given the difference in scale between bacteria and algae [21] and also given that the bacterial strain used is not photosynthetic and is dependent on the micro-community (culture) to sustain its viability through searching for nutrients or escaping toxins through chemotaxis, which is reflected in the emitted light from its bioluminescence activity [29,30].
The efficiency of the proposed design based on the field topology was proven by the differential efficiency of some parts of the tile compared to other parts, as reported in the Results section. P1 achieved the longest and most visible light, maintaining the hosted bacterial culture’s viability and bioluminescence activity for 10 days without refilling the media. This is justified by the denser curvilinear peaks in this part, P1, of the tile compared to the other parts, which incorporate fewer peaks and wider wells. As reported in the Results section, higher light intensity was observed at the thin curvilinear peaks of the tile parts. Two reasons justify this: 1. The scale of the bacterial colonies requires minimal distance (shortest path) between their agents (bacterium) in the colony to activate and maintain the bioluminescence activity most economically in terms of exerted energy and consumption of media [31,32,33]; 2. The inhibition of motility and, likely, the growth of the bacterial culture in the wells was due to the physical density of the layered media (five layers) above the seeded bacterial culture bioink on the tiles, imposing a three-dimensional challenge for the bacteria to travel not only horizontally to accumulate and densify the culture for more intense and durable bioluminescence but also to travel vertically from its original location on the micro-textured surface of the tile to top over these layers of media to acquire nutrition and oxygen, which are mandatory to sustain the culture and activate the bioluminescence reaction [34]. These findings play a crucial role in optimizing the urban screen panel design and the seeding procedure of the bioluminescent bacterial culture. The optimization trajectories include the following: 1. Minimizing the depth and width of the wells of the field pattern and focusing more on a curvilinear peaks with minimum thickness to follow the shortest path strategy of the bacterial migration mechanism [30] and on a scale more compatible with the bacterial culture. This would minimize the physical resistance on the tile topology in 2D and 3D, resulting in enhanced culture viability, density, biofilm formation, and bioluminescence intensity and duration. 2. Altering the order of the seeding steps by covering the tile first with the optimized media slurry and then seeding the bacterial culture bioink on top of it; this would solve the three-dimensional challenge of the media on the bacterial migration; however, there would still be 2D resistance from the distance between the evenly distributed agents (bacterium) of the bioluminescent bacterial bioink over the tile topology, as well as 3D resistance when new media slurry is sprayed over the tiles to replenish the nutrients. Thus, the third trajectory combines the first two solutions by optimizing the tile design, focusing on the minimum thickness of the curvilinear peaks, seeding the bacterial bioink on top of the optimized media hosted on the urban screen tiles, and minimizing the number applied media layers to less than five and limiting spraying the refill media to 1–2 layers only.
In the current work, Aliivibrio fischeri CECT 524, a marine species found in temperate and subtropical waters [35], was employed as a naturally bioluminescent agent, as it is known for an intensity of its bioluminescence at ~490 nm in the blue–green range, as documented in the literature [22]. A. fischeri produces bioluminescence only when its cell density exceeds a critical threshold; therefore, the optimized media and scaffold design play crucial role in boosting culture viability, density, and bioluminescence behavior. This bioluminescence is a form of chemiluminescence catalyzed by enzymes, differing from fluorescence and phosphorescence in that light emission is not reliant on the absorption of photons. This made it the best choice for the current work objective of developing self-sufficient bioluminescent urban screens that can emit light in darkness without an auxiliary source of light or electricity. In A. fischeri, the process involves two substrates: luciferin, a reduced form of flavin mononucleotide (FMNH2), and a long-chain fatty aldehyde (RCHO), typically tetra decanal. An external reductant facilitates the reduction of flavin mononucleotide (FMN) to FMNH2 via flavin mono-oxygenase oxidoreductase. The reduced flavin (FMNH2) binds to the enzyme and reacts with oxygen to form a 4a-peroxy-flavin intermediate. This intermediate oxidizes the aldehyde into the corresponding acid (RCOOH). It produces a stable luciferase–hydroxyflavin complex in an excited state, which slowly decays to its ground state, emitting blue–green light with a peak intensity around 490 nm [36]. The activity of the electron transport system is crucial for producing the reduced substrates necessary for bioluminescence. This process is driven by central carbon and energy metabolism reactions, and any disturbance in these processes or in electron transport itself impacts the bioluminescence. Thus, monitoring changes in bioluminescence offers a direct and rapid means of assessing the effects of perturbations (physical or chemical) on microbial metabolism [37,38]. Therefore, the other side of optimizing the bioluminescence activity of the culture is through optimizing the culture media.
The Marine Broth media yield the highest growth and bioluminescence activity of the bacterial culture compared to the LB and TSB media tested in the current study. This is justified by its composition, which is rich in nutrients, salt, and trace elements, all of which are required for the growth of the marine bacteria A. fischeri, simulating its natural environment [39].
Meanwhile, the Tryptone Soy Broth (TSB) media failed to record any visible bioluminescence activity of the culture, since it is a general-purpose broth used for the growth of a wide range of microorganisms. It is not customized for culturing marine bacteria [40].
The media-to-agar concentration ranging from a 40% to 50% agar concentration for the solid media applied on the urban screen panels achieved the best results in terms of enabling the bioluminescent chemotaxis behavior based on the enhanced mobility of A. fischeri at lower agar concentrations, which ensures better nutrient availability. This is congruent with the reported results of Tyse, et al., 2022 [17], which justified having diminishing lights at the peaks with more light intensity in the wells due to deferential media drying, which is congruent with the bioluminescence reaction mechanism and requirements as a colony-based chemotaxis behavior, where the motility of the surface or topology is essential to activating the bioluminescence reaction. In the current work, the 3D altitude of the media expressed in the wells submerged with five layers of slurry media imposed physical resistance on the bacterial culture in terms of gliding in search for nutrients, migration, and clustering to perform the chemiluminescence behavior, which is the typical case in [17], where a bacterial bioink was 3D-bioprinted, where the cells have to move against gravity towards the 3D pattern peaks, justifying the results obtained in the current work, where the thin curvilinear peaks were more intense in terms of bioluminescence than the wells, since the bacterial cells were seeded on the 3D-printed PLA scaffold first and then covered with the media. This means that in this work’s case, the peaks carry less media volume than the wells, proving the efficiency of the scaffold-based bioluminescent material integration in architecture applications in terms of sustainability in materials and processes. The method also uses less media and delivers more durable bioluminescence activity, as achieved by the lower 3D media resistance to cell migration and clustering.
In the current work, it was not possible to perform typical bioluminescent light and duration intensity assays due to their incompatibility with the objective or the 3D culture topology of the bioluminescent urban panels (tiles). Usual methods focus on testing the bioluminescence of a bacterial culture in liquid medium or at a specific given point in a solid medium. This was not the case in the current work, as testing the bioluminescence intensity and duration on the bioluminescent urban panel requires diluting a sample taken from a specific zone on the 3D culture immobilized on the 3D-printed scaffolds (panels). This dilution by any solution (for example, PBS) would have resulted in a diluted and localized quantification of the bioluminescence intensity on the scale of the cellular level within the microculture. This is not the objective of these bioluminescent panels, which are designed for architectural and urban applications that require a different methodology of spatial quantification of the bioluminescence intensity and duration within the space. Another reason is the differential topology design of the customized bioreceptive panels (tiles) following the field-based pattern, making it impossible to depend on a singular or even multiple local samples taken from the 3D bioluminescent culture as a representative of the bioluminescent intensity, as they represent the microscale and not the architectural scale of the application. Therefore, the current work did not use the cellular bioluminescence intensity (BLcell) or population bioluminescence intensity due to the uneven 3D topology of the tiles and the need for a holistic and spatial estimation of the emitted light from the tile in the space.
Spatial luminometry is being currently conducted by testing the emitted light from the full tile at varying distances, starting from 1cm3 and reaching 1 m3 away from the tile´s imaginary bounding box to record the luminous spatial effect of these tiles in the architectural space and determine their functional applications in various activities. This will be published in a following paper that will compare the micro-scale cell-based bioluminescence intensity to the architecture-scale bioluminescence intensity to deduce a factor for scaling this application of the bioluminescent 3D-printed panels in architecture and urban applications.
The proposed stand-alone system of bioluminescent urban tiles is considered safe for applications on an architectural and urban scale, since Aliivibrio fischeri is a marine bacterium that generally does not cause pathology. Instead, it is known for its beneficial symbiotic relationship with marine animals [41]. Moreover, the Marine Broth Media used in the current study is a specialized medium for growing and studying marine bacteria, including various species. Its composition, which mimics the salinity and mineral content of seawater, provides the necessary nutrients for these specialized microorganisms that often do not thrive in standard lab media [42]. This reduces the likelihood that the Marine Broth Media used in the current study will be contaminated with other microbial strains that naturally occur in the air, soil, or water. This was also demonstrated by the observations in the current work, with 10-day sustained bioluminescent tiles that required no recharge and that maintained their morphology. While the agar used in the preparation of the slurry media of the bioluminescent bioink is just a solidifying and nutrient transmission agent. Further analysis of the extracellular matrix ECM and the microenvironment within the tiles is still required to further understand the bioluminescent bacterial growth kinetics and their possible contamination with other unwanted microbial strains, such as fungi or bacteria. Such a study would require varying and comparing the incubation environment across architectural and urban scales and varying the environmental conditions, including temperature, humidity, aeration, light and dark cycles, and orientation, which will be explored in a future study due to the current work’s scope and length.
Nevertheless, as a proof-of-concept step in the current work, the proposed bioluminescent tiles are safe to use for an average user, since they are contained inside closed petri dishes and are proposed to be enclosed within an architectural system that will offer chemically and physically customized enclosures of the system, as mentioned before, through ion-exchange membranes.
5. Conclusions
The current work proposes bioluminescent urban screen panels as a sustainable, passive lighting system for architectural- and urban-scale applications by employing a developed bioink from the bioluminescent bacterial strain Aliivibrio fischeri CECT 524 and the optimized growth media seeded on a customized bioreceptive scaffold (3D-printed panel or tile) as the building block of these urban screens. The panel design employed a field-diffusion pattern to generate a smooth transitional topology with varied categories between peaks, projected strips, and wells to increase the receptivity of the tile for the designated bioluminescent strain whilst allowing the anchorage of the bacteria to the surface, allowing the cells to proliferate, migrate, and cluster in order to boost the bioluminescence intensity and duration. The experimental methodology was based on successive and parallel optimization of the bacterial liquid and solid growth media composition, bioink formation by tuning the solid media-to-agar concentration, and testing differential topologies of the customized design of the 3D-printed panel (tile), cut and labelled in four parts (P1, P2, P3, and P4). The results revealed that the Marine Broth (MB) media yield the highest bioluminescence activity in terms of visible blue–green light intensity and in terms of its duration of 10 days. The concentration of 40% agar to 50% solid MB media was the optimal concentration of the developed bioluminescent bioink applied to the 3D-printed panel parts. The results revealed that P1 was the most efficient design in terms of supporting the bioluminescence behavior of the bacterial bioink thanks to its topology that contained more peaks > 0.5 cm, which were the most luminescent parts in P1, and tight wells that were less than 0.5 cm in width, facilitating cell-to-cell signaling and bacterial migration in the shortest path, which is more economic in terms of the energy exerted and media consumed by the local colony. Thus, short-path-based patterns are recommended for designing a bioreceptive scaffold system to host bioluminescent bacteria. It should be emphasized that the transition in scale from the micro- to the meso-culture is a key point in boosting the bioluminescent bacterial culture density and the consequent bioluminescence activity. Furthermore, it is proven that a scaffold–bioluminescent material system is more applicable on an architectural scale for stand-alone applications, achieving sustainability in terms of materials and processes and achieving enhanced and prolonged bioluminescence activity in comparison to micro-culture-scale applications in previous studies that failed to achieve or prove the feasibility of the economic scaling of a typical petri dish micro-culture.
Author Contributions
A.T.E.: Project Management; Funding Acquisition (Main Researcher); Conceptualization; Design Concept; Research Methodology; Data Analysis and Curation; First Draft Writing and Revision; Figures. Y.K.A.: Computational Design; 3D Printing; Research Methodology; Experimental Procedures; Data Analysis and Curation; First Draft Writing; Figures. A.B.M.: Experimental methodology; Data Analysis and Curation; Figures. M.S.S.: Experimental methodology; Data Analysis and Curation; Figures. All authors have read and agreed to the published version of the manuscript.
Funding
This work is funded by Fritz und Trude Fortmann Stiftung (Germany), under the BioLumCity project, 2024–2026.
Data Availability Statement
Images were made by the authors. Data are provided upon request to Alberto T. Estevez: estevez@uic.es.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Atasu, A.; Duran, S.; Van Wassenhove, L.N. The Dark Side of Solar Power. Harvard Business Review. 2021. Available online: https://hbr.org/2021/06/the-dark-side-of-solar-power (accessed on 18 June 2025).
- Kuby Renewable Energy Ltd. The Positive and Negative Environmental Impacts of Solar Panels. [online] Kubye-nergy.ca. 2019. Available online: https://kubyenergy.ca/blog/the-positive-and-negative-environmental-impacts-of-solar-panels (accessed on 18 June 2025).
- Wind Energy Technologies Office. Advantages and Challenges of Wind Energy. [accessed on 18 June 2025] Energy.gov. 2024. Available online: https://www.energy.gov/eere/wind/advantages-and-challenges-wind-energy (accessed on 18 June 2025).
- Statkraft.com. Mythbusting: ‘Wind Power is Unreliable, Inefficient and Harmful to Nature’. 2024. Available online: https://www.statkraft.com/newsroom/explained/mythbusting-wind-power-is-unreliable-inefficient-and-harmful-to-nature/ (accessed on 18 June 2025).
- Wood, J. Wind Power Costs: Why the Industry is Facing Cost Headwinds. [online] World Economic Forum. 2023. Available online: https://www.weforum.org/stories/2023/11/why-offshore-wind-cost-pressures-rising/ (accessed on 18 June 2025).
- Estévez, A.T. Towards Genetic Posthuman Frontiers in Architecture & Design. In ACADIA 2016 Posthuman Frontiers: Data, Designers, and Cognitive Machines; Velikov, K., Ahlquist, S., del Campo, M., Thün, G., Eds.; ACADIA/Taubman College of Architecture and Urban Planning; University of Michigan: Ann Arbor, MI, USA, 2016; pp. 450–459. ISBN 978-0-692-77095-5. Available online: https://www.papers.cumincad.org (accessed on 5 August 2025).
- Abdallah, Y.K.; Estevez, A.T.; Tantawy, D.E.D.M.; Ibraheem, A.M.; Khalil, N.M. Employing Laccase-Producing Aspergillus sydowii NYKA 510 as a Cathodic Biocatalyst in Self-Sufficient Lighting Microbial Fuel Cell. J. Microbiol. Biotechnol. 2019, 29, 1861–1872. [Google Scholar] [CrossRef] [PubMed]
- Jaafari, A.A.Q.; Roznowski, V.; Estévez, A.T.; Abdullah, Y.K. Self-Sufficient Bioelectricity Systems in Architec-ture: Employing Spirulina Platensis in Photosynthetic Microbial Fuel Cells for the Generation of Domestic and Urban Bio-Electricity through a Diffusion-Limited Aggregation Pattern. In Sustainable Engineering Technologies and Architectures; AIP Publishing LLC.: Melville, NY, USA, 2021; pp. 1–18. [Google Scholar] [CrossRef]
- Hassan, M.; Kanwal, S.; Singh, R.S.; Sa, M.A.; Anwar, M.; Zhao, C. Current challenges and future perspectives associated with configuration of microbial fuel cell for simultaneous energy generation and wastewater treatment. Int. J. Hydrogen Energy 2023, 50, 323–350. [Google Scholar] [CrossRef]
- Abdallah, Y.K.; Estevez, A.T. Bioactive Devices as Self-Sufficient Systems for Energy Production in Architecture. J. Green Build. 2021, 16, 3–22. [Google Scholar] [CrossRef]
- Mitiouchkina, T.; Mishin, A.S.; Somermeyer, L.G.; Markina, N.M.; Chepurnyh, T.V.; Guglya, E.B.; Karataeva, T.A.; Palkina, K.A.; Shakhova, E.S.; Fakhranurova, L.I.; et al. Plants with genetically encoded autoluminescence. Nat. Biotechnol. 2020, 38, 944–946. [Google Scholar] [CrossRef]
- Kotlobay, A.A.; Sarkisyan, K.S.; Mokrushina, Y.A.; Marcet-Houben, M.; Serebrovskaya, E.O.; Markina, N.M.; Somermeyer, L.G.; Gorokhovatsky, A.Y.; Vvedensky, A.; Purtov, K.V.; et al. Genetically encodable bioluminescent system from fungi. Proc. Natl. Acad. Sci. USA 2018, 115, 12728–12732. [Google Scholar] [CrossRef]
- Estévez, A.T.; Dollens, D. The Genetic Creation of Bioluminescent Plants for Urban and Domestic Use. Leonardo 2007, 40, 18. Available online: https://muse.jhu.edu/article/209698 (accessed on 5 August 2025). [CrossRef]
- Royaldanishacademy.com. BioLum|Det Kongelige Akademi. 2024. Available online: https://royaldanishacademy.com/en/case/biolum (accessed on 21 December 2024).
- Malekpour, A.; Chen, X. Printability and Cell Viability in Extrusion-Based Bioprinting from Experimental, Computational, and Machine Learning Views. J. Funct. Biomater. 2022, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.; Tamke, M.; Mosse, A.; Jakob, S.; Bradshaw, H.; Buchwald, F.; Mosshammer, M. Imprimer la Lumiere: 3d Printing Bioluminescence for Architectural Materiality. In Proceedings of the 2021 DigitalFUTURES, Shanghai, China, 3–4 July 2021. Zenodo (CERN European Organization for Nuclear Research). [Google Scholar] [CrossRef]
- Tyse, G.; Tamke, M.; Thomsen, M.R.; Mosse, A.F. Bioluminescent micro-architectures: Planning design in time, an eco-metabolistic approach to biodesign. Arch. Struct. Constr. 2022, 2, 471–479. [Google Scholar] [CrossRef]
- Abdallah, Y.K.; Estévez, A.T. Biomaterials and Architecture, a Possible Future: Bioprinting Architecture. J. Regen. Med. 2022, 11, 1000210. Available online: https://spanish.scitechnol.com/abstract/biomaterials-architecture-a-possible-future-bio-printing-architecture-18989.html (accessed on 25 August 2025).
- Estevez, A.T.; Abdallah, Y.K. Biomimetic Approach for Enhanced Mechanical Properties and Stability of Self-Mineralized Calcium Phosphate Dibasic–Sodium Alginate–Gelatine Hydrogel as Bone Replacement and Structural Building Material. Processes 2024, 12, 944. [Google Scholar] [CrossRef]
- Chen, J.; Fan, Y.; Dong, G.; Zhou, H.; Du, R.; Tang, X.; Ying, Y.; Li, J. Designing biomimetic scaffolds for skin tissue engineering. Biomater. Sci. 2023, 11, 3051–3076. [Google Scholar] [CrossRef]
- Abdallah, Y.K.; Estévez, A.T. 3D-Printed Bioreceptive Tiles of Reaction–Diffusion (Gierer–Meinhardt Model) for Multi-Scale Algal Strains’ Passive Immobilization. Buildings 2023, 13, 1972. [Google Scholar] [CrossRef]
- Scheerer, S.; Gomez, F.; Lloyd, D. Bioluminescence of Vibrio fischeri in continuous culture: Optimal conditions for stability and intensity of photoemission. J. Microbiol. Methods 2006, 67, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Xu, T. Ion exchange membranes: State of their development and perspective. J. Membr. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Olemskoi, A.; Khomenko, A.; Olemskoi, D. Field theory of self-organization. Phys. A Stat. Mech. Appl. 2004, 332, 185–206. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Liu, P.; Eisenberg, B. Field theory of reaction-diffusion: Law of mass action with an energetic variational approach. Phys. Rev. E 2020, 102, 062147. [Google Scholar] [CrossRef]
- Aschtgen, M.-S.; Brennan, C.A.; Nikolakakis, K.; Cohen, S.; McFall-Ngai, M.; Ruby, E.G. Insights into flagellar function and mechanism from the squid–vibrio symbiosis. npj Biofilms Microbiomes 2019, 5, 32. [Google Scholar] [CrossRef]
- Scheidweiler, D.; Bordoloi, A.D.; Jiao, W.; Sentchilo, V.; Bollani, M.; Chhun, A.; Engel, P.; de Anna, P. Spatial structure, chemotaxis and quorum sensing shape bacterial biomass accumulation in complex porous media. Nat. Commun. 2024, 15, 191. [Google Scholar] [CrossRef] [PubMed]
- de Anna, P.; Pahlavan, A.A.; Yawata, Y.; Stocker, R.; Juanes, R. Chemotaxis under flow disorder shapes microbial dispersion in porous media. Nat. Phys. 2020, 17, 68–73. [Google Scholar] [CrossRef]
- DeLoney-Marino, C.R. Observing Chemotaxis in Vibrio fischeri Using Soft Agar Assays in an Undergraduate Microbiology Laboratory. J. Microbiol. Biol. Educ. 2013, 14, 271–272. [Google Scholar] [CrossRef][Green Version]
- Zhuang, X.-Y.; Tseng, C.-K.; Lo, C.-J. Viscosity-dependent swimming patterns assist Aliivibrio fischeri for symbiont partner finding. Phys. Rev. Res. 2024, 6, 033214. [Google Scholar] [CrossRef]
- Millikan, D.S.; Ruby, E.G. Vibrio fischeri Flagellin A Is Essential for Normal Motility and for Symbiotic Competence during Initial Squid Light Organ Colonization. J. Bacteriol. 2004, 186, 4315–4325. [Google Scholar] [CrossRef]
- Sanchez, S.; Ng, W.-L. Motility Control as a Possible Link Between Quorum Sensing to Surface Attachment in Vibrio Species. Adv. Exp. Med. Biol. 2023, 1404, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Millikan, D.S.; Ruby, E.G. Alterations in Vibrio fischeri Motility Correlate with a Delay in Symbiosis Initiation and Are Associated with Additional Symbiotic Colonization Defects. Appl. Environ. Microbiol. 2002, 68, 2519–2528. [Google Scholar] [CrossRef]
- Nelson, C.M.; Chen, C.S. Cell-cell signaling by direct contact increases cell proliferation via a PI3K-dependent signal. FEBS Lett. 2002, 514, 238–242. [Google Scholar] [CrossRef]
- Ruby, E.G.; Lee, K.-H. The Vibrio fischeri-Euprymna scolopes Light Organ Association: Current Ecological Paradigms. Appl. Environ. Microbiol. 1998, 64, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Meighen, E.A. Molecular biology of bacterial bioluminescence. Microbiol. Rev. 1991, 55, 123–142. [Google Scholar] [CrossRef] [PubMed]
- Erzinger, G.S.; Schmoeller, F.; Pinto, L.H.; Américo, L.; Hemmersbach, R.; Hauslage, J.; Häder, D.-P. Biolumines-cence systems in environmental biosensors. Bioassays 2018, 241–262. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780128118610000127?via%3Dihub (accessed on 25 August 2025).
- Futra, D.; Heng, L.Y.; Surif, S.; Ahmad, A.; Ling, T.L. Microencapsulated Aliivibrio fischeri in Alginate Microspheres for Monitoring Heavy Metal Toxicity in Environmental Waters. Sensors 2014, 14, 23248–23268. [Google Scholar] [CrossRef]
- Mirjani, M.; Soleimani, M.; Salari, V. Toxicity assessment of total petroleum hydrocarbons in aquatic environments using the bioluminescent bacterium Aliivibrio fischeri. Ecotoxicol. Environ. Saf. 2021, 207, 111554. [Google Scholar] [CrossRef]
- Cálix-Lara, T.; Duong, T.; Taylor, T. Addition of a surfactant to tryptic soy broth allows growth of a Lactic Acid Bacteria food antimicrobial, Escherichia coli O157:H7, and Salmonella enterica. Lett. Appl. Microbiol. 2012, 54, 392–397. [Google Scholar] [CrossRef]
- Aizawa, S.-I. Aliivibrio fischeri—Light-Organ Symbiont in the Bobtail Squid. In Flagellar World; Academic Press: Cambridge, MA, USA, 2014; pp. 16–17. [Google Scholar] [CrossRef]
- Huang, Z.; Mo, S.; Yan, L.; Wei, X.; Huang, Y.; Zhang, L.; Zhang, S.; Liu, J.; Xiao, Q.; Lin, H.; et al. A Simple Culture Method Enhances the Recovery of Culturable Actinobacteria from Coastal Sediments. Front. Microbiol. 2021, 12, 675048. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).