The Vaginal Microbiome during Pregnancy in Health and Disease
Abstract
:1. Introduction
1.1. Scope of the Study
1.2. United Nations Goals on Maternal and Child Health
1.3. Adverse Pregnancy Outcomes
1.4. Health Risks in Pregnancy
2. Types of Infections during Pregnancy
2.1. Viral Infections
2.2. Bacterial Infections
2.3. Fungal Infections
2.4. Parasitic Infections
3. The Human Microbiome
3.1. The Human Microbiome Project
3.2. Methods of Investigation
3.2.1. 16S rRNA Sequencing
3.2.2. Metagenomics Sequencing
3.2.3. Omics Techniques
4. The Human Vaginal Microbiome
Lactobacillus ssp. Protective Role
5. Healthy and Abnormal Vaginal Microbiomes in Non-Pregnant Women
5.1. Normal Flora
5.2. Abnormal Flora
6. The Vaginal Microbiome during Pregnancy
6.1. Cross-Sectional Studies
6.1.1. Healthy Pregnancies
6.1.2. Pregnancies with Adverse Outcomes
Miscarriage
Stillbirth
Preterm Birth
Other Adverse Outcomes
6.2. Longitudinal Studies
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- UN Millennium Development Goals. Available online: https://www.un.org/millenniumgoals/ (accessed on 15 July 2022).
- UN Department of Economic and Social Affairs. Transforming Our World: The 2030 Agenda for Sustainable Development UN General Assembly A/RES/70/1; UN: New York, NY, USA, 2015; Available online: https://sdgs.un.org/2030agenda (accessed on 15 July 2022).
- UN Inter-agency Group for Child Mortality Estimation. Levels & Trends in Child Mortality, Report 2021; UNICEF: New York, NY, USA, 2021; Available online: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 15 July 2022).
- Editorial: Miscarriage-Worldwide reform of care is needed. Lancet 2021, 397, 1507.
- UN Inter-Agency Group for Child Mortality Estimation. A Neglected Tragedy: The Global Burden of Stillbirth Report 2020; UNICEF: New York, NY, USA, 2020; Available online: https://www.unicef.org/media/84851/file/UN-IGME-the-global-burden-of-stillbirths-2020.pdf (accessed on 30 July 2022).
- Walani, S.R. Global burden of preterm birth. Int. J. Gynecol. Obstet. 2020, 150, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Kaforau, L.S.K.; Tessema, G.A.; Jancey, J.; Dhamrait, G.; Bugoro, H.; Pereira, G. Prevalence and risk factors of adverse birth outcomes in the Pacific Island Region: A scoping review. Lancet Reg. Health-West. Pac. 2022, 21, 100402. [Google Scholar] [CrossRef] [PubMed]
- Baskaradoss, J.K.; Geevarghese, A.; Al Dosari, A.A.F. Causes of adverse pregnancy outcomes and the role of maternal periodontal status. Open Dent. J. 2012, 6, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Hussein, J.; Ugwumadu, A.; Witkin, S.S. Editor’s Choice. Br. J. Obst. Gynaecol. 2011, 118, i–ii. [Google Scholar] [CrossRef]
- Zeng, M.; Yang, L.; Mao, Y.; Yang He, Y.; Li, M.; Liu, J.; Zhu, Q.; Chen, L.; Zhou, W. Preconception reproductive tract infections status and adverse pregnancy outcomes: A population-based retrospective cohort study. BMC Pregnancy Childbirth 2022, 22, 501. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Child Health and Human Development. What Infections Can Affect Pregnancy? Available online: https://www.nichd.nih.gov/health/topics/pregnancy/conditioninfo/infections# (accessed on 15 July 2022).
- Pomar, L.; Baud, D. Emerging virus infections in adverse pregnancy outcomes. Viruses 2022, 14, 285. [Google Scholar] [CrossRef]
- Chaudhry, S.A.; Koren, G. Hepatitis A infection during pregnancy. Can. Fam. Physician 2015, 61, 963–964. [Google Scholar]
- Sirilert, S.; Tongsong, T. Hepatitis B virus infection in pregnancy: Immunological response, natural course and pregnancy outcomes. J. Clin. Med. 2021, 10, 2926. [Google Scholar] [CrossRef]
- Wu, C.; Wu, X.; Xia, J. Hepatitis E virus infection during pregnancy. Virol. J. 2020, 17, 73. [Google Scholar] [CrossRef]
- Wang, R.; Yan, W.; Du, M.; Tao, L.; Liu, J. The effect of influenza virus infection on pregnancy outcomes: A systematic review and meta-analysis of cohort studies. Int. J. Infect. Dis. 2021, 105, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, R.; Platania, A.; Cuccia, M.; Zappalà, G.; Giorgianni, G.; D’Agati, P.; Bellia, M.A.; Marranzano, M. Measles and pregnancy: Immunity and immunization-What can be learned from observing complications during an epidemic year. J. Pregnancy 2020, 2020, 6532868. [Google Scholar] [CrossRef] [PubMed]
- Canadian Paediatric Society. Rubella (German measles) in Pregnancy. Paediatr. Child Health 2007, 12, 798. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Pregnancy and Rubella; National Center for Immunixation and Respiratory Disease (NCIRD), Division of Viral Diseases: Atlanta, GA, USA, 2020. Available online: https://www.cdc.gov/rubella/pregnancy.html#:~:text=Pregnant%20women%20who%20contract%20rubella (accessed on 30 July 2022).
- Shi, T.-L.; Huang, L.-J.; Xiong, Y.-Q.; Zhong, Y.-Y.; Yang, J.-J.; Fu, T.; Lei, X.-F.; Chen, Q. The risk of herpes simplex virus and human cytomegalovirus infection during pregnancy upon adverse pregnancy outcomes: A meta-analysis. J. Clin. Virol. 2018, 104, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Njue, A.; Coyne, C.; Margulis, A.V.; Wang, D.; Marks, M.A.; Russell, K.; Das, R.; Sinha, A. The role of congenital cytomegalovirus infection in adverse birth outcomes: A review of the potential mechanisms. Viruses 2021, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Condrat, C.E.; Filip, L.; Gherghe, M.; Cretoiu, D.; Suciu, N. Maternal HPV infection: Effects on pregnancy outcome. Viruses 2021, 13, 2455. [Google Scholar] [CrossRef]
- World Health Organisation. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. 2013. Available online: https://www.who.int/hiv/pub/guidelines/arv2013/download/en/ (accessed on 30 July 2022).
- Tukei, V.J.; Hoffman, H.J.; Greenberg, L.; Thabelo, R.; Nchephe, M.; Mots’oane, T.; Masitha, M.; Chabela, M.; Mokone, M.; Mofenson, L.; et al. Adverse pregnancy outcomes among HIV-positive women in the era of universal antiretroviral therapy remain elevated compared with HIV-negative women. Pediatr. Infect. Dis. J. 2021, 40, 821–826. [Google Scholar] [CrossRef]
- Ko, J.Y.; DeSisto, C.L.; Simeone, R.M.; Ellington, S.; Galang, R.R.; Oduyebo, T.; Gilboa, S.M.; Lavery, A.M.; Gundlapalli, A.V.; Shapiro-Mendoza, C.K. Adverse pregnancy outcomes, maternal complications, and severe illness among US delivery hospitalizations with and without a coronavirus disease 2019 (COVID-19) Diagnosis. Clin. Infect. Dis. 2021, 73 (Suppl. S1), S24–S31. [Google Scholar] [CrossRef]
- Yeruva, T.; Rajkumar, H.; Donugama, V. Vaginal lactobacilli profile in pregnant women with normal & abnormal vaginal flora. Indian J. Med. Res. 2017, 146, 534–540. [Google Scholar]
- Godoy-Vitorino, F.; Romaguera, J.; Zhao, C.; Vargas-Robles, D.; Ortiz-Morales, G.; Vázquez-Sánchez, F.; Sanchez-Vázquez, M.; de la Garza-Casillas, M.; Martinez-Ferrer, M.; White, J.R.; et al. Cervicovaginal fungi and bacteria associated with cervical intraepithelial neoplasia and high-risk human papillomavirus infections in a Hispanic population. Front. Microbiol. 2018, 9, 2533. [Google Scholar] [CrossRef]
- Maki, Y.; Fujisaki, M.; Sato, Y.; Sameshima, H. Candida chorioamnionitis leads to preterm birth and adverse fetal-neonatal outcome. Infect. Dis. Obstet. Gynecol. 2017, 2017, 9060138. [Google Scholar] [CrossRef] [PubMed]
- MotherSafe Royal Hospital for Women. Thrush and Pregnancy. NSW Health 2021. Available online: https://www.seslhd.health.nsw.gov.au/sites/default/files/groups/Royal_Hospital_for_Women/Mothersafe/documents/thrushpreg2021 (accessed on 30 July 2022).
- Garrison, A.; Boivin, M.; Khoshnood, B.; Courtin, D.; Alao, J.; Mireku, M.; Ibikounle, M.; Massougbodji, A.; Cot, M.; Bodeau-Livinec, F. Soil-transmitted helminth infection in pregnancy and long-term child neurocognitive and behavioral development: A prospective mother-child cohort in Benin. PLoS Negl. Trop. Dis. 2021, 15, e0009260. [Google Scholar] [CrossRef] [PubMed]
- Murenjekwa, W.; Makasi, R.; Ntozini, R.; Chasekwa, B.; Mutasa, K.; Moulton, L.H.; Tielsch, J.M.; Humphrey, J.H.; Smith, L.E.; Prendergast, A.J.; et al. Determinants of urogenital schistosomiasis among pregnant women and its association with pregnancy outcomes, neonatal deaths, and child growth. J. Infect. Dis. 2021, 223, 1433–1444. [Google Scholar] [CrossRef]
- Weill, A.; Bernigaud, C.; Mokni, M.; Gil, S.; Elefant, E.; Chosidow, O. Scabies-infested pregnant women: A critical therapeutic challenge. PLoS Negl. Trop. Dis. 2021, 15, e0008929. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.L.L.; Hasang, W.; Rogerson, S.J.; Teo, A. Poor birth outcomes in malaria in pregnancy: Recent insights into mechanisms and prevention approaches. Front. Immunol. 2021, 12, 621382. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.M.; Eick, S.M.; Dailey, C.; Dale, A.P.; Mehta, M.; Nair, A.; Cordero, J.F.; Welton, M. Relationship between pregnancy-associated malaria and adverse pregnancy outcomes: A systematic review and meta-analysis. J. Trop. Pediatr. 2020, 66, 327–338. [Google Scholar] [CrossRef]
- Kwizera, A.; Ntasumumuyange, D.; Small, M.; Rulisa, S.; Moscovitz, A.N.; Magriples, U. Assessment of perinatal outcomes of pregnant women with severe versus simple malaria. PLoS ONE 2021, 16, e0247053. [Google Scholar] [CrossRef] [PubMed]
- Arranz-Solis, D.; Mukhopadhyay, D. Toxoplasma effectors that affect pregnancy outcomes. Trends Parasitol. 2021, 27, 283–295. [Google Scholar] [CrossRef]
- Li, X.L.; Wei, H.X.; Zhang, H.; Peng, H.J.; Lindsay, D.S. A meta-analysis on risks of adverse pregnancy outcomes in Toxoplasma gondii infection. PLoS ONE 2014, 9, e97775. [Google Scholar] [CrossRef]
- Van Gerwen, O.T.; Craig-Kuhn, M.C.; Jones, A.T.; Schroeder, J.A.; Deaver, J.; Buekens, P.; Kissinger, P.J.; Muzny, C.A. Trichomoniasis and adverse birth outcomes: A systematic review and meta-analysis. Br. J. Obst. Gynaecol. 2021, 128, 1907–1915. [Google Scholar] [CrossRef]
- Human Microbiome Project Phase I. 2007. Available online: https://hmpdacc.org/hmp/ (accessed on 30 July 2022).
- NIH Human Microbiome Portfolio Analysis Team. A review of 10 years of human microbiome research activities at the US National Institutes of Health, Fiscal Years 2007–2016. Microbiome 2019, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- MOMS-PI. 2014. Available online: https://www.vmc.vcu.edu/projects.html (accessed on 30 July 2022).
- Nakama, C.; Thompson, B.; Szybala, C.; McBeth, A.; Dobner, P.; Zwickey, H. The continuum of microbial ecosystems along the female reproductive tract: Implications for health and fertility. Pathogens 2022, 11, 1244. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Park, S.D.; Jang, I.H.; Uh, Y.; Lee, A. The prevalence of vaginal microorganisms in pregnant women with preterm labor and preterm birth. Ann. Lab. Med. 2012, 32, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Wen, A.; Srinivasan, U.; Goldberg, D.; Owen, J.; Marrs, C.F.; Misra, D.; Wing, A.; Ponnaluri, S.; Miles-Jay, A.; Bucholz, B.; et al. Selected vaginal bacteria and risk of preterm birth: An ecological perspective. J. Infect. Dis. 2014, 209, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Woese, C.R.; Fox, G.E. Phylogenetic structure of the prokaryotic domain: The primary kingdoms. Proc. Natl. Acad. Sci. USA 1977, 74, 5088–5090. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Hoffman, N.G.; Morgan, M.T.; Matsen, F.A.; Fiedler, T.L.; Hall, R.W.; Ross, F.J.; McCoy, C.O.; Bumgarner, R.; Marrazzo, J.M.; et al. Bacterial communities in women with bacterial vaginosis: High resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS ONE 2012, 7, e37818. [Google Scholar] [CrossRef] [PubMed]
- Fettweis, J.M.; Serrano, M.G.; Sheth, N.U.; Mayer, C.M.; Glascock, A.L.; Brooks, J.P.; Jefferson, K.K. Species-level classification of the vaginal microbiome. BMC Genom. 2012, 13 (Suppl. S8), S17. [Google Scholar] [CrossRef]
- Gotschlich, E.C.; Colbert, R.A.; Gill, T. Methods in microbiome research: Past, present, and future. Best Pract. Res. Clin. Rheumatol. 2019, 3, 101498. [Google Scholar] [CrossRef]
- Babu, M.; Snyder, M. Multi-Omics profiling for health. Mol. Cell. Proteomics 2023, 22, 100561. [Google Scholar] [CrossRef]
- Wang, C.; Segal, L.N.; Hu, J.; Zhou, B.; Hayes, R.B.; Ahn, J.; Li, H. Microbial risk score for capturing microbial characteristics, integrating multi-omics data, and predicting disease risk. Microbiome 2022, 10, 121. [Google Scholar] [CrossRef]
- Kwoji, I.D.; Aiyegoro, O.A.; Okpeku, M.; Adeleke, M.A. Multi-omics’ data integration: Applications in probiotics studies. NPJ Sci. Food 2023, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Pekmezovic, M.; Mogavero, S.; Naglik, J.R.; Hube, B. Host-pathogen interactions during female genital tract infections. Trends Microbiol. 2019, 27, 982–996. [Google Scholar] [CrossRef] [PubMed]
- Linhares, I.M.; Summers, P.R.; Larsen, B.; Giraldo, P.C.; Witkin, S.S. Contemporary perspectives on vaginal pH and lactobacilli. Am. J. Obstet. Gynecol. 2011, 204, 120.e1–120.e5. [Google Scholar] [CrossRef] [PubMed]
- Lehtoranta, L.; Ala-Jaakkola, R.; Laitila, A.; Maukonen, J. Healthy vaginal microbiota and influence of probiotics across the female life span. Front. Microbiol. 2022, 13, 819958. [Google Scholar] [CrossRef] [PubMed]
- Bayigga, L.; Kateete, D.P.; Anderson, D.J.; Sekikubo, M.; Nakanjako, D. Diversity of vaginal microbiota in sub-Saharan Africa and its effects on HIV transmission and prevention. Am. J. Obstet. Gynecol. 2019, 220, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Monin, L.; Whettlock, E.M.; Male, V. Immune responses in the human female reproductive tract. Immunology 2020, 160, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Villa, P.; Cipolla, C.; D’Ippolito, S.; Amar, I.D.; Shachor, M.; Ingravalle, F.; Scaldaferri, F.; Puca, P.; Di Simone, N.; Scambia, G. The interplay between immune system and microbiota in gynecological diseases: A narrative review. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5676–5690. [Google Scholar] [PubMed]
- Alonzo Martinez, M.C.; Cazorla, E.; Canovas, E.; Martinez-Blanch, J.F.; Chenoll, E.; Climent, E.; Navarro-Lopez, V. Study of the vaginal microbiota in healthy women of reproductive age. Microorganisms 2021, 9, 1069. [Google Scholar] [CrossRef]
- Subramanyam, D. Recent findings of Lactobacillus diversity and their functional role in vaginal ecosystems. In Recent Developments in Applied Microbiology and Biochemistry; Buddolla, V., Ed.; Academic Press: London, UK, 2019; Chapter 1; pp. 3–12. [Google Scholar]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Factories 2020, 19, 203. [Google Scholar] [CrossRef]
- He, Y.; Niu, X.; Wang, B.; Na, R.; Xiao, B.; Yang, H. Evalution of the inhibitory effects of Lactobacillus gasseri and Lactobacillus crispatus on the adhesion fo seven common lower genital tract infection-causing pathogens to vaginal epithelial cells. Front. Med. 2020, 7, 284. [Google Scholar] [CrossRef]
- McKloud, E.; Delaney, C.; Sherry, L.; Kean, R.; Williams, S.; Metcalfe, R.; Thomas, R.; Richardson, R.; Gerasimidis, K.; Nile, C.J.; et al. Recurrent vulvovaginal candidiasis: A dynamic interkingdom biofilm disease of Candida and Lactobacillus. mSystems 2021, 6, e0062221. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.I.; Lievens, E.; Malik, S.; Imholz, N.; Lebeer, S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front. Physiol. 2015, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.-H.; Shin, J.; Rhee, M.-S.; Park, K.-R.; Cho, B.-K.; Lee, S.-K.; Kim, B.-C. Vaginal microbiota profiles of native Korean women and associations with high-risk pregnancy. J. Microbiol. Biotechnol. 2020, 30, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Scillato, M.; Spitale, A.; Mongelli, G.; Privitera, G.F.; Mangano, K.; Cianci, A.; Stefani, S.; Santagati, M. Antimicrobial properties of Lactobacillus cell-free supernatants against multidrug-resistant urogenital pathogens. Microbiol. Open 2021, 10, e1173. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, A.; Lledo, B.; Díaz, M.C.; Lozano, F.M.; Ruiz, V.; Fuentes, A.; Lopez-Pineda, A.; Moliner, B.; Castillo, J.C.; Ortiz, J.A.; et al. Effect of the vaginal microbiome on the pregnancy rate in women receiving assisted reproductive treatment. J. Assist. Reprod. Genet. 2019, 6, 2111–2119. [Google Scholar] [CrossRef]
- Liu, D.; Shao, L.; Zhang, Y.; Kang, W. Safety and efficacy of Lactobacillus for preventing necrotizing enterocolitis in preterm infants. Int. J. Surg. 2020, 76, 79–87. [Google Scholar] [CrossRef]
- Gerson, K.D.; McCarthy, C.; Elovitz, M.A.; Ravel, J.; Sammel, M.D.; Burris, H.H. Cervicovaginal microbial communities deficient in Lactobacillus species are associated with second trimester short cervix. Am. J. Obs. Gyn. 2020, 222, 491.e1–491.e8. [Google Scholar] [CrossRef]
- Kosti, I.; Lyalina, S.; Pollard, K.S.; Butte, A.J.; Sirota, M. Meta-analysis of vaginal microbiome data provides new insights into preterm birth. Front. Microbiol. 2020, 8, 11476. [Google Scholar] [CrossRef]
- Redelinghuys, M.; Geldenhuys, J.; Jung, H.; Kock, M.M. Bacterial vaginosis: Current diagnostic avenues and future opportunities. Front. Cell. Infect. Microbiol. 2020, 10, 354. [Google Scholar] [CrossRef]
- Chen, H.; Min, S.; Wang, L.; Zhao, L.; Luo, F.; Lei, W.; Wen, Y.; Luo, L.; Zhou, Q.; Peng, L.; et al. Lactobacillus modulates Chlamydia infectivity and genital tract pathology in vitro and in vivo. Front. Microbiol. 2022, 13, 877223. [Google Scholar] [CrossRef]
- Foschi, C.; Salvo, M.; Cevenini, R.; Parolin, C.; Vitali, B.; Marangoni, A. Vaginal lactobacilli reduce Neisseria gonorrhoeae viability through multiple strategies: An in vitro study. Front. Cell. Infect. Microbiol. 2017, 7, 502. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, E.; Makvandi, M.; Teimoori, A.; Ataei, A.; Ghafari, S.; Samarbaf-Zadeh, A. Antiviral effects of Lactobacillus crispatus against HSV-2 in mammalian cell lines. J. Chin. Med. Assoc. 2018, 81, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.P.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The vaginal microbiome and preterm birth. Nat. Med. 2019, 25, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, K.K.; Parikh, H.I.; Garcia, E.M.; Edwards, D.J.; Serrano, M.G.; Hewison, M.; Shary, J.R.; Powell, A.M.; Hollis, B.W.; Fettweis, J.M.; et al. Relationship between vitamin D status and the vaginal microbiome during pregnancy. J. Perinatol. 2019, 39, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Campisciano, G.; Iebba, V.; Zito, G.; Luppi, S.; Martinelli, M.; Fischer, L.; De Seta, F.; Basile, G.; Ricci, G.; Comar, M. Lactobacillus iners and gasseri, Prevotella bivia and HPV belong to the microbiological signature negatively affecting human reproduction. Microorganisms 2021, 9, 39. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Z.; Chen, T. Role of vaginal microbiota dysbiosis in gynecological diseases and the potential interventions. Front. Microbiol. 2021, 12, 643422. [Google Scholar] [CrossRef]
- Santiago, G.L.D.S.; Cools, P.; Verstraelen, H.; Trog, M.; Missine, G.; El Aila, N.; Verhelst, R.; Tency, I.; Claeys, G.; Temmerman, M.; et al. Longitudinal study of the dynamics of vaginal microflora during two consecutive menstrual cycles. PLoS ONE 2011, 6, e28180. [Google Scholar]
- Mls, J.; Stranik, J.; Kacerovsky, M. Lactobacillus iners-dominated vaginal microbiota in pregnancy. Ceska Gynekol. 2019, 84, 463–467. [Google Scholar]
- Wells, J.; Chandler, R.; Dunn, A.; Brewster, G. The vaginal microbiome in U.S. black women: A systematic review. J. Womens Health 2020, 29, 362–375. [Google Scholar] [CrossRef]
- Borgdorfff, H.; van der Veer, C.; van Houdt, R.; Alberts, C.J.; de Vries, H.J.; Bruisten, S.M.; Snijder, M.B.; Prins, M.; Geerlings, S.E.; van der Loeff, M.F.S.; et al. The association between ethnicity and vaginal microbiota composition in Amsterdam, the Netherlands. PLoS ONE 2017, 12, e0181135. [Google Scholar] [CrossRef]
- Petrova, M.I.; Reid, G.; Vaneechoutte, M.; Lebeer, S. Lactobacillus iners: Friend or foe? Trends Microbiol. 2017, 25, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Vaneechoutte, M. Lactobacillus iners, the unusual suspect. Res. Microbiol. 2017, 168, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.A.; Fischer, M.D.; Petrova, M.I.; Balkus, J.E. Epidemiologic evidence on the role of Lactobacillus iners in sexually transmitted infections and bacterial vaginosis: A series of systematic reviews and meta-analyses. Sex. Transm. Dis. 2022, 50, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Kwak, W.; Han, Y.H.; Seol, D.; Kim, H.; Ahn, H.; Jeong, M.; Kang, J.; Kim, H.; Kim, T.H. Complete genome of Lactobacillus iners KY using Flongle provides insight into the genetic background of optimal adaption to vaginal econiche. Front. Microbiol. 2020, 11, 1048. [Google Scholar] [CrossRef] [PubMed]
- McMillan, A.; Macklaim, J.M.; Burton, J.P.; Reid, G. Adhesion of Lactobacillus iners AB-1 to human fibronectin: A key mediator for persistence in the vagina? Reprod. Sci. 2013, 20, 791–796. [Google Scholar] [CrossRef]
- Macklaim, J.M.; Fernandes, A.D.; Di Bella, J.M.; Hammond, J.-A.; Reid, G.; Gloor, G.B. Comparative meta-RNA-seq of the vaginal microbiota and differential expression by Lactobacillus iners in health and dysbiosis. Microbiome 2013, 1, 12. [Google Scholar] [CrossRef]
- Rampersaud, R.; Lewis, E.L.; LaRocca, T.J.; Ratner, A.J. Environmental pH modulates inerolysin activity via post-binding blockade. Sci. Rep. 2018, 8, 1542. [Google Scholar] [CrossRef]
- Ragaliauskas, T.; Plečkaitytė, M.; Jankunec, M.; Labanauskas, L.; Baranauskiene, L.; Valincius, G. Inerolysin and vaginolysin, the cytolysins implicated in vaginal dysbiosis, differently impair molecular integrity of phospholipid membranes. Sci. Rep. 2019, 9, 10606. [Google Scholar] [CrossRef]
- Zheng, N.; Guo, R.; Wang, J.; Zhou, W.; Ling, Z. Contribution of Lactobacillus iners to vaginal health and diseases: A systematic review. Front. Cell. Infect. Microbiol. 2021, 11, 792787. [Google Scholar] [CrossRef]
- Tozetto-Mendoza, T.R.; Mendes-Correa, M.C.; Moron, A.F.; Forney, L.J.; Linhares, I.M.; Ribeiro da Silva, A., Jr.; Layla Honorato, L.; Steven, S.; Witkin, S.S. The vaginal Torquetenovirus titer varies with vaginal microbiota composition in pregnant women. PLoS ONE 2022, 17, e0262672. [Google Scholar] [CrossRef]
- Castanheira, C.P.; Sallas, M.L.; Nunes, R.A.L.; Lorenzi, N.P.C.; Termini, L. Microbiome and cervical cancer. Pathobiology 2021, 88, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Norenhag, J.; Du, J.; Olovsson, M.; Verstraelen, H.; Engstrand, L.; Brusselaers, N. The vaginal microbiota, human papillomavirus and cervical dysplasia: A systematic review and network meta-analysis. Br. J. Obst. Gyn. 2020, 27, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Zeber-Lubecka, N.; Kulecka, M.; Lindner, B.; Krynicki, R.; Paziewska, A.; Nowakowski, A.; Bidzinski, M.; Ostrowski, J. Increased diversity of a cervical microbiome associates with cervical cancer. Front. Oncol. 2022, 12, 1005537. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Feng, Y.; Qin, T.; Zhao, X.; Lu, J.; Ma, C. Characteristics of vaginal microbiome in reproductive-age females with HPV infection in Xinjiang, China. J. Evid. Based Complement. Altern. Med. 2022, 2022, 7332628. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, S.; Visconti, S.; Gentili, M.; Lusenti, E.; Nunzi, E.; Ronchetti, S.; Perito, S.; Gaziano, R.; Monari, C. Lactobacillus iners cell-free supernatant enhances biofilm formation and hyphal/pseudohyphal growth by Candida albicans vaginal isolates. Microorganisms 2021, 9, 2577. [Google Scholar] [CrossRef]
- Ponomarova, O.; Gabrielli, N.; Sévin, D.C.; Mülleder, M.; Zirngibl, K.; Bulyha, K.; Andrejev, S.; Kafkia, E.; Typas, A.; Uwe Sauer, U.; et al. Yeast creates a niche for symbiotic lactic acid bacteria through nitrogen overflow. Cell Syst. 2017, 5, 345–357. [Google Scholar] [CrossRef]
- Vitale, S.G.; Ferrari, F.; Ciebiera, M.; Zgliczyńska, M.; Rapisarda, A.M.C.; Vecchio, G.M.; Pino, A.; Angelico, G.; Knafel, A.; Riemma, G.; et al. The role of genital tract microbiome in fertility: A systematic review. Int. J. Mol. Sci. 2022, 23, 180. [Google Scholar] [CrossRef]
- Zhang, F.; Dai, J.; Chen, T. Role of Lactobacillus in female infertility via modulating sperm agglutination and immobilization. Front. Cell. Infect. Microbiol. 2020, 10, 620529. [Google Scholar] [CrossRef]
- Babu, G.; Singaravelu, B.G.; Srikumar, R.; Reddy, S.V.; Kokan, A. Comparative study on the vaginal flora and incidence of asymptomatic vaginosis among healthy women and in women with Infertility Problems of Reproductive Age. J. Clin. Diagn. Res. 2017, 11, DC18–DC22. [Google Scholar] [CrossRef]
- Chen, H.; Wang, L.; Zhao, L.; Luo, L.; Min, S.; Wen, Y.; Lei, W.; Shu, M.; Li, Z. Alterations of vaginal microbiota in women with infertility and Chlamydia trachomatis infection. Front. Cell. Infect. Microbiol. 2021, 11, 698840. [Google Scholar] [CrossRef]
- Auriemma, R.S.; Scairati, R.; Del Vecchio, G.; Liccardi, A.; Verde, N.; Pirchio, R.; Pivonello, R.; Ercolini, D.; Colao, A. The vaginal microbiome: A long urogenital colonization throughout woman life. Front. Cell. Infect. Microbiol. 2021, 11, 686167. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4680–4687. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Singh, M.P.; Goyal, K. Diversity of vaginal microbiome in pregnancy: Deciphering the obscurity. Front. Public Health 2020, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, K.; Riehle, K.; Ma, J.; Segata, N.; Mistretta, T.-A.; Coarfa, C.; Raza, S.; Rosenbaum, S.; Van den Veyver, I.; Aleksandar Milosavljevic, A.; et al. A Metagenomic Approach to Characterization of the Vaginal Microbiome Signature in Pregnancy. PLoS ONE 2012, 7, e36466. [Google Scholar] [CrossRef] [PubMed]
- De Seta, F.; Campisciano, G.; Zanotta, N.; Ricci, G.; Comar, M. The Vaginal Community State Types Microbiome-Immune Network as Key Factor for Bacterial Vaginosis and Aerobic Vaginitis. Front. Microbiol. 2019, 10, 2451. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef] [PubMed]
- Anukam, K.C.; Osazuwa, E.O.; Ahonkhai, I.; Reid, G. Lactobacillus vaginal microbiota of women attending a reproductive health care service in Benin City, Nigeria. Sex. Transm. Dis. 2006, 33, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Jespers, V.; Kyongo, J.; Joseph, S.; Hardy, L.; Cools, P.; Crucitti, T.; Mwaura, M.; Ndayisaba, G.; Delany-Moretlwe, S.; Buyze, J.; et al. A longitudinal analysis of the vaginal microbiota and vaginal immune mediators in women from sub-Saharan Africa. Sci. Rep. 2017, 7, 11974. [Google Scholar] [CrossRef]
- Anahtar, M.N.; Byrne, E.H.; Doherty, K.E.; Bowman, B.A.; Yamamoto, H.S.; Soumillon, M.; Padavattan, N.; Ismail, N.; Moodley, A.; Sabatini, M.E.; et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 2015, 42, 965–976. [Google Scholar] [CrossRef]
- Lennard, K.; Dabee, S.; Barnabas, S.L.; Havyarimana, E.; Blakney, A.; Jaumdally, S.Z.; Botha, G.; Mkhize, N.N.; Bekker, L.G.; Lewis, D.A.; et al. Microbial composition predicts genital tract inflammation and persistent bacterial vaginosis in South African adolescent females. Infect. Immun. 2017, 86, e00410-17. [Google Scholar] [CrossRef]
- Gosmann, C.; Anahtar, M.N.; Handley, S.A.; Farcasanu, M.; Abu-Ali, G.; Bowman, B.A.; Padavattan, N.; Desai, C.; Droit, L.; Moodley, A.; et al. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in Young South African Women. Immunity 2017, 46, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Hummelen, R.; Fernandes, A.D.; Macklaim, J.M.; Dickson, R.J.; Changalucha, J.; Gloor, G.B.; Reid, G. Deep sequencing of the vaginal microbiota of women with HIV. PLoS ONE. 2010, 5, e12078. [Google Scholar] [CrossRef] [PubMed]
- Odogwu, N.M.; Olayemi, O.O.; Omigbodun, A.O. The vaginal microbiome of sub-Saharan African women: Revealing important gaps in the era of next-generation sequencing. PeerJ 2020, 8, e9684. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.L.; Gilbert, N.M. Roles of the vagina and the vaginal microbiota in urinary tract infection: Evidence from clinical correlations and experimental models. GMS Infect. Dis. 2020, 8, Doc02. [Google Scholar] [PubMed]
- Meštrović, T.; Matijašić, M.; Perić, M.; Paljetak, H.C.; Barešić, A.; Verbanac, D. The Role of Gut, Vaginal, and Urinary Microbiome in Urinary Tract Infections: From Bench to Bedside. Diagnostics 2020, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Parvaiz, F.; Manzoor, S. Bacterial vaginosis: An insight into the prevalence, alternative treatments regimen and it’s associated resistance patterns. Microb Pathog. 2019, 127, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Rosca, A.S.; Castro, J.; Sousa, L.G.V.; França, A.; Vaneechoutte, M.; Cerca, N. In vitro interactions within a biofilm containing three species found in bacterial vaginosis (BV) support the higher antimicrobial tolerance associated with BV recurrence. J. Antimicrob. Chemother. 2022, 77, 2183–2190. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Rosca, A.S.; Cools, P.; Vaneechoutte, M.; Cerca, N. Gardnerella vaginalis enhances Atopobium vaginae viability in an in vitro model. Front. Cell. Infect. Microbiol. 2020, 10, 83. [Google Scholar] [CrossRef]
- Muzny, C.A.; Łaniewski, P.; Schwebke, J.R.; Herbst-Kralovetz, M.M. Host-vaginal microbiota interactions in the pathogenesis of bacterial vaginosis. Curr. Opin. Infect. Dis. 2020, 33, 59–65. [Google Scholar] [CrossRef]
- Gustin, A.T.; Thurman, A.R.; Chandra, N.; Schifanella, L.; Alcaide, M.; Fichirova, R.; Doncel, G.F.; Gale, M., Jr. Recurrent bacterial vaginosis following metronidazole treatment is associated with microbiota richness at diagnosis. Am. J. Obs. Gynecol. 2022, 226, 225.E1–225.E15. [Google Scholar] [CrossRef]
- McClelland, R.; Lingappa, J.R.; Srinivasan, S.; Kinuthia, J.; John-Stewart, G.; C Jaoko, W.; Richardson, B.A.; Yuhas, K.; Fiedler, T.L.; Mandaliya, K.N.; et al. Evaluation of the association between the concentrations of key vaginal bacteria and the increased risk of HIV acquisition in African women from five cohorts: A nested case-control study. Lancet Infect. Dis. 2018, 18, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Torcia, M.G. Interplay among vaginal microbiome, immune response and sexually transmitted viral infections. Int. J. Mol. Sci. 2019, 20, 266. [Google Scholar] [CrossRef] [PubMed]
- Alisoltani, A.; Manhanzva, M.T.; Potgieter, M.; Balle, C.; Bell, L.; Ross, E.; Iranzadeh, A.; du Plessis, M.; Radzey, N.; McDonald, Z.; et al. Microbial function and genital inflammation in young South African women at high risk of HIV infection. Microbiome 2020, 8, 165. [Google Scholar] [CrossRef] [PubMed]
- Papon, N.; Van Dijck, P. A complex microbial interplay underlies recurrent vulvovaginal candidiasis pathobiology. mSystems 2021, 6, e0106621. [Google Scholar] [CrossRef] [PubMed]
- Kalia, N.; Singh, J.; Kaur, M. Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: A critical review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- de Cássia Orlandi Sardi, J.; Silva, D.R.; Anibal, P.C.; Carvalho Moraes de Campos Baldin, J.J.; Rodrigues Ramalho, S.R.; Rosalen, P.L.; Rodrigues Macedo, M.L.; Hofling, J.F. Vulvovaginal candidiasis: Epidemiology and risk factors, pathogenesis, resistance, and new therapeutic options. Curr. Fungal Infect. Rep. 2021, 15, 32–40. [Google Scholar] [CrossRef]
- Denning, D.W.; Kneale, M.; Sobel, J.D.; Rautemaa-Richardson, R. Global burden of recurrent vulvovaginal candidiasis: A systematic review. Lancet Infect. Dis. 2018, 18, e339–e347. [Google Scholar] [CrossRef]
- Tortelli, B.A.; Lewis, W.G.; Allsworth, J.E.; Member-Meneh, N.; Foster, L.R.; Reno, H.E.; Peipert, J.F.; Fay, J.C.; Lewis, A.L. Associations between the vaginal microbiome and Candida colonization in women of reproductive age. Am. J. Obstet. Gynecol. 2020, 222, 471.e1–471.e9. [Google Scholar] [CrossRef]
- Ceccarani, C.; Foschi, C.; Parolin, C.; D’Antuono, A.; Gaspari, V.; Consolandi, C.; Laghi, L.; Camboni, T.; Vitali, B.; Severgnini, M.; et al. Diversity of vaginal microbiome and metabolome during genital infections. Sci. Rep. 2019, 9, 14095. [Google Scholar] [CrossRef]
- Ncib, K.; Bahia, W.; Leban, N.; Mahdhi, A.; Trifa, F.; Mzoughi, R.; Haddad, A.; Jabeur, C.; Donders, G. Microbial diversity and pathogenic properties of microbiota associated with aerobic vaginitis in women with recurrent pregnancy loss. Diagnostics 2022, 12, 2444. [Google Scholar] [CrossRef]
- Oerlemans, E.F.M.; Wuyts, S.; Bellen, G.; Wittouck, S.; De Boeck, I.; Ruban, K.; Allonsius, C.N.; van den Broek, M.F.L.; Donders, G.G.G.; Lebeer, S. The dwindling microbiota of aerobic vaginitis, an inflammatory state enriched in pathobionts with limited TLR stimulation. Diagnostics 2020, 10, 879. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Dong, A.; Zhao, J.; Wang, C.; Griffin, C.; Gragnoli, C.; Xue, F.; Wu, R. Vaginal microbiota networks as a mechanistic predictor of aerobic vaginitis. Front. Microbiol. 2022, 13, 998813. [Google Scholar] [CrossRef]
- Qi, W.; Li, H.; Wang, C.; Li, H.; Zhang, B.; Dong, M.; Fan, A.; Han, C.; Xue, F. Recent advances in presentation, diagnosis and treatment for mixed vaginitis. Front. Cell. Infect. Microbiol. 2021, 11, 759795. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; A, D.; Qin, H.; Mi, L.; Zhang, D. Correlation analysis of vaginal microbiome changes and bacterial vaginosis plus vulvovaginal candidiasis mixed vaginitis prognosis. Front. Cell. Infect. Microbiol. 2022, 12, 860589. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Anumba, D.O.C. The vaginal microenvironment: The physiologic role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- González-Sánchez, A.; Reyes-Lagos, J.J.; Peña-Castillo, M.A.; Nirmalkar, N.; García-Mena, J.; Pacheco-López, G. Vaginal microbiota is stable and mainly dominated by Lactobacillus at third trimester of pregnancy and active childbirth: A longitudinal study of ten Mexican women. Curr. Microbiol. 2022, 79, 230. [Google Scholar] [CrossRef]
- Serrano, M.G.; Parikh, H.I.; Brooks, J.P.; Edwards, D.J.; Arodz, T.J.; Edupuganti, L.; Huang, B.; Girerd, P.H.; Bpkhari, Y.A.; Bradley, S.P.; et al. Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat. Med. 2019, 25, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, Y.; Chen, T.; Li, R. The female vaginal microbiome in health and bacterial vaginosis. Front. Cell. Infect. Microbiol. 2021, 11, 631972. [Google Scholar] [CrossRef]
- van der Veer, C.; Hertzberger, R.Y.; Bruisten, S.M.; Tytgat, H.L.P.; Swanenburg, J.; de Kat Angelino-Bart, A.; Schuren, F.; Molenaar, D.; Reid, G.; de Vries, H.; et al. Comparative genomics of human Lactobacillus crispatus isolates reveals genes for glycosylation and glycogen degradation: Implications for in vivo dominance of the vaginal microbiota. Microbiome 2019, 7, 49. [Google Scholar] [CrossRef]
- Edwards, V.L.; Smith, S.B.; McComb, E.J.; Tamarelle, J.; Ma, B.; Humphrys, M.S.; Gajer, P.; Gwilliam, K.; Schaefer, A.M.; Lai, S.K.; et al. The cervicovaginal microbiota-host interaction modulates Chlamydia trachomatis infection. mBio 2019, 10, e01548-19. [Google Scholar] [CrossRef]
- Lee, S.; Oh, K.Y.; Hong, H.; Jin, C.H.; Shim, E.; Kim, S.H.; Kim, B.-Y. Community state types of vaginal microbiota and four types of abnormal vaginal microbiota in pregnant Korean women. Front. Public Health 2020, 8, 507024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhai, Q.; Wang, J.; Ma, X.; Xing, B.; Fan, H.; Gao, Z.; Zhao, F.; Liu, W. Variation of the vaginal microbiome during and after pregnancy in Chinese women. Genom. Proteom. Bioinform. 2022, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Al-Memar, M.; Bobdiwala, S.; Fourie, H.; Mannino, R.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; Timmerman, D.; Bourne, T.; Bennett, P.R.; et al. The association between vaginal bacterial composition and miscarriage: A nested case–control study. Br. J. Obst. Gynaecol. 2020, 127, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Kanmaz, A.G.; İnan, A.H.; Beyan, E.; Budak, A. The effects of threatened abortions on pregnancy outcomes. Ginekol. Pol. 2019, 90, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Grewal, K.; Lee, Y.S.; Smith, A.; Brosens, J.J.; Bourne, T.; Al-Memar, M.; Kundu, S.; MacIntyre, D.A.; Bennett, P. Chromosomally normal miscarriage is associated with vaginal dysbiosis and local inflammation. BMC Med. 2022, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, A.; Laghi, L.; Zagonari, S.; Patuelli, G.; Zhu, C.; Foschi, C.; Morselli, S.; Pedna, M.F.; Sambri, V. New insights into vaginal environment during pregnancy. Front. Mol. Biosci. 2021, 8, 656844. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.B.; Hanlon, A.L.; Wu, G.; Liu, C.; Fredricks, D.N. First trimester levels of BV-associated bacteria and risk of miscarriage among women early in pregnancy. Matern. Child Health J. 2015, 19, 2682–2687. [Google Scholar] [CrossRef]
- Xu, L.; Huang, L.; Lian, C.; Xue, H.; Lu, Y.; Chen, X.; Xia, Y. Vaginal microbiota diversity of patients with embryonic miscarriage by using 16S rDNA High-Throughput Sequencing. Int. J. Genom. 2020, 2020, 1764959. [Google Scholar] [CrossRef]
- Christiansen, O.B.; Steffensen, R.; Nielsen, H.S.; Varming, K. Multifactorial etiology of recurrent miscarriage and its scientific and clinical implications. Gynecol. Obstet. Investig. 2008, 66, 257–267. [Google Scholar] [CrossRef]
- Giakoumelou, S.; Wheelhouse, N.; Cuschieri, K.; Entrican, G.; Howie, S.E.; Horne, A.W. The role of infection in miscarriage. Hum. Reprod. Update 2016, 22, 116–133. [Google Scholar] [CrossRef]
- Kuon, R.J.; Togawa, R.; Vomstein, K.; Weber, M.; Goeggl, T.; Strowitzki, T.; Markert, U.R.; Zimmermann, S.; Daniel, V.; Dalpke, A.H.; et al. Higher prevalence of colonization with Gardnerella vaginalis and Gram-negative anaerobes in patients with recurrent miscarriage and elevated peripheral natural killer cells. J. Reprod. Immunol. 2017, 120, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, T.; Ma, Y.; Huang, Z.; He, Y.; Pan, H.; Fang, M.; Ding, H. Alteration of vaginal microbiota in patients with unexplained recurrent miscarriage. Exp. Ther. Med. 2019, 17, 3307–3316. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Quinlivan, J.A.; Peek, M.; Castaño-Rodríguez, N.; Mendz, G.L. Is there an association between the vaginal microbiome and first trimester miscarriage? A prospective observational study. J. Obs. Gyn. Res. 2022, 48, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zhang, X.; Liang, Y.; Lin, S.; Qian, W.; Fan, S. Alterations in vaginal microbiota and associated metabolome in women with recurrent implantation failure. mBio 2020, 1, e03242-19. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of lactobacilli and lactoferrin in the mucosal cervicovaginal defense. Front. Immunol. 2018, 9, 376. [Google Scholar] [CrossRef] [PubMed]
- Nija, R.J.; Sanju, S.; Sidharthan, N.; Mony, U. Extracellular trap by blood cells: Clinical implications. Tissue Eng. Regen. Med. 2020, 17, 141–153. [Google Scholar] [CrossRef]
- Tong, M.; Abrahams, V.M. Neutrophils in preterm birth: Friend or foe? Placenta 2020, 102, 17–20. [Google Scholar] [CrossRef]
- Omeljaniuk, W.J.; Jabłońska, E.; Garley, M.; Pryczynicz, A.; Ratajczak-Wrona, W.; Socha, K.; Borawska, M.H.; Charkiewicz, A.E. Biomarkers of neutrophil extracellular traps (NETs) and nitric oxide-(NO)-dependent oxidative stress in women who miscarried. Sci. Rep. 2020, 10, 13088. [Google Scholar] [CrossRef]
- Hug, L.; You, D.; Blencowe, H.; Mishra, A.; Wang, Z.; Fix, M.J.; Wakefield, J.; Moran, A.C.; Gaigbe-Togbe, V.; Suzuki, E.; et al. Global, regional, and national estimates and trends in stillbirths from 2000 to 2019: A systematic assessment. Lancet 2021, 398, 772–785. [Google Scholar] [CrossRef]
- Eunice Kennedy Shriver National Institute of Child Health and Human Development. What Are Possible Causes of Stillbirth? Available online: https://www.nichd.nih.gov/health/topics/stillbirth/topicinfo/causes# (accessed on 22 December 2022).
- Megli, C.J.; Coyne, C.B. Infections at the maternal–fetal interface: An overview of pathogenesis and defence. Nat. Rev. Microbiol. 2022, 20, 67–82. [Google Scholar] [CrossRef]
- Stillbirth, Cleveland Clinic. 2023. Available online: https://my.clevelandclinic.org/health/diseases/9685-stillbirth (accessed on 15 February 2023).
- Seale, A.C.; Blencowe, H.; Bianchi-Jassir, F.; Embleton, N.; Bassat, Q.; Ordi, J.; Menéndez, C.; Cutland, C.; Briner, C.; Berkley, J.A.; et al. Stillbirth with Group B Streptococcus disease worldwide: Systematic review and meta-analyses. Clin. Infect. Dis. 2017, 65 (Suppl. S2), S125–S132. [Google Scholar] [CrossRef] [PubMed]
- Page, J.M.; Bardsley, T.; Thorsten, V.; Allshouse, A.A.; Varner, M.W.; Debbink, M.P.; Dudley, D.J.; Saade, G.R.; Goldenberg, R.L.; Stoll, B.; et al. Stillbirth associated with infection in a diverse U.S. cohort. Obstet. Gynecol. 2019, 134, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Aguinaga, M.; Valdespino, Y.; Medina, D.; Espino y Sosa, S.; Sevilla, R.; Miranda, O.; Acevedo, S.; Monroy, I.E.; Helguera, A.C.; Pérez, J.; et al. Causal analysis of fetal death in high-risk pregnancies. J. Perinat. Med. 2021, 49, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.J.; Embleton, N.D.; Clark, J.E.; Bythell, M.; Ward Platt, M.P.; Berrington, J.E. Viral infections: Contributions to late fetal death, stillbirth, and infant death. J. Pediatr. 2013, 163, 424–428. [Google Scholar] [CrossRef]
- Herrera-Salazar, A.; Flores-Hernández, L.A.; Valdespino-Vázquez, M.Y.; Fonseca-Coronado, S.; Moreno-Verduzco, E.R. Viral infections in stillbirth: A contribution underestimated in Mexico? J. Perinat. Med. 2022, 50, 786–795. [Google Scholar] [CrossRef]
- Joseph, A.; Mahida, N.; Clark, G.; Irving, W.; Soo, S. Congenital citomegalovirus infection. Paediatr. Child Health. 2018, 28, 6. [Google Scholar] [CrossRef]
- Stonoga, E.T.S.; de Almeida, L.A.; Zadorosnei, R.P.; Permegiani de Oliveira, A.L.; Chiste, J.A.; Arias Fugaça, C.; Prá, D.M.M.; Percicote, A.P.; Rossoni, A.; Nogueira, M.B.; et al. Intrauterine transmission of SARS-CoV-2. Emerg. Infect. Dis. 2021, 27, 638–641. [Google Scholar] [CrossRef]
- Calvert, C.; Brockway, M.; Zoega, H.; Miller, J.E.; Been, J.V.; Amegah, A.K.; Racine-Poon, A.; Oskoui, S.E.; Abok, I.I.; Aghaeepour, N.; et al. Changes in preterm birth and stillbirth during COVID-19 lockdowns in 26 countries. Nat. Hum. Behav. 2023, 7, 529–544. [Google Scholar] [CrossRef]
- Cao, G.; Liu, J.; Liu, M. Global, regional, and national incidence and mortality of neonatal preterm birth, 1990–2019. JAMA Pediatr. 2022, 176, 787–796. [Google Scholar] [CrossRef]
- Ohuma, E.O.; Moller, A.-B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: A systematic analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef]
- Cappelletti, M.; Presicce, P.; Kallapur, S.G. Immunobiology of acute chorioamnionitis. Front. Immunol. 2020, 11, 649. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, N.; Romero, R.; Tarca, A.L.; Miller, D.; Panaitescu, B.; Schwenkel, G.; Gudicha, D.W.; Hassan, S.S.; Pacora, P.; Jung, E.; et al. Gasdermin D: Evidence of pyroptosis in spontaneous preterm labor with sterile intra-amniotic inflammation or intra-amniotic infection. Am. J. Reprod. Immunol. 2019, 82, e13184. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.R.; Brown, R.G.; MacIntyre, D.A. Vaginal microbiome in preterm rupture of membranes. Obstet. Gynecol. Clin. N. Am. 2020, 47, 503–521. [Google Scholar] [CrossRef]
- dos Anjos, L.G.; Pastuschek, J.; Heimann, Y.; Dawczynski, K.; PEONS study group; Schleußner, E.; Pieper, D.H.; Zöllkau, J. Vaginal and neonatal microbiota in pregnant women with preterm premature rupture of membranes and consecutive early onset neonatal sepsis. BMC Med. 2023, 21, 92. [Google Scholar] [CrossRef]
- Sun, S.; Serrano, M.G.; Fettweis, J.M.; Basta, P.; Rosen, E.; Ludwig, K.; Sorgen, A.A.; Blakley, I.C.; Wu, M.C.; Nancy Dole, N. Race, the vaginal microbiome, and spontaneous preterm birth. mSystems 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, A.L.; Satten, G.A.; Hu, Y.-J.; Knight, A.K.; Hill, C.C.; Wright, M.L.; Smith, A.K.; Read, T.D.; Pearce, B.D.; Corwin, E.J. Vaginal microbiome composition in early pregnancy and risk of spontaneous preterm and early term birth among African-American women. Front. Cell. Infect. Microbiol. 2021, 11, 641005. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.C.; Bocking, A.; Hill, J.E.; Money, D.M. Increased richness and diversity of the vaginal microbiota and spontaneous preterm birth. Microbiome 2018, 6, 117. [Google Scholar] [CrossRef] [PubMed]
- Bunyan, I.A.; Lamees, A.; Abdul-Lateef, L.A.; Al-Rubaeaee, A.A. Molecular Detection of Gardnerella vaginalis isolated from preterm labor patients and study of some factors on biofilm formation in Al-Hilla City, Iraq. J. Glob. Pharm. Technol. 2017, 9, 265–270. [Google Scholar]
- Chen, S.; Xue, X.; Zhang, Y.; Zhang, H.; Huang, X.; Chen, X.; Deng, G.; Luo, S.; Gao, J. Vaginal Atopobium is Associated with Spontaneous Abortion in the First Trimester: A Prospective Cohort Study in China. Microbiol. Spectr. 2022, 10, e0203921. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Suga, S.; Sugimi, S.; Kurata, N.; Yamashita, H.; Yasuhi, I. Vaginal Ureaplasma urealyticum or Mycoplasma hominis and preterm delivery in women with threatened preterm labor. J. Matern. Fetal Neonatal Med. 2022, 35, 878–883. [Google Scholar] [CrossRef]
- Alinezhad, S.; Bakhshandehnosrat, S.; Baniaghil, A.S.; Livani, S.; Bazouri, M.; Shafipour, M.; Behnampour, N.; Ghaemi, E.A. The role of genital mycoplasmas in preterm labor. J. Reprod. Infertil. 2022, 23, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.P.; Procter, S.R.; Paul, P.; Chandna, J.; Lewin, A.; Seedat, F.; Koukounari, A.; Dangor, Z.; Leahy, S.; Santhanam, S.; et al. Group B streptococcus infection during pregnancy and infancy: Estimates of regional and global burden. Lancet Glob. Health 2022, 10, e807–e819. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Yin, J.; Wang, W.; Zhao, F.; Ding, X.; Yu, H.; Zhang, X.; Huang, B. The associations between low abundance of Mycoplasma hominis and female fecundability: A pregnancy-planning cohort study. BMC Microbiol. 2022, 22, 121. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chi, X.-Z.; Zhang, L.; Chen, R.; Cao, J.-R.; Sun, X.-Y.; Yang, H.-Q.; Liao, Q.-P. Vaginal microbiome analysis of healthy women during different periods of gestation. Biosci. Rep. 2020, 40, BSR20201766. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, D.B.; Callahan, B.J.; McMurdie, P.J.; Costello, E.K.; Lyella, D.J.; Robaczewska, A.; Suna, C.L.; Goltsman, D.S.A.; Wong, R.J.; Shawa, G.; et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. USA 2015, 112, 11060–11065. [Google Scholar] [CrossRef]
- Nunn, K.L.; Witkin, S.S.; Schneider, G.M.; Boester, A.; Nasioudis, D.; Minis, E.; Gliniewicz, K.; Forney, L.J. Changes in the vaginal microbiome during the pregnancy to postpartum transition. Reprod. Sci. 2021, 28, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Theis, K.R.; Gomez-Lopez, N.; Winters, A.D.; Panzer, J.; Lin, H.; Galaz, J.; Greenberg, J.M.; Shaffer, Z.; Kracht, D.J.; et al. The vaginal microbiota of pregnant women varies with gestational age, maternal age, and parity. bioRxiv 2023, 11, e03429-22. [Google Scholar] [CrossRef] [PubMed]
- Feyaerts, D.; Joosten, I.; van der Molen, R.G. A pregnancy to remember: Trained immunity of the uterine mucosae. Mucosal Immunol. 2021, 14, 539–541. [Google Scholar] [CrossRef]
- Doyle, R.; Gondwe, A.; Fan, Y.-M.; Maleta, K.; Ashorn, P.; Klein, N.; Harris, K.A. A Lactobacillus-deficient vaginal microbiota dominates postpartum women in rural Malawi. Appl. Environ. Microbiol. 2018, 84, e02150-17. [Google Scholar] [CrossRef]
- Avershina, E.; Slangsvold, S.; Simpson, M.R.; Storrø, O.; Johnsen, R.; Øien, T.; Rudi, K. Diversity of vaginal microbiota increases by the time of labor onset. Sci. Rep. 2017, 7, 17558. [Google Scholar] [CrossRef]
- Celik, E.; Ozcan, G.; Vatansever, C.; Paerhati, E.; Kuşkucu, M.A.; Dogan, O.; Cekic, S.G.; Ergonul, O.; Gürsoy, A.; Keskin, Ö.; et al. Alterations in vaginal microbiota among pregnant women with COVID-19. J. Med. Virol. 2022, 95, e28132. [Google Scholar] [CrossRef]
- Nguyen, A.T.C.; Le Nguyen, N.T.; Hoang, T.T.A.; Nguyen, T.T.; Tran, T.T.Q.; Tran, D.N.T.; Nguyen, A.T.K.; Tran, L.M.; Nguyen, D.H.C.; Le, T.M.; et al. Aerobic vaginitis in the third trimester and its impact on pregnancy outcomes. BMC Pregnancy Childbirth 2022, 22, 432. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wu, M.; Wang, C.; Li, H.; Fan, A.; Wang, Y.; Cha, H.; Xue, F. The pathogenesis of prevalent aerobic bacteria in aerobic vaginitis and adverse pregnancy outcomes: A narrative review. Reprod. Health 2022, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Price, J.T.; Vwalika, B.; Hobbs, M.; Nelson, J.A.E.; Stringer, E.M.; Zou, F.; Rittenhouse, K.J.; Azcarate-Peril, A.; Kasaro, M.P.; Stringer, J.S.A. Highly diverse anaerobe-predominant vaginal microbiota among HIV-infected pregnant women in Zambia. PLoS ONE 2021, 14, e0223128. [Google Scholar] [CrossRef] [PubMed]
- Holliday, M.; Uddipto, K.; Castillo, G.; Vera, L.E.; Quinlivan, J.A.; Mendz, G.L. Insights into the Genital Microbiota of Women Who Experienced Fetal Death in Utero. Microorganisms 2023, 11, 1877. [Google Scholar] [CrossRef]
- Łaniewski, P.; Ilhan, Z.E.; Herbst-Kralovetz, M.M. The microbiome and gynaecological cancer development, prevention and therapy. Nat. Rev. Urol. 2020, 17, 232–250. [Google Scholar] [CrossRef]
- Sakabe, Y.; Nishizawa, H.; Kato, A.; Noda, Y.; Ohwaki, A.; Yoshizawa, H.; Kato, T.; Sekiya, T.; Fujii, T.; Kurahashi, H. Longitudinal study of the vaginal microbiome in pregnancies involving preterm labor. Fujita Med. J. 2022, 8, 96–101. [Google Scholar]
- Yao, Y.; Cai, X.; Chen, C.; Fang, H.; Zhao, Y.; Fei, W.; Chen, F.; Zheng, C. The Role of microbiomes in pregnant women and offspring: Research progress of recent years. Front. Pharmacol. 2020, 11, 643. [Google Scholar] [CrossRef]
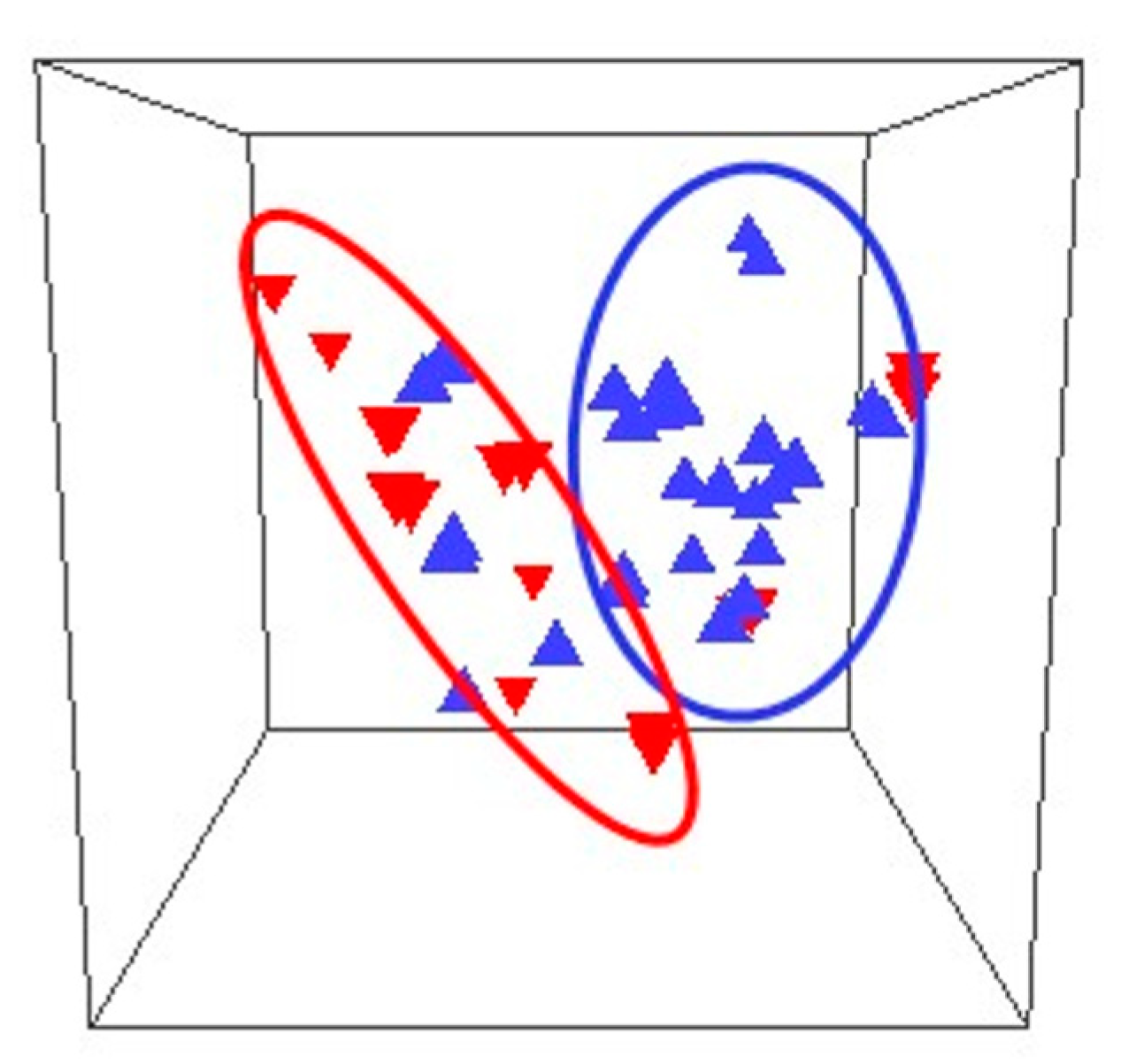
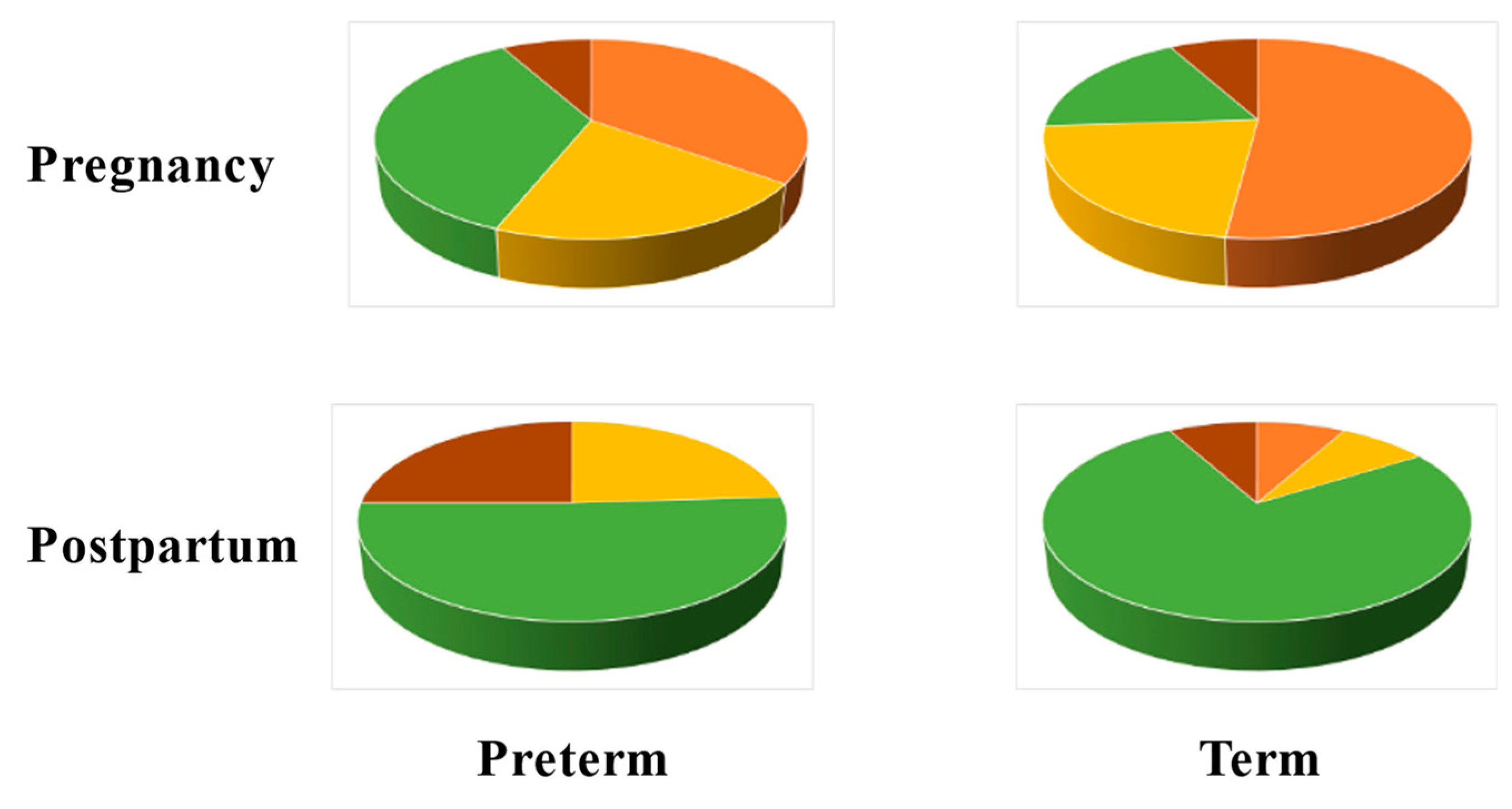
| Virus | Disease Risk/Manifestation |
|---|---|
| Arboviruses | FD, FM, PTB, TE, NI |
| Chikungunya | MI, NI, IM |
| Cytomegalovirus | SI, PTB, BD |
| Coronavirus disease-19 | PE, PTB |
| Dengue | MI, SB, PTB, LBW, NI |
| Hepatitis A | PPROM, PA, PTB |
| Hepatitis B | GDM, PE, PTB |
| Hepatitis E | FD, FHF |
| Herpes simplex | SB, AB, PTB |
| Human immunodeficiency | SI, PTB, LBW |
| Influenza | SB, FD, LBW |
| Measles | MI, SB, PTB, LBW |
| Rubella | MI, SB, LBW, BD, ID |
| Venezuelan equine encephalitis | MI, SB, PTB |
| West Nile | FM, FD |
| Zika | FM, FD, TE |
| Pathogen | Disease Risk/Manifestation |
|---|---|
| Fungi | |
| Aspergillus | MI, SB, SI, PTB, LBW, NJ |
| Candida | SB, AB, PTB, NI |
| Saccharomyces | GDM |
| Sporobolomyces | GDM |
| Parasites | |
| Helminths | PTB, LBW, NI, CI, MS, AM, ST |
| Scabies | BI, NI |
| Malaria | FD, PI, SI, PTB, LBW, CI |
| Toxoplasmosis | SB, SI, FD, BD, ID, MS |
| Trichomoniasis | PPROM, PTB, LBW, ID |
| Vagitype | Predominant Taxon |
|---|---|
| I | L. crispatus |
| II | L. gasseri |
| III | L. iners |
| IV | Various obligate anaerobes |
| V | L. jensenii |
| Term Birth | Preterm Birth |
|---|---|
| 1. L. iners | 1. L. iners |
| 2. L. crispatus | 2. No specific type |
| 3. Lachnospiraceae BVAB1 | 3. Lachnospiraceae BVAB1 |
| 4. G. vaginalis | 4. G. vaginalis |
| 5. No specific type | 5. L. crispatus |
| 6. A. vaginae | 6. A. vaginae |
| 7. L. gasseri | 7. Prevotella cluster 2 |
| 8. L. delbrueckii | 8. S. amnii |
| 9. Streptococcus cluster 29 | 9. S. agalactiae |
| 10. S. amnii | 10. L. gasseri |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendz, G.L. The Vaginal Microbiome during Pregnancy in Health and Disease. Appl. Microbiol. 2023, 3, 1302-1338. https://doi.org/10.3390/applmicrobiol3040089
Mendz GL. The Vaginal Microbiome during Pregnancy in Health and Disease. Applied Microbiology. 2023; 3(4):1302-1338. https://doi.org/10.3390/applmicrobiol3040089
Chicago/Turabian StyleMendz, George L. 2023. "The Vaginal Microbiome during Pregnancy in Health and Disease" Applied Microbiology 3, no. 4: 1302-1338. https://doi.org/10.3390/applmicrobiol3040089
APA StyleMendz, G. L. (2023). The Vaginal Microbiome during Pregnancy in Health and Disease. Applied Microbiology, 3(4), 1302-1338. https://doi.org/10.3390/applmicrobiol3040089





