Reduced Infestation Levels of Lepeophtheirus salmonis in Atlantic Salmon (Salmo salar) following Immersion Exposure to Probiotic Aliivibrio spp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Cultivation of Probiotic Bacteria for Infection Trials
2.2. Fish, Identification and Facilities
2.3. Administration of Probiotic Bacteria
2.4. Salmon Lice
2.5. Lice Challenges and Sampling
2.6. Cultivation and Application of Bacteria to Pre-Adult Salmon Lice
2.7. Statistics
3. Results
3.1. Lice Counts
3.2. Application of Bacteria to Pre-Adult Salmon Lice
4. Discussion
4.1. Lice Attachment
4.2. Bacterial Composition
4.3. Administration Method
4.4. Mechanism
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skern-Mauritzen, R.; Torrissen, O.; Glover, K.A. Pacific and Atlantic Lepeophtheirus salmonis (Krøyer, 1838) are allopatric subspecies: Lepeophtheirus salmonis salmonis and L. salmonis oncorhynchi subspecies novo. BMC Genet 2014, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Dawson, L.H.J.; Mcvicar, A.H. Comparison of the susceptibility of sea trout and Atlantic salmon for sea lice. ICES J. Mar. Sci. 1997, 54, 1129–1139. [Google Scholar] [CrossRef]
- Finstad, B.; Bjørn, P.A.; Grimnes, A.; Hvidsten, N.A. Laboratory and field investigations of salmon lice [Lepeophtheirus salmonis (Krøyer)] infestation on Atlantic salmon (Salmo salar L.) post-smolts. Aquac. Res. 2000, 31, 795–803. [Google Scholar] [CrossRef]
- Murray, A.G. Using observed load distributions with a simple model to analyse the epidemiology of sea lice (Lepeophtheirus salmonis) on sea trout (Salmo trutta). Pest Manag. Sci. 2002, 58, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Heuch, P.A.; Nordhagen, J.R.; Schram, T.A. Egg production in the salmon louse [Lepeophtheirus salmonis (Krøyer)] in relation to origin and water temperature. Aquac. Res. 2000, 31, 805–814. [Google Scholar] [CrossRef]
- Tucker, C.S.; Sommerville, C.; Wootten, R. An Investigation into the Larval Energetics and Settlement of the Sea Louse, Lepeophtheirus salmonis, an Ectoparasitic Copepod of Atlantic salmon, Salmo salar. Fish Pathol. 2000, 35, 137–143. [Google Scholar] [CrossRef]
- Hamre, L.A.; Eichner, C.; Caipang, C.M.A.; Dalvin, S.T.; Bron, J.E.; Nilsen, F.; Boxshall, G.; Skern-Mauritzen, R. The Salmon Louse Lepeophtheirus salmonis (Copepoda: Caligidae) Life Cycle Has Only Two Chalimus Stages. PLoS ONE 2013, 8, e73539. [Google Scholar] [CrossRef]
- Jones, M.W.; Sommerville, C.; Bron, J. The histopathology associated with the juvenile stages of Lepeophtheirus salmonis on the Atlantic salmon, Salmo salar L. J. Fish Dis. 1990, 13, 303–310. [Google Scholar] [CrossRef]
- Johnson, S.C. The Biology of Lepeophtheirus salmonis. Ph.D. Thesis, Simon Fraser University, Burnaby, BC, Canada, 1991. [Google Scholar]
- Bjørn, P.A.; Finstad, B. The development of salmon lice (Lepeophtheirus salmonis) on artificially infected post smolts of sea trout (Salmo trutta). Can. J. Zool. 1998, 76, 970–977. [Google Scholar] [CrossRef]
- Heggland, E.I.; Dondrup, M.; Nilsen, F.; Eichner, C. Host gill attachment causes blood-feeding by the salmon louse (Lepeophtheirus salmonis) chalimus larvae and alters parasite development and transcriptome. Parasites Vectors 2020, 13, 225. [Google Scholar] [CrossRef]
- Fast, M.D.; Muise, D.M.; Easy, R.E.; Ross, N.W.; Johnson, S.C. The effects of Lepeophtheirus salmonis infections on the stress response and immunological status of Atlantic salmon (Salmo salar). Fish Shellfish. Immunol. 2006, 21, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Ugelvik, M.S.; Mæhle, S.; Dalvin, S. Temperature affects settlement success of ectoparasitic salmon lice (Lepeophtheirus salmonis) and impacts the immune and stress response of Atlantic salmon (Salmo salar). J. Fish Dis. 2022, 45, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Schreck, C.B.; Tort, L. The concept of stress in fish. In Fish Physiology, 1st ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 35, pp. 1–34. [Google Scholar]
- Eissa, N.; Wang, H.P. Transcriptional stress responses to environmental and husbandry stressors in aquaculture species. Rev. Aquac. 2016, 8, 61–88. [Google Scholar] [CrossRef]
- Braden, L.M.; Monaghan, S.J.; Fast, M.D. Salmon immunological defence and interplay with the modulatory capabilities of its ectoparasite Lepeophtheirus salmonis. Parasite Immunol. 2020, 42, e12731. [Google Scholar] [CrossRef]
- Johnson, S.C.; Albright, L.J. Comparative susceptibility and histopathology of the response of naive Atlantic. Chinook and coho salmon to experimental infection with Lepeophtheirus salmonis (Copepoda: Caligidae). Dis. Aquat. Org. 1992, 14, 179–193. [Google Scholar] [CrossRef]
- Barker, S.E.; Bricknell, I.R.; Covello, J.; Purcell, S.; Fast, M.D.; Wolters, W.; Bouchard, D.A. Sea lice, Lepeophtheirus salmonis (Krøyer 1837), infected Atlantic salmon (Salmo salar L.) are more susceptible to infectious salmon anemia virus. PLoS ONE 2019, 14, e0209178. [Google Scholar] [CrossRef] [PubMed]
- Lhorente, J.P.; Gallardo, J.A.; Villanueva, B.; Carabaño, M.J.; Neira, R. Disease Resistance in Atlantic Salmon (Salmo salar): Coinfection of the Intracellular Bacterial Pathogen Piscirickettsia salmonis and the Sea Louse Caligus rogercresseyi. PLoS ONE 2014, 9, e95397. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, M.S.; Leadbeater, S.; Garcia, C.; Sylvain, F.-É.; Custodio, M.; Ang, K.P.; Powell, F.; Carvalho, G.R.; Creer, S.; Elliot, J.; et al. Parasitism perturbs the mucosal microbiome of Atlantic Salmon. Sci. Rep. 2017, 7, srep43465. [Google Scholar] [CrossRef]
- Gonçalves, A.T.; Collipal-Matamal, R.; Valenzuela-Muñoz, V.; Nuñez-Acuña, G.; Valenzuela-Miranda, D.; Gallardo-Escárate, C. Nanopore sequencing of microbial communities reveals the potential role of sea lice as a reservoir for fish pathogens. Sci. Rep. 2020, 10, 2895. [Google Scholar] [CrossRef]
- Gallardo-Escárate, C.; Valenzuela-Muñoz, V.; Núñez-Acuña, G.; Carrera, C.; Gonçalves, A.T.; Valenzuela-Miranda, D.; Benavente, B.P.; Roberts, S. Catching the complexity of salmon-louse interactions. Fish Shellfish. Immunol. 2019, 90, 199–209. [Google Scholar] [CrossRef]
- Bailey, R.J.E.; Birkett, M.A.; Ingvarsdóttir, A.; Mordue, A.J.; Mordue, W.; O’Shea, B.; Pickett, J.A.; Wadhams, L.J. The role of semiochemicals in host location and non-host avoidance by salmon louse (Lepeophtheirus salmonis) copepodids. Can. J. Fish. Aquat. Sci. 2006, 63, 448–456. [Google Scholar] [CrossRef]
- Devine, J.G.; Ingvarsdóttir, A.; Mordue, W.; Pike, W.A.; Pickett, J.; Duce, I.; Mordue, A.J. Salmon Lice, Lepeophtheirus salmonis, Exhibit Specific Chemotactic Responses to Semiochemicals Originating from the Salmonid, Salmo salar. J. Chem. Ecol. 2000, 26, 1833–1847. [Google Scholar] [CrossRef]
- Ingvarsdóttir, A.; Birkett, M.A.; Duce, I.; Genna, R.L.; Mordue, W.; Pickett, J.A.; Wadhams, L.J.; Mordue, A.J. Semiochemical strategies for sea louse control: Host location cues. Pest Manag. Sci. 2002, 58, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Mordue, A.J.; Birkett, M.A. A review of host finding behaviour in the parasitic sea louse, Lepeophtheirus salmonis(Caligidae: Copepoda). J. Fish Dis. 2009, 32, 3–13. [Google Scholar] [CrossRef]
- Komisarczuk, A.Z.; Grotmol, S.; Nilsen, F. Ionotropic receptors signal host recognition in the salmon louse (Lepeophtheirus salmonis, Copepoda). PLoS ONE 2017, 12, e0178812. [Google Scholar] [CrossRef]
- Núñez-Acuña, G.; Gallardo-Escárate, C.; Fields, D.M.; Shema, S.; Skiftesvik, A.B.; Ormazábal, I.; Browman, H.I. The Atlantic salmon (Salmo salar) antimicrobial peptide cathelicidin-2 is a molecular host-associated cue for the salmon louse (Lepeophtheirus salmonis). Sci. Rep. 2018, 8, 13738. [Google Scholar] [CrossRef] [PubMed]
- Difford, G.F.; Haugen, J.-E.; Aslam, M.L.; Johansen, L.H.; Breiland, M.W.; Hillestad, B.; Baranski, M.; Boison, S.; Moghadam, H.; Jacq, C. Variation in volatile organic compounds in Atlantic salmon mucus is associated with resistance to salmon lice infection. Sci. Rep. 2022, 12, 4839. [Google Scholar] [CrossRef]
- Iversen, A.; Hermansen, Ø.; Nystøyl, R.; Hess, E.J. Kostnadsutvikling i lakseoppdrett, Nofima, Tromsø, Norway, Report 24/2017, pp. 1–47. Available online: https://nofima.no/publikasjon/1523319/ (accessed on 3 November 2023).
- Berle, H.; Rim, S.Y.; Thesis, M. The Cost of Sea Lice and Its Implications for the Future of the Norwegian Aquaculture Industry—A Study on Sea Lice and Recommendations. Master’s Thesis, Norwegian School of Economics, Bergen, Norway, 2018. [Google Scholar]
- Fiskehelserapporten 2022. Norwegian Veterinary Institute’s Report Series, 5a/2023, Ås, Norway. Available online: https://www.vetinst.no/rapporter-og-publikasjoner/rapporter/2023/norwegian-fish-health-report-2022 (accessed on 3 November 2023).
- Overton, K.; Dempster, T.; Oppedal, F.; Kristiansen, T.S.; Gismervik, K.; Stien, L.H. Salmon lice treatments and salmon mortality in Norwegian aquaculture: A review. Rev. Aquac. 2018, 11, 1398–1417. [Google Scholar] [CrossRef]
- Sviland Walde, C.; Bang Jensen, B.; Stormoen, M.; Asche, F.; Misund, B. The economic impact of decreased mortality and increased growth associated with preventing, replacing or improving current methods for delousing farmed Atlantic salmon in Norway. Prev. Vet. Med. 2023, 221, 106062. [Google Scholar] [CrossRef]
- FAO/WHO. Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria; Fao & Who Report; FAO/WHO: Córdoba, Argentina, 2001; pp. 1–34. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Gómez, G.D.; Balcázar, J.L. A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunol. Med. Microbiol. 2008, 52, 145–154. [Google Scholar] [CrossRef]
- Naik, S.; Bouladoux, N.; Wilhelm, C.; Molloy, M.J.; Salcedo, R.; Kastenmuller, W.; Deming, C.; Quinones, M.; Koo, L.; Conlan, S.; et al. Compartmentalized control of skin immunity by resident commensals. Science 2012, 337, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Singh, R. Probiotics in aquaculture: A promising emerging alternative approach. Symbiosis 2018, 77, 99–113. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Sun, Y.-Z.; Wang, A.; Zhou, Z. Probiotics as means of diseases control in aquaculture, a review of current knowledge and future perspectives. Front. Microbiol. 2018, 9, 2429. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.; Pereira, W.A.; Mendonça, C.M.N.; Urquiza, A.V.; Leblanc, J.G.; Cotter, P.D.; Romero, J.; Oliveira, R.P.S. Use of Probiotic Bacteria and Bacteriocins as an Alternative to Antibiotics in Aquaculture. Microorganisms 2022, 10, 1705. [Google Scholar] [CrossRef]
- Simón, R.; Docando, F.; Nuñez-Ortiz, N.; Tafalla, C.; Díaz-Rosales, P. Mechanisms Used by Probiotics to Confer Pathogen Resistance to Teleost Fish. Front. Immunol. 2021, 12, 653025. [Google Scholar] [CrossRef] [PubMed]
- Talukder Shefat, S.H. Probiotic Strains Used in Aquaculture. Int. Res. J. Microbiol. 2018, 7, 43–55. [Google Scholar] [CrossRef]
- Pieters, N.; Brunt, J.; Austin, B.; Lyndon, A.R. Efficacy of in-feed probiotics against Aeromonas bestiarum and Ichthyophthirius multifiliis skin infections in rainbow trout (Oncorhynchus mykiss, Walbaum). J. Appl. Microbiol. 2008, 105, 723–732. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Tovar-Ramírez, D.; Ascencio-Valle, F.; Civera-Cerecedo, R.; Gracia-López, V.; Barbosa-Solomieu, V. Effects of dietary live yeast Debaryomyces hansenii on the immune and antioxidant system in juvenile leopard grouper Mycteroperca rosacea exposed to stress. Aquaculture 2008, 280, 39–44. [Google Scholar] [CrossRef]
- Barrett, L.T.; Oppedal, F.; Robinson, N.; Dempster, T. Prevention not cure: A review of methods to avoid sea lice infestations in salmon aquaculture. Rev. Aquac. 2020, 12, 2527–2543. [Google Scholar] [CrossRef]
- Klakegg, Ø.; Myhren, S.; Juell, R.A.; Aase, M.; Salonius, K.; Sørum, H. Improved health and better survival of farmed lumpfish (Cyclopterus lumpus) after a probiotic bath with two probiotic strains of Aliivibrio. Aquaculture 2019, 518, 734810. [Google Scholar] [CrossRef]
- Klakegg, Ø.; Salonius, K.; Nilsen, A.; Fülberth, M.; Sørum, H. Enhanced growth and decreased mortality in Atlantic salmon (Salmo salar) after probiotic bath. J. Appl. Microbiol. 2020, 129, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Bron, J.E.; Sommerville, C.; Jones, M.; Rae, G.H. The settlement and attachment of early stages of the salmon louse, Lepeophtheirus salmonis (Copepoda: Caligidae) on the salmon host, Salmo salar. J. Zool. 1991, 224, 201–212. [Google Scholar] [CrossRef]
- Dalvin, S.; Oppedal, F. The effect of temperature and light on development of salmon lice. Norwegian Institute of Marine Research, Bergen, Norway, Report 2019-13, pp. 1–19.
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; Philippart de Foy, J.M.; Dequenne, I.; de Timary, P.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Jiang, S.; Huo, D.; Allaband, C.; Estaki, M.; Cantu, V.; Belda-Ferre, P.; Vázquez-Baeza, Y.; Zhu, Q.; Ma, C.; et al. Candidate probiotic Lactiplantibacillus plantarum HNU082 rapidly and convergently evolves within human, mice, and zebrafish gut but differentially influences the resident microbiome. Microbiome 2021, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, S.; Nilsen, T.O.; Kolarevic, J.; Ebbesson, L.O.E.; Pedrosa, C.; Fivelstad, S.; Hosfeld, C.; Stefansson, S.O.; Terjesen, B.F.; Takle, H.; et al. Stocking density limits for post-smolt Atlantic salmon (Salmo salar L.) with emphasis on production performance and welfare. Aquaculture 2017, 468, 363–370. [Google Scholar] [CrossRef]
- Hosfeld, C.D.; Hammer, J.; Handeland, S.O.; Fivelstad, S.; Stefansson, S.O. Effects of fish density on growth and smoltification in intensive production of Atlantic salmon (Salmo salar L.). Aquaculture 2009, 294, 236–241. [Google Scholar] [CrossRef]
- Delfosse, C.; Pageat, P.; Lafont-Lecuelle, C.; Asproni, P.; Chabaud, C.; Cozzi, A.; Bienboire-Frosini, C. Effect of handling and crowding on the susceptibility of Atlantic salmon (Salmo salar L.) to Lepeophtheirus salmonis (Krøyer) copepodids. J. Fish Dis. 2020, 44, 327–336. [Google Scholar] [CrossRef]
- Krasnov, A.; Skugor, S.; Todorcevic, M.; Glover, K.A.; Nilsen, F. Gene expression in Atlantic salmon skin in response to infection with the parasitic copepod Lepeophtheirus salmonis, cortisol implant, and their combination. BMC Genomics 2012, 13, 130. [Google Scholar] [CrossRef]
- Thompson, F.L.; Iida, T.; Swings, J. Biodiversity of Vibrios. Microbiol. Mol. Biol. Rev. 2004, 68, 403–431. [Google Scholar] [CrossRef]
- Hjerde, E.; Karlsen, C.; Sørum, H.; Parkhill, J.; Willassen, N.P.; Thomson, N.R. Co-cultivation and transcriptome sequencing of two co-existing fish pathogens Moritella viscosa and Aliivibrio wodanis. BMC Genom. 2015, 16, 447. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.A.; Whyte, S.K.; Braden, L.M.; Purcell, S.L.; Manning, A.J.; Muckle, A.; Fast, M.D. Impact of co-infection with Lepeophtheirus salmonis and Moritella viscosa on inflammatory and immune responses of Atlantic salmon (Salmo salar). J. Fish Dis. 2020, 43, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, C.; Vanberg, C.; Mikkelsen, H.; Sørum, H. Co-infection of Atlantic salmon (Salmo salar), by Moritella viscosa and Aliivibrio wodanis, development of disease and host colonization. Vet. Microbiol. 2014, 171, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, C.; Sørum, H.; Willassen, N.P.; Åsbakk, K. Moritella viscosa bypasses Atlantic salmon epidermal keratocyte clearing activity and might use skin surfaces as a port of infection. Vet. Microbiol. 2012, 154, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Kashulin, A.; Sørum, H. A novel in vivo model for rapid evaluation of Aliivibrio salmonicida infectivity in Atlantic salmon. Aquaculture 2014, 420–421, 112–118. [Google Scholar] [CrossRef]
- Travers, M.-A.; Florent, I.; Kohl, L.; Grellier, P. Probiotics for the Control of Parasites: An Overview. J. Parasitol. Res. 2011, 2011, 610769. [Google Scholar] [CrossRef]
- Austin, B. The bacterial microflora of fish, revised. Sci. World J. 2006, 6, 931–945. [Google Scholar] [CrossRef]
- Uribe, C.; Folch, H.; Enriquez, R.; Moran, G. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 2011, 56, 486–503. [Google Scholar] [CrossRef]
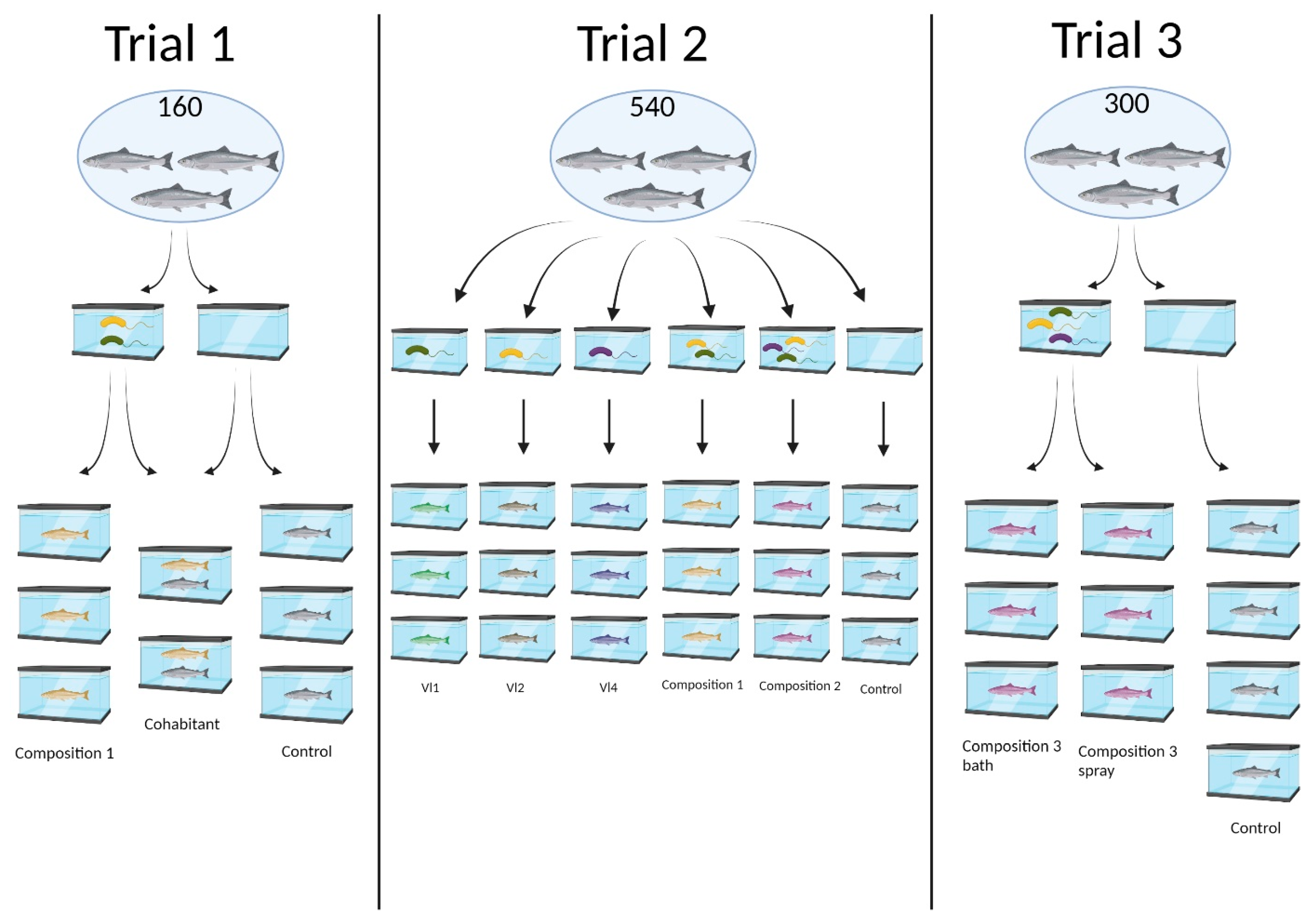
| Trials | Composition | Effective Dose (CFU Bacteria mL) | Administration | Challenge |
|---|---|---|---|---|
| Trial 1 | Composition 1 (Aliivibrio spp. VI1/VI2 co-culture) | 6 × 108 | 2 min immersion | 30 copepodids/fish Day 155 |
| Trial 2 | Aliivibrio sp. Vl1 Aliivibrio sp. Vl2 Aliivibrio sp. Vl4 Composition 1 (Aliivibrio spp. VI1/VI2 co-culture) Composition 2 (Aliivibrio spp. VI1/VI2/VI3 mixed) | Vl1: 1.4 × 108 Vl2: 2.1 × 108 Vl4: 3.5 × 106 Composition 1: 1.2 × 108 Composition 2: 1.3 × 108 | 2 min immersion | 30 copepodids/fish Day 58 |
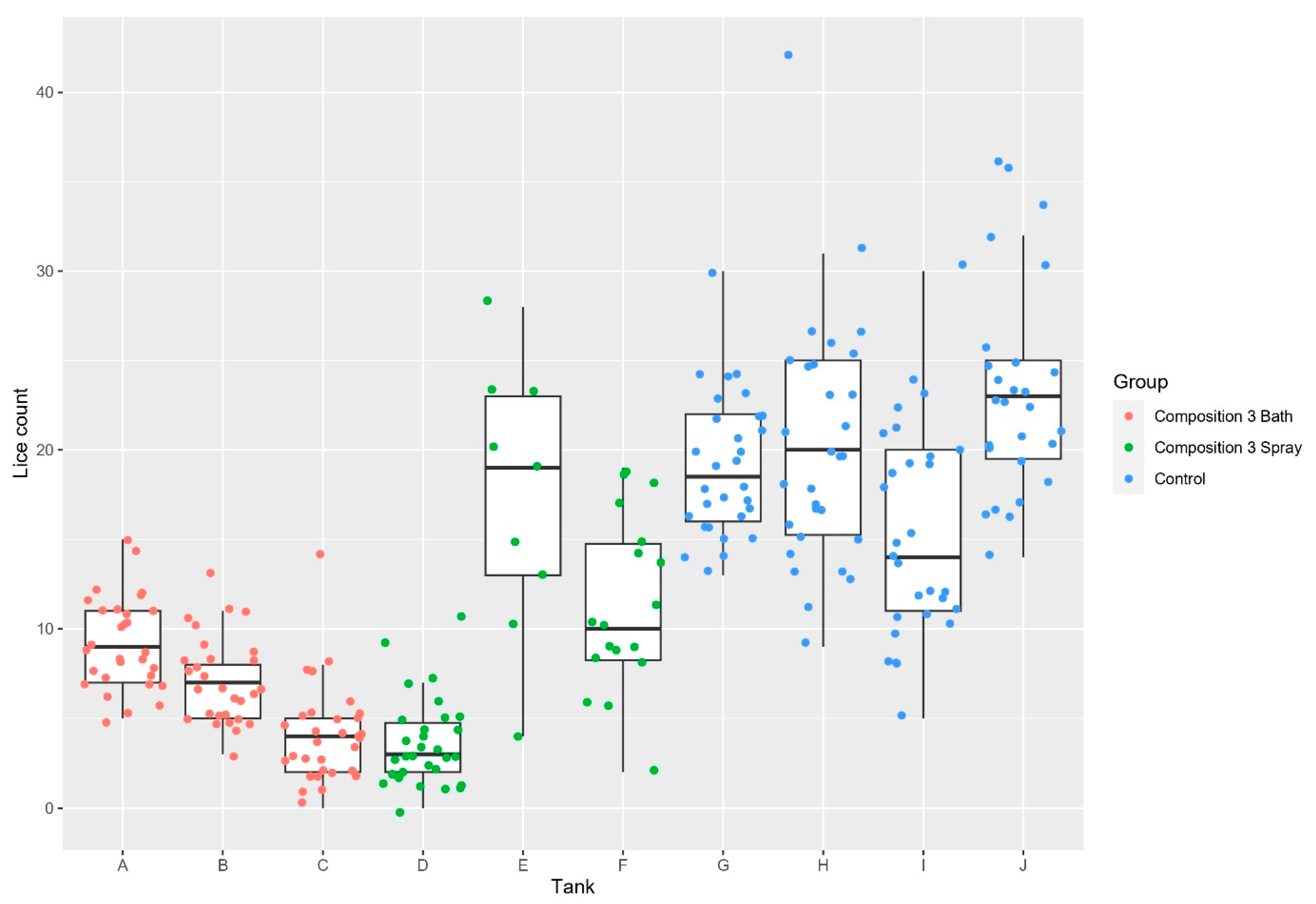
| Trial 3 | Composition 3 (Aliivibrio spp. Vl1/Vl2 co-culture mixed with Vl4) | 7.1 × 107 | 2 min immersion or spray | 30 copepodids/fish Day 91 |
| Trial | Group | Attached Lice Probiotic Group | Attached Lice Control Group | Reduction |
|---|---|---|---|---|
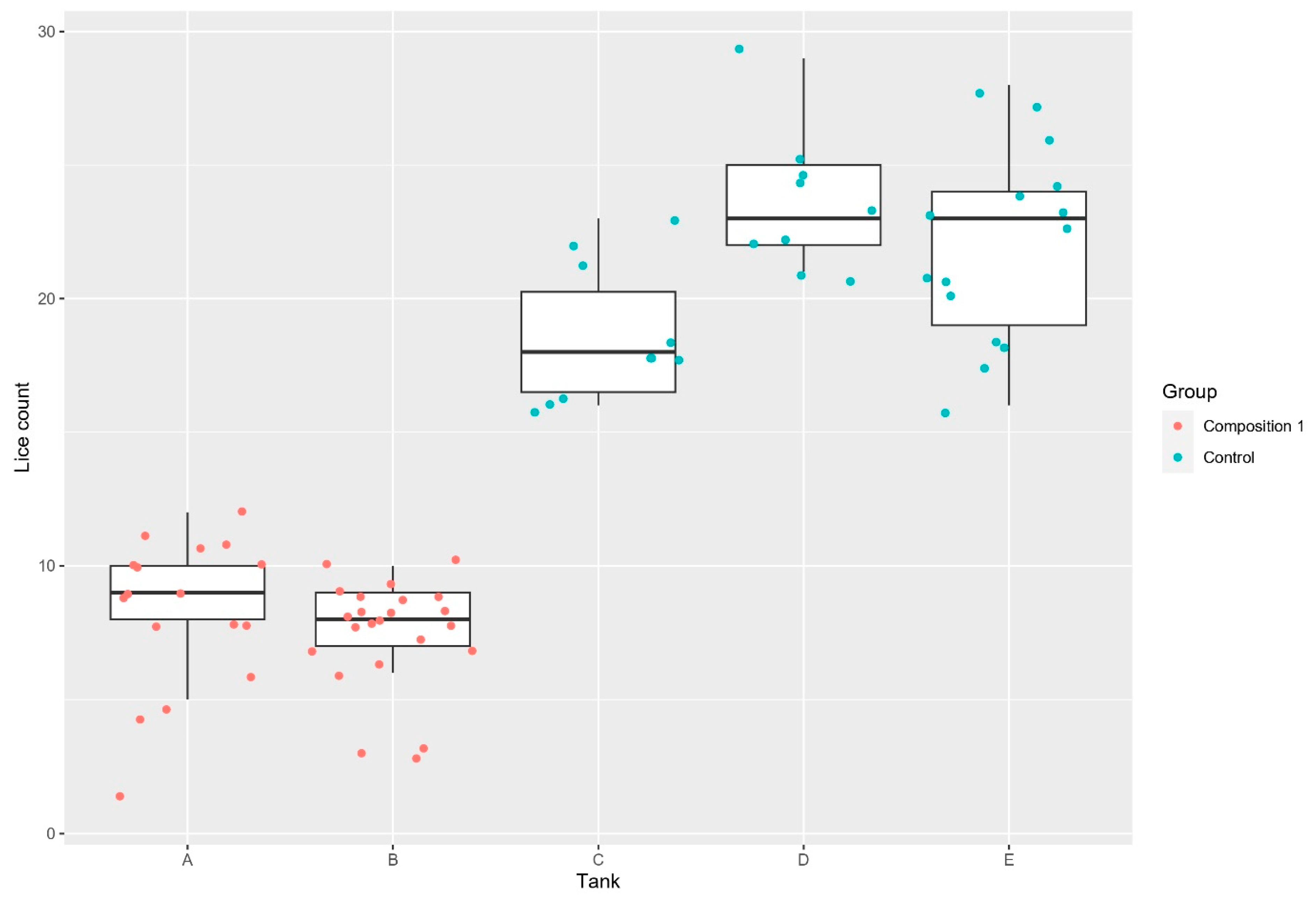
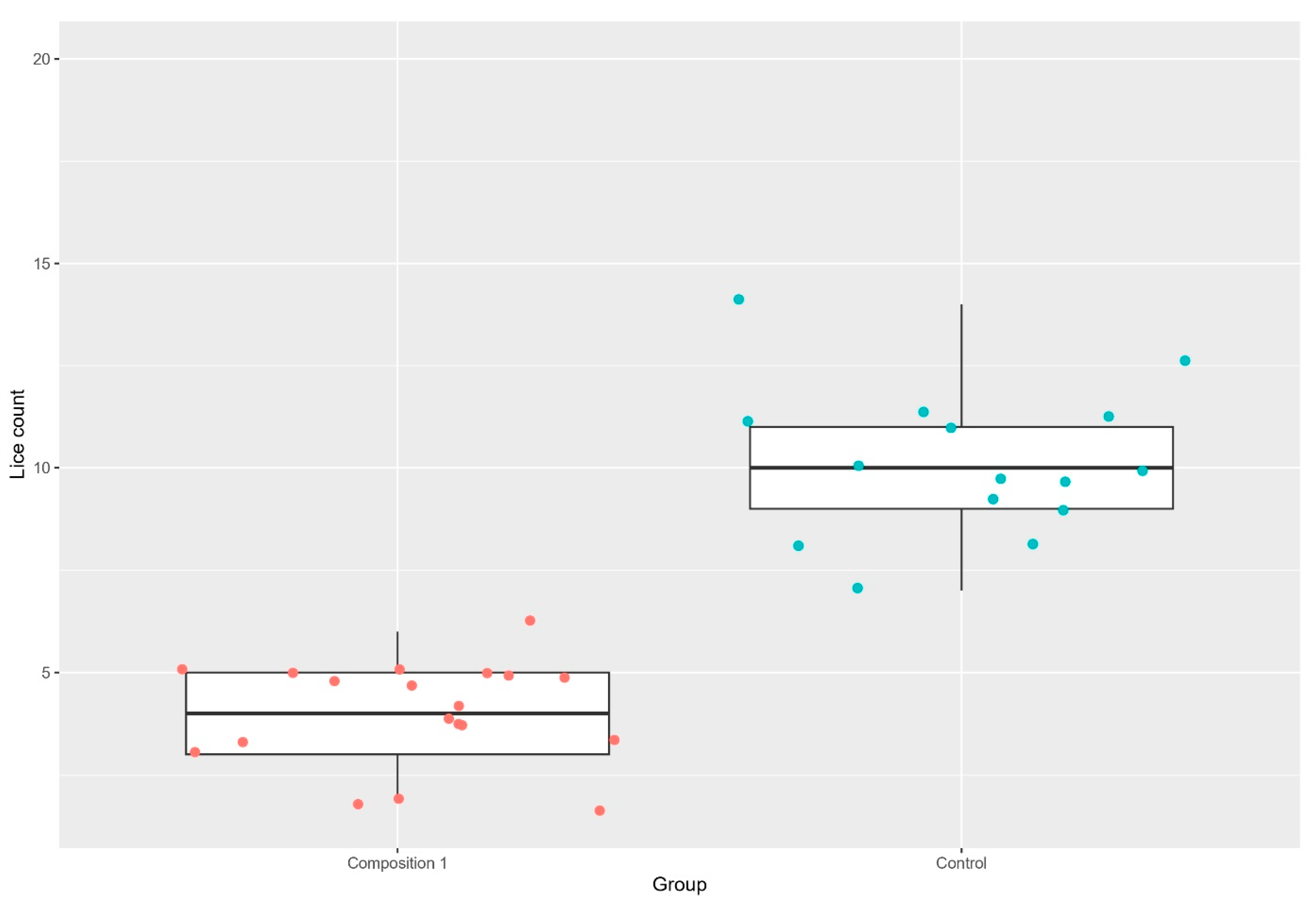
| Trial 1 | Composition 1, separate tanks Composition 1, cohabitation tanks | 7.83 (2.4) 4.05 (1.2) | 21.38 (3.6) 10.14 (1.8) | 63.38% 60.06% |
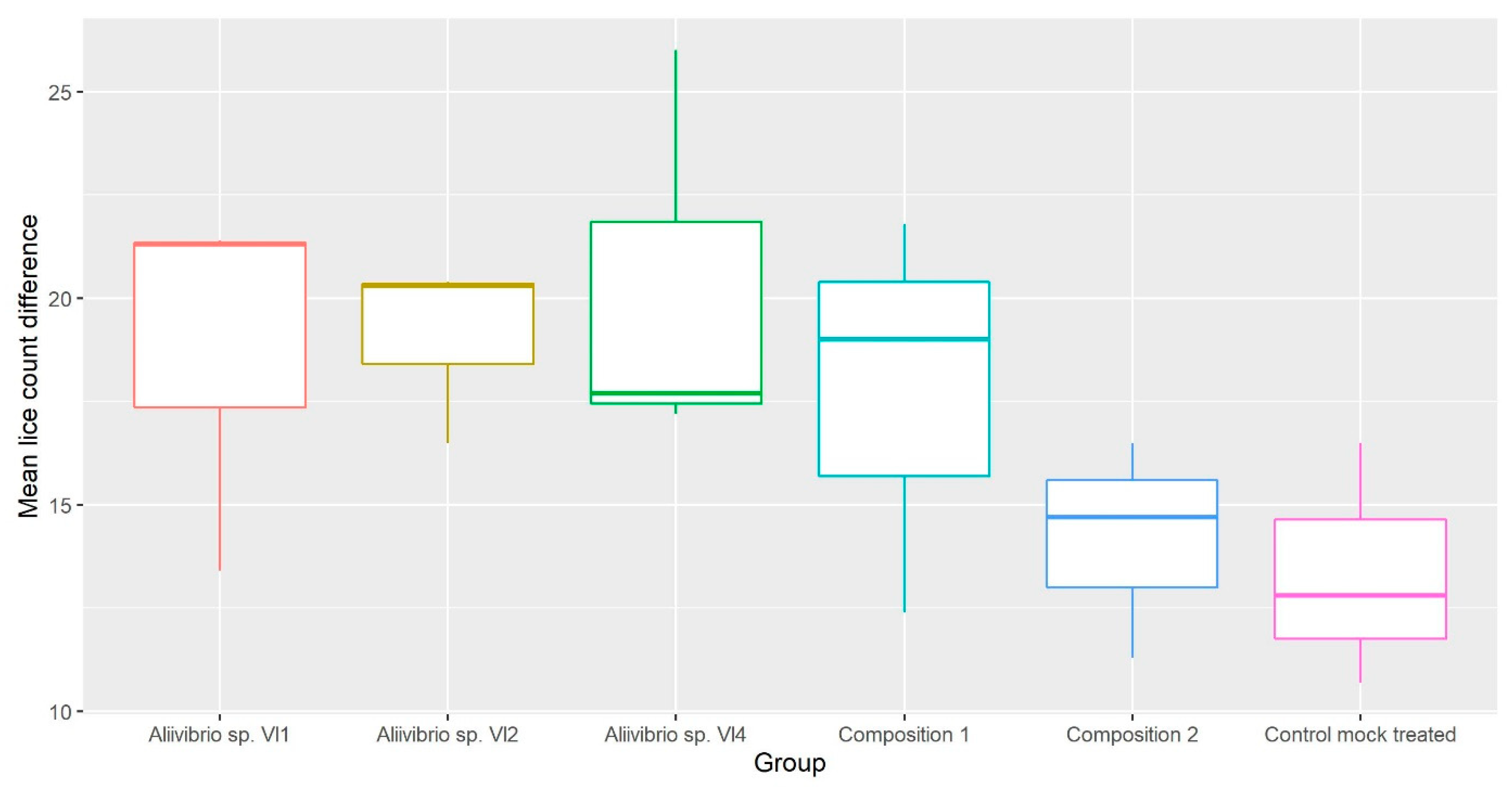
| Trial 2 | Aliivibrio sp. Vl1 Aliivibrio sp. Vl2 Aliivibrio sp. Vl4 Composition 1 Composition 2 | Vl1 first count: 23.49 (6.6) Vl1 second count: 6.69 (3.4) Vl2 first count: 22.51 (6.1) Vl2 second count: 5.53 (2.8) Vl4 first count: 20.61 (6.4) Vl4 second count: 2.43 (2.1) Composition 1 first count: 18.02 (7.9) Composition 1 second count: 2.79 (2.6) Composition 2 first count: 15.56 (4.4) Composition 2 second count: 3.14 (2.3) | First count: 15.38 (5.2) Second count: 4.06 (2.5) | First count: No reduction. Vl1 second count: No reduction. Vl2 second count: No reduction. Vl4 second count: 40.15% Composition 1 second count: 31.28% Composition 2 second count: 22.67% Difference in reduction compared to control (δ): Vl1: 31.62% Vl2: 33.34% Vl4: 37.74% Composition 1: 25.72% Composition 2: 8.86% |
| Trial 3 | Composition 3 bath Composition 3 spray | 6.77 (3.3) 8.18 (6.8) | 19.39 (6.3) | Bath: 65.06% Spray: 57.82% |
| Attached Lice at 0 h | Dead Lice at 24 h | |
|---|---|---|
| Container | 250 mL without air | 250 mL without air |
| Aliivibrio sp. Vl1 | 10 | 0 |
| Aliivibrio sp. Vl2 | 10 | 0 |
| Aliivibrio sp. Vl3 | 10 | 2 |
| Aliivibrio spp. Vl1, Vl2, Vl3 | 10 | 0 |
| A. salmonicida | 5 | 5 |
| A. wodanis Ft 5426 | 10 | 0 |
| M. viscosa Ft 5427 | 5 | 5 |
| Aliivibrio sp. NCIMB 42181 | 10 | 10 |
| Aliivibrio spp. NCIMB 42953, 42952, 42954 | 10 | 10 |
| Negative control | 10 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steen Dobloug, M.; Skagen-Sandvik, C.; Evensen, Ø.; Gadan, K.; Bakke, M.J.; Sørum, H.; Salonius, K. Reduced Infestation Levels of Lepeophtheirus salmonis in Atlantic Salmon (Salmo salar) following Immersion Exposure to Probiotic Aliivibrio spp. Appl. Microbiol. 2023, 3, 1339-1354. https://doi.org/10.3390/applmicrobiol3040090
Steen Dobloug M, Skagen-Sandvik C, Evensen Ø, Gadan K, Bakke MJ, Sørum H, Salonius K. Reduced Infestation Levels of Lepeophtheirus salmonis in Atlantic Salmon (Salmo salar) following Immersion Exposure to Probiotic Aliivibrio spp. Applied Microbiology. 2023; 3(4):1339-1354. https://doi.org/10.3390/applmicrobiol3040090
Chicago/Turabian StyleSteen Dobloug, Marius, Camilla Skagen-Sandvik, Øystein Evensen, Koestan Gadan, Marit Jørgensen Bakke, Henning Sørum, and Kira Salonius. 2023. "Reduced Infestation Levels of Lepeophtheirus salmonis in Atlantic Salmon (Salmo salar) following Immersion Exposure to Probiotic Aliivibrio spp." Applied Microbiology 3, no. 4: 1339-1354. https://doi.org/10.3390/applmicrobiol3040090
APA StyleSteen Dobloug, M., Skagen-Sandvik, C., Evensen, Ø., Gadan, K., Bakke, M. J., Sørum, H., & Salonius, K. (2023). Reduced Infestation Levels of Lepeophtheirus salmonis in Atlantic Salmon (Salmo salar) following Immersion Exposure to Probiotic Aliivibrio spp. Applied Microbiology, 3(4), 1339-1354. https://doi.org/10.3390/applmicrobiol3040090






