Abstract
Biosurfactants are amphiphilic molecules with surface tension reducing activities. Among biosurfactant producers, fungi have been identified as promising organisms. While many studies have investigated biosurfactant production in fungal species from the Ascomycota and Basidiomycota phyla, less is known concerning species from the Mucoromycota phylum. In this context, the aim of this study was to screen and optimize biosurfactant production in 24 fungal strains, including seven Mucor, three Lichtheimia, and one Absidia species. After cultivation in a medium stimulating surfactant production, the surface activity of cell-free supernatants was measured using both oil spreading and parafilm M tests. Among them, five Mucor strain cell-free supernatants belonging to M. circinelloides, M. lanceolatus, M. mucedo, M. racemosus, and M. plumbeus, showed oil repulsion. Then, the impact of the medium composition on surfactant production was evaluated for eight strains. Three of them, i.e., Mucor circinelloides UBOCC-A-109190, Mucor plumbeus UBOCC-A-111133, and Mucor mucedo UBOCC-A-101353 showed an interesting surfactant production potential, reducing the medium surface tension to 36, 31, and 32 mN/m, respectively. A preliminary characterization of the surfactant molecules produced by these strains was performed and showed that these compounds belonged to the glycolipid family.
1. Introduction
Surfactants are defined as amphiphilic molecules that are able to reduce tension at phase interfaces, emulsify oil in water and water in oil, as well as help in stable gel and foam formation [1,2]. Synthetic and chemical surfactants are produced from petrochemicals and are increasingly used, even though they may have an impact on the environment and human health. Hence, there is a need to develop alternative molecules such as biosurfactants [3].
When it comes to surfactants produced from biological sources, e.g., bacteria and fungi, they can be divided into biosurfactants and bioemulsifiers. Biosurfactants are low molecular weight compounds produced by microorganisms, which possess high surface tension reducing and low emulsification activities [2]. They can be differentiated from bioemulsifiers, which are of a high molecular weight and possess a high emulsification activity, but little or negligible surface tension reducing properties [2]. Because of their functional properties as well as their biodegradability and low toxicity for the environment, these compounds are of great interest to replace existing synthetic surfactants for different sectors such as the pharmaceutical, cosmetic, and food industries, as well as in the field of pollutant remediation [4,5,6]. They are divided into a large number of molecules, but the best known are glycolipids, which include rhamnolipids, sophorolipids, and mannosylerythritol lipids. Biosurfactants are already present on the market and many companies produce glycolipids, mainly rhamnolipids and sophorolipids, which confirm the renewed interest in these molecules [7].
Bacteria are currently the most studied biosurfactant producers, with Pseudomonas and Bacillus spp. being among the highest surfactant producers [8]. In contrast, fungi have been less studied than bacteria [9]. Nevertheless, a quite large number of studies have highlighted biosurfactant production in yeasts and filamentous fungi, mostly belonging to the Ascomycota phylum, e.g., Aspergillus, Candida, Fusarium, and Penicillium spp. [3]. Sophorolipids, which are notably produced by the yeast Starmerella bombicola (syn. Candida bombicola) [10] and other Starmerella spp., are probably the class of fungal surfactant that has attracted the most attention during the last decades. Other biosurfactant-producing filamentous fungi include Aspergillus ustus [11], Ustilago maydis [12], Fusarium fujikuroi, and Penicillium chrysogenum [13], producing biosurfactants from the glycolipoprotein, glycolipid, trehalolipid, and lipopeptide families.
Among other fungal phyla, the potent ability of members of the Mucoromycota phylum, including Mucor, Lichtheimia, and Absidia spp., to produce surfactant has not been studied to a large extent. The Mucor, Lichtheimia, and Absidia genera all belong to the Mucorales order, of which Mucor is the largest genus with 63 currently recognized species [14,15]. Mucor, Lichtheimia, and Absidia spp. are often ubiquitous and some species are known for their positive and negative impact on human activities, such as their involvement in the manufacture of fermented foods (e.g., cheese and cereal-based fermented foods) or their role in food spoilage and as causative mycosis agents, respectively [16,17]. In addition, Mucor, Lichtheimia, and Absidia spp. are also interesting organisms for biotechnological applications such as metabolite production, e.g., enzymes (lipases and proteases), polyunsaturated fatty-acids and biofuel production, and biotransformation (e.g., terpenoid production and the transformation thereof) [17,18]. Biosurfactant production in the Mucor genus has been examined, but not to a great extent. Among Mucor spp., Mucor circinelloides [19], Mucor hiemalis [20], and Mucor indicus [21] were found as efficient biosurfactant producers, while to the best of our knowledge, there are no data available concerning Lichtheimia and Absidia spp. In this context, the present study aimed to provide insight into the production of biosurfactants/bioemulsifiers by filamentous fungi belonging to the Mucoromycotina phylum. Twenty-four strains, including seven Mucor species, three Lichtheimia, and one Absidia species were screened for their potential to produce surfactant molecules. Then, the impact of medium composition was studied for eight strains presenting surfactant activities. Finally, emulsifying and surfactant activities of the three most promising strains were further investigated and a preliminary characterization of the produced molecules was performed. This study highlights the production of surface- active compounds by Mucor species that have been little studied for this feature.
2. Materials and Methods
2.1. Fungal Strains and Spore Suspension Preparation
Twenty-four fungal strains (Table 1), belonging to the Mucoromycotina phylum, were obtained from the Université de Bretagne Occidentale Culture Collection (UBOCC, Plouzané, France). For preparing the spore suspension, each strain was cultivated for 7 days at 25 °C on potato dextrose agar (PDA) slants. Then, spores were harvested using 3 mL of sterile water and the spore concentration enumerated using a Malassez Cell. After centrifugation for 5 min at 5000× g, a sufficient sterile water volume was added to get a calibrated spore suspension at 107 spores/mL.

Table 1.
Fungal strains used in this study.
2.2. Cultivation Media and Growth Conditions
2.2.1. Preliminary Screening of Surfactant Production
All of the tested isolates were first cultivated in BSF5 medium (see composition in Table 2), a pre-optimized medium for surfactant production, previously autoclaved for 15 min at 121 °C. Fungal isolates were grown in 100 mL of medium in a 250-mL Erlenmeyer flask with an initial spore concentration of 5 × 105 spores/mL and were incubated for 96 h at 25 °C on a rotary shaker (Infors AG, Bottmingen, Switzerland) at 150 rpm. After incubation, culture broth was centrifuged at 8000× g for 20 min at 4 °C. The supernatant was filtered on 1.2 µM pore size fiberglass filters (Grosseron, Couëron, France) followed by filtration on 0.45 µm pore size cellulose acetate filters and then used for screening tests, i.e., oil spreading (OST) and Parafilm M test (PMT) tests, as described below. The cultures were carried out in triplicate.

Table 2.
Composition of the tested media for biosurfactant production.
2.2.2. Impact of Medium Composition on Surfactant Production,
Seven additional media, whose composition is shown in Table 2, were then tested for biosurfactant production on eight strains, yielding positive results for either one or both the OST and PMT, using the same culture conditions and cell-free supernatant preparation procedure as described above, and subjected to OST and PMT. Furthermore, the effect of prolonged incubation time (144 h) during growth on the supernatant surfactant activity was evaluated in the BSF5 medium. For all these tested conditions, the dry biomass was also determined at the end of cultivation for a single culture after drying cells for 72 h at 55 °C in an oven (Carbolite-Gero, Eragny, France).
2.3. Detection of Surface-Active Compounds in Culture Supernatants
Different methodologies were applied to detect surface active compounds in cell-free supernatants (CFS), including OST, PMT, emulsion index after 24 h (E24), and surface tension (ST) measurements. OST and PMT were applied as initial screening tests on all tested isolates, as well as to assess the impact of the medium composition and incubation time on a subset of selected strains. E24 and ST were applied on three selected strains for further characterization of CFS. All tests were performed in triplicate.
2.3.1. Oil Spreading Test (OST)
OST was performed as described previously [22], with slight modifications. Briefly, 25 mL of distilled water was placed in a 9-cm-diameter polystyrene Petri dish. Then, 15 µL of crude oil was deposited on the water surface in the Petri dish center, and after 2 min, 15 µL of CFS was placed on the oil surface. OST was considered as positive when a halo was observed on the surface, indicating the presence of surface-active compounds in the CFS. SDS 20% and distilled water were used as a positive and negative control, respectively. Repulsion diameters were measured for comparing the obtained values for each tested strain and medium.
2.3.2. Parafilm M Test
Parafilm M tests were performed as described previously [23]. First, 15 µL of CFS was deposited on parafilm (Parafilm MⓇ, Bemis Company, Inc., Neenah, WI, USA), previously fixed on graph paper. Then, drop diameters were manually measured. To allow for comparison between the Parafilm M test results of the strains cultivated in different media, the drop diameter of the uninoculated culture medium was subtracted from that of the CFS.
2.3.3. Emulsification Index (E24)
The emulsification index was determined as described previously [24], on Mucor circinelloides MM1, Mucor mucedo MC1, and Mucor plumbeus MP1 CFS. Briefly, 1 mL of diesel and 1 mL of cell free supernatant were placed in a test tube, followed by vortexing for 2 min at maximum speed. The tubes were left to stand for 24 h and the emulsion height was measured. The index was calculated according to the following formula:
Emulsification index = (Height of the emulsion layer/Total height) × 100
2.3.4. Surface Tension Measurement
The surface tension of CFS was determined as described previously [25]. For this, the CFS density was determined using a density meter (DMA4500M, Anton Paar, Graz, Austria) and was integrated for measuring surface tension using the du Nouy ring type method on a TD2 tensiometer (Lauda, Lauda-Königshofen, Germany). Calibration on ethanol and pure water was made and the control was set on the non-inoculated BSF5 medium. All measurements were done at room temperature after carefully dipping the platinum ring until equilibrium was reached. The standard deviation was set to 0.01.
2.4. Biosurfactant Extraction
Biosurfactant extraction was performed as described elsewhere [11,26]. Supernatants were acidified to pH 2 with 1 M HCl and stored at 4 °C for 24 h. They were then centrifuged at 8000× g for 20 min and the precipitate was recovered. The precipitate was then neutralized with 0.067 M phosphate buffer at pH 7. After placing this solution in a separatory funnel, an equal volume of ethyl acetate was added. After gentle stirring and decantation for 1 h, the organic phase was set aside and the process was repeated a second time. The organic phase was recovered and pooled with the first recovered solution. Ethyl acetate was then evaporated using a SpeedVac vacuum evaporator (Thermo Fisher Scientific, Waltham, MA, USA) and the active fractions were stored at −20 °C until further use.
2.5. Chemical Characterization by Thin Layer Chromatography (TLC)
TLC analysis was applied on crude extracts, as described previously [26,27]. Briefly, 4 µL of crude extracts were manually spotted on a silica gel plate 60 (Merck, Darmstadt, Germany) and eluted using chloroform/methanol/water (60/30/4; v/v/v) as a mobile phase. Primulin, ninhydrin, and Molisch reagents were used for lipid, amino-acid, and carbohydrate revelation, respectively. Carbohydrate and amino-acid revelations were performed after placing TLC plates for 5 min at 100 °C in an oven. Primulin revelation was performed under UV light at 366 nm. The retention factors were then measured and compared.
2.6. Statistical Analysis
Statistical analyses were performed using Rcommander [28]. After verifying the normal distribution (Shapiro−Wilk test) of the data and the homogeneity of variances (Bartlett test), a one-way analysis of variance was used to detect significant differences among means. A Tukey’s honestly significant difference (HSD, α = 0.05) test was then applied to compare the mean values in the different tested media for each single strain and between the strains for each medium.
3. Results
3.1. Qualitative Screening of Surface Active Compound Production
As shown in Table 3, 10 out of the 24 tested strains, all belonging to the Mucor genus, were positive to at least one screening test (i.e., MC1, MC3, ML2, ML3, MS1, MM1, MM2, MM3, MR3, and MP1), while 5 of those (i.e., MC1, ML2, MM3, MR3, and MP1) were positive to both OST and PMT, indicative of a potent surface-active compound production. With the exception of M. brunneogriseus, each tested Mucor species harbored at least one strain showing surface active supernatants. However, within the same species, surface-active compound production was not detected in all tested strains in both tests, indicating intraspecific variability. It is also worth mentioning that more tested strains were positive to PMT than OST, highlighting the interest in applying two different tests for surface-active compound production screening.

Table 3.
Qualitative screening of surface-active compound production in 24 Mucor, Lichtheimia and Absidia spp. based on oil spreading and Parafilm M test results. Strains selected for further experimentations are shown in bold.
Five strains (MC1, ML2, MS1, MM3, and MP1) showing repulsion in OST and three additional strains (MS1, MS3, and MM3) showing a surfactant with a high hydrophobicity in PMT, especially MS1 and MS3, were selected for further experimentation.
3.2. Impact of Medium Composition
In the second part of this study, PMT and OST quantitative results were used as proxies to evaluate and compare surfactant production for eight selected strains grown in eight different media (Figure 1, and Supplementary Tables S1 and S2). Noteworthy, all strains grew well in the tested media, yielding dry biomass ranging between 4.5 and 19.2 g/L (mean biomass = 10.2 g/L), depending on the studied strain and tested media (data not shown). As shown in Figure 1, the medium used in which yeast extract concentration, glucose concentration, oil nature, and canola oil concentration were set at different levels, strongly affected PMT (Figure 1A) and OST (Figure 1B) values, independently of the studied strain. Indeed, despite a thorough evaluation of the effect of each individual factor was not performed in the present study, several observations could be made based on the obtained results. Concerning the effect of the yeast extract concentration, decreasing its concentration from 5 g/L (BSF1) to 2.5 g/L (BSF2), while keeping the other medium components constant, significantly reduced the PMT and OST values for most of the tested strains, with the exception of MR3, as well as ML2 and MR3 strains, for which a significant increase was observed for the PMT and OST values, respectively. Moreover, substitution of canola oil (BSF4) by groundnut oil (BSF7) or linseed oil (BSF8) significantly reduced the OST values of most strain CFS (i.e., MM1, MM3, ML2, MC3, MC1, and MS1), while the PMT values were significantly decreased when groundnut oil was used (i.e., MM1, MP1, MS1, ML2, MC1, and MC3). In addition, a slight increase in PMT values was observed in the presence of linseed oil. Concerning the effect of canola oil concentration, an increase from 10 (BSF3) to 15 (BSF4) and 20 (BSF5) g/L resulted in increased PMT values at 20 g/L, but not at 15 g/L. In contrast, at the latter two concentrations, OST values were significantly more increased for most of the tested strains in the media, with increased canola oil concentrations set to 15 or 20 g/L as compared to 10 g/L, while there were little differences between the media containing 15 or 20 g/L. Finally, combining high glucose and canola oil concentrations (BSF6) yielded significantly lower PMT and OST values for all tested strains, with the exception of the MP1 strain, compared to conditions with low glucose and high canola oil concentrations (BSF5).
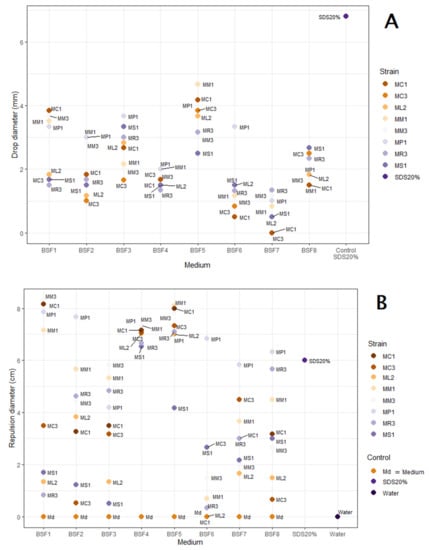
Figure 1.
PMT (A) and OST (B) values of eight Mucor spp. (MC, M. circinelloides; ML, M. lanceolatus; MM, M. mucedo; MP, M. plumbeus; MR, M. racemosus; MS, M. spinosus) grown in eight different media (see Table 2 for medium composition).
Altogether, these results showed that the BSF5 medium yielded significantly higher PMT and OST values for most of the studied strains (MM1 and MC1 showing the strongest activities). In addition, while PMT values of CFS obtained from BSF5 were significantly lower than SDS 20%, which was used as a positive control, the CFS OST values of all selected strains, with the exception of MS1 strain, were significantly higher than that of SDS 20%. Finally, extension of cultivation time from 96 h to 144 h did not have any significant effect on CFS OST and PMT values (data not shown). Based on these results, three strains, i.e., M. circinelloides MC1, M. mucedo MM1, and M. plumbeus MP1, were selected for further characterization of their surfactant activities and preliminary characterization of the surfactant molecules.
3.3. Surface-Tension Reducing and Emulsifying Activities
In the third part of the present study, surface-tension reducing and emulsifying activities of BSF5 CFS obtained from M. circinelloides MC1, M. mucedo MM1, and M. plumbeus MP1 were determined using ST measurements and the E24 test. The three Mucor CFS did not show mucedo MM1 and M. plumbeus MP1, and reduced the surface tension of BSF5 medium from 46.76 ± 0.01 mN/m to 32 ± 0.01 mN/m and 31 ± 0.01 mN/m, respectively while M. circinelloides MC1 reduced the surface tension to 36 mN/m (Figure 2) for any emulsifying activity (E24 < 6.6%), but reduced surface tension.
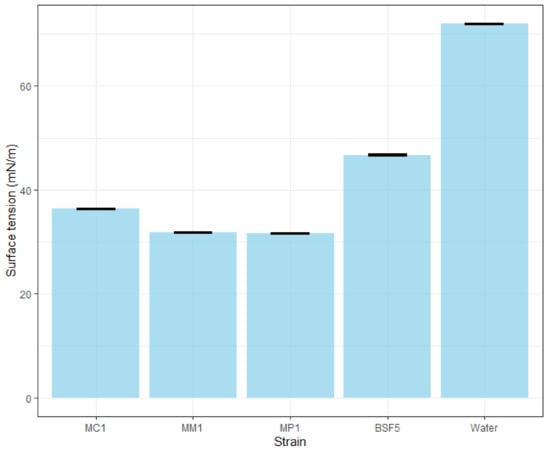
Figure 2.
Surface-tension reduction of BSF5 cell-free supernatants obtained from M. circinelloides MC1, M. mucedo MM1, and M. plumbeus MP1. The BSF5 medium and water were used as the negative controls.
3.4. Chemical Characterization by TLC
After extraction, surface-active compounds produced by M. circinelloides MC1, M. plumbeus MP1, and M. mucedo MM1 were analyzed by TLC, which is a simple, fast, and low-cost method allowing for their preliminary characterization. The combination of silica as the stationary phase and chloroform/methanol/water (60/30/4; v/v/v) as the mobile phase enabled compound separation following their polarity, with Rf increasing as the polarity of analytes decreased. Thus, the same spot, at an Rf value of 0.76, obtained from the three extracts MC1, MP1, and MM1 (Figure 3A,B), indicated the presence of compounds of a low polarity. The revelation with primulin (Figure 3A) proves that lipids were contained in this spot. Moreover, the (light) brown coloration observed with the Molisch reagent (Figure 3B) also suggested the presence of carbohydrates. All these results coincided with to the fact that the three extracts contained glycolipids. The ninhydrin revelation shows that amino-acids were present in all three extracts and the BSF5 medium at the same Rf values (Figure 3C). Thus, the extracts had no peptidic compounds less polar than those contained in the BSF5 medium. From these preliminary results, we conclude that extracts did not contain lipopeptides that were expected to be less polar than the component amino-acids of the BSF5 medium.
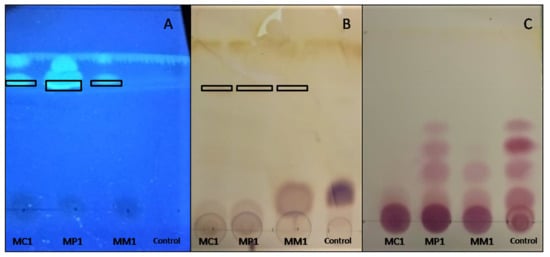
Figure 3.
TLC of crude extracts of M. circinelloides MC1, M. plumbeus MP1, and M. mucedo MM1 and BSF5 medium (control), revealed with Primulin at 366 nm (A), Molisch (B) and Ninhydrin (C) reagents.
4. Discussion
In the present study, 24 strains belonging to seven Mucor, three Lichtheimia, and one Absidia species were screened using OST and PMT for their potential to produce surface-active compounds. Among the three tested Mucor spp., one to two strains of each species, with the exception of M. brunneogriseus, were positive to at least one test, highlighting that surface-active compound production is widespread in Mucor spp. This is not surprising from an ecological point of view, as Mucor spp. produce lipases in significant quantities [29] and surfactant production can increase lipid bioavailability. Noteworthy, lipase activities can also lead to the production of mono- and di-glycerides that also possess surfactant activities and may participate in the observed effect of CFS during phenotypic tests. In contrast to Mucor spp., none of the four tested strains of Lichtheimia and Absidia spp. were positive to OST and PMT in the studied conditions. Nevertheless, further work on a higher number of strains would be necessary to assess whether surfactant production exists in members of this genus, as surfactant production is also strain-dependent.
After this qualitative screening, PMT and OST quantitative results were used as proxies to evaluate and compare the surface-active compound production for eight selected strains grown in eight different media. To the best of our knowledge, this is the first time that these two simple and rapid tests have been used to assess the medium effect on the surfactant activity of CFS of different strains. However, it is worth mentioning that this approach only gives an indirect phenotypic evaluation of surface-active compound production in contrast to other methods such as surface tension measurement or direct quantification of surfactant molecules.
This preliminary approach led to the selection of a BSF5 medium containing glucose (5 g/L) and canola oil (20 g/L) in a 1:4 ratio, as well as 5 g/L yeast extract. Both these factors, i.e., oil type (and its associated fatty acid profile), as well as the ratio between primary carbon source (e.g., glucose) and hydrophobic inducer (e.g., vegetable oil), can modulate biosurfactant yield at the species and intra-species level, as recently reviewed by de Oliveira Schmidt et al. (2021) [30]. Other factors including hydrophilic inducers (e.g., metals) are also known to impact surfactant production. Therefore, it would be of interest to further optimize the medium composition using an experimental design in order to maximize surfactant production using cheap renewable substrates (e.g., glycerol) and to assess the effect of each factor.
Further characterization of CFS from three selected Mucor spp. showed that they harbored a poor emulsifying activity, but considerably reduced surface-tension, suggesting that the produced surface-active compounds could be classified as a biosurfactant per se. Noteworthy, in the tested conditions, M. mucedo MM1 and M. plumbeus MP1 were the most effective at reducing CFS surface-tension, with similar surface-tension values as that reported for M. hiemalis UCP 0039 (i.e., 32 mN/m) [20]. In contrast, these values were higher than that reported for M. circinelloides M-06 (i.e., 26 mN/m), while the surface-tension of M. indicus (no strain number) and M. circinelloides UCP 001 CFS were not reported in the studies of Oje et al. (2016) and Marques et al. (2020), respectively [21,24]. Furthermore, in the present study, CFS from selected Mucor spp. did not show any emulsifying activity in contrast to Mucor spp. selected in the aforementioned studies, which harbored a high emulsifying activity with emulsifying index values after 24 h higher than 50% and up to 100%. This result may indicate that surface-active compounds produced by the three Mucor spp. selected in the present study are different from those reported in other studies.
A preliminary characterization of the surfactant molecules produced by these strains was performed and showed that these compounds belonged to the glycolipid family. Similar conclusions were drawn concerning M. hiemalis [20] and M. indicus [21] surfactants, while a surfactant from the lipopeptide class was reported for M. circinelloides [24]. Further work should involve purification of the produced compounds for further characterization using more powerful techniques such as LC-MS, GC-MS, and/or NMR.
5. Conclusions
In the present study, three strains of M. circinelloides, M. plumbeus, and M. mucedo were shown to produce biosurfactants yielding reduced-surface tension of the cultivation medium, but no emulsifying activity. Preliminary chemical analyses tend to classify these molecules in the glycolipid class, but additional analyses must be conducted to accurately characterize them. Further work could also involve their toxicity assessment and production at a higher scale, as well as exploring their application and efficiency in the depollution field, for example, to replace synthetic surfactants currently used in petroleum dispersants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/applmicrobiol2010018/s1, Table S1: PMT mean values obtained for 8 Mucor spp. grown in 8 different media; Table S2: OST mean values obtained for 8 Mucor spp. grown in 8 different media.
Author Contributions
Conceptualization, M.-E.L., S.L.F. and J.M. funding acquisition, M.-E.L. and S.L.F. formal analysis, M.C., J.M. and M.-E.L. investigation, M.C., R.M. and C.P. supervision, M.-E.L., J.M., S.L.F., B.C., D.D.S. and R.N. visualization, M.C. writing—original draft preparation, M.C. and J.M.; writing—review and editing, S.L.F., M.-E.L., J.M., B.C., D.D.S. and R.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Brest Métropole and the Cedre (PhD studentship for M.C.). The authors also acknowledge the financial support of the EGAAL doctoral school and Université de Bretagne Occidentale.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank the Université de Bretagne Occidentale Culture Collection (UBOCC, Plouzané, France, www.univ-brest.fr/ubocc, 25 January 2022) facility and team for providing the strains used in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Naughton, P.; Marchant, R.; Naughton, V.; Banat, I. Microbial biosurfactants: Current trends and applications in agricultural and biomedical industries. J. Appl. Microbiol. 2019, 127, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Twigg, M.S.; Baccile, N.; Banat, I.M.; Déziel, E.; Marchant, R.; Roelants, S.; Van Bogaert, I.N.A. Microbial biosurfactant research: Time to improve the rigour in the reporting of synthesis, functional characterization and process development. Microb. Biotechnol. 2020, 14, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Sanches, M.A.; Luzeiro, I.G.; Cortez, A.C.A.; de Souza, S.; Albuquerque, P.M.; Chopra, H.K.; de Souza, J.V.B. Production of Biosurfactants by Ascomycetes. Int. J. Microbiol. 2021, 2021, 6669263. [Google Scholar] [CrossRef] [PubMed]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Fayaz, F.; Alara, O.R. Biosurfactants—A new frontier for social and environmental safety: A mini review. Biotechnol. Res. Innov. 2018, 2, 81–90. [Google Scholar] [CrossRef]
- Dell’Anno, F.; Sansone, C.; Ianora, A. Biosurfactant-induced remediation of contaminated marine sediments: Current knowledge and future perspectives. Mar. Environ. Res. 2018, 137, 196–205. [Google Scholar] [CrossRef]
- Jahan, R.; Bodratti, A.M.; Tsianou, M.; Alexandridis, P. Biosurfactants, natural alternatives to synthetic surfactants: Physicochemical properties and applications. Adv. Colloid Interface Sci. 2019, 275, 102061. [Google Scholar] [CrossRef]
- Moutinho, L.F.; Moura, F.R.; Silvestre, R.C.; Romão-Dumaresq, A.S. Microbial biosurfactants: A broad analysis of properties, applications, biosynthesis, and techno-economical assessment of rhamnolipid production. Biotechnol. Prog. 2021, 37, e3093. [Google Scholar] [CrossRef]
- Perfumo, A.; Smyth, T.J.P.; Marchant, R.; Banat, I.M. Production and Roles of biosurfactants and bioemulsifiers in accessing hydrophobic substrates. In Handbook of Hydrocarbon and Lipid Microbiology; Springer: Cham, Switzerland, 2010; pp. 1501–1512. [Google Scholar] [CrossRef]
- Banat, I.; Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial biosurfactant production, applications and future potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. [Google Scholar] [CrossRef]
- Drakontis, C.E.; Amin, S. Biosurfactants: Formulations, Properties, and Applications. Curr. Opin. Colloid Interface Sci. 2020, 48, 77–90. [Google Scholar] [CrossRef]
- Kiran, S.; Hema, T.A.; Gandhimathi, R.; Selvin, J.; Thomas, T.A.; Ravji, T.R.; Natarajaseenivasan, K. Optimization and production of a biosurfactant from the sponge-associated marine fungus Aspergillus ustus MSF3. Colloids Surf. B Biointerfaces 2009, 73, 250–256. [Google Scholar] [CrossRef]
- Bhardwaj, G.; Cameotra, S.S.; Chopra, H.K. Biosurfactants from Fungi: A Review. J. Pet. Environ. Biotechnol. 2013, 4, 1–6. [Google Scholar] [CrossRef]
- Luft, L.; Confortin, T.C.; Todero, I.; Zabot, G.L.; Mazutti, M.A. An overview of fungal biopolymers: Bioemulsifiers and biosurfactants compounds production. Crit. Rev. Biotechnol. 2020, 40, 1059–1080. [Google Scholar] [CrossRef]
- Walther, G.; Pawłowska, J.; Alastruey-Izquierdo, A.; Wrzosek, M.; Rodriguez-Tudela, J.; Dolatabadi, S.; Chakrabarti, A.; de Hoog, G. DNA barcoding in Mucorales: An inventory of biodiversity. Pers.—Mol. Phylogeny Evol. Fungi 2013, 30, 11–47. [Google Scholar] [CrossRef]
- Wagner, L.; Stielow, J.; De Hoog, G.; Bensch, K.; Schwartze, V.U.; Voigt, K.; Alastruey-Izquierdo, A.; Kurzai, O.; Walther, G. A new species concept for the clinically relevant Mucor circinelloides complex. Pers.—Mol. Phylogeny Evol. Fungi 2020, 44, 67–97. [Google Scholar] [CrossRef]
- Lee Taylor, D.; Sinsabaugh, R.L. Chapter 4—The soil fungi: Occurrence, phylogeny, and ecology. In Soil Microbiology, Ecology and Biochemistry, 4th ed.; Paul, E.A., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 77–109. [Google Scholar]
- Morin-Sardin, S.; Nodet, P.; Coton, E.; Jany, J.-L. Mucor: A Janus-faced fungal genus with human health impact and industrial applications. Fungal Biol. Rev. 2017, 31, 12–32. [Google Scholar] [CrossRef]
- Kosa, G.; Zimmermann, B.; Kohler, A.; Ekeberg, D.; Afseth, N.K.; Mounier, J.; Shapaval, V. High-throughput screening of Mucoromycota fungi for production of low- and high-value lipids. Biotechnol. Biofuels 2018, 11, 66. [Google Scholar] [CrossRef]
- Hasani Zadeh, P.; Moghimi, H.; Hamedi, J. Biosurfactant production by Mucor circinelloides: Environmental applications and surface-active properties. Eng. Life Sci. 2018, 18, 317–325. [Google Scholar] [CrossRef]
- Ferreira, I.N.S.; Rodríguez, D.M.; Campos-Takaki, G.M.; Andrade, R.F.D.S. Biosurfactant and bioemulsifier as promising molecules produced by Mucor hiemalis isolated from Caatinga soil. Electron. J. Biotechnol. 2020, 47, 51–58. [Google Scholar] [CrossRef]
- Oje, O.A.; Okpashi, V.E.; Uzor, J.C.; Uma, U.O.; Irogbolu, A.O.; Onwurah, I.N. Effect of Acid and Alkaline Pretreatment on the Production of Biosurfactant from Rice Husk Using Mucor indicus. Res. J. Environ. Toxicol. 2016, 10, 60–67. [Google Scholar] [CrossRef][Green Version]
- Morikawa, M.; Hirata, Y.; Imanaka, T. A study on the structure–function relationship of lipopeptide biosurfactants. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2000, 1488, 211–218. [Google Scholar] [CrossRef]
- Yalçın, H.T.; Ergin-Tepebaşı, G.; Uyar, E. Isolation and molecular characterization of biosurfactant producing yeasts from the soil samples contaminated with petroleum derivatives. J. Basic Microbiol. 2018, 58, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Marques, N.S.A.A.; Da Silva, I.G.S.; Cavalcanti, D.L.; Maia, P.C.S.V.; Santos, V.P.; Andrade, R.F.S.; Campos-Takaki, G.M. Eco-Friendly Bioemulsifier Production by Mucor circinelloides UCP0001 Isolated from Mangrove Sediments Using Renewable Substrates for Environmental Applications. Biomolecules 2020, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Arshad, M.; Asghar, H.N.; Sheikh, M.A.; Crowley, D.E.; Ali, S.; Malik, M.A.; Ansar, M.; Qureshi, R. Isolation, Screening and Functional Characterization of Biosurfactant Producing Bacteria Isolated from Crude Oil Contaminated Site. Int. J. Agric. Biol. 2016, 18, 542–548. [Google Scholar] [CrossRef]
- Schulz, D.; Passeri, A.; Schmidt, M.; Lang, S.; Wagner, F.; Wray, V.; Gunkel, W. Marine Biosurfactants, I. Screening for Biosurfactants among Crude Oil Degrading Marine Microorganisms from the North Sea. Z. Naturforsch. C J. Biosci. 1991, 46, 197–203. [Google Scholar] [CrossRef]
- Satpute, S.K.; Banpurkar, A.G.; Dhakephalkar, P.K.; Banat, I.M.; Chopade, B.A. Methods for investigating biosurfactants and bioemulsifiers: A review. Crit. Rev. Biotechnol. 2010, 30, 127–144. [Google Scholar] [CrossRef]
- Fox, J. The R Commander: A Basic Statistics Graphical User Interface to R. J. Stat. Softw. 2005, 14, 1–42. [Google Scholar] [CrossRef]
- Alves, M.; Campos-Takaki, G.M.; Porto3, A.L.F.; Milanez, A.I. Screening of Mucor spp. for the production of amylase, lipase, polygalacturonase and protease. Braz. J. Microbiol. 2002, 33, 325–330. [Google Scholar] [CrossRef]
- De Oliveira Schmidt, V.K.; Carvalho, J.D.S.; De Oliveira, D.; De Andrade, C.J. Biosurfactant inducers for enhanced production of surfactin and rhamnolipids: An overview. World J. Microbiol. Biotechnol. 2021, 37, 21. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).